Evaluation of Short-Term Toxicity and Cholesterol-Lowering Effects in Rats Exposed to Dietary Fiber Derived from Cassava Pulp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Dietary Fiber Preparation
2.3. Short-Term Toxicity Study of Dietary Fiber
2.3.1. Diet Preparation
2.3.2. Experimental Protocol
2.3.3. Hematological Assay
2.3.4. Blood Chemistry
2.3.5. Organ Weights
2.3.6. Histopathological Examination
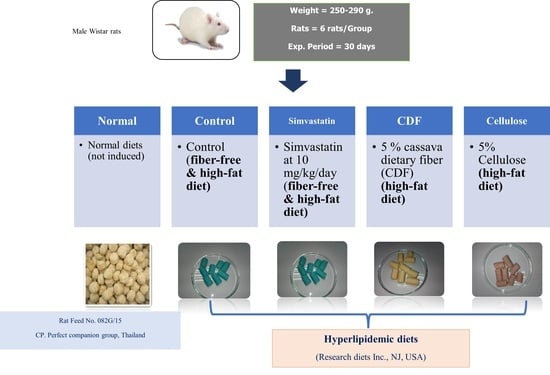
2.4. Cholesterol-Lowering Properties of Dietary Fiber
2.4.1. Diet Preparation
2.4.2. Experimental Design
2.4.3. Biochemical Assays of Serum Cholesterol, Triglyceride, and Glucose
2.4.4. Liver Total Lipids and Cholesterol
