Identification of Polyphenols in Sea Fennel (Crithmum maritimum) and Seaside Arrowgrass (Triglochin maritima) Extracts with Antioxidant, ACE-I, DPP-IV and PEP-Inhibitory Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Processing
2.3. Preparation of the Extract
2.4. Determination of Total Phenol Content
2.5. Scavenging Activity of ABTS Radical Cation
2.6. Ferric Reducing Antioxidant Power (FRAP)
2.7. Determination of ACE Inhibitory Activity
2.8. Determination of PEP Inhibitory Activity
2.9. Determination of DPP-IV Inhibitory Activity
2.10. Chromatographic Fractionation of the Extracts
2.11. Tentative Identification of Polyphenols by HPLC-ESI-QTOF-MS
2.12. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Content and Bioactivity of the Extracts
3.1.1. Phenolic Content
3.1.2. Antioxidant Activity
3.1.3. Enzyme Inhibitory Capacity of the Ethanolic Extracts
3.2. Characterization and Bioactivity of the Fractions
3.2.1. Identification of Potential Bioactive Polyphenols Present in the Factions
3.2.2. Antioxidant Activity of the Fractions
3.2.3. Enzyme Inhibitory Activities of the Bioactive Fractions
ACE-Inhibitory Activity
DPP-IV Inhibitory Activity
PEP Inhibitory Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faustino, M.V.; Faustino, M.A.F.; Pinto, D.C.G.A. Halophytic grasses, a new source of nutraceuticals? A review on their secondary metabolites and biological activities. Int. J. Mol. Sci. 2019, 20, 1067. [Google Scholar] [CrossRef]
- Kafi, M.; Khan, M.A. Crop and Forage Production Using Saline Waters; Centre for Science & Technology of Non-Aligned and other Developing Countries (NAM S&T Centre); Daya Publishing House: New Delhi, India, 2008; Volume 1. [Google Scholar]
- Li, L.; Zhao, Y.; Han, G.; Guo, J.; Meng, Z.; Chen, M. Progress in the study and use of seawater vegetables. J. Agric. Food Chem. 2020, 68, 5998–6006. [Google Scholar] [CrossRef]
- Flowers, T.J.; Galal, H.K.; Bromham, L. Evolution of halophytes: Multiple origins of salt tolerance in land plants. Funct. Plant Biol. 2010, 37, 604–612. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Majdoub, H. Pectic polysaccharides from edible halophytes: Insight on extraction processes, structural characterizations and immunomodulatory potentials. Int. J. Biol. Macromol. 2021, 173, 554–579. [Google Scholar] [CrossRef]
- Lefèvre, G.; Rivière, C. Amaranthaceae halophytes from the French Flanders coast of the North Sea: A review of their phytochemistry and biological activities. Phytochem. Rev. 2020, 19, 1263–1302. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Pinto, D.C.G.A.; Cunha, Â.; Silva, H. Halophytes as Medicinal Plants against human infectious diseases. Appl. Sci. 2022, 12, 7493. [Google Scholar] [CrossRef]
- Lopes, M.; Sanches-Silva, A.; Castilho, M.; Cavaleiro, C.; Ramos, F. Halophytes as source of bioactive phenolic compounds and their potential applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 1078–1101. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Halophytic herbs of the Mediterranean basin: An alternative approach to health. Food Chem. Toxicol. 2018, 114, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magne, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Kraouia, M.; Nartea, A.; Maoloni, A.; Osimani, A.; Garofalo, C.; Fanesi, B.; Ismaiel, L.; Aquilanti, L.; Pacetti, D. Sea Fennel (Crithmum maritimum L.) as an emerging crop for the manufacturing of innovative foods and nutraceuticals. Molecules 2023, 28, 4741. [Google Scholar] [CrossRef]
