Impact of a Starch Hydrolysate on the Production of Exopolysaccharides in a Fermented Plant-Based Dessert Formulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starch Hydrolysis
2.1.1. Enzyme Selection
2.1.2. Hydrolysis Method
2.2. Hydrolysate Characterisation
2.2.1. Determination of Starch and Sugar Content
2.2.2. Conductivity Measurement
2.3. Preparation of Fermented Products
2.4. Characterization of the Fermented Products
2.4.1. Kinetics of Acidification, Determination of pH, and Titratable Acidity
2.4.2. Texture Analysis
2.4.3. Water-Holding Capacity
2.4.4. Microbiological Analysis
2.4.5. Quantification of Exopolysaccharides
2.5. Statistical Analysis
3. Results and Discussion
3.1. Hydrolysate Characteristics
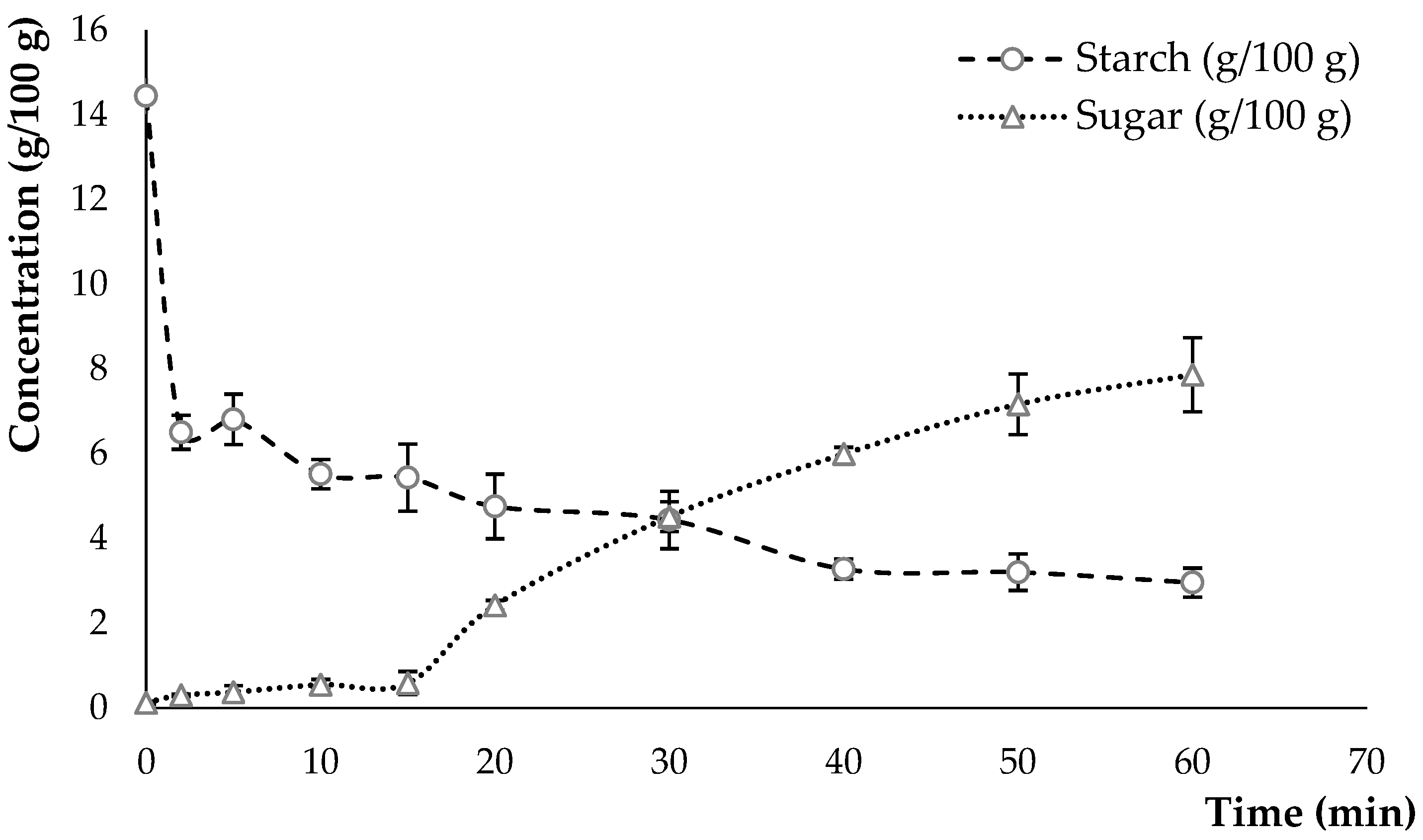
3.1.1. Composition of the Hydrolysate
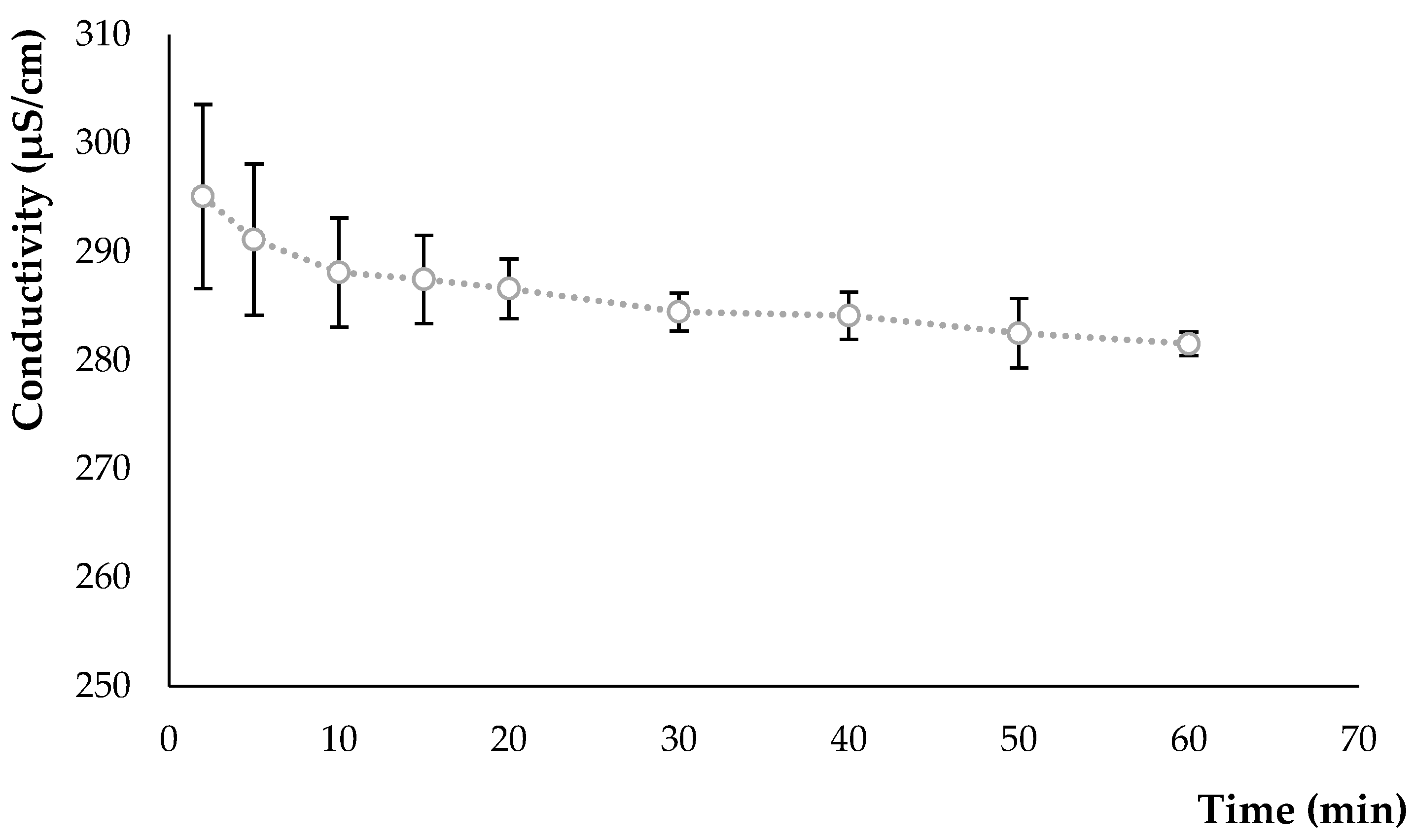
3.1.2. Conductivity Variation
3.2. Characterisation of the Fermented Product
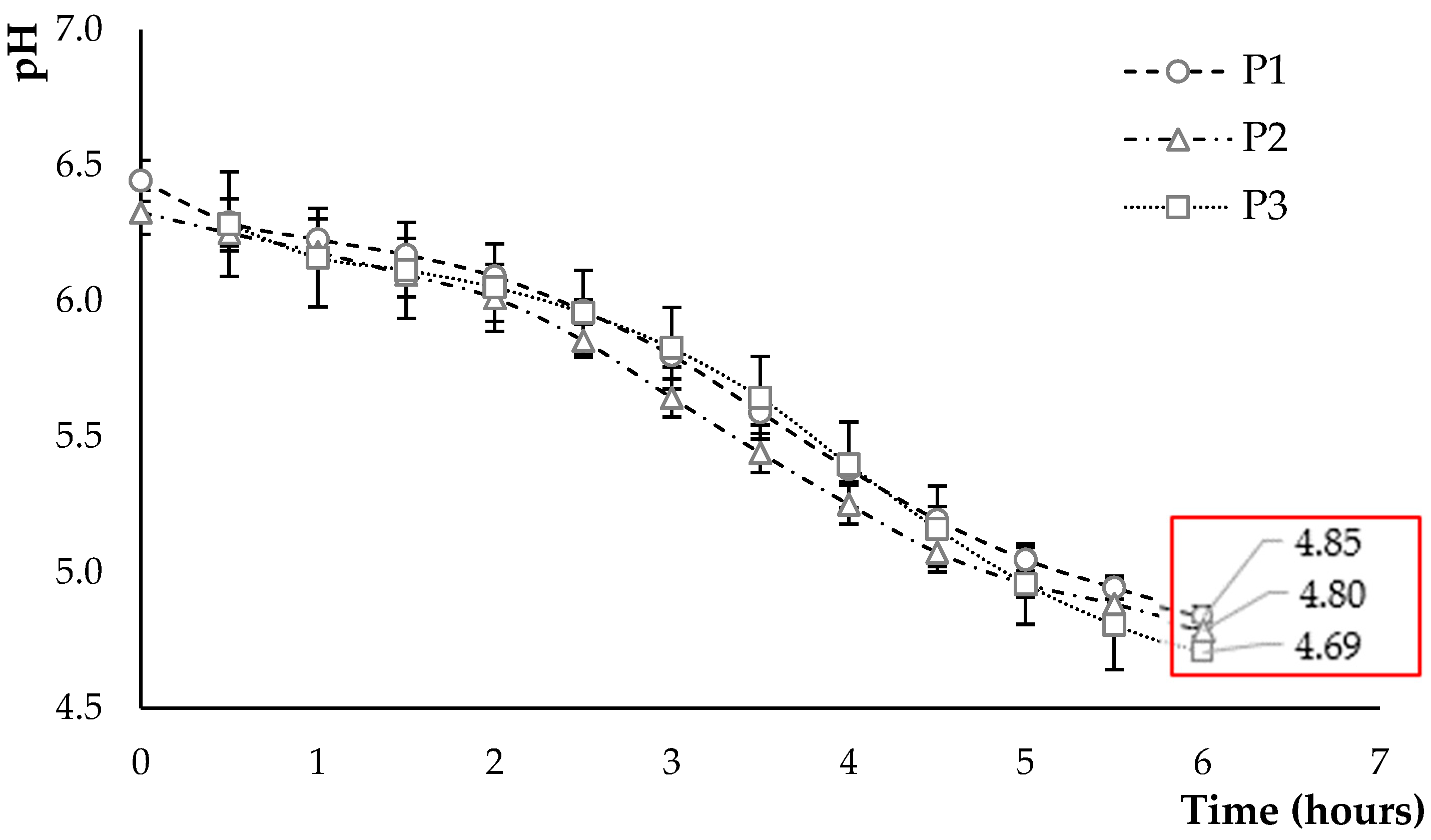
3.2.1. Kinetics of Acidification
3.2.2. Post-Acidification of Fermented Products
3.2.3. Texture Analysis
3.2.4. Water-Holding Capacity
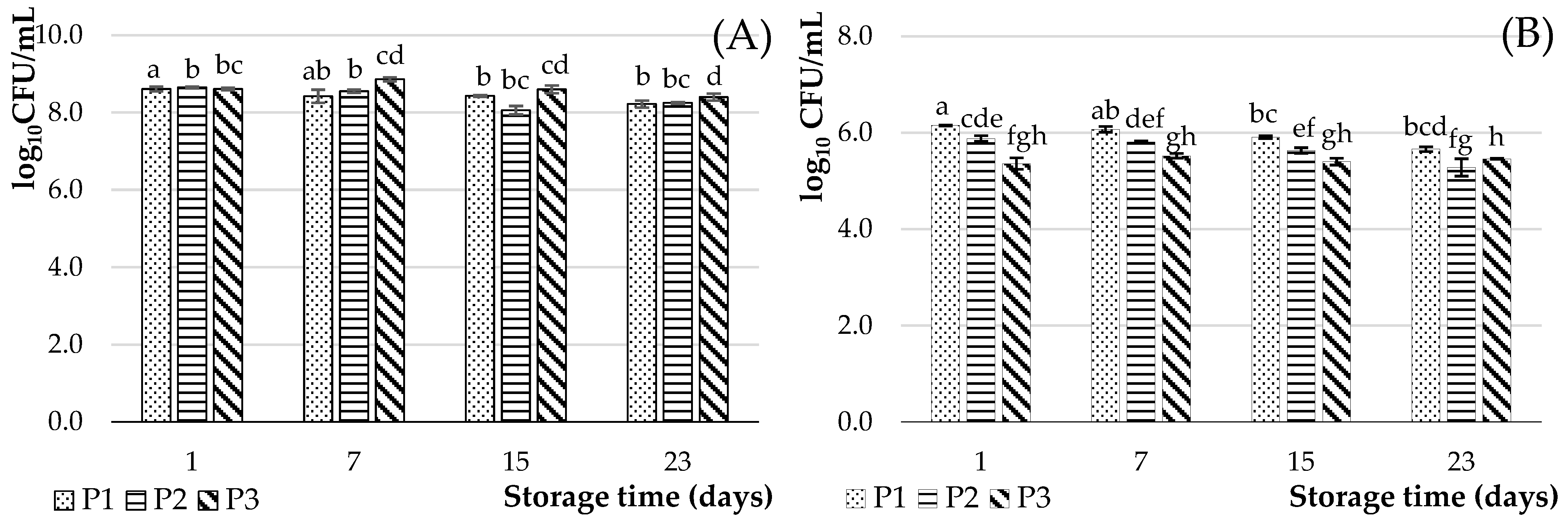
3.2.5. Microbiological Analysis
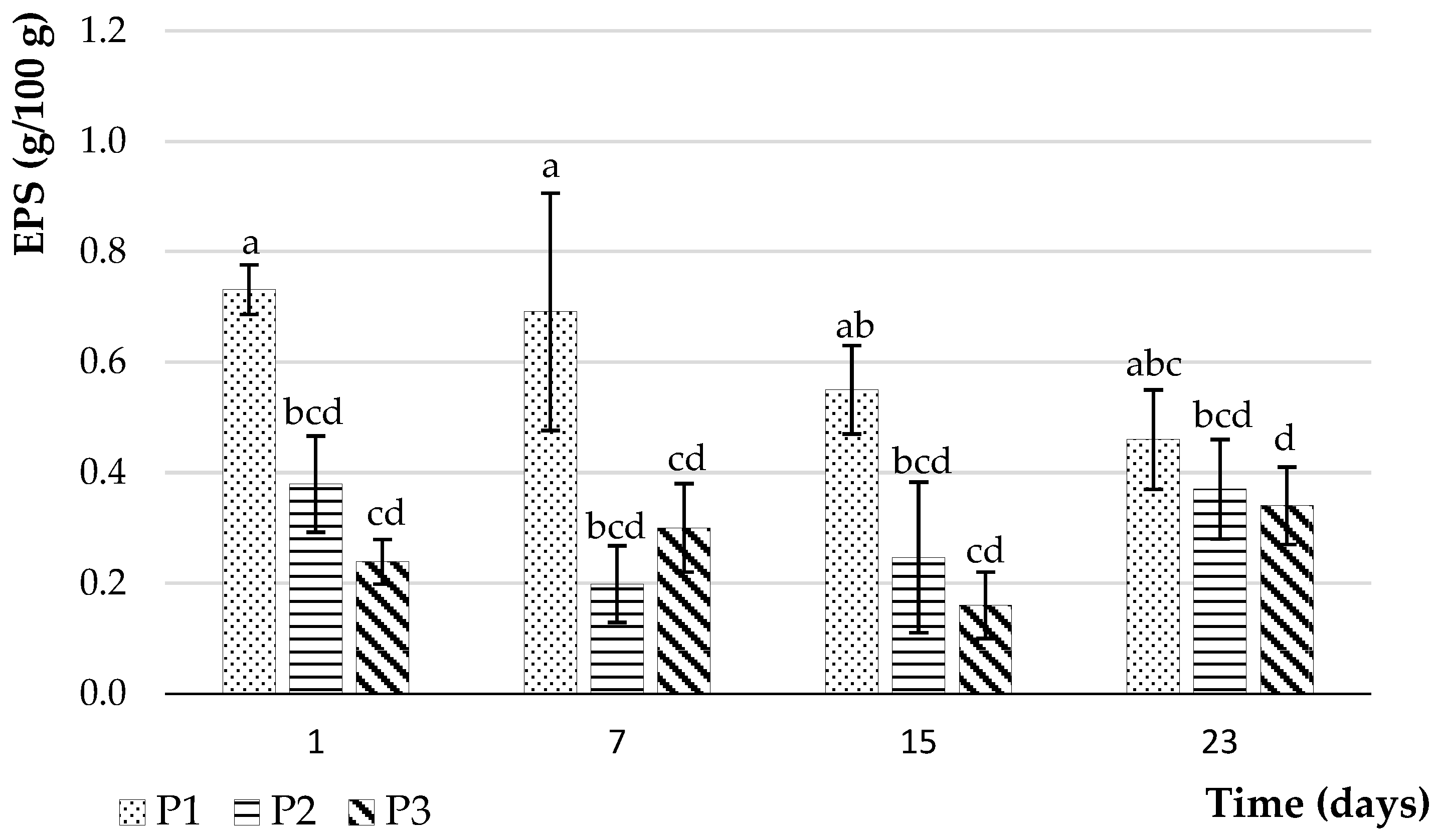