2.4.5. Fecal Total Lipids and Cholesterol
2.4.6. Determination of Short-Chain Fatty Acids (SCFAs) in Feces
2.5. Statistical Analysis
3. Results
3.1. Chemical Compositions
3.2. Short-Term Toxicity of Dietary Fiber
3.2.1. Clinical Observation, Feed Consumption, and Body Weight
3.2.2. Hematology Parameters
3.2.3. Blood Chemistry
3.2.4. Organ Weights
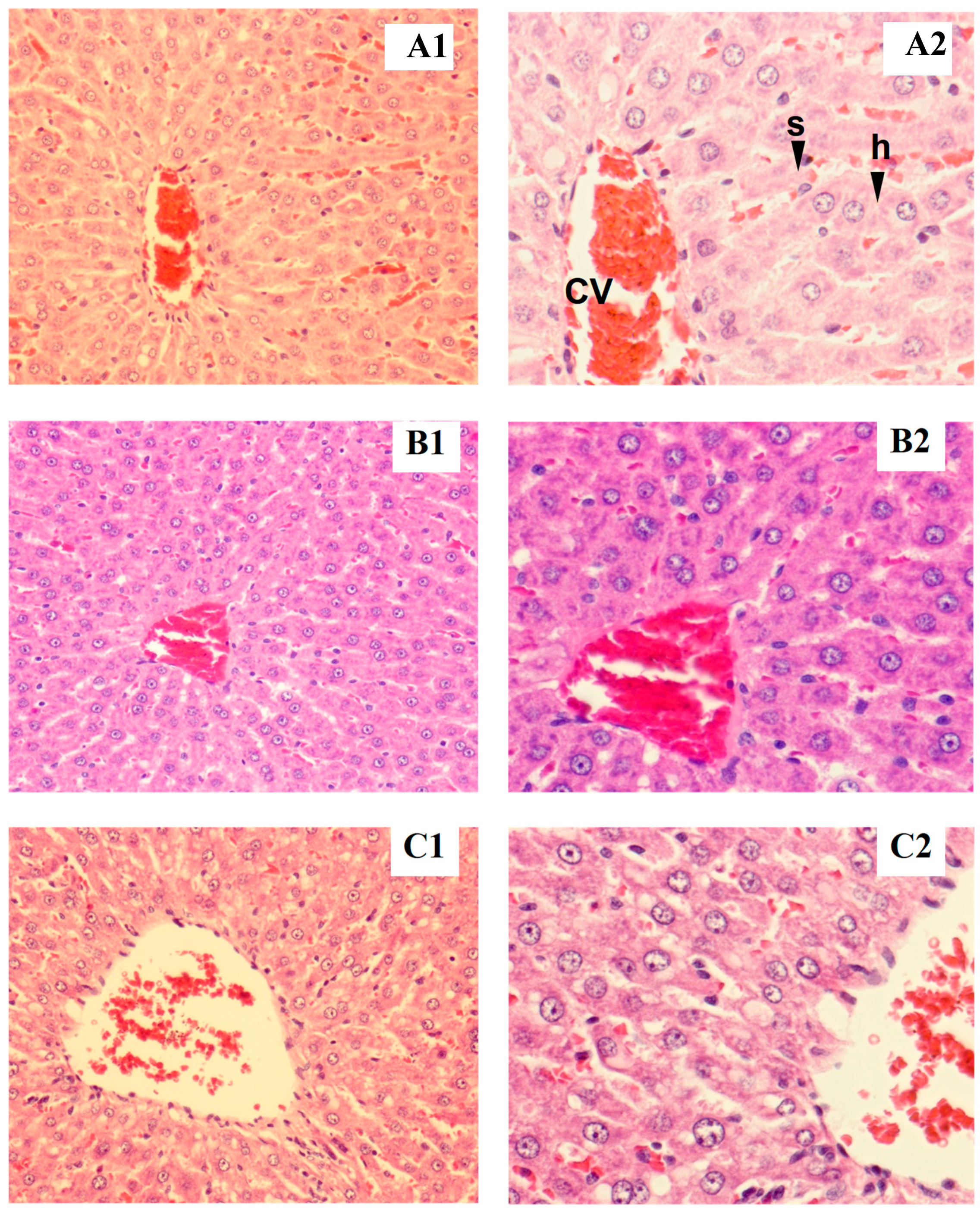
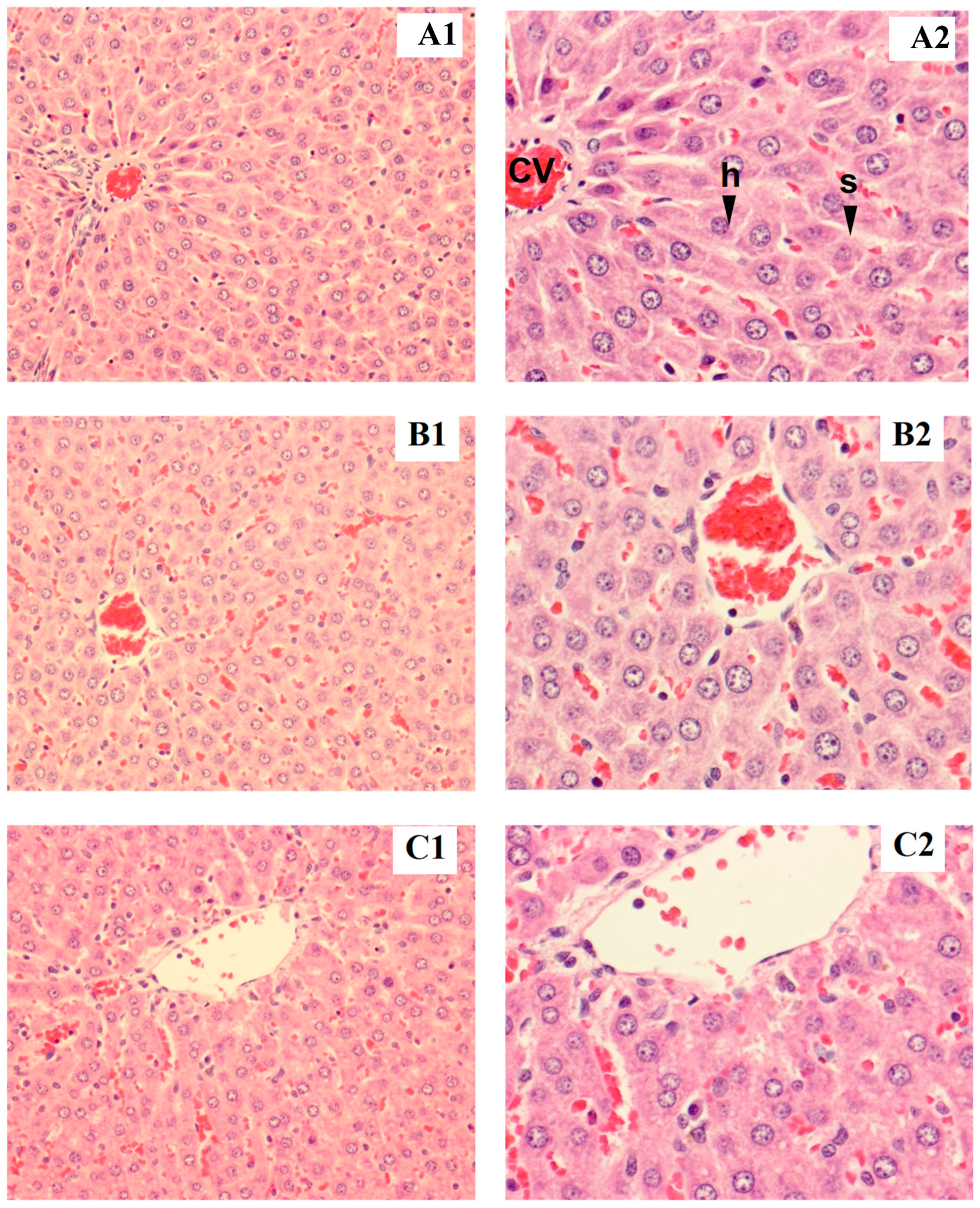
3.2.5. Histopathological Examination
3.3. Cholesterol-Lowering Properties of Dietary Fiber
3.3.1. Food Intake, Body Weight Gain, and Fresh Fecal Mass
3.3.2. Biochemical Assays of Serum Cholesterol, Triglyceride, and Glucose
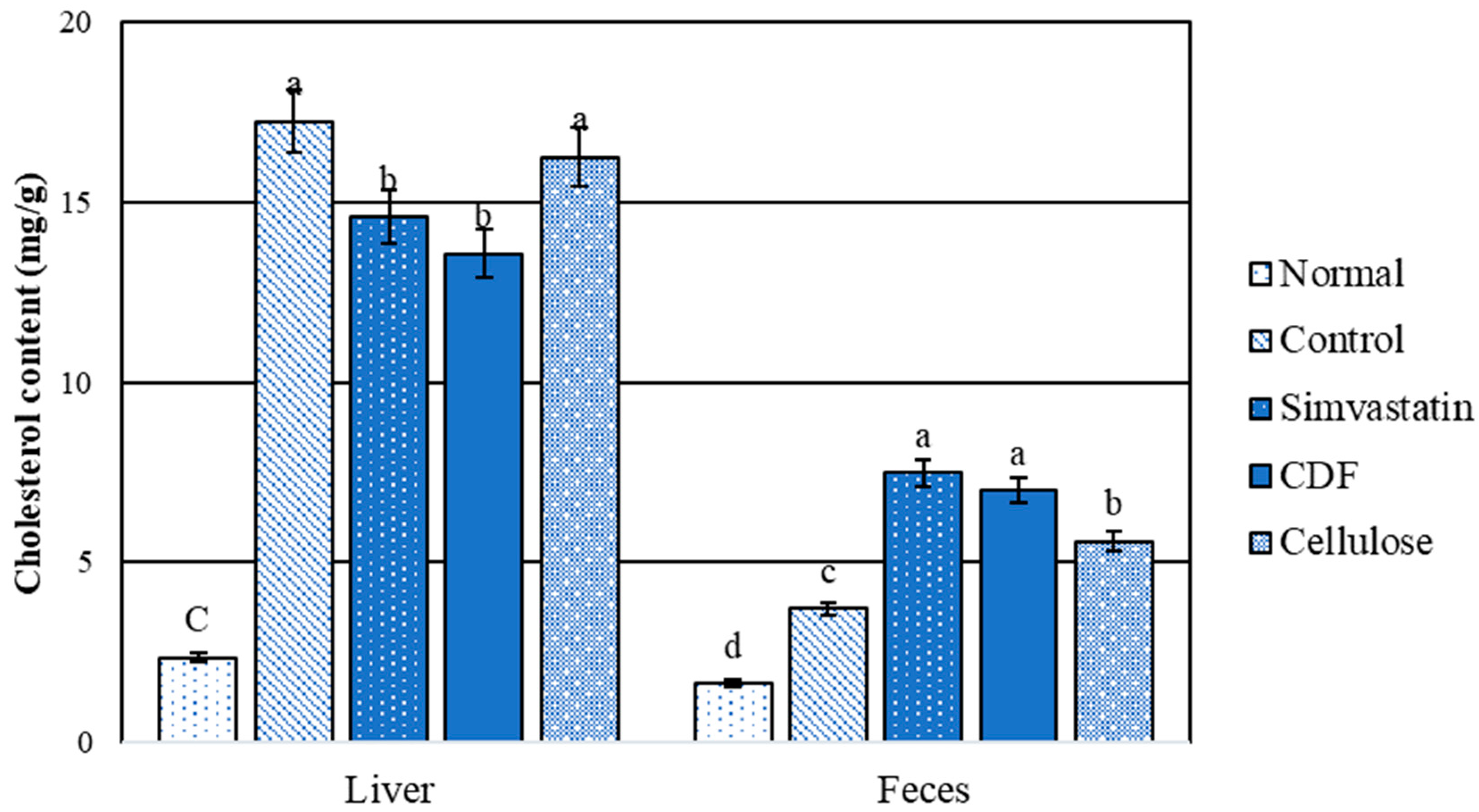
3.3.3. Total Lipid Content in Liver and Feces
3.3.4. Cholesterol Content in Liver and Feces
3.3.5. Short-Chain Fatty Acids in Feces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sriroth, K.; Chollakup, R.; Chotineeranat, S.; Piyachomkwan, K.; Oates, C.G. Processing of cassava waste for improved biomass utilization. Bioresour. Technol. 2000, 71, 63–69. [Google Scholar] [CrossRef]
- Thailand Topioca Starch. Treatment and Utilization of Root, Stem, and Pulp, in Treatment and Utilization of Wastewater and Solid Wastes. 2010. Available online: https://sustainablecassava.org/information-hub/cassava-value-chain/waste-treatment/ (accessed on 1 May 2018).
