Optimization of a Novel Tyrosinase Inhibitory Peptide from Atrina pectinata Mantle and Its Molecular Inhibitory Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Atrina pectinata Mantle Gelatin Peptides (APGPs)
2.3. Optimization of Enzymatic Parameters via Response-Surface Methodology (RSM)
2.4. Analysis of the Tyrosinase Inhibitory Activity
2.5. Assay of DH of APGP
2.6. Examination of Amino Acids
2.7. Analysis of Molecular-Weight Distribution (MWD)
2.8. Ultrafiltration Separation
2.9. Identification of the Tyrosinase Inhibitory Peptides
2.10. Molecular-Docking Studies
2.11. Synthesis of Tyrosinase Inhibitory Peptides
2.12. Enzyme Inhibition Kinetic Assay
2.13. Statistical Analysis
3. Results
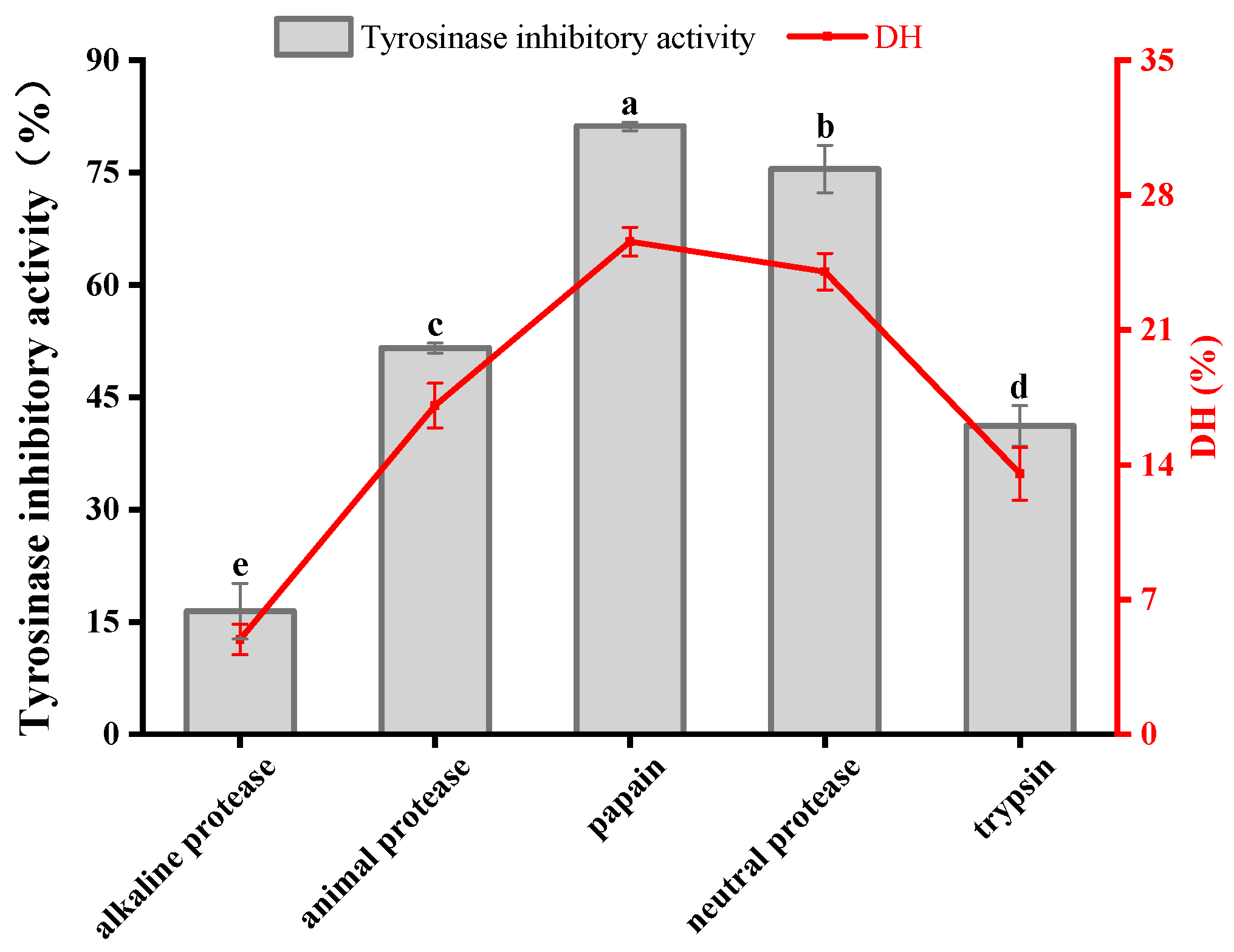
3.1. Protease Screening
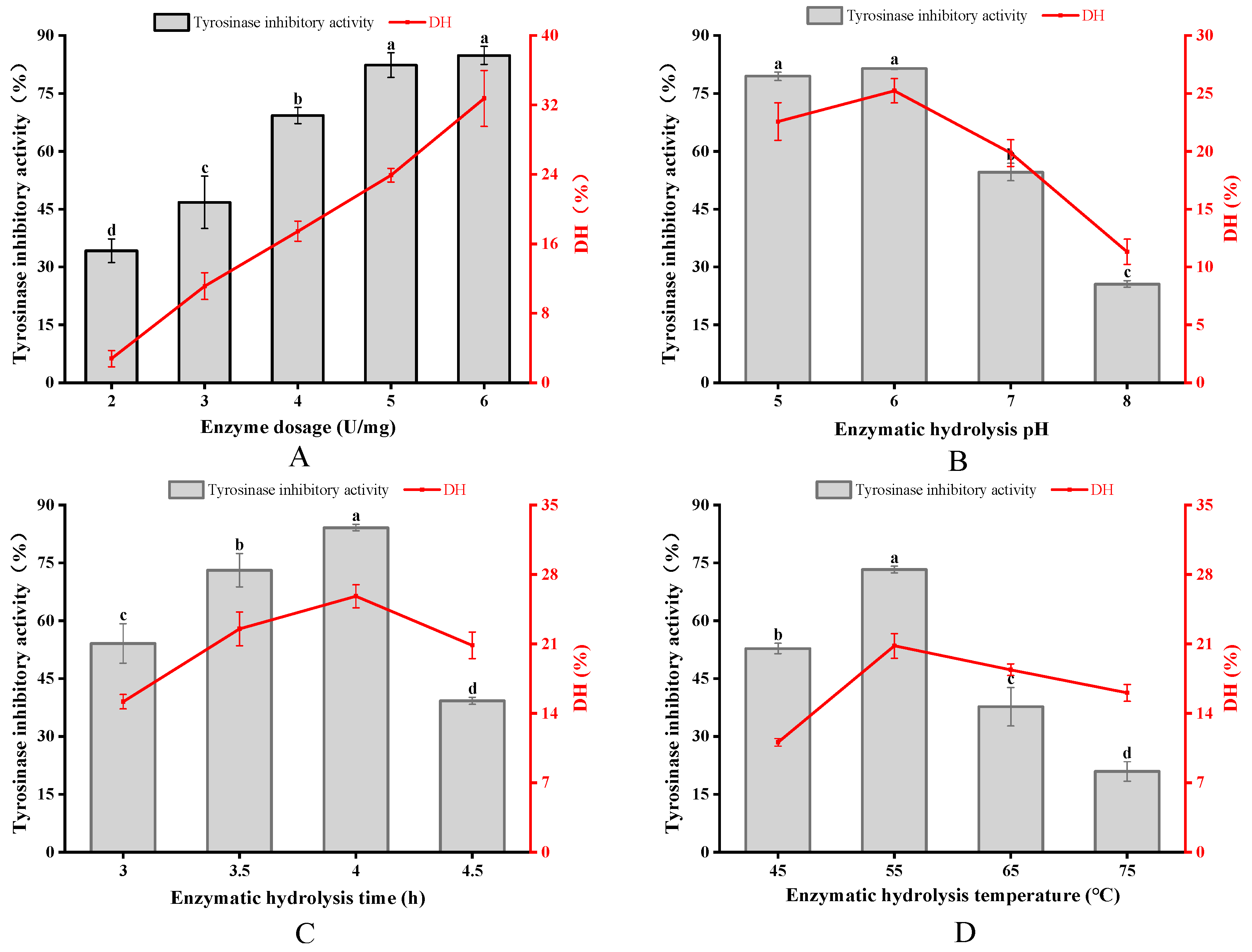
3.2. Optimization of APGP
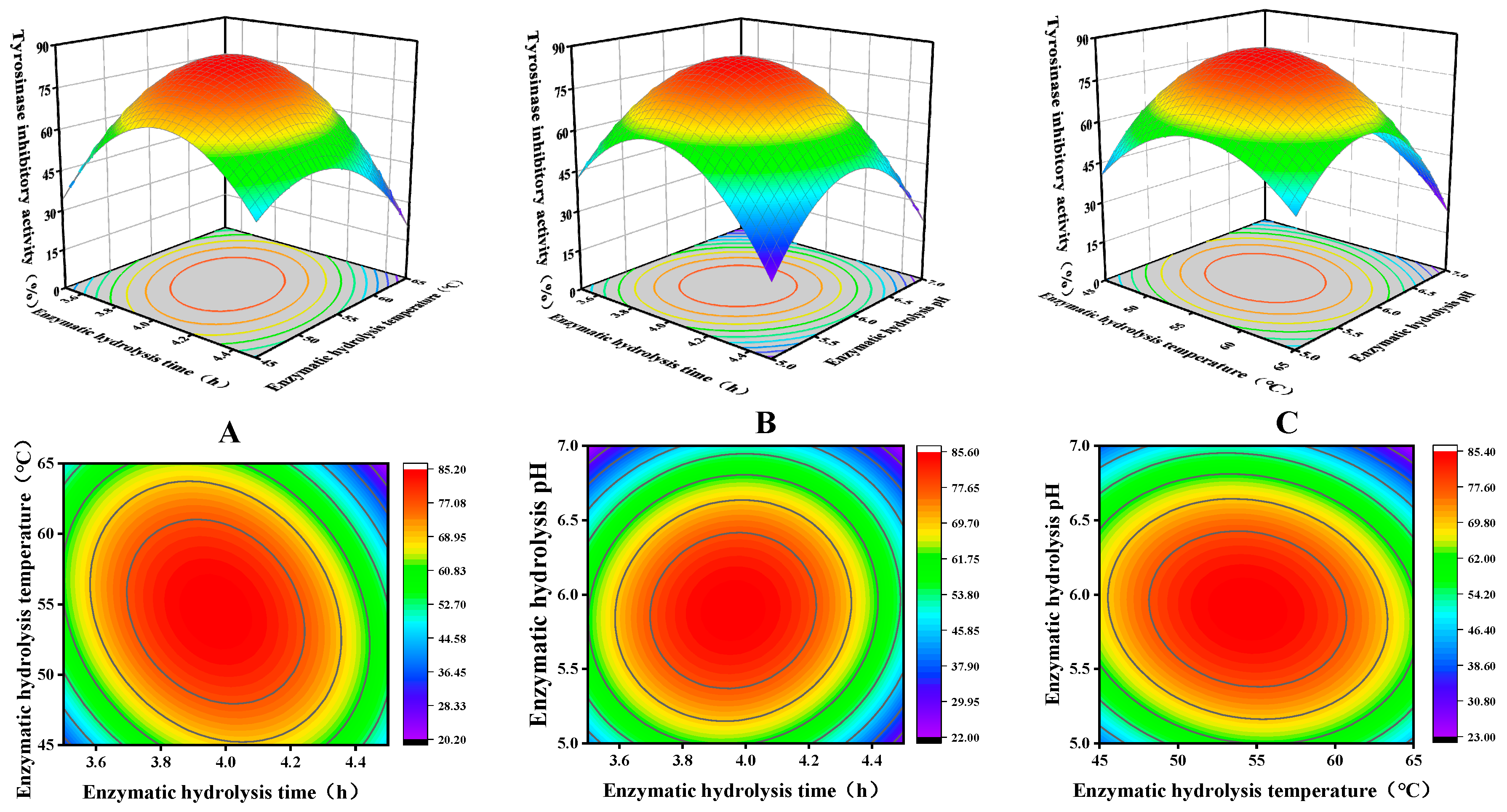
3.3. Optimization Analysis of RSM
3.4. Amino Acid Composition and MWD of APGP
3.5. Enrichment and Identification of Potential APGPs
3.6. Mechanism of Tyrosinase Inhibition
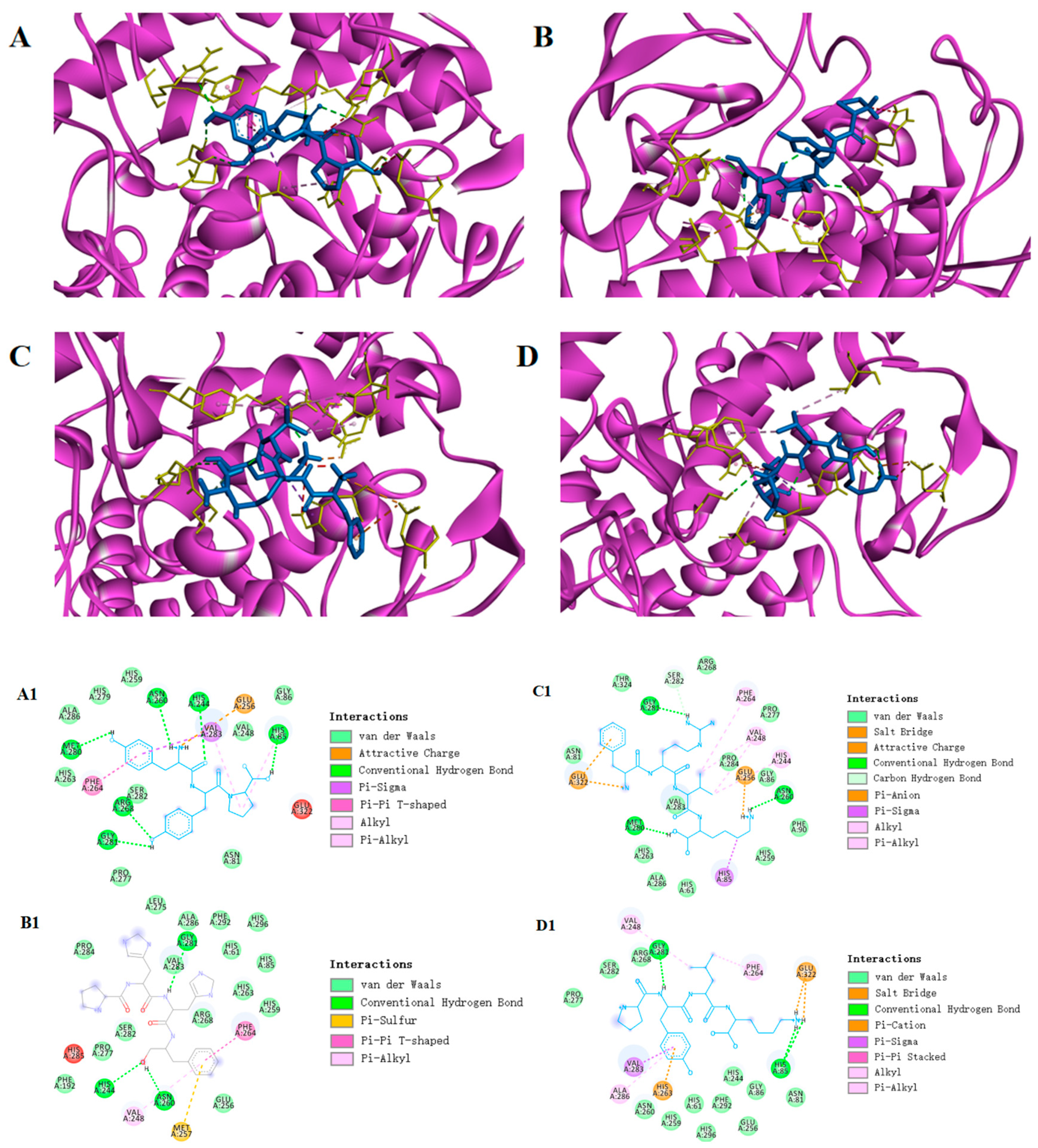
3.6.1. Molecular Docking
3.6.2. Synthetic Peptide Activity Validation
3.6.3. Detection of Inhibitory Effect, Mechanism and Type
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tayier, N.; Qin, N.Y.; Zhao, L.N.; Zeng, Y.; Wang, Y.; Hu, G.; Wang, Y.Q. Theoretical exploring of a molecular mechanism for melanin inhibitory activity of calycosin in zebrafish. Molecules 2021, 26, 6998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.E.; Chu, M.Y.; Jiang, T.; Song, X.H.; Hou, J.F.; Cheng, L.Y.; Feng, Y.; Chen, C.B.; Wang, E.P. By-Product of the Red Ginseng Manufacturing Process as Potential Material for Use as Cosmetics: Chemical Profiling and In Vitro Antioxidant and Whitening Activities. Molecules 2022, 27, 8202. [Google Scholar] [CrossRef] [PubMed]
- Im, S.B.; Mun, S.K.; Ha, N.I.; Jang, H.Y.; Kang, K.Y.; Park, K.W.; Seo, K.S.; Kim, K.J.; Yee, S.T. Whitening Activity of Acteoside from Stachys sieboldii Fermented with Hericium erinaceus Mycelia on Melanocytes. Fermentation 2023, 9, 697. [Google Scholar] [CrossRef]
- Song, X.; Hu, X.; Zhang, Y.; Pan, J.; Gong, D.; Zhang, G. Inhibitory mechanism of epicatechin gallate on tyrosinase: Inhibitory interaction, conformational change and computational simulation. Food Funct. 2020, 11, 4892–4902. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.J.; Zhang, Z.Y.; Jin, J.; Han, J.X.; Wang, Y.; Yang, K.; Yang, Y.Y.; Wang, H.Q.; Dai, X.T.; Yao, C.; et al. Salidroside can target both P4HB-mediated inflammation and melanogenesis of the skin. Theranostics 2020, 10, 11110–11126. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Z.A.; Gheida, S.F.; El Maghraby, G.M.; Farag, Z.E. Evaluation of the efficacy and safety of combinations of hydroquinone, glycolic acid, and hyaluronic acid in the treatment of melasma. J. Cosmet. Dermatol. 2015, 14, 113–123. [Google Scholar] [CrossRef]
