Effect of Extraction Methods on Aroma Profile, Antioxidant Activity and Sensory Acceptability of Specialty Coffee Brews
Abstract
1. Introduction
2. Materials and Methods
2.1. Coffee Beans
2.2. Chemicals and Reagents
2.3. Roasting and Grinding of Coffee Beans
2.4. Particle Size Determination by Sieve Analysis
2.5. Coffee Brews Preparation
2.6. DPPH Radical Scavenging Activity Measurement
2.7. Total Polyphenols Content (TPC) Assay by Folin–Ciocalteu Method
2.8. Caffeine and Chlorogenic Acid Contents by HPLC Analysis
2.9. Aroma Profile of Coffee Brews by HS-SPME/GC-MS
2.10. Sensory Analysis of Coffee Brews
2.11. Statistical Analysis
3. Results and Discussion
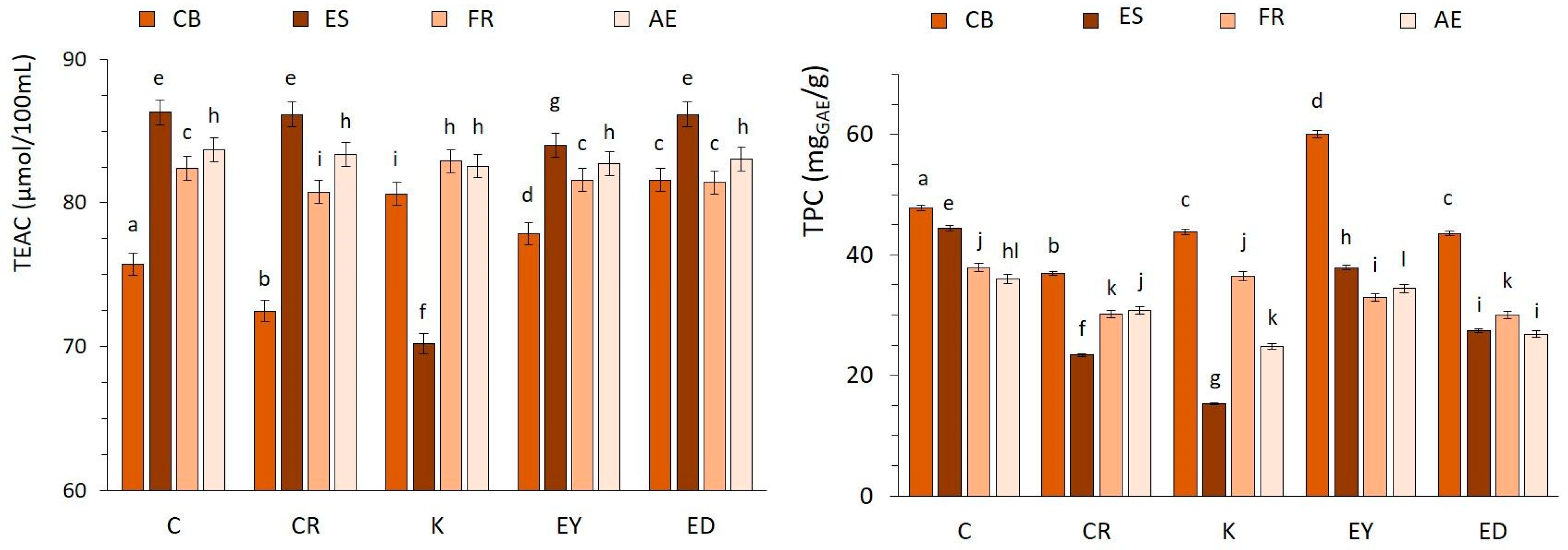
3.1. Antioxidant Activity of Coffee Brews
3.2. Total Polyphenols Content (TPC) Assay by Folin–Ciocalteu Method
3.3. Caffeine and Chlorogenic Acid Contents
3.4. Aroma Profile of Coffee Brews by HS-SPME/GC-MS
3.5. Sensory Evaluation of Coffee Brews
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parenti, A.; Guerrini, L.; Masella, P.; Spinelli, S.; Calamai, L.; Spugnoli, P. Comparison of Espresso Coffee Brewing Techniques. J. Food Eng. 2014, 121, 112–117. [Google Scholar] [CrossRef]
- Zhai, X.; Yang, M.; Zhang, J.; Zhang, L.; Tian, Y.; Li, C.Y.; Bao, L.; Ma, C.; Abd El-Aty, A.M. Feasibility of Ultrasound-Assisted Extraction for Accelerated Cold Brew Coffee Processing: Characterization and Comparison with Conventional Brewing Methods. Front. Nutr. 2022, 9, 849811. [Google Scholar] [CrossRef]
- Claassen, L.; Rinderknecht, M.; Porth, T.; Röhnisch, J.; Seren, H.Y.; Scharinger, A.; Gottstein, V.; Noack, D.; Schwarz, S.; Winkler, G.; et al. Cold Brew Coffee—Pilot Studies on Definition, Extraction, Consumer Preference, Chemical Characterization and Microbiological Hazards. Foods 2021, 10, 865. [Google Scholar] [CrossRef] [PubMed]
- Kwok, R.; Lee Wee Ting, K.; Schwarz, S.; Claassen, L.; Lachenmeier, D.W. Current Challenges of Cold Brew Coffee—Roasting, Extraction, Flavor Profile, Contamination, and Food Safety. Challenges 2020, 11, 26. [Google Scholar] [CrossRef]
- Rao, N.Z.; Fuller, M.; Grim, M.D. Physiochemical Characteristics of Hot and Cold Brew Coffee Chemistry: The Effects of Roast Level and Brewing Temperature on Compound Extraction. Foods 2020, 9, 902. [Google Scholar] [CrossRef]
- Nosal, B.M.; Sakaki, J.R.; Kim, D.; Chun, O.K. Impact of Coffee Preparation on Total Phenolic Content in Brewed Coffee Extracts and their Contribution to the Body’s Antioxidant Status. Food Sci. Biotechnol. 2022, 31, 1081–1088. [Google Scholar] [CrossRef]
- Janda, K.; Jakubczyk, K.; Baranowska-Bosiacka, I.; Kapczuk, P.; Kochman, J.; Rębacz-Maron, E.; Gutowska, I. Mineral Composition and Antioxidant Potential of Coffee Beverages Depending on the Brewing Method. Foods 2020, 9, 121. [Google Scholar] [CrossRef]
- Gloess, A.N.; Schönbächler, B.; Klopprogge, B.; D’Ambrosio, L.; Chatelain, K.; Bongartz, A.; Strittmatter, A.; Rast, M.; Yeretzian, C. Comparison of Nine Common Coffee Extraction Methods: Instrumental and Sensory Analysis. Eur. Food Res. Technol. 2013, 236, 607–627. [Google Scholar] [CrossRef]