- Sánchez-Faure, A.; Calvo, M.M.; Pérez-Jiménez, J.; Martín-Diana, A.B.; Rico, D.; Montero, M.P.; Gómez-Guillén, M.C.; López-Caballero, M.E.; Martínez-Alvarez, O. Exploring the potential of common iceplant, seaside arrowgrass and sea fennel as edible halophytic plants. Food Res. Int. 2020, 137, 109613. [Google Scholar] [CrossRef] [PubMed]
- Maoloni, A.; Cardinali, F.; Mila, V. Microbiological safety and stability of novel green sauces made with sea fennel (Crithmum maritimum L.). Food Res. Inter. 2022, 157, 111463. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.; Alve, M.I.; Neves, M.; Tecelão, C.; Ferreira-Dias, S. Enrichment of sunflower oil with ultrasound-assisted extracted bioactive compounds from Crithmum maritimum L. Foods 2022, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Piatti, D.; Angeloni, S.; Maggi, F.; Caprioli, G.; Ricciutelli, M.; Arnoldi, L.; Bosisio, S.; Mombelli, G.; Drenaggi, E.; Sagratini, G. Comprehensive characterization of phytochemicals in edible sea fennel (Crithmum maritimum L., Apiaceae) grown in central Italy. J. Food Comp. Anal. 2023, 115, 104884. [Google Scholar] [CrossRef]
- Politeo, O.; Popović, M.; Bratinčević, M.V.; Kovačević, K.; Urlić, B.; Mekinić, I.G. Chemical profiling of Sea Fennel (Crithmum maritimum L., Apiaceae) essential oils and their isolation residual waste-waters. Plants 2023, 12, 214. [Google Scholar] [CrossRef]
- Zafeiropoulou, V.; Tomou, E.-M.; Douros, A.; Skaltsa, H. The effect of successive harvesting on the volatile constituents of two essential oils of cultivated populations of sea fennel (Crithmum maritimum L.) in Greece. J. Essent. Oil Bear. Plants 2021, 24, 1–11. [Google Scholar] [CrossRef]
- Souid, A.; Croce, C.M.D.; Frassinetti, S.; Gabriele, M.; Pozzo, L.M.; Ciardi, M.; Abdelly, C.; Ben Hamed, K.; Magné, C.; Longo, V. Nutraceutical potential of leaf hydro-ethanolic extract of the edible halophyte Crithmum maritimum L. Molecules 2021, 26, 5380. [Google Scholar] [CrossRef]
- Mekinić, I.G.; Blazěvić, I.; Mudnić, I.; Burčul, F.; Grga, M.; Skroza, D.; Jerčić, I.; Ljubenkov, I.; Boban, M.; Milos, M.; et al. Sea fennel (Crithmum maritimum L.): Phytochemical profile, antioxidative, cholinesterase inhibitory and vasodilatory activity. J. Food Sci. Technol. 2016, 53, 3104–3112. [Google Scholar] [CrossRef]
- Jallali, I.; Zaouali, Y.; Missaoui, I.; Smeoui, A.; Abdelly, C.; Ksouri, R. Variability of antioxidant and antibacterial effects of essential oils and acetonic extracts of two edible halophytes: Crithmum maritimum L. and Inula crithmoїdes L. Food Chem. 2014, 145, 1031–1038. [Google Scholar] [CrossRef]
- Lee, J.M.; Min-Jin, Y.; Grace, C.; Lee, M.S.; Park, Y.G.; Dae-Sung, L. Antioxidant and anti-inflammatory activity of six halophytes in Korea. Nat. Prod. Sci. 2018, 24, 40–46. [Google Scholar] [CrossRef]
- Boestfleisch, C.; Papenbrock, J. Changes in secondary metabolites in the halophytic putative crop species Crithmum maritimum L., Triglochin maritima L. and Halimione portulacoides (L.) Aellen as reaction to mild salinity. PLoS ONE 2017, 12, e0176303. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Lamba, S.; Kumar, A.; Kumar, P.; Mann, A.; Devi, D.S.; Pooja, P.; Kumari, A.; Rani, B. Antioxidant defence in halophytes under high salinity. In Halophytes and Climate Change: Adaptive Mechanisms and Potential Uses; Hasanuzzaman, E.M., Shabala, S., Fujita, M., Eds.; CAB International: Wallingford, UK, 2019. [Google Scholar]