- Park, J.J.; Hwang, S.J.; Kang, Y.S.; Jung, J.; Park, S.; Hong, J.E.; Park, Y.; Lee, H.J. Synthesis of arbutin-gold nanoparticle complexes and their enhanced performance for whitening. Arch. Pharmacal. Res. 2019, 42, 977–989. [Google Scholar] [CrossRef]
- Karakaya, G.; Türe, A.; Ercan, A.; Öncül, S.; Aytemir, M.D. Synthesis, computational molecular docking analysis and effectiveness on tyrosinase inhibition of kojic acid derivatives. Bioorg. Chem. 2019, 88, 102950. [Google Scholar] [CrossRef]
- Chen, Q.; Kou, L.; Wang, F.; Wang, Y. Size-dependent whitening activity of enzyme-degraded fucoidan from laminaria japonica. Carbohyd. Polym. 2019, 225, 115211. [Google Scholar] [CrossRef]
- Qiu, Y.T.; Wang, Y.M.; Yang, X.R.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Gelatin and antioxidant peptides from gelatin hydrolysate of skipjack tuna (Katsuwonus pelamis) scales: Preparation, identification and activity evaluation. Mar. Drugs 2019, 17, 565. [Google Scholar] [CrossRef]
- Xiao, F.; Chen, S.; Li, L.; He, J.; Cheng, W.; Ren, G. In Vitro Antioxidant activity of peptides from simulated gastro-intestinal digestion products of Cyprinus carpio haematopterus scale gelatin. Foods 2019, 8, 618. [Google Scholar] [CrossRef] [PubMed]
- Dara, P.K.; Elavarasan, K.; Shamasundar, B.A. Improved utilization of croaker skin waste and freshwater carps visceral waste: Conversion of waste to health benefitting peptides. J. Pept. Res. Ther. 2020, 26, 2641–2651. [Google Scholar] [CrossRef]
- Mirzapour Kouhdasht, A.; Moosavi Nasab, M.; Kim, Y.M.; Eun, J.B. Antioxidant mechanism, antibacterial activity, and functional characterization of peptide fractions obtained from barred mackerel gelatin with a focus on application in carbonated beverages. Food Chem. 2021, 342, 128339. [Google Scholar] [CrossRef] [PubMed]
- O’keeffe, M.B.; Norris, R.; Alashi, M.A.; Aluko, R.E.; Fitzgerald, R.J. Peptide identification in a porcine gelatin prolyl endoproteinase hydrolysate with angiotensin converting enzyme (ACE) inhibitory and hypotensive activity. J. Funct. Foods 2017, 34, 77–88. [Google Scholar] [CrossRef]