- Klikarová, J.; Řeháková, B.; Česlová, L. Evaluation of Regular and Decaffeinated (Un)Roasted Coffee Beans using HPLC and Multivariate Statistical Methods. J. Food Compos. Anal. 2022, 114, 104841. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, J.; Hong, Q.; Dong, W.; Chen, X.; Wu, G.; Zhang, Z. Identification of Changes in the Volatile Compounds of Robusta Coffee Beans during Drying Based on HS-SPME/GC-MS and E-Nose Analyses with the Aid of Chemometrics. LWT 2022, 161, 113317. [Google Scholar] [CrossRef]
- Lopes, G.R.; Petronilho, S.; Ferreira, A.S.; Pinto, M.; Passos, C.P.; Coelho, E.; Rodrigues, C.; Figueira, C.; Rocha, S.M.; Coimbra, M.A. Insights on Single-Dose Espresso Coffee Capsules’ Volatile Profile: From Ground Powder Volatiles to Prediction of Espresso Brew Aroma Properties. Foods 2021, 10, 2508. [Google Scholar] [CrossRef] [PubMed]
- Gancarz, M.; Dobrzański, B., Jr.; Oniszczuk, T.; Combrzyński, M.; Ćwikła, D.; Rusinek, R. Detection and Differentiation of Volatile Compound Profiles in Roasted Coffee Arabica Beans from Different Countries using an Electronic Nose and GC-MS. Sensors 2020, 20, 2124. [Google Scholar] [CrossRef]
- Rahman, N.A.A.; Muharram, S.H.; Abiola, O. Antibacterial Activity of NESCAFÉ Instant Coffee Beverages and Pharyngitis-Causing Streptococcus Species. Brunei Darussalam J. Health 2014, 5, 70–79. [Google Scholar]
- Ruiz-Diaz, C.P.; Verle Rodrigues, J.C.; Miro-Rivera, E.; Diaz-Vazquez, L.M. Impact of the Coffee Berry Borer on the Volatile and Semi-Volatile Compounds; Qualitative Profile of Coffea Arabica Berries. Food Chem. Adv. 2023, 2, 100154. [Google Scholar] [CrossRef]
- Kussmann, M.; Abe Cunha, D.H.; Berciano, S. Bioactive Compounds for Human and Planetary Health. Front. Nutr. 2023, 10, 1193848. [Google Scholar] [CrossRef] [PubMed]
- Bastian, F.; Hutabarat, O.S.; Dirpan, A.; Nainu, F.; Harapan, H.; Emran, T.B.; Simal-Gandara, J. From Plantation to Cup: Changes in Bioactive Compounds during Coffee Processing. Foods 2021, 10, 2827. [Google Scholar] [CrossRef]
- Kučera, L.; Papoušek, R.; Kurka, O.; Barták, P.; Bednář, P. Study of Composition of Espresso Coffee Prepared from various Roast Degrees of Coffea arabica L. Coffee Beans. Food Chem. 2016, 199, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Gancarz, M.; Dobrzański, B.; Malaga-Toboła, U.; Tabor, S.; Combrzyński, M.; Ćwikła, D.; Strobel, W.R.; Oniszczuk, A.; Karami, H.; Darvishi, Y.; et al. Impact of Coffee Bean Roasting on the Content of Pyridines Determined by Analysis of Volatile Organic Compounds. Molecules 2022, 27, 1559. [Google Scholar] [CrossRef] [PubMed]
- Freitas, V.V.; Rodrigues Borges, L.L.; Dias Castro, G.A.; Henrique dos Santos, M.; Teixeira Ribeiro Vidigal, M.C.; Fernandes, S.A.; Stringheta, P.C. Impact of Different Roasting Conditions on the Chemical Composition, Antioxidant Activities, and Color of Coffea canephora and Coffea arabica L. Samples. Heliyon 2023, 9, e19580. [Google Scholar] [CrossRef]
- Wachter, I.; Rantuch, P.; Drienovský, M.; Martinka, J.; Ház, A.; Štefko, T. Determining the Activation Energy of Spent Coffee Grounds by the Thermogravimetric Analysis. J. Chem. Technol. Metall. 2022, 57, 1006–1018. [Google Scholar]