- Chakraborty, R.; Roy, S. Angiotensin-converting enzyme inhibitors from plants: A review of their diversity, modes of action, prospects, and concerns in the management of diabetes-centric complications. J. Integr. Med. 2021, 19, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Cicero, A.F.; Agnoletti, D.; Fiorini, G. Pathophysiology of cough with angiotensin-converting enzyme inhibitors: How to explain within-class differences? Eur. J. Intern. Med. 2023, 110, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, L.; Shu, G.; Yuan, J.; Zhang, J.; Qin, S.; Li, J. Enhanced antihypertensive potential of fermented pomegranate juice: The contribution of phenolic compounds biotransformation and the resultant angiotensin-I-converting enzyme inhibition mechanism. Food Chem. 2023, 404, 134745. [Google Scholar] [CrossRef]
- Calvo, M.M.; Martín-Diana, A.B.; Rico, D.; López-Caballero, M.E.; Martínez-Álvarez, O. Antioxidant, antihypertensive, hypoglycaemic and nootropic activity of a polyphenolic extract from the halophyte Ice Plant (Mesembryanthemum crystallinum). Foods 2022, 11, 1581. [Google Scholar] [CrossRef]
- Song, F.; Tang, M.M.; Wang, H.; Zhang, Y.F.; Zhu, K.X.; Chen, X.A.; Chen, H.; Zhao, X.M. UHPLC-MS/MS identification, quantification of flavonoid compounds from Areca catechu L. extracts and in vitro evaluation of antioxidant and key enzyme inhibition properties involved in hyperglycemia and hypertension. Ind. Crops. Prod. 2022, 189, 115787. [Google Scholar] [CrossRef]
- Wijesekara, I.; Kim, S.-K. Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: Prospects in the pharmaceutical industry. Mar. Drugs 2010, 8, 1080–1093. [Google Scholar] [CrossRef]
- Fan, W.Z.; Tezuka, Y.; Komatsu, K.; Namba, T.; Kadota, S. Prolyl endopeptidase inhibitors from the underground part of Rhodiola sacra S.H. Fu. Biol. Pharm. Bull. 1999, 22, 157–161. [Google Scholar] [CrossRef][Green Version]
- Singh, A.-K.; Yadav, D.; Sharma, N.; Jin, J.-O. Dipeptidyl Peptidase (DPP)-IV inhibitors with antioxidant potential isolated from natural sources: A novel approach for the management of diabetes. Pharmaceuticals 2021, 14, 586. [Google Scholar] [CrossRef]
- Farias, D.D.; de Araujo, F.F.; Neri-Numa, I.A.; Pastore, G. M Antidiabetic potential of dietary polyphenols: A mechanistic review. Food Res. Int. 2021, 145, 110383. [Google Scholar] [CrossRef]
- Soukup, O.; Stralkova, R.; Mistova, T.; Popov, M.; Hejtmankova, A.; Lachman, J.; Klimova, B.; Kuca, K. Polyphenolic compound from Vitis vinifera L. have potential for the Alzheimer disease treatment. Lett. Drug. Des. Discov. 2017, 14, 853–859. [Google Scholar] [CrossRef]
- Sila, A.; Martinez-Alvarez, O.; Haddar, A.; Gómez-Guillén, M.C.; Nasri, M.; Montero, M.P.; Bougatef, A. Recovery, viscoelastic and functional properties of Barbel skin gelatine: Investigation of anti-DPP-IV and anti-prolyl endopeptidase activities of generated gelatine polypeptide. Food Chem. 2015, 168, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.R.; Paik, Y.S. Antioxidative and prolyl endopeptidase inhibitory activities of the phenolic constituents isolated from Phellinus linteus. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 652–656. [Google Scholar] [CrossRef]
- Gass, J.; Khosla, C. Prolyl endopeptidases. Cell. Mol. Life Sci. 2007, 64, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Sentandreu, M.A.; Toldrá, F.A. Rapid, simple and sensitive fluorescence method for the assay of angiotensin-I converting enzyme. Food Chem. 2006, 97, 546–554. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Houta, O.; Akrout, A.; Neffati, M.; Amri, H. Phenolic contents, antioxidant and antimicrobial potentials of Crithmum maritimum cultivated in Tunisia arid zones. J. Biol. Active Prod. Nature 2011, 1, 138–143. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Magné, C. Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiol. Biochem. 2009, 47, 37–41. [Google Scholar] [CrossRef]