- Xu, Q.; Zheng, L.; Huang, M.; Zhao, M. Exploring structural features of potent dipeptidyl peptidase iv (DPP-IV) inhibitory peptides derived from tilapia (Oreochromis niloticus) skin gelatin by an integrated approach of multivariate analysis and gly-pro-based peptide library. Food Chem. 2022, 397, 133821. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Lu, W.; Liu, J.; Zhang, W.; Wang, Y.; Ma, M.; Cao, X.; Guo, Y. Cell proliferation stimulation ability and osteogenic activity of low molecular weight peptides derived from bovine gelatin hydrolysates. J. Agric. Food Chem. 2020, 68, 7630–7640. [Google Scholar] [CrossRef]
- Hu, Z.Z.; Sha, X.M.; Zhang, L.; Zha, M.J.; Tu, Z.C. From fish scale gelatin to tyrosinase inhibitor: A novel peptides screening approach application. Front. Nutr. 2022, 9, 853442. [Google Scholar] [CrossRef]
- Li, H.L.; Li, M.J.; Xiong, G.Q.; Cai, J.; Liao, T.; Zu, X.Y. Silver carp (Hypophthalmichthys molitrix) scale collagen peptides-1 (SCPs1) inhibit melanogenesis through downregulation of the cAMP-CREB signaling pathway. Nutrients 2023, 15, 2449. [Google Scholar] [CrossRef]
- Lee, H.J.; Roy, V.C.; Ho, T.C.; Park, J.S.; Jeong, Y.R.; Lee, S.C.; Kim, S.Y.; Chun, B.S. Amino acid profiles and biopotentiality of hydrolysates obtained from comb penshell (Atrina pectinata) viscera using subcritical water hydrolysis. Mar. Drugs 2021, 19, 137. [Google Scholar] [CrossRef]
- Hashimoto, K.; Yamada, K.; Sekino, M.; Kobayashi, M.; Sasaki, T.; Fujinami, Y.; Yamamoto, M.; Choi, K.S.; Henmi, Y. Population genetic structure of the pen shell atrina pectinata sensu lato (bivalvia: Pinnidae) throughout east asia. Reg. Stud. Mar. Sci. 2021, 48, 102024. [Google Scholar] [CrossRef]
- Hong, S.Y.; Kim, D.G.; Kim, Y.O.; Park, J.Y.; Seo, J.K.; Nam, B.H.; Hong, Y.K. Purification and cdna cloning of the antimicrobial peptide apmolluscidin from the pen shell, Atrina pectinata. Fish Shellfish Immun. 2018, 81, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Feng, C.; Lin, H.S.; Qin, X.M.; Cao, W.H.; Zhang, C.H.; Gao, J.L.; Zheng, H.N.; Chen, Y.B. Characterization of collagen from mantle of scallop Chlamys nobilis by different extraction methods. J. Dalian Ocean Univ. 2022, 37, 858–865. [Google Scholar]
- Yu, Q.; Li, J.; Fan, L. Effect of drying methods on the microstructure, bioactivity substances, and antityrosinase activity of asparagus stems. J. Agric. Food Chem. 2019, 67, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.W.; Zhang, C.H.; Lin, H.S.; Qin, X.M.; Cao, W.H.; Yang, Y.R. Optimization of Preparation Process of Oyster Oligopeptides by Response Surface Methodology. J. Guangdong Ocean Univ. 2019, 39, 85–92. [Google Scholar]
- Chen, X.; Wu, J.; Li, L.; Wang, S. Cryoprotective activity and action mechanism of antifreeze peptides obtained from tilapia scales on Streptococcus thermophilus during cold stress. J. Agric Food Chem. 2019, 67, 1918–1926. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal Structure of Agaricus bisporus Mushroom Tyrosinase: Identity of the Tetramer Subunits and Interaction with Tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef]