- Wandee, Y.; Uttapap, D.; Puncha-arnon, S.; Puttanlek, C.; Rungsardthong, V.; Wetprasit, N. Enrichment of rice noodles with fibre-rich fractions derived from cassava pulp and pomelo peel. Int. J. Food Sci. Technol. 2014, 49, 2348–2355. [Google Scholar] [CrossRef]
- IUPAC. Acute Toxicity in the Gold Book, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 2006. [Google Scholar] [CrossRef]
- Marques, F.Z.; Nelson, E.M.; Chu, P.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-osta, A.; et al. High-fiber diet and acetated supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, R.; Hara, H.; Aoyama, Y.; Takahashi, T.; Furuta, H. Ingestion of water-soluble soybean fiber prevents osteopenia and hypercholesterolemia induced by ovariectomy in rats. J. Agric. Food Chem. 2003, 51, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Dabour, N.; El-Saadany, K.; Shoukry, E.; Hamdy, S.; Taïbi, A.; Kheadr, E. The ability of yoghurt supplemented with dietary fibers or brans eatracted from wheat or rice to reduce serum lipids and enhance liver function in male hypercholesterolemic rats. J. Food Biochem. 2022, 46, 12. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Deakins, D.A.; Floore, T.L.; Smith, B.H.; Whitis, S.E. Dietary fiber and coronary heart disease. Crit. Rev. Food. Sci. Nutr. 1990, 29, 95–147. [Google Scholar] [CrossRef] [PubMed]
- Esmael, O.; Sonbul, S.; Kumosani, T.A.; Mosalhy, S.S. Hypolipiddemic effect of fruit fibers in rats fed with high dietary fat. Toxicol. Ind. Health 2013, 31, 281–288. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M. Effect of feeding on some dietary fibers for reducing blood cholesterol. J. Food Diary Sci. 2013, 4, 13–25. [Google Scholar] [CrossRef]
- Kachenpukdee, N.; Santerre, C.; Ferruzzi, M.; Oonsivilai, R. Enzymatic digestion optimization of dietary fiber from cassava pulp and their effect on mercury bioaccessibility and intestinal uptake from fish using an in vitro digestion/Caco-2 model. Int. Food Res. J. 2016, 23, 660–666. [Google Scholar]
- OECD. OECD Guideline for Testing of Chemical: Acute Oral Toxicity-Acute Toxicity Class Method; Test No. 423., Adopted 22nd March 1996, and Revised Method Adopted 17th December, 2001; OECD: Paris, France, 2022. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues. J. Biol. Chem. 1957, 226, 496–509. [Google Scholar] [CrossRef]
- Searcy, R.L.; Bergquist, L.M. A new color reaction for the quantitation of serum cholesterol. Clin. Chim. Acta 1960, 5, 192–199. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Jackix, E.; Monteiro, E.B.; Raposo, H.F.; Amaya-Farfán, J. Cholesterol reducing and bile-acid binding properties of taioba (Xanthosoma sagittifolium) leaf in rats fed a high-fat diet. Food Res. Int. 2013, 51, 886–891. [Google Scholar] [CrossRef]
- Henningsson, Å.; Björck, I.; Nyman, M. Short-chain fatty acid formation at fermentation of indigestible carbohydrates. Food Nutr. Res. 2001, 45, 165–168. [Google Scholar] [CrossRef]
- Sembries, S.; Dongowski, G.; Mehrländer, K.; Will, F.; Dietrich, H. Physiological effects of extraction juices from apple, grape, and red beet pomaces in rats. J. Agric. Food Chem. 2006, 54, 10269–10280. [Google Scholar] [CrossRef]
- Wong, J.M.; De Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Ali, D.; Soewarno, N.; Sumarno, P.D.; Sumaryo, W. Cassava pulp as a biofuel feedstock of an enzymatic hydrolysis process. Makara J. Technol. 2011, 15, 183–192. [Google Scholar] [CrossRef]
- Kosoom, W.; Charoenwattanasakun, N.; Ruangpanit, Y.; Rattanatabtimtong, S.; Attamangkune, S. Physical, chemical and biological properties of cassava pulp. In Proceedings of the 47th Kasetsart University Annual Conference, Kasesart, Bangkok, Thailand, 17–20 March 2009; pp. 17–20. Available online: https://kukr.lib.ku.ac.th/kukr_es/kps_agri/search_detail/dowload_digital_file/11358/101030 (accessed on 1 May 2018).