3.2.6. Exopolysaccharide Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grasso, N.; Roos, Y.H.; Crowley, S.V.; Arendt, E.K.; O’Mahony, J.A. Composition and Physicochemical Properties of Commercial Plant-Based Block-Style Products as Alternatives to Cheese. Future Foods 2021, 4, 100048. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Cardello, A.V.; Jin, D.; Ryan, G.S.; Giacalone, D. Consumer Perception of Plant-Based Yoghurt: Sensory Drivers of Liking and Emotional, Holistic and Conceptual Associations. Food Res. Int. 2023, 167, 112666. [Google Scholar] [CrossRef] [PubMed]
- Blandino, A.; Al-Aseeri, M.E.; Pandiella, S.S.; Cantero, D.; Webb, C. Cereal-Based Fermented Foods and Beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Jeske, S.; Zannini, E.; Arendt, E.K. Past, Present and Future: The Strength of Plant-Based Dairy Substitutes Based on Gluten-Free Raw Materials. Food Res. Int. 2018, 110, 42–51. [Google Scholar] [CrossRef]
- Zhu, F. Structures, Properties, Modifications, and Uses of Oat Starch. Food Chem. 2017, 229, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Demir, H.; Simsek, M.; Yıldırım, G. Effect of Oat Milk Pasteurization Type on the Characteristics of Yogurt. LWT 2021, 135, 110271. [Google Scholar] [CrossRef]
