Effect of Enzymatic Biotransformation on the Hypotensive Potential of Red Grape Pomace Extract
Abstract
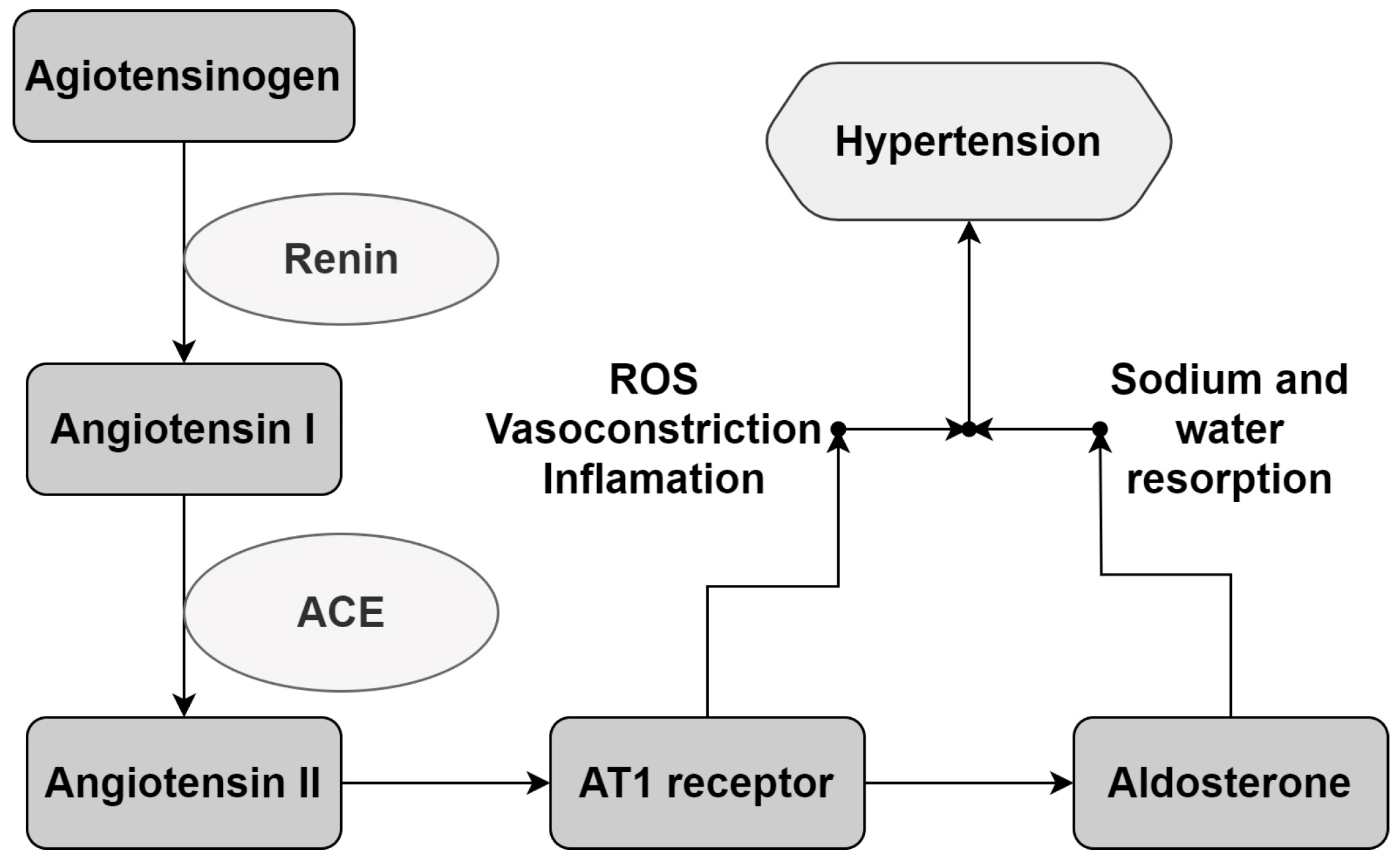
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples and Enzymes
2.3. Extracts Preparation
2.4. Total Phenolic Content
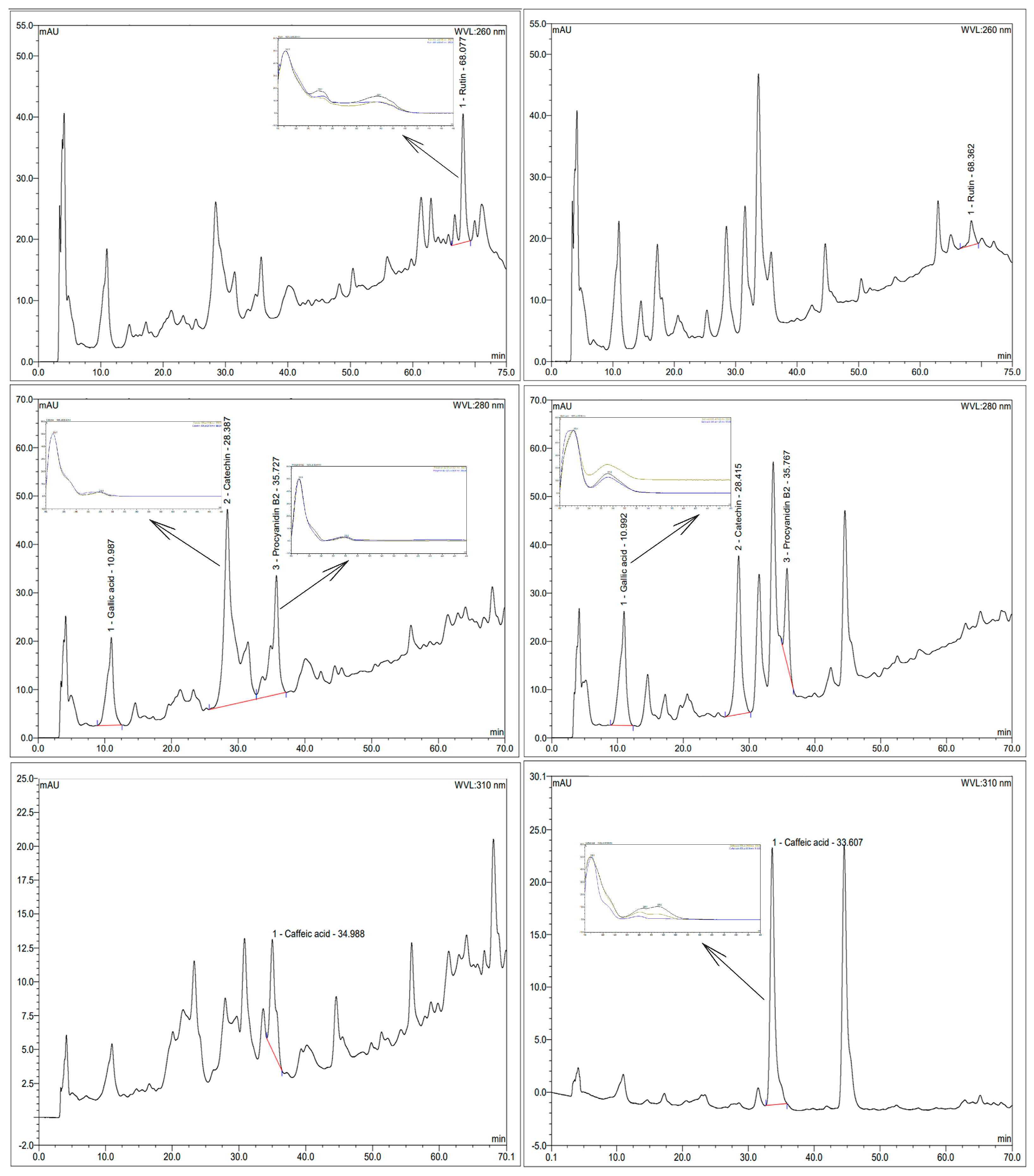
2.5. Phenolic Identification by HPLC Analysis
2.6. Antioxidant Capacity
2.7. In Vitro ACE Inhibitory Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Enzymatic Treatment on Extraction Yield and TPC
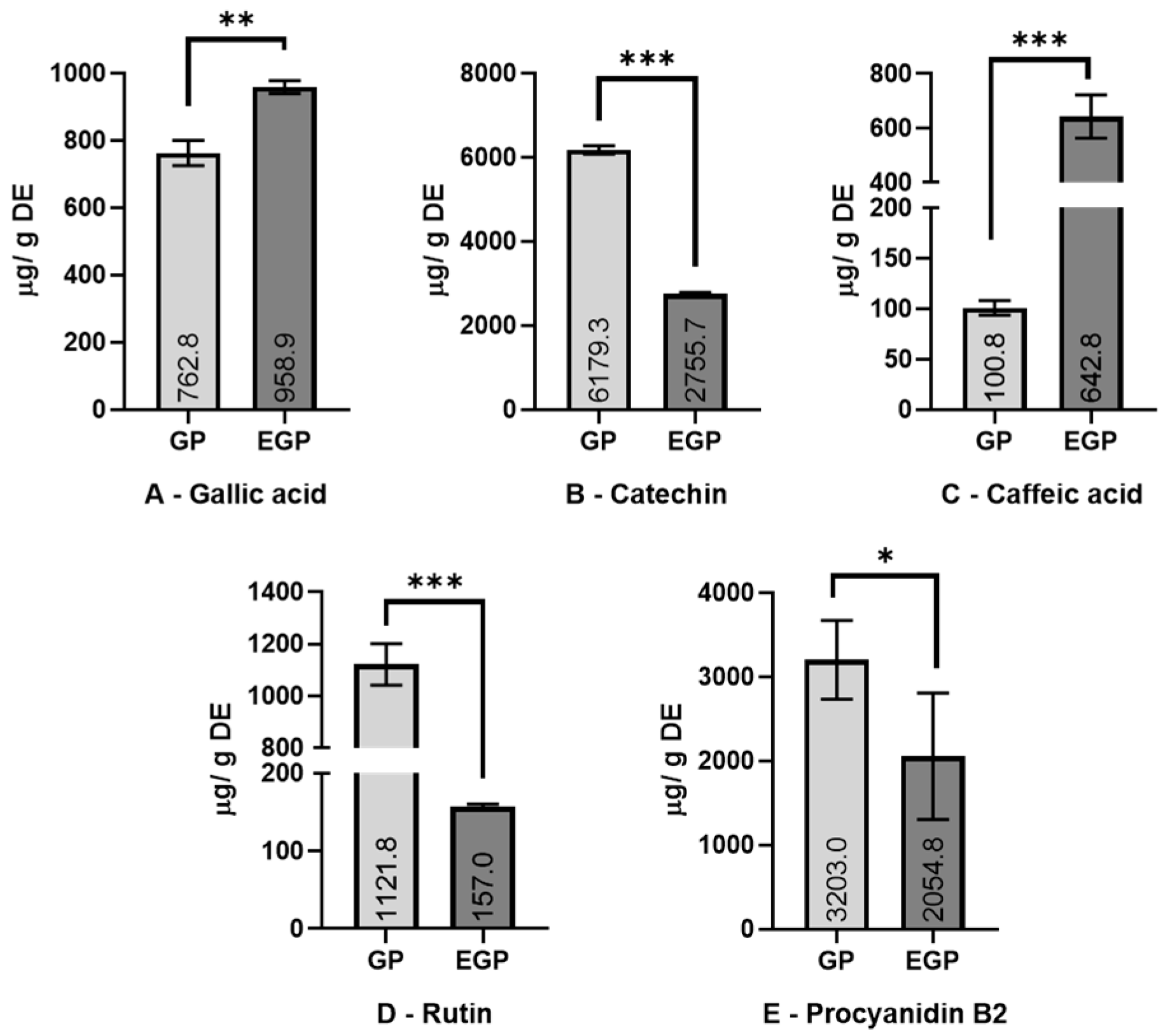
3.2. Phenolic Identification by HPLC Analysis
3.3. Antioxidant Capacity of Extracts
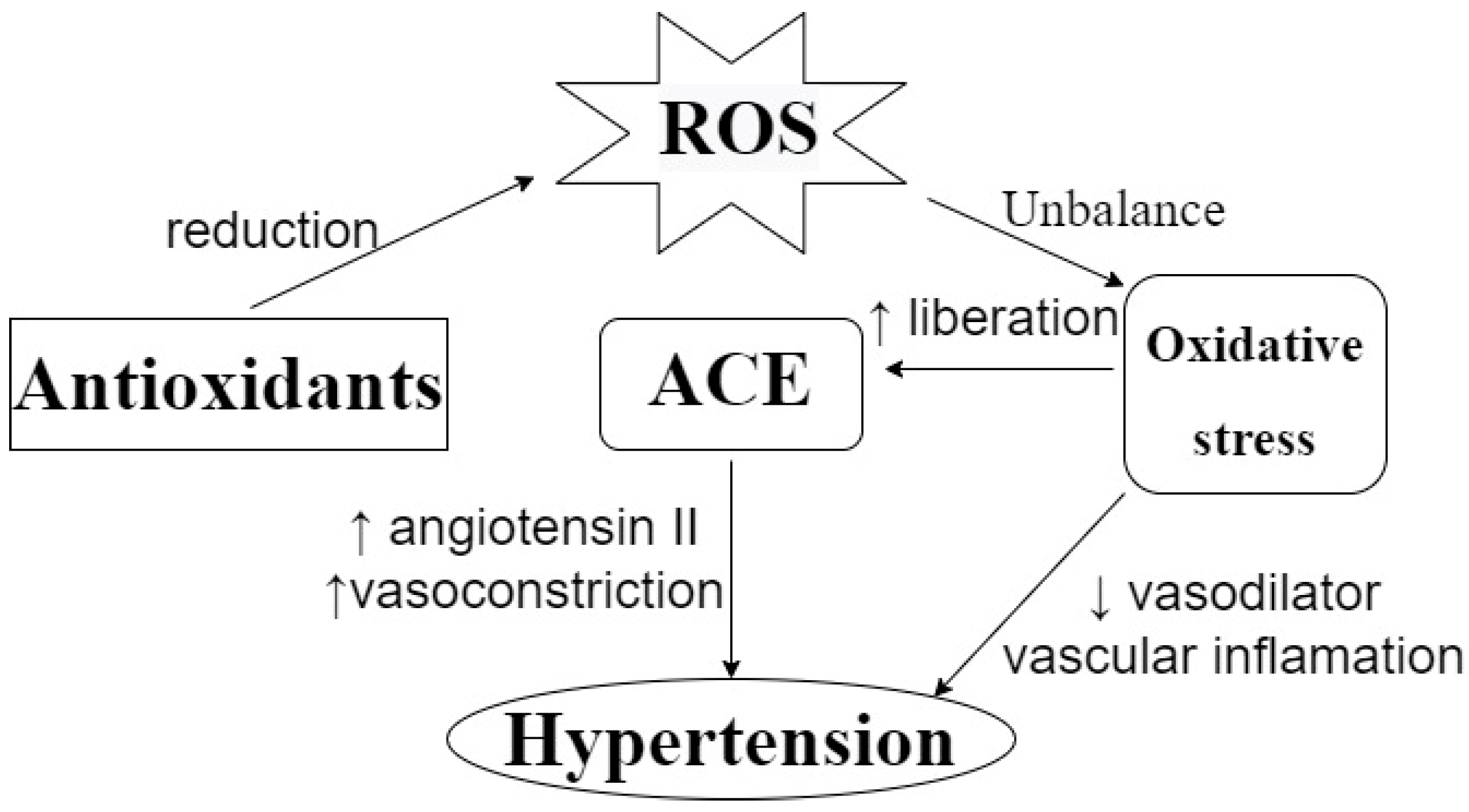
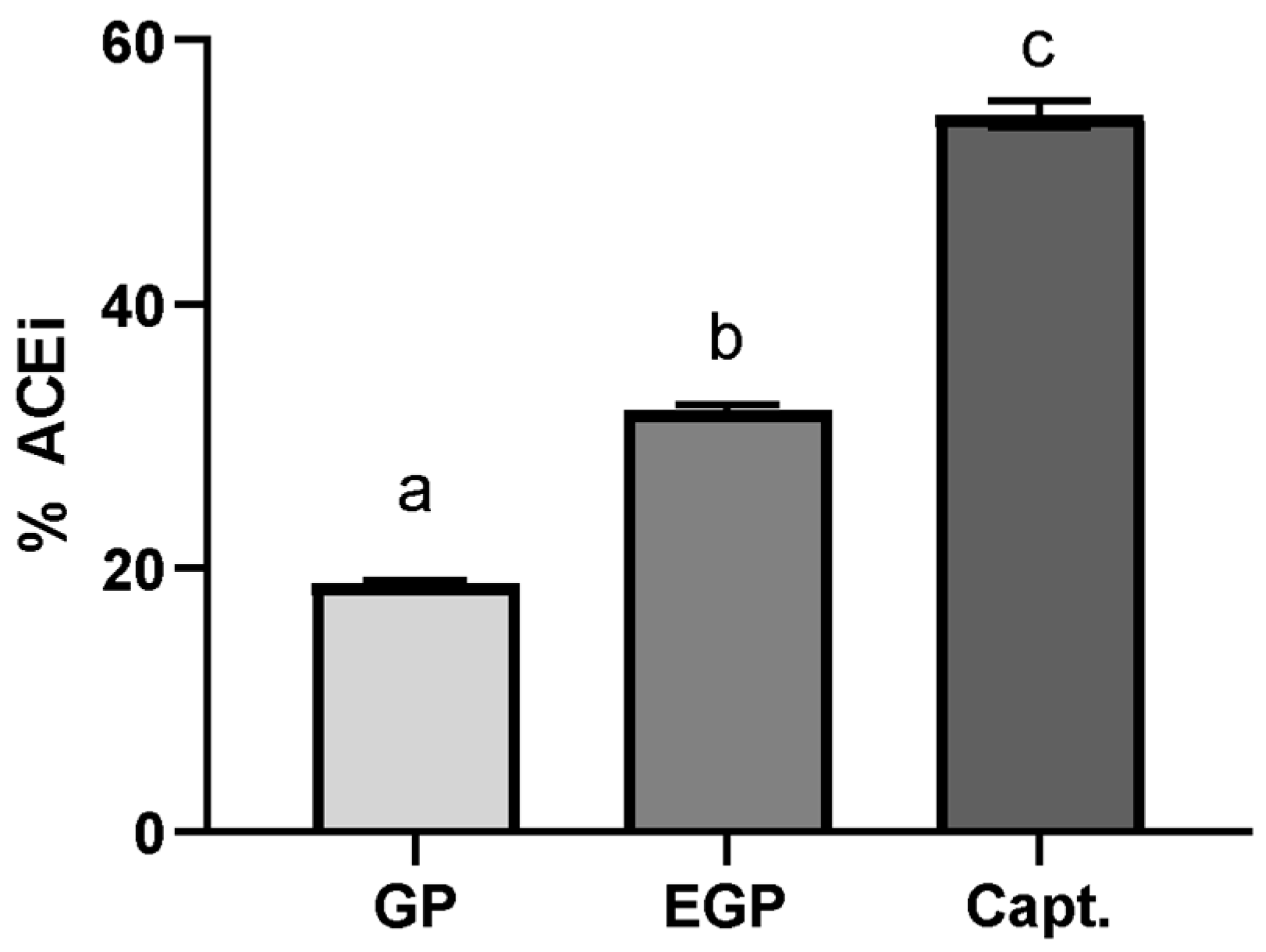
3.4. In Vitro ACE Inhibitory Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Blood Pressure. Available online: https://apps.who.int/gho/data/view.main.2464ESTSTANDARD?lang=en (accessed on 1 October 2021).