- Veršić Bratinčević, M.; Kovačić, R.; Popović, M.; Radman, S.; Generalić Mekinić, I. Comparison of Conventional and Green Extraction Techniques for the Isolation of Phenolic Antioxidants from Sea Fennel. Processes 2023, 11, 2172. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, H.; Yin, G.Y.; Chen, L.Y.; Hu, C.Z. Extraction of polysaccharides from pumpkin by ultrasonic method and it’s primary antioxidant activities research. Adv. Mat. Res. 2011, 183–185, 1970–1974. [Google Scholar] [CrossRef]
- Kadoglidou, K.; Irakli, M.; Boutsika, A.; Mellidou, I.; Maninis, N.; Sarrou, E.; Georgiadou, V.; Tourvas, N.; Krigas, N.; Moysiadis, T.; et al. Metabolomic fingerprinting and molecular characterization of the rock samphire germplasm collection from the Balkan Botanic Garden of Kroussia, northern Greece. Plants 2022, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Nabet, N.; Boudries, H.; Chougui, N.; Loupassaki, S.; Souagui, S.; Burló, F.; Hernández, F.; Carbonell-Barrachina, A.A.; Madani, K.; Larbat, R. Biological activities and secondary compound composition from Crithmum maritimum aerial parts. Int. J. Food Prop. 2017, 20, 1843–1855. [Google Scholar] [CrossRef]
- Zengin, G.; Sinan, K.I.; Ak, G.; Mahomoodally, M.F.; Paksoy, M.Y.; Picot-Allain, C.; Glamocilja, J.; Sokovic, M.; Jekő, J.; Cziákyg, Z.; et al. Chemical profile, antioxidant, antimicrobial, enzyme inhibitory, and cytotoxicity of seven Apiaceae species from Turkey: A comparative study. Ind. Crops Prod. 2020, 153, 112572. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Said, A.; Tundis, R.; Rashed, K.; Statti, G.A.; Hufner, A.; Menichini, F. Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excelsa (Roxb) (Simaroubaceae). Phytother. Res. 2007, 21, 32–36. [Google Scholar] [CrossRef]
- Men, R.; Li, N.; Xing, Y.; Tang, Y.; Tan, C.; Meng, F.; Zhang, J.; Ni, H.; Jia, X. Chemical constituents and ACE inhibitory activity of desert plant Suaeda physophora. Pall. Acta Pharm. Sin. B 2013, 3, 328–332. [Google Scholar] [CrossRef]
- Shukor, N.A.; Van Camp, J.; Gonzales, G.B.; Staljanssens, D.; Struijs, K.; Zotti, M.J.; Raes, K.; Smagghe, G. Angiotensin-converting enzyme inhibitory effects by plant phenolic compounds: A study of structure activity relationships. J. Agric. Food Chem. 2013, 61, 11832–11839. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Hu, S.; Gong, G.; Zhao, J. Recent advances in understanding dietary polyphenols protecting against hypertension. Trends Food Sci. Technol. 2023, 138, 685–696. [Google Scholar] [CrossRef]
- Li, H.F.; Chen, S.A.; Wu, S.N. Evidence for the stimulatory effect of resveratrol on Ca(2+)-activated K+ current in vascular endothelial cells. Cardiovasc. Res. 2000, 45, 1035–1045. [Google Scholar] [CrossRef]
- Sharifi, A.M.; Darabi, R.; Akbarloo, N. Study of antihypertensive mechanism of Tribulus terrestris in 2K1C hypertensive rats: Role of tissue ACE activity. Life Sci. 2003, 73, 2963–2971. [Google Scholar] [CrossRef]
- Phillips, O.A.; Mathew, K.T.; Oriowo, M.A. Antihypertensive and vasodilator effects of methanolic and aqueous extracts of Tribulus terrestris in rats. J. Ethnopharmacol. 2006, 104, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Murshid, S.S.A.; Atoum, D.; Abou-Hussein, D.R.; Abdallah, H.M.; Hareeri, R.H.; Almukadi, H.; Edrada-Ebel, R. Genus Salsola: Chemistry, Biological Activities and Future Prospective—A Review. Plants 2022, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Gattringer, J.; Ndogo, O.E.; Retzl, B.; Ebermann, C.; Gruber, C.W.; Hellinger, R. Cyclotides isolated from violet plants of Cameroon are inhibitors of human Prolyl Oligopeptidase. Front. Pharmacol. 2021, 12, 707596. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.R.; Stuker, C.; Kichik, N.; Tarrago, T.; Giralt, E.; Morel, A.F.; Dalcol, I.I. Flavonoids with prolyl oligopeptidase inhibitory activity isolated from Scutellaria racemosa Pers. Fitoterapia 2010, 81, 552–556. [Google Scholar] [CrossRef]