- Kohajdová, Z. Fermented Cereal Products. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 91–117. ISBN 978-0-444-63666-9. [Google Scholar]
- Bakr, S.A. Nutritional and Therapeutical Values of Chickpea Water Extract Enriched Yogurt Made from Cow and Camel Milk. Am. J. Drug Discov. Dev. 2013, 3, 47–59. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, L.; Zheng, Y.; Shen, J. Rheological, Textural, and Enzymatic Hydrolysis Properties of Chickpea Starch from a Chinese Cultivar. Food Hydrocoll. 2016, 54, 23–29. [Google Scholar] [CrossRef]
- Wang, S.; Chelikani, V.; Serventi, L. Evaluation of Chickpea as Alternative to Soy in Plant-Based Beverages, Fresh and Fermented. LWT 2018, 97, 570–572. [Google Scholar] [CrossRef]
- Trinidad, T.P.; Mallillin, A.C.; Valdez, D.H.; Loyola, A.S.; Askali-Mercado, F.C.; Castillo, J.C.; Encabo, R.R.; Masa, D.B.; Maglaya, A.S.; Chua, M.T. Dietary Fiber from Coconut Flour: A Functional Food. Innov. Food Sci. Emerg. Technol. 2006, 7, 309–317. [Google Scholar] [CrossRef]
- Adeloye, J.B.; Osho, H.; Idris, L.O. Defatted Coconut Flour Improved the Bioactive Components, Dietary Fibre, Antioxidant and Sensory Properties of Nixtamalized Maize Flour. J. Agric. Food Res. 2020, 2, 100042. [Google Scholar] [CrossRef]