- Ogedegbe, G.; Pickering, T.G. Epidemiology of hypertension. In Hurst’s the Heart, 13th ed.; Fuster, V., Walsh, R.A., Harrington, R.A., Eds.; McGrawHill: New York, NY, USA, 2011; Volume 2, Chapter 68; pp. 1533–1605. [Google Scholar]
- WHO. A Global Brief on Hypertension; WHO Press: Geneva, Switzerland, 2013; Available online: http://www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en/ (accessed on 7 August 2018).
- Santos, R.A.S.; Ferreira, A.J.; Pinheiro, S.V.B. Papel do sistema renina-angiotensina. In Hipertensão; Brandão, A., Amodeo, C., Nobre, F., Fuchs, F.D., Eds.; Elsevier: Rio de Janeiro, Brazil, 2006; Chapter 3.6; pp. 66–75. [Google Scholar]
- Victor, R.G.; Kaplan, N.M. Hipertensão Sistêmica: Mecanismos e diagnósticos. In Braunwald: Tratado de Medicina Cardiovascular, 8th ed.; Libby, P., Bonow, R., Mann, D., Zipes, D., Eds.; Elsevier: Rio de Janeiro, Brazil, 2010; Volume 1, Chapter 40; pp. 1027–1048. [Google Scholar]
- Caribe, J.; Campos, J.M. Plantas Que Ajudam o Homem: Guia Prático Para a Época Atual, 5th ed.; Pensamento-Cultrix: São Paulo, Brazil, 1997; 321p. [Google Scholar]
- Duke, J.A. CRC Handbook of Medicinal Herbs; CRC Press: Boca Rato, FL, USA, 1987; 677p. [Google Scholar]
- WHO. WHO Monographs on Selected Medicinal Plants; WHO Graphics: Geneva, Switzerland, 1999; Volome 1, 289p. [Google Scholar]
- Hung, W.C.; Ling, X.H.; Chang, C.C.; Hsu, H.F.; Wang, S.W.; Lee, Y.C.; Luo, C.; Lee, Y.T.; Houng, J.Y. Inhibitory Effects of Siegesbeckia orientalis Extracts on Advanced Glycation End Product Formation and Key Enzymes Related to Metabolic Syndrome. Molecules 2017, 22, 1785. [Google Scholar] [CrossRef] [PubMed]
