Effects of Blanching, Freezing and Canning on the Carbohydrates in Sweet Corn
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
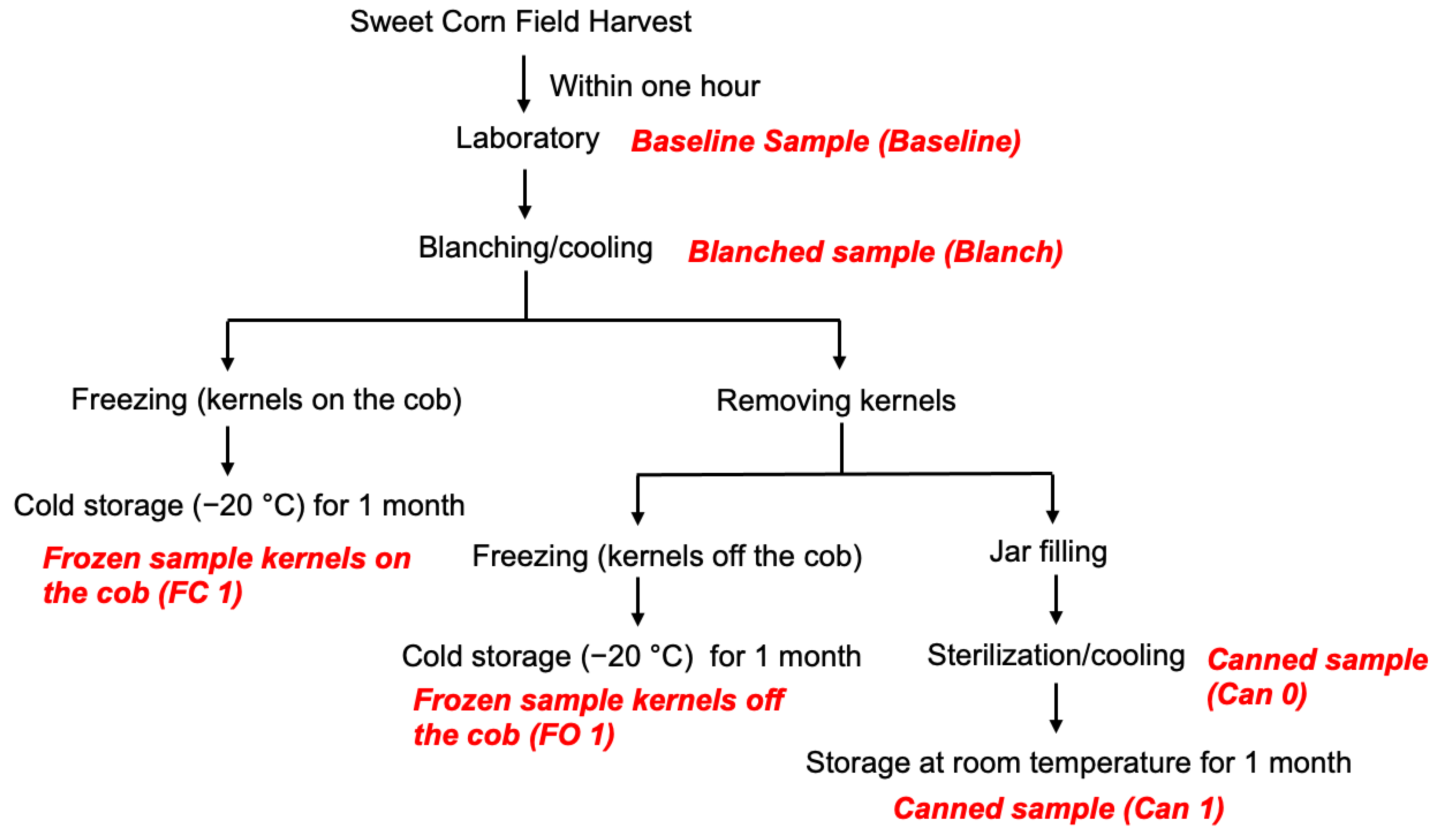
2.3. Sweet Corn Processing Methods
2.4. Analyses by AOAC Methods
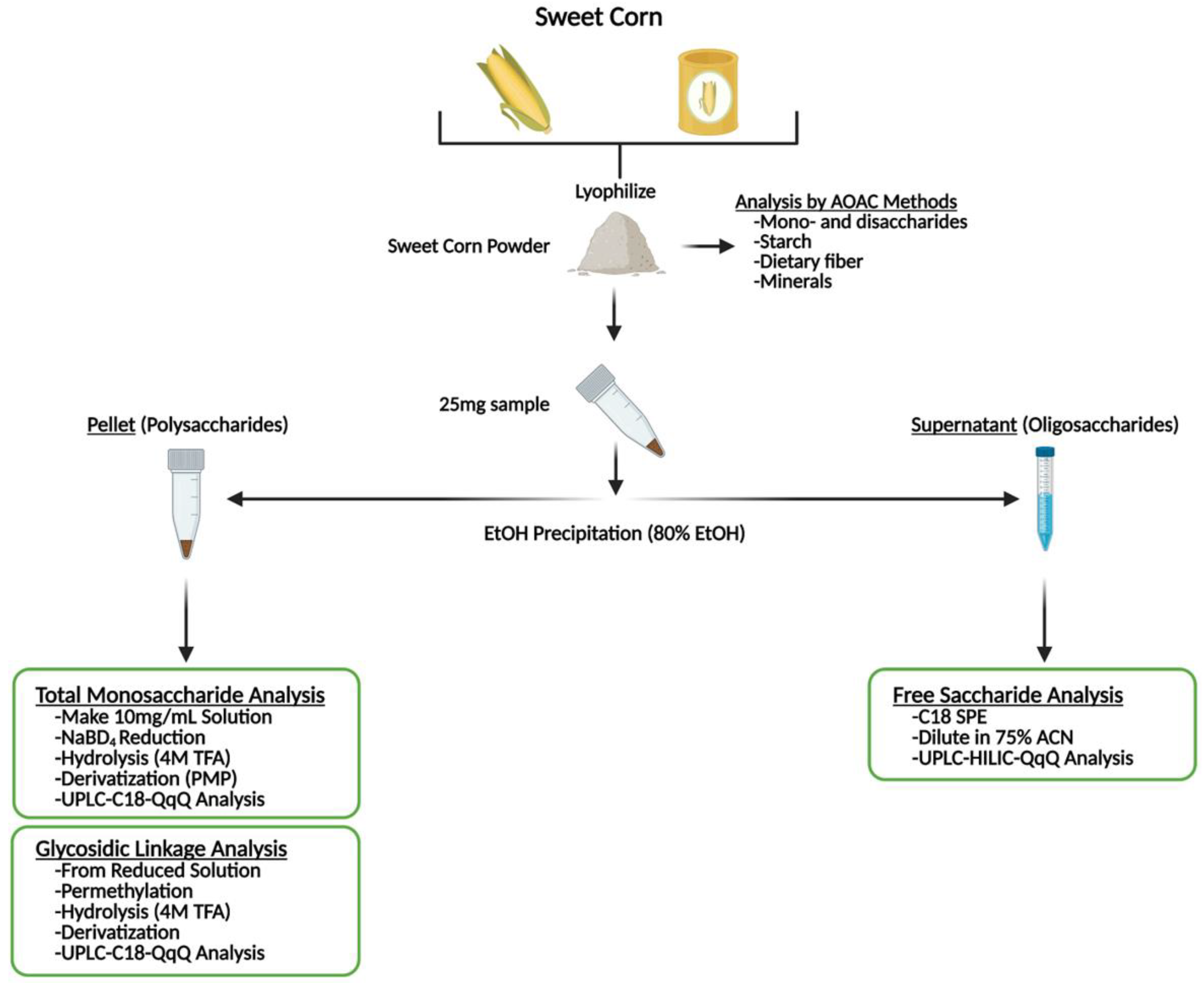
2.5. Sample Preparation for Glycomic Analysis
2.6. Analysis of Oligosaccharides
2.7. Analysis of Monosaccharide Composition of Total Polysaccharides (MCTP)