- Makinde, F.M.; Eyitayo, A.O. The Evaluation of Nutritional Composition and Functional and Pasting Properties of Wheat Flour-Coconut Flour Blends. Croat. J. Food Sci. Technol. 2019, 11, 21–29. [Google Scholar] [CrossRef]
- Dhakal, D.; Younas, T.; Bhusal, R.P.; Devkota, L.; Henry, C.J.; Dhital, S. Design Rules of Plant-Based Yoghurt-Mimic: Formulation, Functionality, Sensory Profile and Nutritional Value. Food Hydrocoll. 2023, 142, 108786. [Google Scholar] [CrossRef]
- Villas-Boas, F.; Yamauti, Y.; Moretti, M.M.S.; Franco, C.M.L. Influence of Molecular Structure on the Susceptibility of Starch to α-Amylase. Carbohydr. Res. 2019, 479, 23–30. [Google Scholar] [CrossRef]
- Acevedo, B.A.; Villanueva, M.; Chaves, M.G.; Avanza, M.V.; Ronda, F. Starch Enzymatic Hydrolysis, Structural, Thermal and Rheological Properties of Pigeon Pea (Cajanus Cajan) and Dolichos Bean (Dolichos Lab-Lab) Legume Starches. Int. J. Food Sci. Technol. 2020, 55, 712–719. [Google Scholar] [CrossRef]