- Shafaei, A.; Sultan Khan, M.S.; Aisha, F.A.A.; Abdul Majid, A.M.; Hamdan, M.R.; Mordi, M.N.; Ismail, Z. Flavonoids-Rich Orthosiphon stamineus Extract as New Candidate for Angiotensin I-Converting Enzyme Inhibition: A Molecular Docking Study. Molecules 2016, 21, 1500. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Souri, E.; Ziai, S.A.; Amin, G.; Amanlou, M. Discovery of new angiotensin converting enzyme (ACE) inhibitors from medicinal plants to treat hypertension using an in vitro assay. DARU J. Pharm. Sci. 2013, 21, 74–81. [Google Scholar] [CrossRef]
- Sultana, R.; Alashi, A.M.; Islam, K.; Saifullah, M.; Haque, C.E.; Aluko, R.E. Inhibitory Activities of Polyphenolic Extracts of Bangladeshi Vegetables against α-Amylase, α-Glucosidase, Pancreatic Lipase, Renin, and Angiotensin-Converting Enzyme. Foods 2020, 9, 844. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ademosun, A.O.; Ademiluyi, A.O.; Omojokun, O.S.; Nwanna, E.E.; Longe, K.O. In Vitro Studies on the Antioxidant Property and Inhibition of alpha-Amylase, alpha-Glucosidase, and Angiotensin I-Converting Enzyme by Polyphenol-Rich Extracts from Cocoa (Theobroma cacao) Bean. Pathol. Res. Int. 2014, 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ademiluyi, A.O.; Agunloye, O.M.; Ademosun, A.O.; Ogunsakin, B.G. Inhibitory Effect of Garlic, Purple Onion, and White Onion on Key Enzymes Linked with Type 2 Diabetes and Hypertension. J. Diet. Suppl. 2018, 16, 105–118. [Google Scholar] [CrossRef]
- Mao, L.M.; Qi, X.W.; Hao, J.H.; Liu, H.F.; Xu, Q.H.; Bu, P.L. In vitro, ex vivo and in vivo anti-hypertensive activity of Chrysophyllum cainito L. extract. Int. J. Clin. Exp. Med. 2015, 8, 17912–17921. [Google Scholar]
- Pinto, P.; Santos, C.N. Worldwide (poly)phenol intake: Assessment methods and identified gaps. Eur. J. Nutr. 2017, 56, 1393–1408. [Google Scholar] [CrossRef]
- Chun, O.K.; Kim, D.-O.; Smith, N.; Schroeder, D.; Han, J.T.; Lee, C.Y. Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the American diet. J. Sci. Food Agric. 2005, 85, 1715–1724. [Google Scholar] [CrossRef]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic Compounds in the Potato and Its Byproducts: An Overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef]
- Doshi, P.; Adsule, P.; Banerjee, K.; Oulkar, D. Phenolic compounds, antioxidant activity and insulinotropic effect of extracts prepared from grape (Vitis vinifera L.) byproducts. J. Food Sci. Technol. 2015, 52, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAOSTAT: Production Quantity. Available online: http://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 4 October 2021).