2.8. Analysis of Glycosidic Linkage of Total Polysaccharides (GLTP)
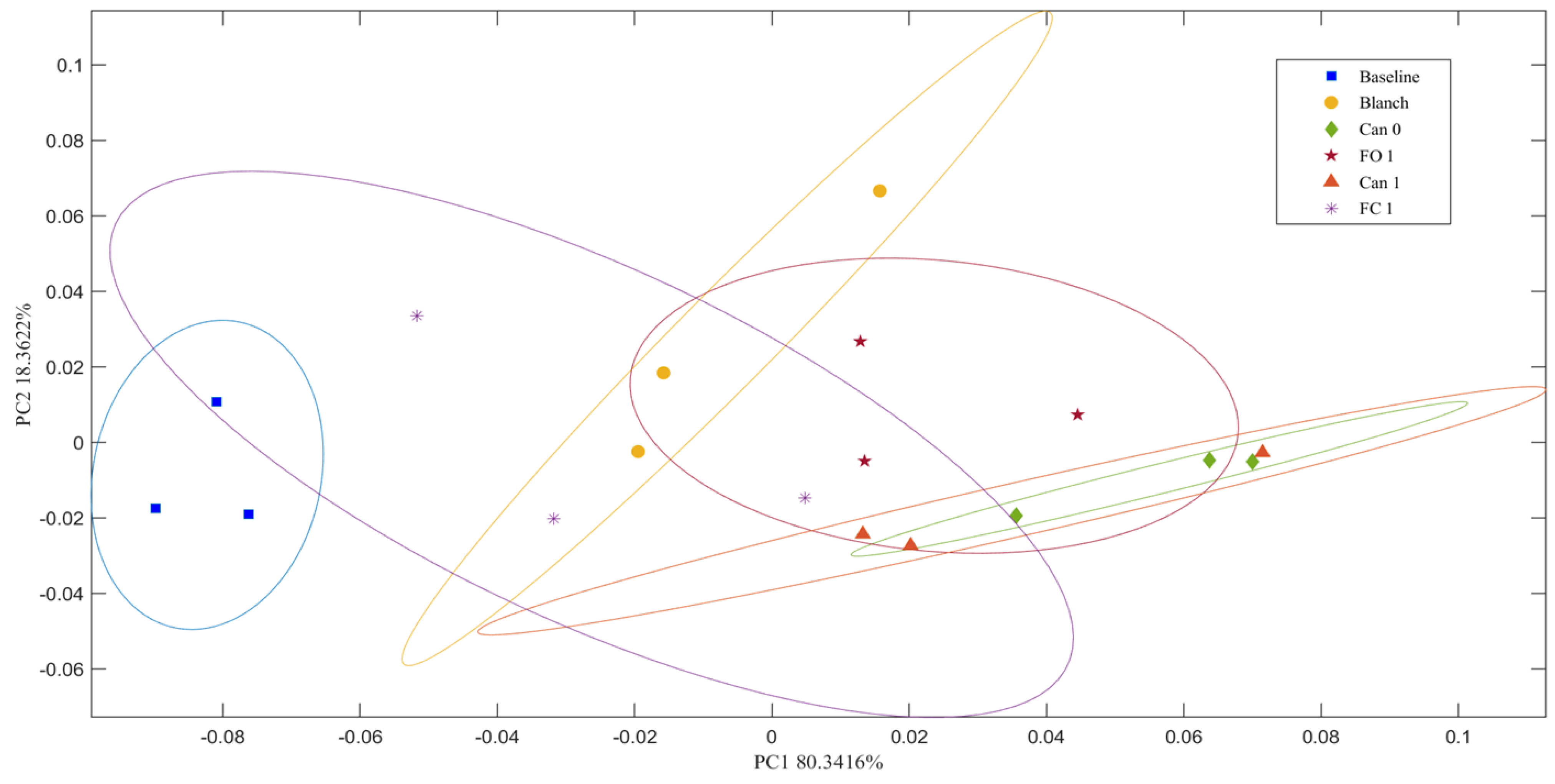
2.9. Principal Component Analysis
2.10. Statistical Analysis
3. Results
3.1. Quality Control for AOAC Methods
3.2. Carbohydrates in Fresh Sweet Corn
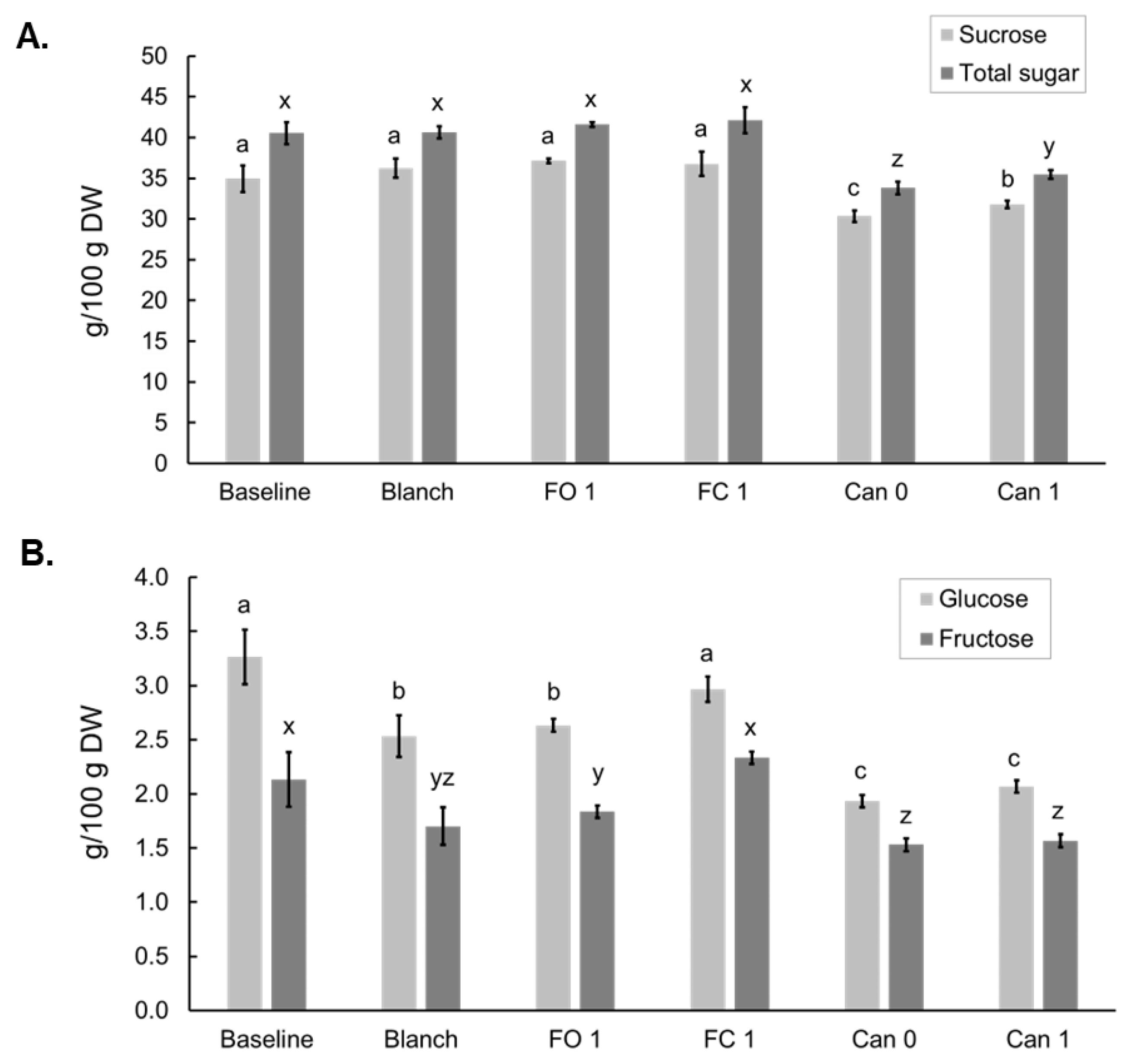
3.3. Effects of Blanching, Freezing and Canning on Simple Sugars
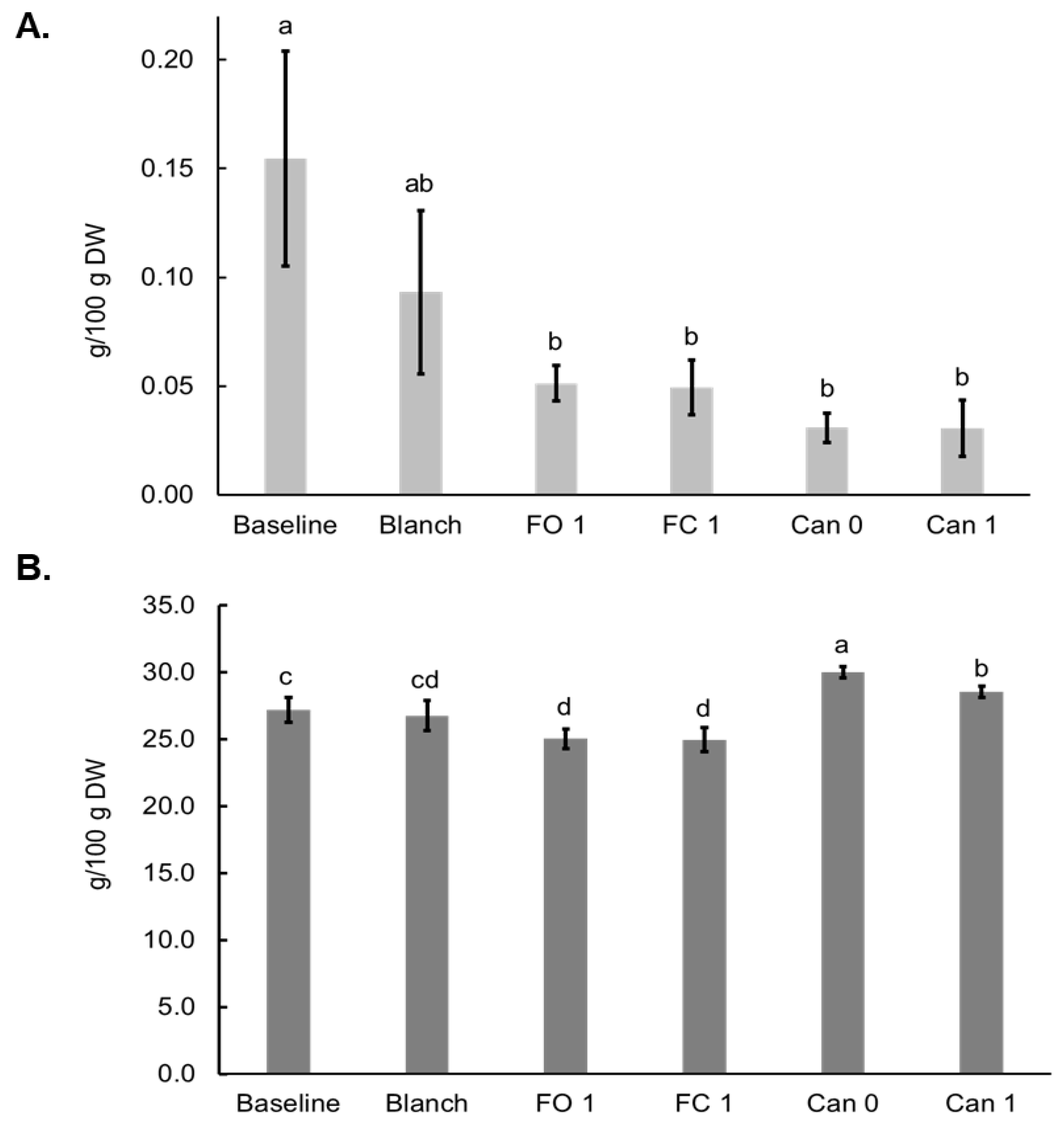