- Toraya-Avilés, R.; Segura-Campos, M.; Chel-Guerrero, L.; Betancur-Ancona, D. Effects of Pyroconversion and Enzymatic Hydrolysis on Indigestible Starch Content and Physicochemical Properties of Cassava (Manihot Esculenta) Starch. Starch Stärke 2017, 69, 1600267. [Google Scholar] [CrossRef]
- Korcz, E.; Varga, L. Exopolysaccharides from Lactic Acid Bacteria: Techno-Functional Application in the Food Industry. Trends Food Sci. Technol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Wang, D.; Hou, F.; Ma, X.; Chen, W.; Yan, L.; Ding, T.; Ye, X.; Liu, D. Study on the Mechanism of Ultrasound-Accelerated Enzymatic Hydrolysis of Starch: Analysis of Ultrasound Effect on Different Objects. Int. J. Biol. Macromol. 2020, 148, 493–500. [Google Scholar] [CrossRef]
- De Souza, I.A.; Orsi, D.C.; Gomes, A.J.; Lunardi, C.N. Enzymatic Hydrolysis of Starch into Sugars Is Influenced by Microgel Assembly. Biotechnol. Rep. 2019, 22, e00342. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.; Malek, M.; Mohamad Puad, N.I. A Review on Acid and Enzymatic Hydrolyses of Sago Starch. Int. Food Res. J. 2017, 24, 265–273. [Google Scholar]
- Peyer, L.C.; Zannini, E.; Arendt, E.K. Lactic Acid Bacteria as Sensory Biomodulators for Fermented Cereal-Based Beverages. Trends Food Sci. Technol. 2016, 54, 17–25. [Google Scholar] [CrossRef]