- Esquivel, P.; Jiménez, V.M. Functional Properties of Coffee and Coffee by-Products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Lee, W.T.; Smith, A.; Arshad, A. Uneven Extraction in Coffee Brewing. Phys. Fluids 2023, 35, 054110. [Google Scholar] [CrossRef]
- Bhumiratana, N.; Adhikari, K.; Chambers, E. Evolution of Sensory Aroma Attributes from Coffee Beans to Brewed Coffee. LWT 2011, 44, 2185–2192. [Google Scholar] [CrossRef]
- Khamitova, G.; Angeloni, S.; Fioretti, L.; Ricciutelli, M.; Sagratini, G.; Torregiani, E.; Vittori, S.; Caprioli, G. The Impact of Different Filter Baskets, Heights of Perforated Disc and Amount of Ground Coffee on the Extraction of Organics Acids and the Main Bioactive Compounds in Espresso Coffee. Food Res. Int. 2020, 133, 109220. [Google Scholar] [CrossRef] [PubMed]
- Council, E.U. Council Directive 98/83 about Water Quality Intended for Human Consumption. OJEC L 1998, 330, 32–54. [Google Scholar]
- Lapčík, L.; Lapčíkova, B.; Gautam, S.; Vašina, M.; Valenta, T.; Řepka, D.; Čépe, K.; Rudolf, O. Acoustic and Mechanical Testing of Commercial Cocoa Powders. Int. J. Food Prop. 2022, 25, 2184–2197. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Latos, M.; Zaborski, M.; Podsedek, A. Antioxidant and Antiradical Properties of Green Tea Extract Compounds. Int. J. Electrochem. Sci. 2017, 12, 6600–6610. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Somporn, C.; Kamtuo, A.; Theerakulpisut, P.; Siriamornpun, S. Effects of Roasting Degree on Radical Scavenging Activity, Phenolics and Volatile Compounds of Arabica Coffee Beans (Coffea Arabica L. Cv. Catimor). Int. J. Food Sci. Tech. 2011, 46, 2287–2296. [Google Scholar] [CrossRef]
- Febrina, L.; Happyana, N.; Syah, Y.M. Metabolite Profiles and Antidiabetic Activity of the Green Beans of Luwak (Civet) Coffees. Food Chem. 2021, 355, 129496. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cheng, C.; Liang, J. Effect of Esterification Condensation on the Folin–Ciocalteu Method for the Quantitative Measurement of Total Phenols. Food Chem. 2015, 170, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Domínguez-Rodríguez, G.; Castro-Puyana, M.; Marina, M.L. Polyphenols analysis and related challenges. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 177–232. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E.; Karpowicz, D.; Leśniewska, B. Antioxidant Properties of Coffee Substitutes Rich in Polyphenols and Minerals. Food Chem. 2019, 278, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Do Coffee Polyphenols have a Preventive Action on Metabolic Syndrome Associated Endothelial Dysfunctions? An Assessment of the Current Evidence. Antioxidants 2018, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Opustilová, K.; Lapčíková, B.; Lapčík, L.; Gautam, S.; Valenta, T.; Li, P. Physico-Chemical Study of Curcumin and its Application in O/W/O Multiple Emulsion. Foods 2023, 12, 1394. [Google Scholar] [CrossRef]
- SCA Protocols. Cupping Specialty Coffee; 16DEC2015; Specialty Coffee Association: Santa Ana, CA, USA, 2015. [Google Scholar]
- Chapko, M.J.; Seo, H. Characterizing Product Temperature-Dependent Sensory Perception of Brewed Coffee Beverages: Descriptive Sensory Analysis. Food Res. Int. 2019, 121, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Di Donfrancesco, B.; Gutierrez Guzman, N.; Chambers IV, E. Comparison of Results from Cupping and Descriptive Sensory Analysis of Colombian Brewed Coffee. J. Sens. Stud. 2014, 29, 301–311. [Google Scholar] [CrossRef]