- Rattanachomsri, U.; Tanapongpipat, S.; Eurwilaichitr, L.; Champreda, V. Simultaneous non-thermal saccharification of cassava pulp by multi-enzyme activity and ethanol fermentation by Candida tropicalis. J. Biosci. Bioeng. 2009, 107, 488–493. [Google Scholar] [CrossRef]
- Suksombat, W.; Lounglawan, P.; Noosen, P. Energy and protein evaluation of five feedstuffs used in diet in which cassava pulp as main energy source for lactating dairy cows. Suranaree J. Sci. Technol. 2006, 14, 99–107. [Google Scholar]
- Jacometti, G.A.; Mello, L.R.; Nascimento, P.H.; Sueiro, A.C.; Yamashita, F.; Mali, S. The physicochemical properties of fibrous residues from the agro industry. LWT-Food Sci. Technol. 2015, 62, 138–143. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T.; Vandenberghe, L.P.; Mohan, R. Biotechnological potential of agro-industrial residues. II: Cassava bagasse. Bioresour. Technol. 2000, 74, 81–87. [Google Scholar] [CrossRef]
- Lacourse, N.L.; Chicalo, K.; Zallie, J.P.; Altieri, P.A. Dietary Fiber Derived from Tapioca and Process Therefor. U.S. Patent US5350593A, 27 September 1994. Available online: https://patents.google.com/patent/US5350593A/en (accessed on 1 May 2018).
- Hong, Y.; Zi-jun, W.; Jian, X.; Ying-jie, D.; Fang, M. Development of the dietary fiber functional food and studies on its toxicological and physiologic properties. Food Chem. Toxicol. 2012, 50, 3367–3374. [Google Scholar] [CrossRef]
- Gao, Y.; Shen, J.; Yin, J.; Li, C.; Fu, C.; Cho, S. A subchronic dietary toxicity study of rice hull fiber in rats. Food Chem. Toxicol. 2013, 51, 137–142. [Google Scholar] [CrossRef]
- Uberoi, S.; Vadhera, S.; Soni, G. Role of dietary fiber from pulses and cereals as hypocholesterolemic and hypolipidemic agent. J. Food Sci. Technol. 1992, 29, 281–283. [Google Scholar]
- Fang, Y.; Ma, J.; Lei, P.; Wang, L.; Qu, J.; Zhao, J.; Liu, F.; Yan, X.; Wu, W.; Jin, L.; et al. Konjac Glucomannan: An Emerging Specialty Medical Food to Aid in the Treatment of Type 2 Diabetes Mellitus. Foods 2023, 12, 363. [Google Scholar] [CrossRef]
- Chirinang, P.; Oonsivilai, R. Physicochemical properties, in-vitro binding capacities for lard, cholesterol, bile acids and assessment of prebiotic potential of dietary fiber from cassava pulp. Int. Food Res. J. 2018, 25, S63–S74. [Google Scholar]

| Ingredients a | Control (Fiber Free) | Cassava DF | Cellulose |
|---|---|---|---|
| Casein | 140 | 140 | 140 |
| DL-methionine | 2 | 2 | 2 |
| Corn starch | 435 | 435 | 435 |
| Maltodextrin | 150 | 150 | 150 |
| Sucrose | 100 | 100 | 100 |
| Cassava DF | - | 50 | - |
| Cellulose | - | - | 50 |
| Soybean oil | 50 | 50 | 50 |
| Coconut oil | 35 | 35 | 35 |
| Mineral mix S10001 | 35 | 35 | 35 |
| Calcium carbonate | 5.5 | 5.5 | 5.5 |
| Sodium chloride | 8 | 8 | 8 |
| Potassium citrate | 10 | 10 | 10 |
| Vitamin mix V10001 | 10 | 10 | 10 |
| Choline bitartrate | 2 | 2 | 2 |