- Hering, A.; Stefanowicz-Hajduk, J.; Dziomba, S.; Halasa, R.; Krzemieniecki, R.; Sappati, S.; Baginski, M.; Ochocka, J.R. Mangiferin affects melanin synthesis by an influence on tyrosinase: Inhibition, mechanism of action and molecular docking studies. Antioxidants 2023, 12, 1016. [Google Scholar] [CrossRef]
- Liu, H.; Ru, G.; Zhang, Z.; Li, Y.; Xia, C.; Lu, C.; Zhang, Q. Experimental study on optimization of initial pH for photo-fermentation bio-hydrogen under different enzymatic hydrolysis of Chlorella vulgaris. Bioresour. Technol. 2021, 338, 125571. [Google Scholar] [CrossRef]
- Tedeschi, T.; Anzani, C.; Ferri, M.; Marzocchi, S.; Caboni, M.F.; Monari, S.; Tassoni, A. Enzymatic digestion of calf fleshing meat by-products: Antioxidant and anti-tyrosinase activity of protein hydrolysates, and identification of fatty acids. Foods 2021, 10, 755. [Google Scholar] [CrossRef]
- Gaviria, G.Y.; Zapata, M.J. Optimization and scale up of the enzymatic hydrolysis of californian red worm protein (Eisenia foetida). Heliyon 2023, 9, e16165. [Google Scholar] [CrossRef]
- Qu, S.J.; Liu, S.J.; Su, Y.C.; Pan, N.; Liao, D.Y.; Xu, M.; Wang, Q.; Liu, Z.Y. Optimization of polypeptides extraction from Takifugu flavidus by response surface methodology. Sci. Techno. Food Ind. 2021, 42, 133–138. [Google Scholar]
- Yin, X.S.; Zhang, X.Q.; Li, D.Q. Simultaneous optimization of ultrasound-assisted extraction of antioxidants and tyrosinase inhibitory activities of Semen Oroxyli flavonoids using response surface methodology. J. Food Meas. Charact. 2020, 14, 694–707. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, F.; He, Z.; Fang, X.; Liu, X. Optimization and Molecular Mechanism of Novel α-Glucosidase Inhibitory Peptides Derived from Camellia Seed Cake through Enzymatic Hydrolysis. Foods 2023, 12, 393. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Liu, L.Y.; Lu, B.Y.; Xia, D.Z.; Zhang, Y. Evaluation of antihypertensive and antihyperlipidemic effects of bamboo shoot angiotensin converting enzyme inhibitory peptide in vivo. J. Agric. Food Chem. 2012, 60, 11351–11358. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.S.; Tsai, K.C.; Chen, W.C.; Wang, Y.T.; Lee, Y.C.; Lu, C.K.; Don, M.J.; Chang, C.Y.; Lee, C.H.; Lin, H.H. Discovery of potent cysteine-containing dipeptide inhibitors against tyrosinase: A comprehensive investigation of 20 × 20 dipeptides in inhibiting dopachrome formation. J. Agric. Food Chem. 2015, 63, 6181–6188. [Google Scholar] [CrossRef]