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.M.; Roberto, B.S.; Blumberg, J.B.; Chen, C.Y.O.; Macedo, G.A. Enzymatic biotransformation of polyphenolics increases antioxidant activity of red and white grape pomace. Food Res. Int. 2016, 89, 533–539. [Google Scholar] [CrossRef]
- Lopes, D.B.; de Queirós, L.D.; de Ávila, A.R.A.; Monteiro, N.E.S.; Macedo, G.A.; Grumezescu, A.M.; Holban, A.M. Chapter 2—The Importance of Microbial and Enzymatic Bioconversions of Isoflavones in Bioactive Compounds. In Food Bioconversion; Grumezescu, A., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 55–93. [Google Scholar] [CrossRef]
- Ruviaro, A.R.; Barbosa, P.d.P.M.; Macedo, G.A. Enzyme-assisted biotransformation increases hesperetin content in citrus juice by-products. Food Res. Int. 2018, 124, 213–221. [Google Scholar] [CrossRef]
- Avila, A.R.A.; Queirós, L.D.; Lopes, D.B.; Barin, C.G.; Ueta, T.M.; Ruiz, A.L.T.G.; Macedo, G.A.; Macedo, J.A. Enhanced estrogenic effects of biotransformed soy extracts. J. Funct. Foods 2018, 48, 117–124. [Google Scholar] [CrossRef]
- Uzuner, S.; Cekmecelioglu, D.; Kuddus, M. Chapter 3—Enzymes in the Beverage Industry. In Enzymes in Food Biotechnology; Academic Press: Cambridge, MA, USA, 2019; pp. 29–43. [Google Scholar] [CrossRef]
- Ruviaro, A.R.; Barbosa, P.d.P.M.; Martins, I.M.; de Ávila, A.R.A.; Nakajima, V.M.; Dos Prazeres, A.R.; Macedo, J.A.; Macedo, G.A. Flavanones biotransformation of citrus by-products improves antioxidant and ACE inhibitory activities in vitro. Food Biosci. 2020, 38, 100787. [Google Scholar] [CrossRef]
- Chamorro, S.; Viveros, A.; Alvarez, I.; Vega, E.; Brenes, A. Changes in polyphenol and polysaccharide content of grape seed extract and grape pomace after enzymatic treatment. Food Chem. 2012, 133, 308–314. [Google Scholar] [CrossRef]
- Wood, T.M.; Garcia-Campayo, V. Enzymology of cellulose degradation. Biodegradation 1990, 1, 147–161. [Google Scholar] [CrossRef]
- Couri, S. Efeito de Cátions na Morfologia do Agregado e na Produção de Poligalacturonase por Aspergillus Niger Mutante 3T5B8. Doutorado Dissertation, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brazil, 1993. [Google Scholar]
- Matsuura, M.; Sasaki, J.; Murao, S. Studies on β-Glucosidases from Soybeans That Hydrolyze Daidzin and Genistin: Isolation and Characterization of an Isozyme. Biosci. Biotechnol. Biochem. 1995, 59, 1623–1627. [Google Scholar] [CrossRef]
- Xu, C.; Yagiz, Y.; Borejsza-Wysocki, W.; Lu, J.; Gu, L.; Ramirez-Rodrigues, M.M.; Marshall, M.R. Enzyme release of phenolics from muscadine grape (Vitis rotundifolia Michx.) skins and seeds. Food Chem. 2014, 157, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Peschel, W.; Sánchez-Rabaneda, F.; Diekmann, W.; Plescher, A.; Gartzía, I.; Jiménez, D.; Lamuela-Raventós, R.; Buxaderas, S.; Codina, C. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem. 2006, 97, 137–150. [Google Scholar] [CrossRef]
- Macedo, J.A.; Battestin, V.; Ribeiro, M.L.; Macedo, G.A. Increasing the antioxidant power of tea extracts by biotransformation of polyphenols. Food Chem. 2011, 126, 491–497. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food. Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- SIGMA-ALDRICH. Angiotensin I Converting Enzyme (ACE) Activity Assay Kit (Fluorometric)—Technical Bulletin. Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/248/077/cs0002bul-mk.pdf (accessed on 2 April 2022).
- The Jamovi Project. 2021. Available online: https://www.jamovi.org/ (accessed on 1 December 2021).