3.4. Effects of Blanching, Canning and Freezing on Oligosaccharide and Starch
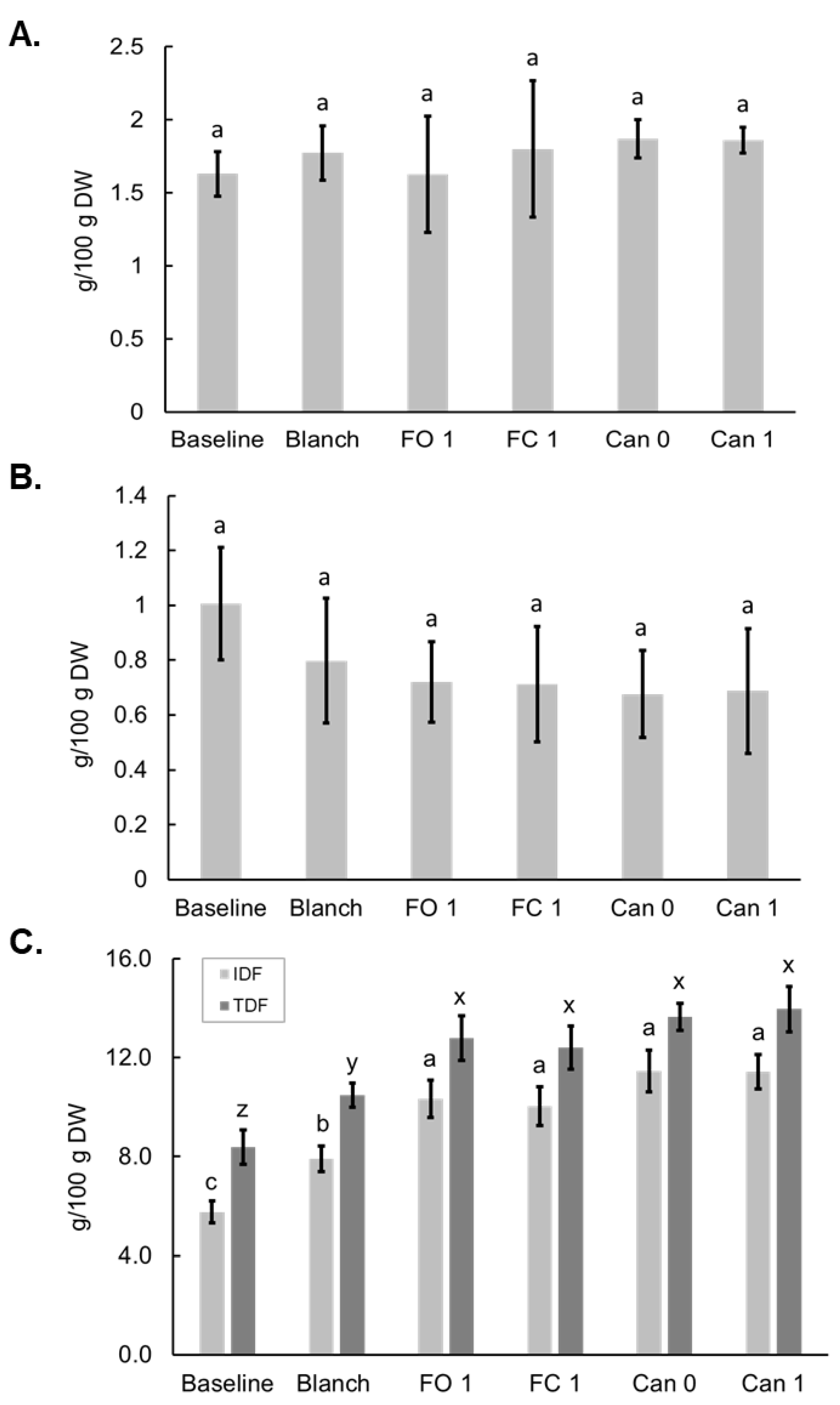
3.5. Effects of Blanching, Freezing and Canning on Dietary Fiber
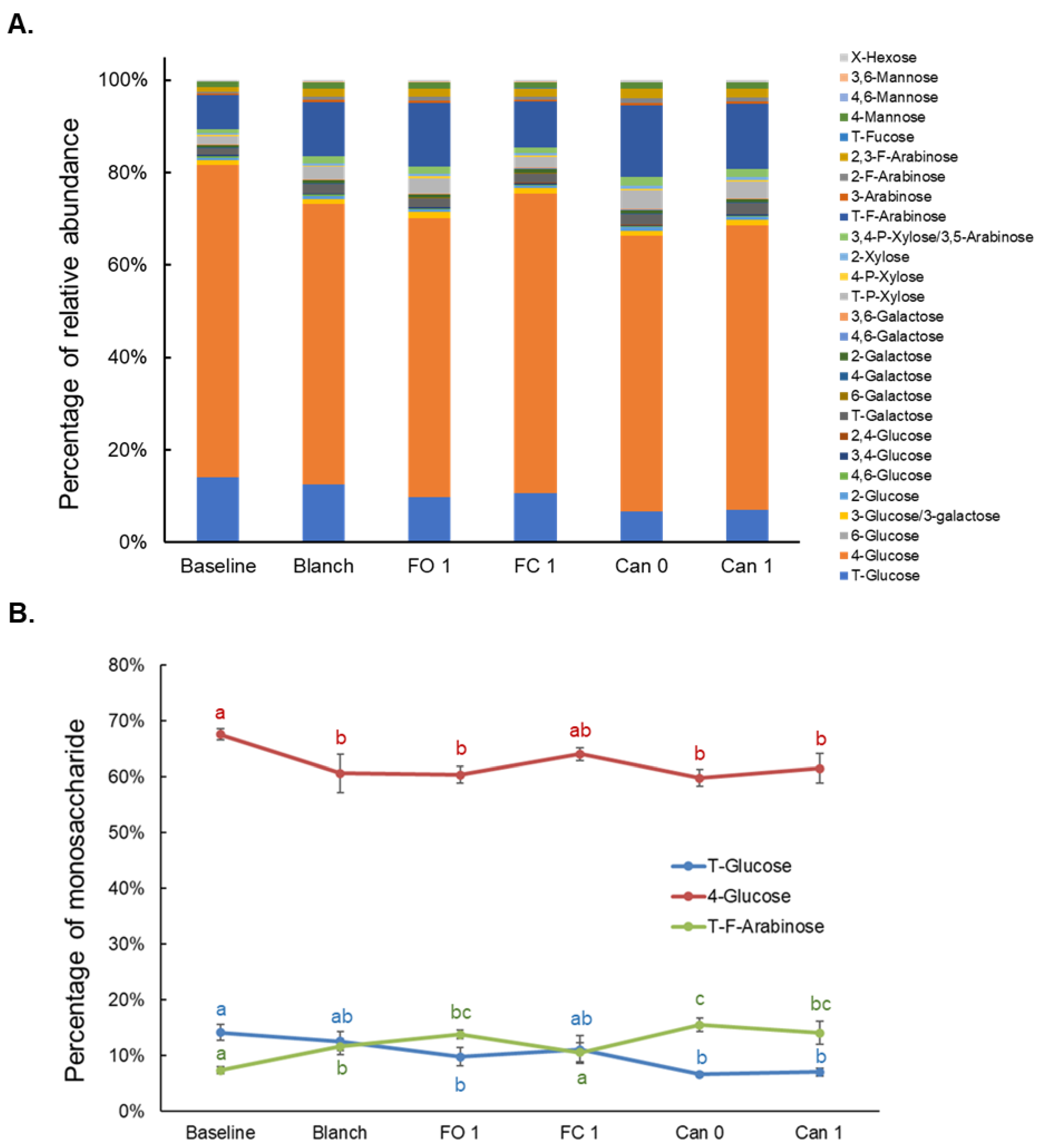
3.6. Effects of Blanching, Freezing and Canning on Monosaccharide Composition of Total Polysaccharides (MCTP)
3.7. Effects of Blanching, Freezing and Canning on Glycosidic Linkages of Total Polysaccharides (GLTP)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DF | Dietary fiber |
| TDF | Total dietary fiber |
| IDF | Insoluble dietary fiber |
| SDF | Soluble dietary fiber |
| SDFP | Dietary fiber soluble in water but not in 78% alcohol |
| SDFS | Dietary fiber soluble in water and 78% alcohol |
| LMWSDF | Low molecular weight dietary fiber |
| HMWSDF | High molecular weight dietary fiber |
| SRM | Standard reference material |
| QC | Quality control |
| MCTP | Monosaccharide composition of total polysaccharides |
| GLTP | Glycosidic linkage of total polysaccharides |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| PCA | Principal component analysis |
References
- USDA ERS. Potatoes and Tomatoes are the Most Commonly Consumed Vegetables. Available online: https://www.ers.usda.gov/data-products/chart-gallery/gallery/chart-detail/?chartId=58340 (accessed on 10 November 2022).