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.S.; Stevens, J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef]
- Gou, L.; Lü, Z.R.; Park, D.; Oh, S.H.; Shi, L.; Park, S.J.; Bhak, J.; Park, Y.D.; Ren, Z.L.; Zou, F. The effect of histidine residue modification on tyrosinase activity and conformation: Inhibition kinetics and computational prediction. J. Biomol. Struct. Dyn. 2008, 26, 395–401. [Google Scholar] [CrossRef]
- Schurink, M.; Van Berkel, W.J.; Wichers, H.J.; Boeriu, C.G. Novel peptides with tyrosinase inhibitory activity. Peptides 2007, 28, 485–495. [Google Scholar] [CrossRef]
- Maqsoudlou, A.; Mahoonak, A.S.; Mora, L.; Mohebodini, H.; Toldrá, F.; Ghorbani, M. Peptide identification in alcalase hydrolysated pollen and comparison of its bioactivity with royal jelly. Food Res. Int. 2019, 116, 905–915. [Google Scholar] [CrossRef]
- Feng, Y.X.; Wang, Z.C.; Chen, J.X.; Li, H.R.; Wang, Y.B.; Ren, D.F.; Lu, J. Separation, identification, and molecular docking of tyrosinase inhibitory peptides from the hydrolysates of defatted walnut (Juglans regia L.) meal. Food Chem. 2021, 353, 129471. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.H. Recent developments on production, purification and biological activity of marine peptides. Food Res. Int. 2021, 147, 110468. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Huang, Y.; Wang, C.; Chen, F.; Yang, L.; Ling, L.; Che, Z.; Chen, X. Recent developments in molecular docking technology applied in food science: A review. Int. J. Food Sci. Tech. 2020, 55, 33–45. [Google Scholar] [CrossRef]
- Jung, H.J.; Noh, S.G.; Park, Y.; Kang, D.; Chun, P.; Chung, H.Y.; Moon, H.R. In vitro and in silico insights into tyrosinase inhibitors with (E)-benzylidene-1-indanone derivatives. Comput. Struct. Biotechnol. J. 2019, 17, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.P.; Gao, C.W.; Feng, H.Y.; Zeng, S.Y.; Chen, S.E. In vitro α-glucosidase inhibitory activity of Rhodiola crenulata extract based on molecular docking and enzyme inhibition kinetics. Nat. Prod. Res. Dev. 2022, 34, 2018–2025. [Google Scholar]
- Qin, C.; Li, Y.; Zhang, Y.; Liu, L.; Wu, Z.; Weng, P. Insights into oat polyphenols constituent against advanced glycation end products mechanism by spectroscopy and molecular interaction. Food Biosci. 2021, 43, 101313. [Google Scholar] [CrossRef]
- Morais, D.V.D.; Costa, M.a.P.D.C.; Santa Bárbara, M.F.; Silva, F.D.L.; Moreira, M.M.; Delerue-Mato, C.; Guimaraes Dias, L.A.; Estevinho, M.L.M.; Carvalho, C.a.L.D. Antioxidant, photoprotective and inhibitory activity of tyrosinase in extracts of Dalbergia ecastaphyllum. PLoS ONE 2018, 13, e0207510. [Google Scholar] [CrossRef]