| Cholesterol | 12.5 | 12.5 | 12.5 |
| Sodium cholic acid | 5 | 5 | 5 |
| FD&C blue dye #1 | 0.1 | - | - |
| FD&C yellow dye #5 | - | 0.1 | - |
| FD&C red dye #40 | - | - | 0.1 |
| Components | Cassava Pulp (%) | Dietary Fiber (%) |
|---|---|---|
| Crude protein | 2.02 ± 0.19 | 1.01 ± 0.10 |
| Fat | 0.21 ± 0.08 | 0.25 ± 0.06 |
| Moisture | 6.63 ± 0.11 | 5.52 ± 0.09 |
| Ash | 3.76 ± 0.05 | 4.52 ± 0.04 |
| Crude fiber | 17.23 ± 0.13 | 40.24 ± 2.22 |
| Carbohydrate | 70.15 | 48.46 |
| Starch | 58.11 ± 0.06 | 8.50 ± 0.31 |
| Neutral detergent fiber (NDF) | 31.40 ± 0.58 | 79.03 ± 0.51 |
| Acid detergent fiber (ADF) | 25.08 ± 0.17 | 70.14 ± 0.40 |
| Acid detergent lignin (ADL) | 4.16 ± 0.10 | 11.59 ± 0.01 |
| Cellulose a | 20.92 | 58.55 |
| Hemicellulose b | 6.32 | 8.89 |
| Functional Properties | Dietary Fiber | Cellulose |
|---|---|---|
| Water-holding capacity (g/g dry weight) | 8.17 ± 0.40 | 4.92 ± 0.33 |
| Water retention capacity (g/g dry weight) | 8.36 ± 0.20 | 5.95 ± 0.49 |
| Swelling capacity (mL/g) | 4.82 ± 0.15 | 1.13 ± 0.15 |
| Oil-holding capacity (g/g) | 3.97 ± 0.14 | 2.66 ± 0.09 |
| Group/Dose % (w/w) | Death Rate a | Side Effect | |
|---|---|---|---|
| Male | Female | ||
| Control | 0/5 | 0/5 | No b |
| 2.5 | 0/5 | 0/5 | No |
| 15.0 | 0/5 | 0/5 | No |
| Sex | Group/Dose % (w/w) | Consumption (g/day) | Body Weight Gain (g) | |
|---|---|---|---|---|
| Day 7 | Day 14 | |||
| Male | Control | 21.04 ± 1.46 | 21.33 ± 1.15 | 20.33 ± 1.53 |
| 2.5 | 20.32 ± 1.50 * | 21.20 ± 1.79 | 18.20 ± 2.17 | |
| 15.0 | 19.95 ± 1.65 * | 18.60 ± 4.83 | 15.40 ± 4.04 | |
| Female | Control | 17.85 ± 1.21 | 12.80 ± 2.28 | 12.40 ± 2.51 |
| 2.5 | 17.16 ± 1.43 * | 13.80 ± 2.17 | 11.00 ± 2.24 | |
| 15.0 | 17.11 ± 1.34 * | 12.00 ± 2.74 | 8.60 ± 1.67 * | |
| Parameters | Relative Organ Weight (g/100 g) | |||||
|---|---|---|---|---|---|---|
| Group/Dose (% w/w) | ||||||
| Male | Female | |||||
| Control | 2.5 | 15.0 | Control | 2.5 | 15.0 | |
| Hb (g/dL) | 16.60 ± 1.60 | 16.38 ± 0.79 | 16.34 ± 0.79 | 16.80 ± 1.32 | 16.03 ± 1.32 | 17.12 ± 0.86 |
| Hct (%) | 49.87 ± 4.06 | 49.30 ± 2.03 | 49.18 ± 2.78 | 48.90 ± 3.65 | 47.28 ± 2.78 | 50.10 ± 2.62 |
| WBC (×103 cell/mm3) | 6.91 ± 2.13 | 6.26 ± 1.88 | 6.31 ± 3.53 | 5.84 ± 1.97 | 6.15 ± 1.61 | 8.72 ± 3.41 |
| PMNs (%) | 22.67 ± 8.08 | 18.00 ± 8.49 | 17.60 ± 4.56 | 22.80 ± 4.60 | 23.50 ± 9.29 | 16.80 ± 5.59 |
| Lymphocyte (%) | 72.67 ± 7.02 | 74.80 ± 6.42 | 77.40 ± 2.41 | 72.40 ± 5.18 | 72.00 ± 9.93 | 82.40 ± 5.18 |
| Monocyte (%) | 4.00 ± 0 | 5.00 ± 1.15 | 3.80 ± 2.28 | 4.00 ± 1.41 | 4.50 ± 1.91 | 3.60 ± 1.67 |
| Eosionphil (%) | 1.33 ± 2.31 | 2.40 ± 0.89 | 0.80 ± 1.10 | 1.60 ± 1.67 | 1.50 ± 1.00 | 1.20 ± 1.10 |
| MCV (fl.) | 54.73 ± 2.40 | 55.26 ± 2.60 | 54.36 ± 2.39 | 53.84 ± 0.74 | 53.78 ± 1.52 | 53.10 ± 1.15 |
| MCH (pg.) | 18.23 ± 0.55 | 18.34 ± 0.75 | 18.04 ± 0.46 | 18.48 ± 0.28 | 18.20 ± 0.34 | 18.14 ± 0.36 |