- Tarrago, T.; Kichik, N.; Segui, J.; Giralt, E. The natural product berberine is a human prolyl oligopeptidase inhibitor. Chem. Med. Chem. 2007, 2, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Maoloni, A.; Pirker, T.; Pferschy-Wenzig, E.M.; Aquilanti, L.; Bauer, R. Characterization of potentially health-promoting constituents in sea fennel (Crithmum maritimum) cultivated in the Conero Natural Park (Marche region, Central Italy). Pharm. Biol. 2023, 61, 1030–1040. [Google Scholar] [CrossRef]
- Alemán, A.; Marín-Peñalver, D.; de Palencia, P.F.; Gómez-Guillén, M.d.C.; Montero, P. Anti-Inflammatory properties, bioaccessibility and intestinal absorption of Sea fennel (Crithmum maritimum) extract encapsulated in soy phosphatidylcholine liposomes. Nutrients 2022, 14, 210. [Google Scholar] [CrossRef]
- Siracusa, L.; Kulisic-Bilusic, T.; Politeo, O.; Krause, I.; Dejanovic, B.; Ruberto, G. Phenolic composition and antioxidant activity of aqueous infusions from Capparis spinosa L. and Crithmum maritimum L. before and after submission to a two-step in vitro digestion model. J. Agric. Food Chem. 2011, 59, 12453–12459. [Google Scholar] [CrossRef]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-Protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and antioxidant assays of polyphenols: A review. J. Fut. Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef]
- Le, K.; Chiu, F.; Ng, K. Identification and quantification of antioxidants in Fructus lycii. Food Chem. 2007, 105, 353–363. [Google Scholar] [CrossRef]
- Kashyap, P.; Shikha, D.; Thakur, M.; Aneja, A. Functionality of apigenin as a potent antioxidant with emphasis on bioavailability, metabolism, action mechanism and in vitro and in vivo studies: A review. J. Food Biochem. 2022, 46, e13950. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-antioxidant activity relationships of luteolin and catechin. J Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef]
- Parellada, J.; Suarez, G.; Guinea, M. Inhibition of zinc metallopeptidases by flavonoids and related phenolic compounds, structure-activity relationships. J. Enzyme Inhib. 1998, 13, 347–359. [Google Scholar] [CrossRef]
- Ende, C.; Gebhardt, R. Inhibition of matrix metalloproteinase-2 and -9 activities by selected flavonoids. Planta Med. 2004, 70, 1006–1008. [Google Scholar] [CrossRef]
- Geng, F.; He, Y.; Yang, L.; Wang, Z. A rapid assay for angiotensin-converting enzyme activity using ultra-performance liquid chromatography-mass spectrometry. Biomed. Chromatogr. 2010, 24, 312–317. [Google Scholar] [CrossRef]
- Jenis, J.; Kim, J.Y.; Uddin, Z.; Song, Y.H.; Lee, H.H.; Park, K.H. Phytochemical profile and angiotensin I converting enzyme (ACE) inhibitory activity of Limonium michelsonii Lincz. J. Nat. Med. 2017, 71, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, L.; Castillo, J.; Quinones, M.; Garcia-Vallve, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Liao, J.; Tang, Y.; Zhang, P.; Tan, C.; Ni, H.; Wu, X.Q.; Li, N.; Jia, X.G. ACE and platelet aggregation inhibitors from Tamarix hohenackeri Bunge (host plant of Herba Cistanches) growing in Xinjiang. Pharmacogn. Mag. 2014, 10, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yang, Q.; Wu, Y.; Ouyang, J. Inhibitory effects of acorn (Quercus variabilis Blume) kernel-derived polyphenols on the activities of α-amylase, α-glucosidase, and dipeptidyl peptidase IV. Food Biosci. 2021, 43, 101224. [Google Scholar] [CrossRef]