- Murata, K.; Suzuki, S.; Miyamoto, A.; Horimoto, M.; Nanko, S.; Mori, D.; Kanamaru, H.; Endo, Y. Tyrosinase inhibitory activity of extracts from Prunus persica. Separations 2022, 9, 107. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; He, X.; Teng, B.; Mcrae, J.M. Valonea tannin: Tyrosinase inhibition activity, structural elucidation and insights into the inhibition mechanism. Molecules 2021, 26, 2747. [Google Scholar] [CrossRef]
- Asebi, N.; Nihei, K.I. Total synthesis of apios isoflavones and investigation of their tyrosinase inhibitory activity. Tetrahedron 2019, 75, 130589. [Google Scholar] [CrossRef]
- Song, W.; Liu, L.L.; Ren, Y.J.; Wei, S.D.; Yang, H.B. Inhibitory effects and molecular mechanism on mushroom tyrosinase by condensed tannins isolation from the fruit of Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex HF Chow. Int. J. Biol. Macromol. 2020, 165, 1813–1821. [Google Scholar] [CrossRef]
- Chen, Q.F.; Shang, C.; Han, M.Q.; Chen, C.; Tang, W.K.; Liu, W.B. Inhibitory mechanism of scutellarein on tyrosinase by kinetics, spectroscopy and molecular simulation. Spectrochim. Acta A 2023, 296, 122644. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Ge, S.; Xu, X.; Zhang, Y.; Wu, P.; Zhang, K.; Xu, X.; Li, C.; Zhao, D.; Tang, X. Design, synthesis and evaluation of cinnamic acid ester derivatives as mushroom tyrosinase inhibitors. MedChemComm 2018, 9, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Wang, C.; Shang, C.; You, X.; Zhang, L.; Liu, W. Integrated study of the mechanism of tyrosinase inhibition by baicalein using kinetic, multispectroscopic and computational simulation analyses. Int. J. Biol. Macromol. 2018, 118, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Nazir, Y.; Rafique, H.; Kausar, N.; Abbas, Q.; Ashraf, Z.; Rachtanapun, P.; Jantanasakulwong, K.; Ruksiriwanich, W. Methoxy-substituted tyramine derivatives synthesis, computational studies and tyrosinase inhibitory kinetics. Molecules 2021, 26, 2477. [Google Scholar] [CrossRef]

| Reagent | Solvent Base Well T1, (μL) | Solvent Reaction Well T2, (μL) | Sample Background Well T3, (μL) | Sample Reaction Well T4, (μL) |
|---|---|---|---|---|
| L-Tyr solutions | 0 | 40 | 0 | 40 |
| Sample solutions | 0 | 0 | 40 | 40 |
| PBS | 110 | 70 | 70 | 30 |
| Mushroom tyrosinase solution | 20 | 20 | 20 | 20 |
| Number | A (Time/h) | B (Temperature/°C) | C (pH) | Inhibition (%) |
|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 83.9 |
| 2 | −1 | 0 | −1 | 41.3 |
| 3 | 0 | 0 | 0 | 85.1 |
| 4 | 0 | 1 | −1 | 45.6 |
| 5 | 0 | 0 | 0 | 82.3 |
| 6 | 0 | 0 | 0 | 89.7 |