- Coda, R.; Montemurro, M.; Rizzello, C.G. Chapter 10—Yogurt-Like Beverages Made With Cereals. In Yogurt in Health and Disease Prevention; Shah, N.P., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 183–201. ISBN 978-0-12-805134-4. [Google Scholar]
- Nouha, K.; Kumar, R.S.; Balasubramanian, S.; Tyagi, R.D. Critical Review of EPS Production, Synthesis and Composition for Sludge Flocculation. J. Environ. Sci. 2018, 66, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for Special Dietary Needs: Non-Dairy Plant-Based Milk Substitutes and Fermented Dairy-Type Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Bancalari, E.; Gatti, M.; Bottari, B.; Mora, D.; Arioli, S. Disclosing Lactobacillus Delbrueckii Subsp. Bulgaricus Intraspecific Diversity in Exopolysaccharides Production. Food Microbiol. 2022, 102, 103924. [Google Scholar] [CrossRef]
- Kunamneni, A.; Singh, S. Response Surface Optimization of Enzymatic Hydrolysis of Maize Starch for Higher Glucose Production. Biochem. Eng. J. 2005, 27, 179–190. [Google Scholar] [CrossRef]
- Almeida, R.L.J.; Dos Santos Pereira, T.; De Andrade Freire, V.; Santiago, Â.M.; Oliveira, H.M.L.; De Sousa Conrado, L.; De Gusmão, R.P. Influence of Enzymatic Hydrolysis on the Properties of Red Rice Starch. Int. J. Biol. Macromol. 2019, 141, 1210–1219. [Google Scholar] [CrossRef]