- Rozano, L.; Zawawi, M.R.A.; Ahmad, M.A.; Jaganath, I.B. Computational analysis of Gynura bicolor bioactive compounds as Dipeptidyl Peptidase-IV inhibitor. Adv. Bioinform. 2017, 3, 154–196. [Google Scholar] [CrossRef]
- Lee, K.-H.; Kwak, J.H.; Lee, K.-B.; Song, K.-S. Prolyl Endopeptidase inhibitors from Caryophylli Flos. Arch. Pharm. Res. 1998, 21, 207–211. [Google Scholar] [CrossRef]
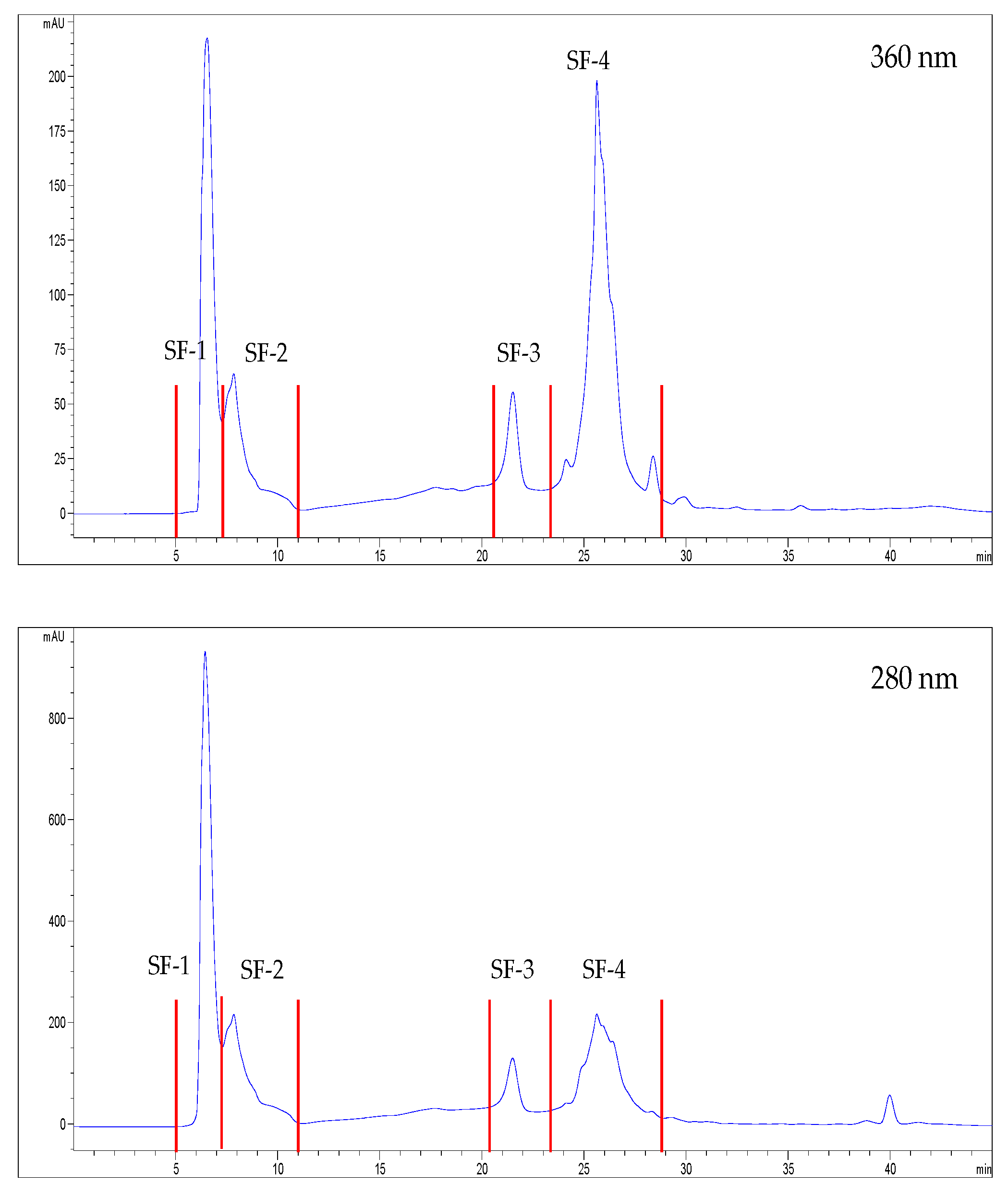
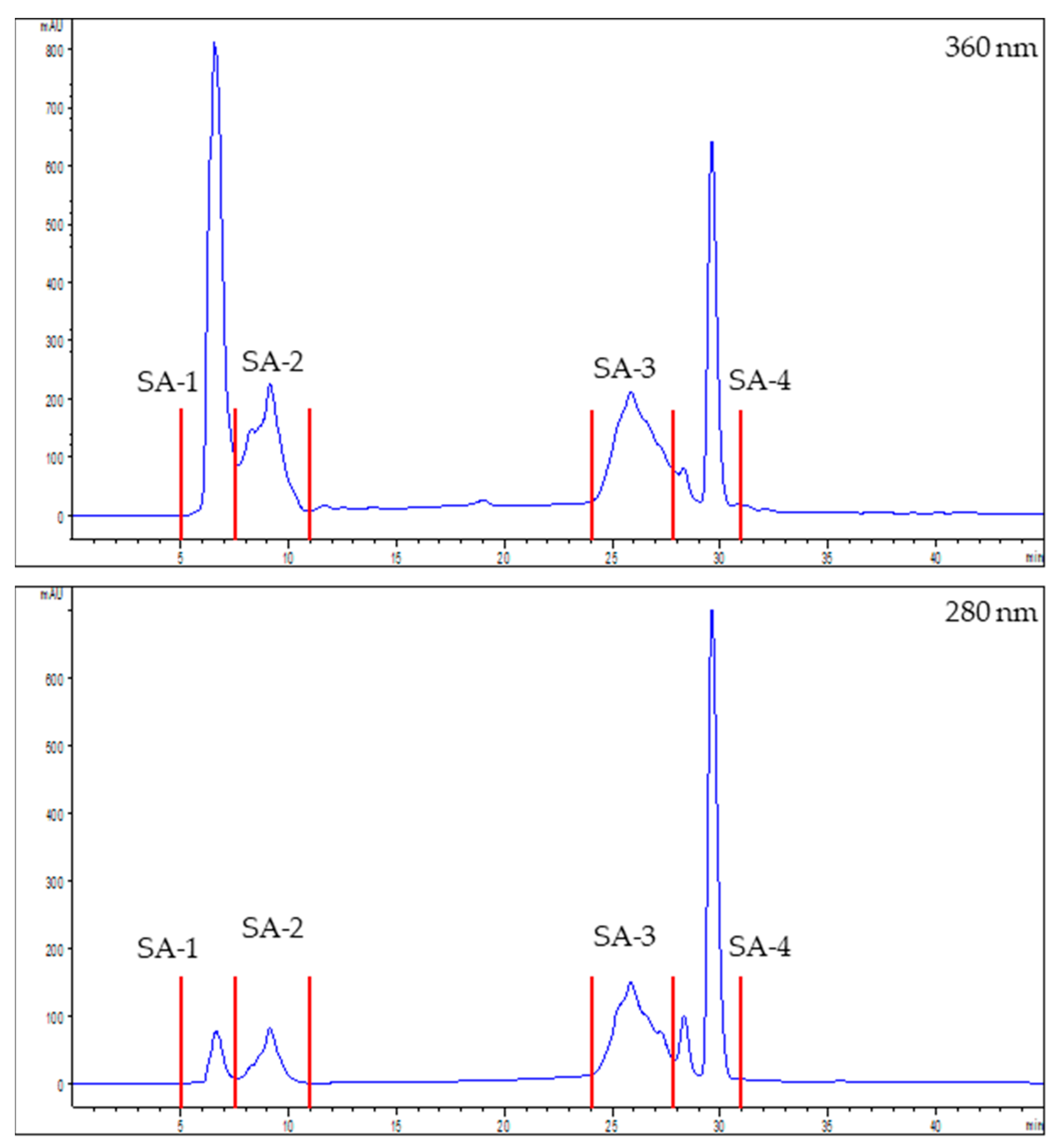
| Sea Fennel | Seaside Arrowgrass | |
|---|---|---|
| Total phenols content (mEq GA/g) | 23.6 ± 0.1 a | 21.2 ± 0.1 b |
| Antioxidant activity | ||
| ABTS (mg Eq ascorbic acid/g) | 20.6 ±0.4 a | 17.4 ± 0.6 b |
| FRAP (mEq Mohr’s salt/g) | 269.0 ± 12.0 a | 169.6 ± 5.8 b |
| Enzymatic activity inhibition | ||
| ACE (%) | 79.6 ± 0.9 a | 90.2 ± 1.3 b |
| PEP (%) | 74.7 ± 3.0 a | 92.8 ± 2.2 b |
| DPP-IV (%) | 72.7 ± 1.1 a | 68.4 ± 1.2 b |
| Assay | Sea Fennel | Seaside Arrowgrass | ||||||
|---|---|---|---|---|---|---|---|---|
| SF-1 | SF-2 | SF-3 | SF-4 | SA-1 | SA-2 | SA-3 | SA-4 | |
| Total phenols content (mEq GA/g) | 9.1 ± 0.97 a | 41.9 ± 4.2 c | 225.1 ± 15.7 g | 155.4 ± 8.4 f | 7.5 ± 4.0 a | 21.3 ± 0.7 b | 104.9 ± 8.4 d | 125.5 ± 7.7 e |
| Antioxidant activity | ||||||||
| ABTS (mg Eq ascorbic acid/g) | 8.8 ± 0.6 b | 32.8 ± 2.6 d | 177.4 ± 11.6 h | 124.4 ± 11.6 g | 4.7 ± 0.6 a | 12.0 ± 0.9 c | 53.6 ± 3.2 e | 90.3 ± 5.5 f |
| FRAP (mEq Mohr’s salt /g) | 248 ± 4 a | 636 ± 14 c | 3233 ± 129 g | 2712 ± 122 f | 248 ± 12 a | 354 ± 13 b | 1048 ± 14 d | 1701 ± 38 e |
| Enzymatic activity inhibition | ||||||||
| ACE (%) | 28.3 ± 2.1 a | 56.7 ± 0.3 c | 92.2 ± 0.1 g | 90.0 ± 1.1 f | 53.8 ± 0.7 b | 68.7 ± 0.7 d | 82.0 ± 1.0 e | 91.0 ± 0.2 f |
| DPP-IV (%) | 8.99 ± 2.7 ab | 26.8 ± 8.6 c | 82.1 ± 2.8 g | 76.1 ± 2.8 f | 5.56 ± 0.3 a | 12.3 ± 1.3 b | 58.2 ± 1.4 e | 49.7 ± 0.8 d |
| PEP (%) | No activity | 7.9 ± 2.8 a | 52.2 ± 2.3 d | 45.4 ± 0.9 c | 12.5 ± 5.2 b | 7.5 ± 1.1 a | 97.3 ± 0.3 e | 98.6 ± 0.2 f |
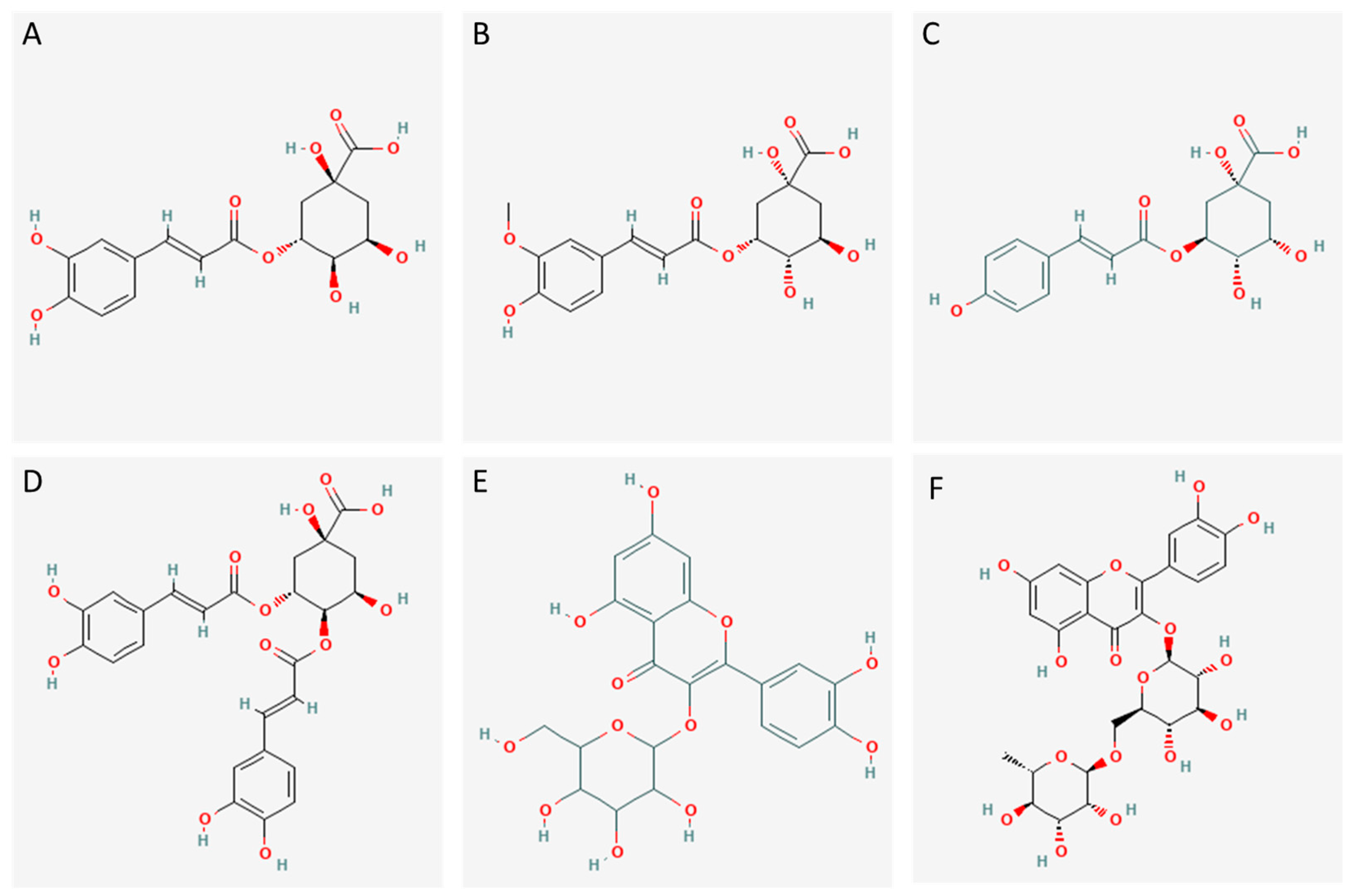
| Compound | Fraction | Experimental Mass | Calculated Mass | Error (ppm) | MS/MS Ions | Rt (min) | Relative Abundance | |
|---|---|---|---|---|---|---|---|---|
| Phenolic acids | ||||||||
| Hydroxybenzoic acid | SF−3 | 137.0238 | 137.0238 | −3.53 | 92, 93, 108, 118, 136, 137 | 8.13 | 3.60 | |
| SF−4 | 137.0246 | −1.63 | 8.15 | 0.30 | ||||
| Hydroxycinnamic acids and derivatives | ||||||||
| Caffeic acid | SF−3 | 179.0350 | 179.0352 | −0.57 | 79, 91, 133, 134, 135 | 11.07 | 1.40 | |
| SF−4 | 179.0355 | 0.48 | 11.09 | 2.23 | ||||
| Caffeoylquinic acid | SF−3 | 353.0878 | 353.0876 | −0.04 | 135, 161, 173, 179, 191 | 8.78 | 1.69 | |
| SF−4 | 353.0883 | −1.46 | 8.48 | 1.24 | ||||
| Caffeoylquinic acid | SF−3 | 353.0878 | 353.0884 | −1.45 | 135, 161, 179, 191, 217 | 9.55 | 20.26 | |
| SF−4 | 353.0886 | −1.79 | 9.57 | 1.36 | ||||
| Caffeoylquinic acid | SF−3 | 353.0878 | 353.0875 | 1.30 | 135, 161, 173, 179, 191, 353 | 10.36 | 7.09 | |
| SF−4 | 353.0886 | −2.64 | 10.53 | 1.28 | ||||
| Caffeoylquinic acid | SF−3 | 353.0878 | 353.0875 | −2.40 | 161, 179, 191, 353 | 12.22 | 12.68 | |
| SF−4 | 353.0875 | 1.11 | 12.25 | 5.48 | ||||
| Dicaffeoylquinic acid | SF−3 | 515.1195 | 515.1210 | −2.12 | 135, 161, 173, 179, 191, 335, 353 | 20.57 | 0.62 | |
| SF−4 | 515.1209 | −2.74 | 20.55 | 3.60 | ||||
| Dicaffeoylquinic acid | SF−3 | 515.1195 | 515.1201 | −0.94 | 135, 179, 191, 335, 353 | 21.37 | 1.43 | |