- Kulapichitr, F.; Borompichaichartkul, C.; Fang, M.; Suppavorasatit, I.; Cadwallader, K.R. Effect of Post-Harvest Drying Process on Chlorogenic Acids, Antioxidant Activities and CIE-Lab Color of Thai Arabica Green Coffee Beans. Food Chem. 2022, 366, 130504. [Google Scholar] [CrossRef]
- Seow, L.; Shamlan, S.; Seow, E. Influence of Roasting Degrees on the Antioxidant and Anti-Angiogenic Effects of Coffea liberica. J. Food Meas. Charact. 2021, 15, 4030–4036. [Google Scholar] [CrossRef]
- Sacchetti, G.; Di Mattia, C.; Pittia, P.; Mastrocola, D. Effect of Roasting Degree, Equivalent Thermal Effect and Coffee Type on the Radical Scavenging Activity of Coffee Brews and their Phenolic Fraction. J. Food Eng. 2009, 90, 74–80. [Google Scholar] [CrossRef]
- Kameya, H. Evaluation of Hydroxyl Radical and Alkyl-Oxy Radical Scavenging Activity of Coffee by ESR Spin Trapping Method. J. Food Sci. Eng. 2017, 7, 305–311. [Google Scholar] [CrossRef][Green Version]
- Laukaleja, I.; Kruma, Z. Influence of the Roasting Process on Bioactive Compounds and Aroma Profile in Specialty Coffee: A Review. In Proceedings of the 13th Baltic Conference on Food Science and Technology and 5th North and East European Congress on Food, Jelgava, Latvia, 2–3 May 2019; pp. 2–3. [Google Scholar]
- Le-Thi, A.; Le, N.; Nguyen, C.; Nguyen, T. Variability of Total Polyphenol Contents in Ground Coffee Products and their Antioxidant Capacities through Different Reaction Mechanisms. Biointerface Res. Appl. Chem. 2022, 12, 4857–4870. [Google Scholar] [CrossRef]
- Fărcaş, A.; Socaci, S.; Bocăniciu, I.; Pop, A.; Tofana, M.; Muste, S.; Feier, D. Evaluation of Biofunctional Compounds Content from Brewed Coffee. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2014, 71, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Bekedam, E.K.; Schols, H.A.; Cämmerer, B.; Kroh, L.W.; van Boekel, M.A.; Smit, G. Electron Spin Resonance (ESR) Studies on the Formation of Roasting-Induced Antioxidative Structures in Coffee Brews at Different Degrees of Roast. J. Agric. Food Chem. 2008, 56, 4597–4604. [Google Scholar] [CrossRef] [PubMed]
- Derossi, A.; Ricci, I.; Caporizzi, R.; Fiore, A.; Severini, C. How Grinding Level and Brewing Method (Espresso, American, Turkish) could affect the Antioxidant Activity and Bioactive Compounds in a Coffee Cup. J. Sci. Food Agric. 2018, 98, 3198–3207. [Google Scholar] [CrossRef]
- Belitz, H.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009; p. 1114. [Google Scholar] [CrossRef]
- Bressanello, D.; Liberto, E.; Cordero, C.; Rubiolo, P.; Pellegrino, G.; Ruosi, M.R.; Bicchi, C. Coffee Aroma: Chemometric Comparison of the Chemical Information Provided by Three Different Samplings Combined with GC–MS to Describe the Sensory Properties in Cup. Food Chem. 2017, 214, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Hu, R.; Long, Y.; Li, H.; Zhang, Y.; Zhu, K.; Chu, Z. Comparative Evaluation of the Volatile Profiles and Taste Properties of Roasted Coffee Beans as Affected by Drying Method and Detected by Electronic Nose, Electronic Tongue, and HS-SPME-GC-MS. Food Chem. 2019, 272, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Mestdagh, F.; Davidek, T.; Chaumonteuil, M.; Folmer, B.; Blank, I. The Kinetics of Coffee Aroma Extraction. Food Res. Int. 2014, 63, 271–274. [Google Scholar] [CrossRef]