- Martins, I.M.; Macedo, G.A.; Macedo, J.A.; Roberto, B.S.; Chen, Q.; Blumberg, J.B.; Chen, C.-Y.O. Tannase enhances the anti-inflammatory effect of grape pomace in Caco-2 cells treated with IL-1β. J. Funct. Foods 2017, 29, 69–76. [Google Scholar] [CrossRef]
- Lekha, P.K.; Lonsane, B.K. Production and application of tannin acyl hydrolase: State of the art. Adv. Appl. Microbiol. 1997, 44, 215–260. [Google Scholar] [CrossRef]
- Rasines-Perea, Z.; Ky, I.; Cros, G.; Crozier, A.; Teissedre, P.L. Grape Pomace: Antioxidant Activity, Potential Effect Against Hypertension and Metabolites Characterization after Intake. Diseases 2018, 6, 60. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Medina-Remón, A.; Pérez-Jiménez, J.; Martínez-González, M.A.; Covas, M.I.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Dietary intake and major food sources of polyphenols in a Spanish population at high cardiovascular risk: The PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.A.; Yuan Yuan, D.; Nawaz, W.; Ze, H.; Zhuo, C.X.; Talal, B.; Taleb, A.; Mais, E.; Qilong, D. Antioxidant therapy for management of oxidative stress induced hypertension. Free Radic. Res. 2017, 51, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Dabla, P.K. Oxidative stress and antioxidants in hypertension-a current review. Curr. Hypertens. Rev. 2015, 11, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef]
- da Costa, C.A.; de Oliveira, P.R.B.; de Bem, G.F.; de Cavalho, L.C.R.M.; Ognibene, D.T.; da Silva, A.F.E.; dos Santos Valença, S.; Pires, K.M.P.; da Cunha Sousa, P.J.; de Moura, R.S.; et al. Euterpe oleracea Mart.-derived polyphenols prevent endothelial dysfunction and vascular structural changes in renovascular hypertensive rats: Role of oxidative stress. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 1199–1209. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oyeleye, S.I.; Oboh, G. Distribution of Phenolic Contents, Antidiabetic Potentials, Antihypertensive Properties, and Antioxidative Effects of Soursop (Annona muricata L.) Fruit Parts In Vitro. Biochem. Res. Int. 2015, 2015, 347673. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, W. Antihypertensive activity of Rosa rugosa Thunb. flowers: Angiotensin I converting enzyme inhibitor. J. Ethnopharmacol. 2012, 144, 562–566. [Google Scholar] [CrossRef]
- Eriz, G.; Sanhueza, V.; Roeckel, M.; Fernández, K. Inhibition of the angiotensin-converting enzyme by grape seed and skin proanthocyanidins extracted from Vitis vinífera L. cv. País. LWT Food Sci. Technol. 2011, 44, 860–865. [Google Scholar] [CrossRef]
- Yu, M.; Kim, H.J.; Heo, H.; Kim, M.; Jeon, Y.; Lee, H.; Lee, J. Comparison of the Antihypertensive Activity of Phenolic Acids. Molecules 2022, 27, 6185. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G. Caffeic acid and chlorogenic acid: Evaluation of antioxidant effect and inhibition of key enzymes linked with hypertension. J. Food Biochem. 2018, 42, e12541. [Google Scholar] [CrossRef]
- Montealegre, R.R.; Peces, R.R.; Vozmediano, J.L.C.; Gascueña, J.M.; Romero, E.G. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Nwanna, E.E.; Ibukun, E.O.; Oboh, G.; Ademosun, A.O.; Boligon, A.A.; Athayde, M. HPLC-DAD Analysis and In-Vitro Property of Polyphenols Extracts from (Solanum aethiopium) Fruits on alpha -Amylase, alpha -Glucosidase and Angiotensin—1-Converting Enzyme Activities. Int. J. Biomed. Sci. 2014, 10, 272–281. [Google Scholar] [CrossRef] [PubMed]
| Extract | Yield | TPC Extract | TPC Sample |
|---|---|---|---|
| (%) | mg GAE/g DE | mg GAE/g DM | |
| GP | 24.5 | 52 ± 1 a | 13 ± 0.2 a |
| EGP | 40.3 | 54 ± 2 a | 22± 1 b |
| Extract | DPPH | ORAC | FRAP |
|---|---|---|---|
| (μmol TE/g DE) | |||
| GP | 644 ± 42 a | 2427 ± 84 a | 503 ± 43 a |
| EGP | 699 ± 62 a | 2461 ± 228 a | 485 ± 35 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, D.; Chiocchetti, G.d.M.e.; Macedo, J.A. Effect of Enzymatic Biotransformation on the Hypotensive Potential of Red Grape Pomace Extract. Foods 2023, 12, 4109. https://doi.org/10.3390/foods12224109
Batista D, Chiocchetti GdMe, Macedo JA. Effect of Enzymatic Biotransformation on the Hypotensive Potential of Red Grape Pomace Extract. Foods. 2023; 12(22):4109. https://doi.org/10.3390/foods12224109
Chicago/Turabian StyleBatista, Daniel, Gabriela de Matuoka e Chiocchetti, and Juliana Alves Macedo. 2023. "Effect of Enzymatic Biotransformation on the Hypotensive Potential of Red Grape Pomace Extract" Foods 12, no. 22: 4109. https://doi.org/10.3390/foods12224109
APA StyleBatista, D., Chiocchetti, G. d. M. e., & Macedo, J. A. (2023). Effect of Enzymatic Biotransformation on the Hypotensive Potential of Red Grape Pomace Extract. Foods, 12(22), 4109. https://doi.org/10.3390/foods12224109