| SF−4 | 515.1218 | −4.32 | 21.37 | 5.58 | ||||
| Dicaffeoylquinic acid | SF−3 | 515.1195 | 515.1198 | −0.71 | 135, 173, 179, 191, 335, 353 | 21.93 | 0.14 | |
| SF−4 | 515.1202 | −2.22 | 21.92 | 1.65 | ||||
| Dicaffeoylquinic acid | SF−3 | 515.1195 | 515.1207 | −1.93 | 135,173,179,191,535 | 22.89 | 0.12 | |
| SF−4 | 515.1212 | −3.33 | 22.87 | 2.29 | ||||
| Coumaroylquinic acid | SF−3 | 337.0929 | 337.0912 | −4.98 | 93, 119, 137, 163, 173, 191, | 11.73 | 1.87 | |
| SF−4 | 337.0928 | −0.06 | 11.73 | 0.57 | ||||
| Coumaroylquinic acid | SF−3 | 337.0929 | 337.0948 | −4.73 | 93, 119, 145, 163, 173, 191 | 12.59 | 14.40 | |
| SF−4 | 337.0935 | −1.70 | 12.59 | 13.91 | ||||
| Coumaroylquinic acid | SF−3 | 337.0929 | 337.0929 | −4.46 | 93, 119, 137, 163, 173, 191 | 13.00 | 1.83 | |
| SF−4 | 337.0939 | −2.29 | 13.00 | 1.46 | ||||
| Coumaroylquinic acid | SF−3 | 337.0929 | 337.0927 | −0.87 | 137, 145, 163, 173, 191 | 15.19 | 12.08 | |
| SF−4 | 337.0939 | −5.92 | 15.19 | 13.96 | ||||
| Feruloylquinic acid | SF−3 | 367.0350 | 367.1042 | −1.84 | 134, 173, 191, 193 | 14.19 | 6.28 | |
| SF−4 | 367.1044 | −2.18 | 14.22 | 8.77 | ||||
| Feruloylquinic acid | SF−3 | 367.1035 | 367.1052 | −4.41 | 134, 173, 191, 193 | 16.34 | 6.28 | |
| SF−4 | 367.1028 | 2.14 | 16.33 | 4.49 | ||||
| Coumaroyl hexoside | SF−3 | 325.0929 | 325.0938 | −2.59 | 119, 120, 163, 164, 165, 325 | 9.77 | 2.86 | |
| Coumarins and derivatives | ||||||||
| Esculetin | SF−3 | 177.0188 | 177.0192 | −0.36 | 105, 107, 121, 133, 149 | 10.63 | 1.10 | |
| SF−4 | 177.0918 | −2.57 | 10.63 | 0.38 | ||||
| Flavonols and derivatives | ||||||||
| Quercetin | SF−4 | 301.0354 | 301.0356 | −0.42 | 65, 83, 107, 121, 151, 179, 301 | 28.91 | 1.16 | |
| Quercetin hexoside | SF−3 | 463.0876 | 463.0883 | −0.07 | 151, 179, 243, 255, 271, 300, 301 | 18.41 | 0.83 | |
| SF−4 | 463.089 | −1.82 | 18.42 | 6.47 | ||||
| Quercetin hexoside | SF−3 | 463.0876 | 463.0895 | −2.72 | 149, 179, 271, 300, 301 | 18.56 | 0.41 | |
| SF−4 | 463.0892 | −1.93 | 18.65 | 5.68 | ||||
| Rutin | SF−3 | 609.1461 | 609.1455 | 1.43 | 151, 271, 300, 301 | 18.02 | 2.37 | |
| SF−4 | 606.1455 | 1.43 | 18.02 | 7.08 | ||||
| Myricetin | SF−4 | 317.0303 | 317.0298 | 1.56 | 109, 125, 151, 152, 163, 179, 227, 271, 317 | 14.03 | 2.10 | |
| Flavones and derivatives | ||||||||
| Apigenin | SF−3 | 269.0455 | 269.0455 | −0.42 | 107, 117, 149, 151, 225, 269 | 33.02 | 0.24 | |
| SF−4 | 269.0466 | −3.77 | 33.13 | 2.61 | ||||
| Apigenin hexoside | SF−3 | 431.0984 | 431.0995 | −1.82 | 270, 283, 311, 312, 341 | 17.90 | 0.17 | |
| SF−4 | 431.0982 | 0.51 | 17.89 | 0.31 | ||||
| Apigenin hexoside | SF−3 | 431.0984 | 431.0984 | 0.58 | No fragment detected | 18.08 | 0.05 | |
| SF−4 | 431.0993 | −2.42 | 18.01 | 0.09 | ||||
| Diosmin | SF−3 | 607.1668 | 607.1681 | −1.98 | 284, 285, 299, 300, 301 | 22.10 | 0.10 | |
| SF−4 | 607.1667 | 0.66 | 22.10 | 1.75 | ||||
| Luteolin | SF−3 | 285.0405 | 285.0408 | 0.04 | 107, 133, 149, 151, 175, 199, 217 | 28.76 | 0.07 | |
| SF−4 | 285.0401 | 1.33 | 28.74 | 3.54 | ||||
| Chrysoeriol | SF−3 | 299.0561 | 299.0556 | 1.12 | 256, 284, 299 | 33.66 | 0.03 | |
| SF−4 | 299.0570 | −2.83 | 33.70 | 0.48 | ||||
| Diosmetin | SF−4 | 299.0561 | 299.0552 | 2.53 | 107, 133, 151, 227, 239, 255, 256, 284 | 33.81 | 0.18 | |
| Compounds | SF-3 (%) | SF-4 (%) |
|---|---|---|
| Phenolic acids (Hydroxybenzoic acid) | 3.60 | 0.30 |
| Hydroxycinnamic acids and derivatives | 91.03 | 67.87 |
| Caffeic acid | 1.40 | 2.23 |
| Caffeoylquinic acids | 41.72 | 9.36 |
| Dicaffeoylquinic acids | 2.31 | 13.12 |
| Coumaroylquinic acids | 30.18 | 29.90 |
| Feruloylquinic acids | 12.56 | 13.26 |