| 7 | 1 | 0 | 1 | 23.0 |
| 8 | 0 | −1 | 1 | 37.5 |
| 9 | 0 | 1 | 1 | 21.1 |
| 10 | −1 | −1 | 0 | 32.5 |
| 11 | 1 | 1 | 0 | 21.4 |
| 12 | −1 | 0 | 1 | 24.2 |
| 13 | 0 | 0 | 0 | 85.1 |
| 14 | 0 | −1 | −1 | 42.1 |
| 15 | 1 | −1 | 0 | 45.7 |
| 16 | 1 | 0 | −1 | 23.8 |
| 17 | −1 | 1 | 0 | 48.0 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 10,466.84 | 9 | 1162.98 | 139.63 | <0.0001 | ** |
| A-time | 128.80 | 1 | 128.80 | 15.46 | 0.0057 | ** |
| B-temperature | 58.86 | 1 | 58.86 | 7.07 | 0.0325 | * |
| C-pH | 276.13 | 1 | 276.13 | 33.15 | 0.0007 | ** |
| AB | 396.01 | 1 | 396.01 | 47.55 | 0.0002 | ** |
| AC | 66.42 | 1 | 66.42 | 7.89 | 0.0256 | * |
| BC | 99.00 | 1 | 99.00 | 11.89 | 0.0107 | * |
| A2 | 3374.55 | 1 | 3374.55 | 405.17 | <0.0001 | ** |
| B2 | 162.36 | 1 | 162.36 | 198.40 | <0.0001 | ** |
| C2 | 3452.48 | 1 | 3452.48 | 414.53 | <0.0001 | ** |
| Residual | 58.30 | 7 | 8.33 | |||
| Lack of fit | 21.29 | 3 | 7.10 | 0.7671 | 0.5691 | |
| Pure error | 37.01 | 4 | 9.25 | |||
| Cor total | 10,525.14 | 16 |
| Amino Acid | Content (g per 100 g) | Amino Acid Ratio (%) | Amino Acid | Content (g per 100 g) | Amino Acid Ratio (%) |
|---|---|---|---|---|---|
| Glu | 11.3 ± 0.14 | 16.32 ± 0.27 | Thr | 3.08 ± 0.02 | 4.44 ± 0.01 |
| Gly | 11.7 ± 0.14 | 16.89 ± 0.27 | Val | 2.94 ± 0.07 | 4.25 ± 0.12 |
| Asp | 7.04 ± 0.07 | 10.17 ± 0.14 | Ser | 3.32 ± 0.01 | 4.80 ± 0.03 |
| Arg | 6.19 ± 0.18 | 8.94 ± 0.23 | Ile | 1.98 ± 0.13 | 2.87 ± 0.18 |
| Leu | 3.52 ± 0.08 | 5.08 ± 0.10 | Tyr | 1.66 ± 0.10 | 2.40 ± 0.13 |
| Pro | 5.38 ± 0.01 | 7.78 ± 0.02 | Phe | 1.44 ± 0.06 | 2.07 ± 0.08 |
| Ala | 4.22 ± 0.01 | 6.09 ± 0.02 | Met | 1.78 ± 0.06 | 2.57 ± 0.07 |
| Lys | 3.07 ± 0.03 | 4.43 ± 0.02 | His | 0.64 ± 0.04 | 0.92 ± 0.05 |
| MW Range (Da) | Rt (min) | Relative Content (%) |
|---|---|---|
| 18,994–5010 | 21.132–23.198 | 16.74 |
| 4989–3010 | 23.206–24.091 | 16.91 |
| 2998–1001 | 24.098–26.302 | 33.50 |
| 998–314 | 26.309–29.304 | 15.57 |
| Component | Tyrosinase Inhibitory Activity (IC50, mg/mL) |
|---|---|
| APGP | 3.268 ± 0.048 c |
| APGP-I (MW > 5 kDa) | 6.863 ± 0.098 d |
| APGP-II (3 kDa < MW < 5 kDa) | 1.372 ± 0.039 b |
| APGP-III (MW < 3 kDa) | 0.608 ± 0.021 a |
| Peptide Sequence | Protein Name | Intensity | m/z | Score | Toxin | Gravy | XP Score (kcal/mol) |
|---|---|---|---|---|---|---|---|
| THG | A0A7R7DZB7 | 56,383,000 | 314.1472 | 150.1 | Non-toxin | −1.43 | −5.4 |
| LGLKNK | L0ET25 | 21,389,000 | 336.7240 | 168.09 | Non-toxin | −0.68 | −6.3 |
| PYNVVHT | A0A7R7DZL1 | 189,410,000 | 829.4233 | 137.91 | Non-toxin | −0.27 | −6.5 |
| VPKLH | A0A7R7DZL1 | 10,988,000 | 297.1922 | 252.12 | Non-toxin | −0.14 | −6.8 |