- Revilla, P.; Anibas, C.M.; Tracy, W.F. Sweet corn research around the world 2015–2020. Agronomy 2021, 11, 534. [Google Scholar] [CrossRef]
- Ai, Y.; Jane, J.-l. Macronutrients in corn and human nutrition. Compr. Rev. Food Sci. Food Saf. 2016, 15, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Haytowitz, D.B.; Ahuja, J.K.C.; Wu, X.; Somanchi, M.; Nickle, M.; Nguyen, Q.A.; Roseland, J.M.; Williams, J.R.; Patterson, K.Y.; Li, Y.; et al. USDA National Nutrient Database for Standard Reference. Available online: https://data.nal.usda.gov/dataset/usda-national-nutrient-database-standard-reference-legacy-release (accessed on 16 November 2022).
- Chambers, E.S.; Byrne, C.S.; Frost, G. Carbohydrate and human health: Is it all about quality? Lancet 2019, 393, 384–386. [Google Scholar] [CrossRef] [PubMed]
- US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; US Department of Agriculture and US Department of Health and Human Services, Ed.; US Government Publishing Office: Washington, DC, USA, 2020.
- Tan, D.; Drewnowski, A.; Lê, K.A. New metrics of dietary carbohydrate quality. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Canfora, E.E.; Blaak, E.E. Gastrointestinal transit time, glucose homeostasis and metabolic health: Modulation by dietary fibers. Nutrients 2018, 10, 275. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Hawkins, M.A.W.; Keirns, N.G.; Helms, Z. Carbohydrates and cognitive function. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 302–307. [Google Scholar] [CrossRef]
- Tao, J.; Li, S.; Gan, R.Y.; Zhao, C.N.; Meng, X.; Li, H.B. Targeting gut microbiota with dietary components on cancer: Effects and potential mechanisms of action. Crit. Rev. Food Sci. Nutr. 2020, 60, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- DeMartino, P.; Cockburn, D.W. Resistant starch: Impact on the gut microbiome and health. Curr. Opin. Biotechnol. 2020, 61, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Rezende, E.S.V.; Lima, G.C.; Naves, M.M.V. Dietary fibers as beneficial microbiota modulators: A proposed classification by prebiotic categories. Nutrition 2021, 89, 111217. [Google Scholar] [CrossRef] [PubMed]
- Quagliani, D.; Felt-Gunderson, P. Closing America’s fiber intake gap: Communication strategies from a food and fiber summit. Am. J. Lifestyle Med. 2017, 11, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Dodson-Swenson, H.G.; Tracy, W.F. Endosperm carbohydrate composition and kernel characteristics of Shrunken2-intermediate (sh2-i/sh2-i Su1/Su1) and Shrunken2-intermediate–sugary1-reference (sh2-i/sh2-i su1-r/su1-r) in sweet corn. Crop Sci. 2015, 55, 2647–2656. [Google Scholar] [CrossRef]
- FAO. Effects of food processing on dietary carbohydrates. In Carbohydrates in Human Nutrition; FAO: Rome, Italy, 1997. [Google Scholar]
- Margareta, E.; Nyman, E.M. Importance of processing for physico-chemical and physiological properties of dietary fibre. Proc. Nutr. Soc. 2003, 62, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Guillon, F.; Champ, M. Structural and physical properties of dietary fibres, and consequences of processing on human physiology. Food Res. Int. 2000, 33, 233–245. [Google Scholar] [CrossRef]
- Brand, J.C.; Nicholson, P.L.; Thorburn, A.W.; Truswell, A.S. Food processing and the glycemic index. Am. J. Clin. Nutr. 1985, 42, 1192–1196. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, B.; Childs, H.; Whent, M.; Yu, L.; Pehrsson, P.R.; Zhao, J.; Wu, X.; Li, S. Effects of boiling and steaming on the carbohydrates of sweet corn. ACS Food Sci. Technol. 2022, 2, 951–960. [Google Scholar] [CrossRef]
- Whent, M.; Huang, J.; Childs, H.; Slavini, M.; Harrison, D.; Novotny, J.; Yu, L.; Pehrsson, P.; Wu, X. Stability of carotenoids in sweet corn: Part 2. Effects of blanching, freezing, and canning. ACS Food Sci. Technol. 2023, 3, 1590–1599. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Method 2018.16. Sugar profile in food, dietary supplements, pet food and animal feeds. In AOAC Official Methods of Analysis; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2018. [Google Scholar]
- AOAC. AOAC Official Method 996.11. Starch (total) in cereal products. In AOAC Official Methods of Analysis; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. AOAC Official Method 2011.25. Insoluble, soluble, and total dietary fiber in foods. In AOAC Official Methods of Analysis; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2011. [Google Scholar]
- McCleary, B.V. Modification to AOAC official methods 2009.01 and 2011.25 to allow for minor overestimation of low molecular weight soluble dietary fiber in samples containing starch. J. AOAC Int. 2014, 97, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.M.; Patterson, K.Y.; Rasor, A.S.; Exler, J.; Haytowitz, D.B.; Holden, J.M.; Pehrsson, P.R. Quality-control materials in the USDA National Food and Nutrient Analysis Program (NFNAP). Anal. Bioanal. Chem. 2006, 384, 1341–1355. [Google Scholar] [CrossRef]
- NIST. Certificate of Analysis. Standard Reference Material 3233 Fortified Breakfast Cereal; National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2020.