| Coumaroyl hexosides | 2.86 | 0.00 |
| Coumarins and derivatives (Esculetin) | 1.10 | 0.38 |
| Flavonols and derivatives | 3.61 | 22.49 |
| Quercetin | 0.00 | 1.16 |
| Quercetin hexosides | 1.24 | 12.15 |
| Rutin | 2.37 | 7.08 |
| Myricetin | 0.00 | 2.10 |
| Flavones and derivatives | 0.66 | 8.96 |
| Apigenin | 0.24 | 2.61 |
| Apigenin hexosides | 0.22 | 0.40 |
| Diosmin | 0.10 | 1.75 |
| Luteolin | 0.07 | 3.54 |
| Chrysoeriol | 0.03 | 0.48 |
| Diosmetin | 0.00 | 0.18 |
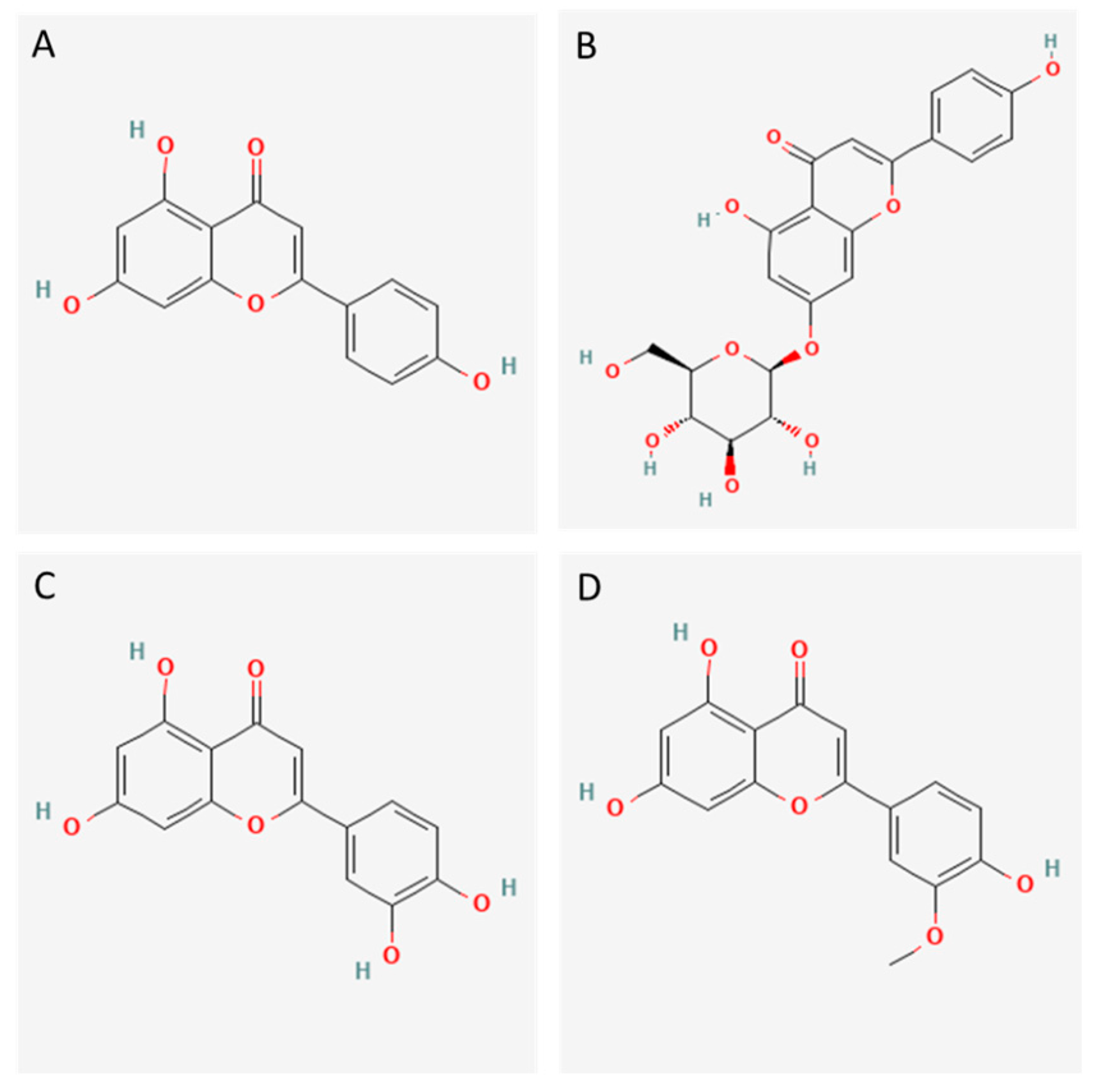
| Compound | Fraction | Experimental Mass | Calculated Mass | Error (ppm) | MS/MS Ions | Rt (min) | Relative Abundance | |
|---|---|---|---|---|---|---|---|---|
| Phenolic acids | ||||||||
| Hydroxybenzoic acid | SA−3 | 137.0238 | 137.0247 | −1.78 | 92, 93, 108, 118, 136, 137 | 8.15 | 0.36 | |
| SA−4 | 137.0251 | −1.02 | 8.17 | 0.01 | ||||
| Hydroxycinnamic acids and derivatives | ||||||||
| Caffeic acid | SA−3 | 179.0350 | 179.0359 | −3.56 | 91, 133, 134, 135 | 11.05 | 0.91 | |
| Coumarins and derivatives | ||||||||
| Esculetin | SA−3 | 177.0188 | 177.0190 | 0.51 | 105, 107, 121, 133, 149 | 10.58 | 0.87 | |
| Flavones and derivatives | ||||||||
| Apigenin | SA−3 | 269.0455 | 269.0458 | −0.71 | 107, 117, 143, 151, 159, 227, 241 | 33.08 | 6.96 | |
| SA−4 | 269.0452 | 0.98 | 33.05 | 52.41 | ||||
| Apigenin−hexoside | SA−3 | 431.0984 | 431.0977 | 1.58 | 270, 283, 311, 312, 341 | 17.89 | 29.85 | |
| SA−4 | 431.0982 | −1.19 | 17.90 | 0.04 | ||||
| Apigenin−hexoside | SA−3 | 431.0984 | 431.0988 | −0.67 | 270, 283, 311, 312, 341 | 18.02 | 50.62 | |
| SA−4 | 431.0994 | −2.23 | 18.02 | 0.07 | ||||
| Luteolin | SA−3 | 285.0405 | 285.0401 | 1.45 | 107, 133, 149, 151, 175, 199, 217 | 28.68 | 8.48 | |
| SA−4 | 285.0399 | 1.92 | 28.66 | 34.66 | ||||
| Chrysoeriol | SA−3 | 299.0561 | 299.0556 | 1.12 | 256, 284, 299 | 33.66 | 1.95 | |
| SA−4 | 299.0563 | −0.78 | 256, 284, 285, 299 | 33.67 | 12.81 | |||
| Compounds | SA-3 (%) | SA-4 (%) |
|---|---|---|
| Phenolic acids (Hydroxybenzoic acid) | 0.36 | 0.01 |
| Hydroxycinnamic Acids and derivatives (Caffeic acid) | 0.91 | 0.00 |
| Coumarins and derivatives (Esculetin) | 0.87 | 0.00 |
| Flavones and derivatives | 97.86 | 99.99 |
| Apigenin | 6.96 | 52.41 |
| Apigenin hexoside | 80.47 | 0.11 |
| Luteolin | 8.48 | 34.66 |
| Chrysoeriol | 1.95 | 12.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo, M.M.; López-Caballero, M.E.; Martínez-Alvarez, O. Identification of Polyphenols in Sea Fennel (Crithmum maritimum) and Seaside Arrowgrass (Triglochin maritima) Extracts with Antioxidant, ACE-I, DPP-IV and PEP-Inhibitory Capacity. Foods 2023, 12, 3886. https://doi.org/10.3390/foods12213886
Calvo MM, López-Caballero ME, Martínez-Alvarez O. Identification of Polyphenols in Sea Fennel (Crithmum maritimum) and Seaside Arrowgrass (Triglochin maritima) Extracts with Antioxidant, ACE-I, DPP-IV and PEP-Inhibitory Capacity. Foods. 2023; 12(21):3886. https://doi.org/10.3390/foods12213886
Chicago/Turabian StyleCalvo, Marta María, María Elvira López-Caballero, and Oscar Martínez-Alvarez. 2023. "Identification of Polyphenols in Sea Fennel (Crithmum maritimum) and Seaside Arrowgrass (Triglochin maritima) Extracts with Antioxidant, ACE-I, DPP-IV and PEP-Inhibitory Capacity" Foods 12, no. 21: 3886. https://doi.org/10.3390/foods12213886
APA StyleCalvo, M. M., López-Caballero, M. E., & Martínez-Alvarez, O. (2023). Identification of Polyphenols in Sea Fennel (Crithmum maritimum) and Seaside Arrowgrass (Triglochin maritima) Extracts with Antioxidant, ACE-I, DPP-IV and PEP-Inhibitory Capacity. Foods, 12(21), 3886. https://doi.org/10.3390/foods12213886