| MCHC (%) | 33.23 ± 0.51 | 33.22 ± 0.37 | 33.26 ± 0.76 | 34.36 ± 0.56 | 33.88 ± 0.43 | 34.16 ± 0.22 |
| Platelet count (×103 cell/mm3) | 832.67 ± 84.01 | 847.20 ± 44.55 | 778.20 ± 113.2 | 804.04 ± 117.1 | 742.50 ± 154.31 | 962.40 ± 7.27 |
| RBC count | 9.14 ± 1.15 | 8.94 ± 0.65 | 9.06 ± 0.60 | 8.09 ± 2.71 | 8.81 ± 0.75 | 9.43 ± 0.35 |
| Parameters | Relative Organ Weight (g/100 g) | |||||
|---|---|---|---|---|---|---|
| Group/Dose (% w/w) | ||||||
| Male | Female | |||||
| Control | 2.5 | 15.0 | Control | 2.5 | 15.0 | |
| ALP (U/L) | 95.00 ± 17.69 | 120.00 ± 19.76 | 114.60 ± 16.09 | 65.60 ± 10.33 | 74.00 ± 20.74 | 60.40 ± 11.01 |
| ALT (U/L) | 43.67 ± 24.99 | 24.80 ± 4.55 | 28.20 ± 2.28 | 24.60 ± 3.97 | 26.40 ± 7.44 | 19.80 ± 3.83 |
| AST (U/L) | 126.00 ± 15.00 | 108.80 ± 17.57 | 120.80 ± 47.92 | 146.00 ± 44.82 | 228.00 ± 96.29 | 179.60 ± 70.22 |
| BUN (mg/dL) | 20.33 ± 1.15 | 20.00 ± 3.54 | 18.80 ± 1.30 | 27.40 ± 3.78 | 21.20 ± 2.39 * | 23.80 ± 5.07 |
| Creatinine (mg/dL) | 0.54 ± 0.02 | 0.50 ± 0.04 | 0.51 ± 0.06 | 0.72 ± 0.10 | 0.62 ± 0.08 | 0.71 ± 0.07 |
| TC (mg/dL) | 82.33 ± 3.51 | 69.60 ± 4.83 * | 75.40 ± 7.99 | 72.80 ± 9.83 | 63.20 ± 10.08 | 65.60 ± 10.43 |
| TG (mg/dL) | 108.00 ± 34.22 | 83.80 ± 12.77 | 71.00 ± 13.00 * | 116.60 ± 16.24 | 82.40 ± 26.71 * | 64.00 ± 7.31 ** |
| Glucose (mg/dL) | 326.00 ± 56.67 | 275.20 ± 93.18 | 305.20 ± 58.0 | 185.20 ± 85.51 | 160.40 ± 68.91 | 131.80 ± 38.66 |
| Organs | Relative Organ Weight (g/100 g) | ||||||
|---|---|---|---|---|---|---|---|
| Group/Dose (% w/w) | |||||||
| Male | Female | ||||||
| Control | 2.5 | 15.0 | Control | 2.5 | 15.0 | ||
| Lung | 0.43 ± 0.03 | 0.51 ± 0.06 | 0.46 ± 0.08 | 0.59 ± 0.07 | 0.54 ± 0.04 | 0.62 ± 0.04 | |
| Heart | 0.33 ± 0.02 | 0.33 ± 0.01 | 0.34 ± 0.05 | 0.40 ± 0.04 | 0.37 ± 0.04 | 0.38 ± 0.02 | |
| Liver | 2.96 ± 0.15 | 3.04 ± 0.04 | 3.04 ± 0.09 | 2.98 ± 0.25 | 3.26 ± 0.30 | 3.09 ± 0.12 | |
| Spleen | 0.22 ± 0.03 | 0.22 ± 0.03 | 0.23 ± 0.06 | 0.29 ± 0.02 | 0.30 ± 0.02 | 0.29 ± 0.03 | |
| Kidney | L | 0.32 ± 0.02 | 0.33 ± 0.02 | 0.33 ± 0.02 | 0.35 ± 0.02 | 0.35 ± 0.01 | 0.34 ± 0.03 |
| R | 0.30 ± 0.00 | 0.33 ± 0.03 * | 0.33 ± 0.02 * | 0.34 ± 0.01 | 0.33 ± 0.02 | 0.34 ± 0.03 | |
| Adrenal gland | L | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| R | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.01 | |
| Testis a/Ovaries b | L | 0.51 ± 0.09 | 0.61 ± 0.06 | 0.57 ± 0.04 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| R | 0.50 ± 0.11 | 0.60 ± 0.07 | 0.58 ± 0.03 | 0.03 ± 0.00 | 0.03 ± 0.01 | 0.03 ± 0.01 | |
| Parameter | Group (Mean ± S.D.) | ||||
|---|---|---|---|---|---|
| Normal | Hyperlipidemia Rats | ||||
| Control | Simvastatin | CDF | Cellulose | ||
| Food intake (g/day) | 20.26 ± 2.71 a | 18.45 ± 4.00 b | 16.48 ± 3.13 c | 19.60 ± 3.90 a | 19.72 ± 3.22 a |
| Body weight gain (g) | 97.50 ± 7.58 a | 99.17 ± 18.0 a | 65.83 ± 13.57 b | 96.67 ± 13.66 a | 100 ± 7.89 a |