| PYLK | A0A7R7DZB4 | 30,099,000 | 260.6599 | 128.3 | Non-toxin | −0.75 | −6.9 |
| FRVK | A0A7R7I1R9 | 23,149,000 | 275.1791 | 231.86 | Non-toxin | −0.35 | −7.0 |
| PHHF | L0ER83 | 49,520,000 | 269.132 | 286.64 | Non-toxin | −1.30 | −7.3 |
| YYP | L0ET20 | 17,004,000 | 442.1976 | 133.49 | Non-toxin | −1.40 | −7.6 |
| PHHF | PYLK | FRVK | YYP | |
|---|---|---|---|---|
| Hydrogen bonds | His244, Asn260, Gly281 | Gly281, His85 | Met280, Gly281, Asn260, Ser282 | Met280, Gly281, Asn260, Arg268, His244, His85 |
| Electrostatic interaction | NO | Glu322, His263 | Glu322, Glu256 | Glu256 |
| Hydrophobic interaction | Val248, Phe264 | Phe264, Val248, His263, Val283, Ala286, | Phe264, Val248, His244, His85 | His85, Phe264, Val283 |
| Peptide Sequence | Tyrosinase Inhibitory Activity (IC50, mM) |
|---|---|
| YYP | 1.764 ± 0.025 |
| FRVK | 5.873 ± 0.068 |
| PHHF | >10 |
| PYLK | >10 |
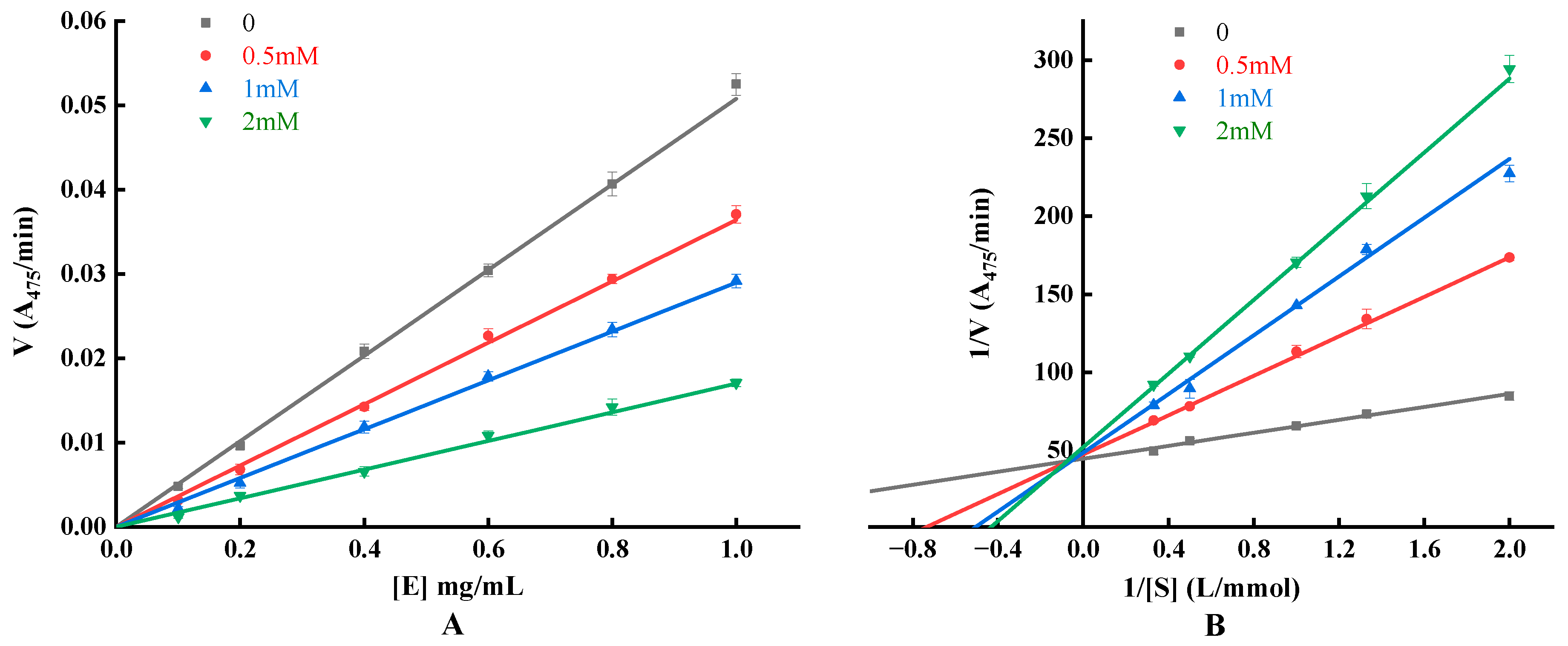
| Concentration (mM) | Fit Equation | Coefficient of Determination R2 | Michaelis’s Constant KM (mmol/L) | Maximum Reaction Speed Vmax (A475/min) | Inhibition Constant KI (mM) | Inhibition Constant KIS (mM) |
|---|---|---|---|---|---|---|
| 0 | y = 20.7837x + 44.6125 | 0.9839 | 0.4659 | 0.0224 | - | - |
| 0.5 | y = 63.1532x + 47.2733 | 0.9992 | 1.3359 | 0.0212 | 0.2453 | 8.3833 |
| 1 | y = 94.1308x + 48.5211 | 0.9928 | 1.9400 | 0.0206 | 0.2834 | 11.4139 |
| 2 | y = 118.0196x + 51.9697 | 0.9968 | 2.2709 | 0.0192 | 0.4275 | 12.1276 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Lin, H.; Shen, W.; Qin, X.; Gao, J.; Cao, W.; Zheng, H.; Chen, Z.; Zhang, Z. Optimization of a Novel Tyrosinase Inhibitory Peptide from Atrina pectinata Mantle and Its Molecular Inhibitory Mechanism. Foods 2023, 12, 3884. https://doi.org/10.3390/foods12213884
Wang W, Lin H, Shen W, Qin X, Gao J, Cao W, Zheng H, Chen Z, Zhang Z. Optimization of a Novel Tyrosinase Inhibitory Peptide from Atrina pectinata Mantle and Its Molecular Inhibitory Mechanism. Foods. 2023; 12(21):3884. https://doi.org/10.3390/foods12213884
Chicago/Turabian StyleWang, Wen, Haisheng Lin, Weiqiang Shen, Xiaoming Qin, Jialong Gao, Wenhong Cao, Huina Zheng, Zhongqin Chen, and Zhishu Zhang. 2023. "Optimization of a Novel Tyrosinase Inhibitory Peptide from Atrina pectinata Mantle and Its Molecular Inhibitory Mechanism" Foods 12, no. 21: 3884. https://doi.org/10.3390/foods12213884
APA StyleWang, W., Lin, H., Shen, W., Qin, X., Gao, J., Cao, W., Zheng, H., Chen, Z., & Zhang, Z. (2023). Optimization of a Novel Tyrosinase Inhibitory Peptide from Atrina pectinata Mantle and Its Molecular Inhibitory Mechanism. Foods, 12(21), 3884. https://doi.org/10.3390/foods12213884