- Córdoba, N.; Moreno, F.L.; Osorio, C.; Velásquez, S.; Ruiz, Y. Chemical and Sensory Evaluation of Cold Brew Coffees using Different Roasting Profiles and Brewing Methods. Food Res. Int. 2021, 141, 110141. [Google Scholar] [CrossRef] [PubMed]
- De Vivo, A.; Genovese, A.; Tricarico, M.C.; Aprea, A.; Sacchi, R.; Sarghini, F. Volatile Compounds in Espresso Resulting from a Refined Selection of Particle Size of Coffee Powder. J. Food Compos. Anal. 2022, 114, 104779. [Google Scholar] [CrossRef]



| Coffee Beans Geographical Origin | |||||
|---|---|---|---|---|---|
| Columbia (C) | Costa Rica (CR) | Kenya (K) | Ethiopia Yirgacheffe (EY) | Ethiopia Daro Kebele (ED) | |
| Variety | Castillo | Catuaí | SL28 | Kurume | Kudhume, Welisho, Dego |
| Cultivation area | Quindio | Tarrazu | Nyeri | Yirgacheffe | Daro Kebele |
| Cultivation altitude (m.a.s.l.) | 1450 | 2200 | 1880–1970 | 2000–2050 | 1700–2200 |
| Processing type | Carbonic maceration | White honey processing | Wet processing (washing) | Wet processing (washing) | Dry (natural) processing |
| Flavour profile | Papaya, strawberry, orange peel | Peach, red apple, caramel | Black currant, hibiscus, orange | Chamomile, bergamot, Earl Grey tea | Violet, strawberry, vanilla |
| Producer | Finca Peurto Alegre | La Pastora, Carlos Montero | Thiriku Cooperative | Tessema Edima | Abado |
| Grinding Degree | Roasting Degree of Coffee Beans | |||||
|---|---|---|---|---|---|---|
| Coffee Brew Type (Abbrev.) | (μm) | C | CR | K | EY | ED |
| Cold brew (CB) | 215 | Omni-roast (light) | Medium | Medium | Medium | Medium |
| Espresso (ES) | 185 | Omni-roast (light) | Medium dark | Medium light | Medium dark | Medium dark |
| French press (FR) | 204 | Omni-roast (light) | Medium | Medium | Medium | Medium |
| Aeropress (AE) | 208 | Omni-roast (light) | Medium | Medium | Medium | Medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapčíková, B.; Lapčík, L.; Barták, P.; Valenta, T.; Dokládalová, K. Effect of Extraction Methods on Aroma Profile, Antioxidant Activity and Sensory Acceptability of Specialty Coffee Brews. Foods 2023, 12, 4125. https://doi.org/10.3390/foods12224125
Lapčíková B, Lapčík L, Barták P, Valenta T, Dokládalová K. Effect of Extraction Methods on Aroma Profile, Antioxidant Activity and Sensory Acceptability of Specialty Coffee Brews. Foods. 2023; 12(22):4125. https://doi.org/10.3390/foods12224125
Chicago/Turabian StyleLapčíková, Barbora, Lubomír Lapčík, Petr Barták, Tomáš Valenta, and Kateřina Dokládalová. 2023. "Effect of Extraction Methods on Aroma Profile, Antioxidant Activity and Sensory Acceptability of Specialty Coffee Brews" Foods 12, no. 22: 4125. https://doi.org/10.3390/foods12224125
APA StyleLapčíková, B., Lapčík, L., Barták, P., Valenta, T., & Dokládalová, K. (2023). Effect of Extraction Methods on Aroma Profile, Antioxidant Activity and Sensory Acceptability of Specialty Coffee Brews. Foods, 12(22), 4125. https://doi.org/10.3390/foods12224125