- Couture, G.; Luthria, D.L.; Chen, Y.; Bacalzo, N.P., Jr.; Tareq, F.S.; Harnly, J.; Phillips, K.M.; Pehrsson, P.R.; McKillop, K.; Fukagawa, N.K.; et al. Multi-glycomic characterization of fiber from aoac methods defines the carbohydrate structures. J. Agric. Food Chem. 2022, 70, 14559–14570. [Google Scholar] [CrossRef]
- Galermo, A.G.; Nandita, E.; Barboza, M.; Amicucci, M.J.; Vo, T.T.; Lebrilla, C.B. Liquid chromatography-tandem mass spectrometry approach for determining glycosidic linkages. Anal. Chem. 2018, 90, 13073–13080. [Google Scholar] [CrossRef]
- Scott, P.; Pratt, R.C.; Hoffman, N.; Montgomery, R. Chapter 10—Specialty Corns. In Corn, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Press: Oxford, UK, 2019; pp. 289–303. [Google Scholar]
- Szymanek, M.; Tanaś, W.; Kassar, F.H. Kernel carbohydrates concentration in Sugary-1, Sugary Enhanced and Shrunken sweet corn kernels. Agric. Agric. Sci. Procedia 2015, 7, 260–264. [Google Scholar] [CrossRef]
- Warman, P.R.; Havard, K.A. Yield, vitamin and mineral contents of organically and conventionally grown potatoes and sweet corn. Agric. Ecosyst. Environ. 1998, 68, 207–216. [Google Scholar] [CrossRef]
- Watanabe, A.; Tochio, T.; Kadota, Y.; Takahashi, M.; Kitaura, Y.; Ishikawa, H.; Yasutake, T.; Nakano, M.; Shinohara, H.; Kudo, T.; et al. Supplementation of 1-kestose modulates the gut microbiota composition to ameliorate glucose metabolism in obesity-prone hosts. Nutrients 2021, 13, 2983. [Google Scholar] [CrossRef] [PubMed]
- McCleary, B.V.; Cox, J. Evolution of a definition for dietary fiber and methodology to service this definition. Luminacoids Res. 2017, 21, 9–21. [Google Scholar]
- Hipsley, E.H. Dietary “fibre” and pregnancy toxaemia. Br. Med. J. 1953, 2, 420–422. [Google Scholar] [CrossRef]
- Trowell, H. Dietary fibre and coronary heart disease. Revue europeenne d’etudes cliniques et biologiques. Rev. Eur. Etud. Clin. Biol. 1972, 17, 345–349. [Google Scholar] [PubMed]
- McCleary, B.V.; Sloane, N.; Draga, A.; Lazewska, I. Measurement of Total Dietary Fiber Using AOAC Method 2009.01 (AACC International Approved Method 32-45.01): Evaluation and Updates. Cereal Chem. 2013, 90, 396–414. [Google Scholar] [CrossRef]
- Kachhadiya, S.; Kumar, N.; Seth, N. Process kinetics on physico-chemical and peroxidase activity for different blanching methods of sweet corn. J. Food Sci. Technol. 2018, 55, 4823–4832. [Google Scholar] [CrossRef] [PubMed]
- Szymanek, M.; Dziwulska-Hunek, A.; Tanaś, W. Influence of blanching time on moisture, sugars, protein, and processing recovery of sweet corn kernels. Processes 2020, 8, 340. [Google Scholar] [CrossRef]
- Barrett, D.M.; Garcia, E.L.; Russell, G.F.; Ramirez, E.; Shirazi, A. Blanch time and cultivar effects on quality of frozen and stored corn and broccoli. J. Food Sci. 2000, 65, 534–540. [Google Scholar] [CrossRef]
- Alan, Ö.; Kinaci, G.; Kinaci, E.; Basciftci, Z.B.; Sonmez, K.; Evrenosoglu, Y.; Kutlu, I. Kernel quality of some sweet corn varieties in relation to processing. Not. Bot. Horti Agrobot. 2014, 42, 414–419. [Google Scholar] [CrossRef][Green Version]
- Yu, X.; Yu, H.; Zhang, J.; Shao, S.; Xiong, F.; Wang, Z. Endosperm structure and physicochemical properties of starches from normal, waxy, and super-sweet maize. Int. J. Food Prop. 2015, 18, 2825–2839. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Peris-Tortajada, M. Chapter 6—Measuring Starch in Food. In Starch in Food, 2nd ed.; Sjöö, M., Nilsson, L., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 255–281. [Google Scholar] [CrossRef]
- McCleary, B.V.; Charmier, L.M.J.; McKie, V.A. Measurement of starch: Critical evaluation of current methodology. Starch 2019, 71, 1800146. [Google Scholar] [CrossRef]
- Makhlouf, J.; Zee, J.; Tremblay, N.; Bélanger, A.; Michaud, M.-H.; Gosselin, A. Some nutritional characteristics of beans, sweet corn and peas (raw, canned and frozen) produced in the province of Quebec. Food Res. Int. 1995, 28, 253–259. [Google Scholar] [CrossRef]
- Rickman, J.C.; Bruhn, C.M.; Barrett, D.M. Nutritional comparison of fresh, frozen, and canned fruits and vegetables II. Vitamin A and carotenoids, vitamin E, minerals and fiber. J. Sci. Food Agric. 2007, 87, 1185–1196. [Google Scholar] [CrossRef]