| Fecal mass (g/day) | 4.68 ± 1.38 a | 2.35 ± 2.12 b | 2.47 ± 2.53 b | 2.76 ± 1.27 b | 3.07 ± 1.28 b |
| Parameter (mg/dL) | Group (Mean ± S.D.) | ||||
|---|---|---|---|---|---|
| Normal | Hyperlipidemic Rats | ||||
| Control | Simvastatin | CDF | Cellulose | ||
| At day 0 | |||||
| Total cholesterol | 84.0 ± 3.46 a | 80.67 ± 6.86 a | 82.17 ± 12.25 a | 73.83 ± 9.15 ab | 68.33 ± 8.7 b |
| Triglyceride | 82.50 ± 12.50 ab | 69.50 ± 5.09 c | 84.17 ± 13.21 a | 89.83 ± 5.15 a | 88.33 ± 7.06 a |
| HDL cholesterol | 55.0 ± 6.0 a | 52.67 ± 3.14 ab | 54.83 ± 7.55 a | 49.33 ± 6.31 ab | 46.17 ± 5.71 b |
| LDL cholesterol | <30 | <30 | <30 | <30 | <30 |
| Glucose | 115.17 ± 4.75 a | 99.83 ± 5.98 bc | 98.50 ± 12.66 c | 110.33 ± 10.01 ab | 100.67 ± 8.12 bc |
| At day 31st | |||||
| Total cholesterol | 77.67 ± 6.59 c | 147.0 ± 12.44 a | 130.83 ± 15.28 b | 133.50 ± 4.93 b | 141.17 ± 9.20 ab |
| Triglyceride | 97.0 ± 12.38 b | 187.17 ± 11.32 a | 95.67 ± 9.33 b | 91.17 ± 8.28 b | 95.50 ± 7.20 b |
| HDL cholesterol | 43.33 ± 7.66 a | 28.17 ± 6.91 c | 36.67 ± 3.67 b | 35.17 ± 4.22 b | 32.83 ± 4.79 bc |
| LDL cholesterol | <30 | <30 | <30 | <30 | <30 |
| Glucose | 189.50 ± 19.59 b | 212.17 ± 13.36 a | 171.33 ± 8.19 c | 203.83 ± 4.96 ab | 202.33 ± 10.33 ab |
| Parameter (mg/g) | Group (Mean ± S.D.) | ||||
|---|---|---|---|---|---|
| Normal | Hyperlipidemia Rats | ||||
| Control | Simvastatin | CDF | Cellulose | ||
| Total SCFA | 3.14 ± 0.58 a | 2.71 ± 1.02 ab | 2.37 ± 0.72 ab | 4.03 ± 0.76 a | 0.85 ± 0.58 b |
| Acetic acid | 1.88 ± 1.0 a | 1.28 ± 1.08 ab | 0.92 ± 0.65 b | 1.95 ± 1.03 a | 0.44 ± 0.54 b |
| Propionic acid | 0.72 ± 0.17 ab | 0.82 ± 0.67 ab | 0.78 ± 0.47 ab | 1.42 ± 0.57 a | 0.24 ± 0.38 b |
| Butyric acid | 0.54 ± 0.12 ab | 0.61 ± 0.33 a | 0.67 ± 0.39 a | 0.66 ± 0.09 a | 0.18 ± 0.28 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posridee, K.; Chirinang, P.; Oonsivilai, A.; Oonsivilai, R. Evaluation of Short-Term Toxicity and Cholesterol-Lowering Effects in Rats Exposed to Dietary Fiber Derived from Cassava Pulp. Foods 2023, 12, 4074. https://doi.org/10.3390/foods12224074
Posridee K, Chirinang P, Oonsivilai A, Oonsivilai R. Evaluation of Short-Term Toxicity and Cholesterol-Lowering Effects in Rats Exposed to Dietary Fiber Derived from Cassava Pulp. Foods. 2023; 12(22):4074. https://doi.org/10.3390/foods12224074
Chicago/Turabian StylePosridee, Kakanang, Pornariya Chirinang, Anant Oonsivilai, and Ratchadaporn Oonsivilai. 2023. "Evaluation of Short-Term Toxicity and Cholesterol-Lowering Effects in Rats Exposed to Dietary Fiber Derived from Cassava Pulp" Foods 12, no. 22: 4074. https://doi.org/10.3390/foods12224074
APA StylePosridee, K., Chirinang, P., Oonsivilai, A., & Oonsivilai, R. (2023). Evaluation of Short-Term Toxicity and Cholesterol-Lowering Effects in Rats Exposed to Dietary Fiber Derived from Cassava Pulp. Foods, 12(22), 4074. https://doi.org/10.3390/foods12224074