- Saha, B.C.; Bothast, R.J. Pretreatment and enzymatic saccharification of corn fiber. Appl. Biochem. Biotechnol. 1999, 76, 65–77. [Google Scholar] [CrossRef]
- Englyst, K.N.; Liu, S.; Englyst, H.N. Nutritional characterization and measurement of dietary carbohydrates. Eur. J. Clin. Nutr. 2007, 61 (Suppl. S1), S19–S39. [Google Scholar] [CrossRef] [PubMed]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch—A review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Faridah, D.N.; Damaiyanti, S.; Indrasti, D.; Jayanegara, A.; Afandi, F.A. Effect of heat moisture treatment on resistant starch content among carbohydrate sources: A meta-analysis. Int. J. Food Sci. Technol. 2021, 57, 1965–1974. [Google Scholar] [CrossRef]
- Asp, N.-G.; Björck, I. Resistant starch. Trends Food Sci. Technol. 1992, 3, 111–114. [Google Scholar] [CrossRef]
- Dong, L.; Qi, X.; Zhu, J.; Liu, C.; Zhang, X.; Cheng, B.; Mao, L.; Xie, C. Supersweet and waxy: Meeting the diverse demands for specialty maize by genome editing. Plant Biotechnol. J. 2019, 17, 1853–1855. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.-h.; Kong, L.-l.; Liu, J.-f.; Zhang, Y.; Yang, Y.-h. Creation method and identification technology for sweet-waxy maize of double recessive genotype. China Veg. 2018, 1, 28–32. [Google Scholar]
- Martens, E.C.; Kelly, A.G.; Tauzin, A.S.; Brumer, H. The devil lies in the details: How variations in polysaccharide fine-structure impact the physiology and evolution of gut microbes. J. Mol. Biol. 2014, 426, 3851–3865. [Google Scholar] [CrossRef] [PubMed]
- Romero Marcia, A.D.; Yao, T.; Chen, M.H.; Oles, R.E.; Lindemann, S.R. Fine carbohydrate structure of dietary resistant glucans governs the structure and function of human gut microbiota. Nutrients 2021, 13, 2924. [Google Scholar] [CrossRef] [PubMed]
- Tuncil, Y.E.; Thakkar, R.D.; Arioglu-Tuncil, S.; Hamaker, B.R.; Lindemann, S.R. Subtle variations in dietary-fiber fine structure differentially influence the composition and metabolic function of gut microbiota. mSphere 2020, 5, e00180-20. [Google Scholar] [CrossRef] [PubMed]
- Magallanes-Cruz, P.A.; Flores-Silva, P.C.; Bello-Perez, L.A. Starch structure influences its digestibility: A review. J. Food Sci. 2017, 82, 2016–2023. [Google Scholar] [CrossRef]
- Amicucci, M.J.; Nandita, E.; Lebrilla, C.B. Function without structures: The need for in-depth analysis of dietary carbohydrates. J. Agric. Food Chem. 2019, 67, 4418–4424. [Google Scholar] [CrossRef] [PubMed]

| Carbohydrate Fraction | Concentration (g/100 g, Fresh Weight) |
|---|---|
| Glucose | 0.83 ± 0.06 |
| Sucrose | 8.85 ± 0.42 |
| Fructose | 0.52 ± 0.08 |
| Total sugars | 10.27 ± 0.34 |
| Starch | 6.89 ± 0.23 |
| Kestose | 0.15 ± 0.05 |
| Insoluble dietary fiber (IDF) | 1.46 ± 0.11 |
| Soluble dietary fiber precipitate (SDFP) | 0.41 ± 0.04 |
| Dietary fiber soluble in water and alcohol (SDFS, LMWDF) | 0.25 ± 0.05 |
| Total soluble fiber (SDFP + SDFS) | 0.67 ± 0.09 |
| HMWDF (IDF + SDFP) | 1.87 ± 0.13 |
| Total dietary fiber (IDF + SDFP + SDFS) | 2.13 ± 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whent, M.M.; Childs, H.D.; Ehlers Cheang, S.; Jiang, J.; Luthria, D.L.; Bukowski, M.R.; Lebrilla, C.B.; Yu, L.; Pehrsson, P.R.; Wu, X. Effects of Blanching, Freezing and Canning on the Carbohydrates in Sweet Corn. Foods 2023, 12, 3885. https://doi.org/10.3390/foods12213885
Whent MM, Childs HD, Ehlers Cheang S, Jiang J, Luthria DL, Bukowski MR, Lebrilla CB, Yu L, Pehrsson PR, Wu X. Effects of Blanching, Freezing and Canning on the Carbohydrates in Sweet Corn. Foods. 2023; 12(21):3885. https://doi.org/10.3390/foods12213885
Chicago/Turabian StyleWhent, Monica M., Holly D. Childs, Shawn Ehlers Cheang, Jiani Jiang, Devanand L. Luthria, Michael R. Bukowski, Carlito B. Lebrilla, Liangli Yu, Pamela R. Pehrsson, and Xianli Wu. 2023. "Effects of Blanching, Freezing and Canning on the Carbohydrates in Sweet Corn" Foods 12, no. 21: 3885. https://doi.org/10.3390/foods12213885
APA StyleWhent, M. M., Childs, H. D., Ehlers Cheang, S., Jiang, J., Luthria, D. L., Bukowski, M. R., Lebrilla, C. B., Yu, L., Pehrsson, P. R., & Wu, X. (2023). Effects of Blanching, Freezing and Canning on the Carbohydrates in Sweet Corn. Foods, 12(21), 3885. https://doi.org/10.3390/foods12213885