- Ait Chekdid, A.; Kahn, C.J.F.; Prévot, E.; Ferrières, M.; Lemois, B.; Choquet, C.; Linder, M. Mixture Design Applied for Formulation and Characterization of Vegetal-Based Fermented Products. LWT 2021, 146, 111336. [Google Scholar] [CrossRef]
- Duru, K.C.; Kovaleva, E.; Danilova, I.; Belousova, A. Production and Assessment of Novel Probiotic Fermented Oat Flour Enriched with Isoflavones. LWT 2019, 111, 9–15. [Google Scholar] [CrossRef]
- Wang, R.; Guo, S. Effects of Endogenous Small Molecular Compounds on the Rheological Properties, Texture and Microstructure of Soymilk Coagulum: Removal of Phytate Using Ultrafiltration. Food Chem. 2016, 211, 521–529. [Google Scholar] [CrossRef]
- Wang, X.; He, Z.; Zeng, M.; Qin, F.; Adhikari, B.; Chen, J. Effects of the Size and Content of Protein Aggregates on the Rheological and Structural Properties of Soy Protein Isolate Emulsion Gels Induced by CaSO4. Food Chem. 2017, 221, 130–138. [Google Scholar] [CrossRef]
- Li, C.; Rui, X.; Zhang, Y.; Cai, F.; Chen, X.; Jiang, M. Production of Tofu by Lactic Acid Bacteria Isolated from Naturally Fermented Soy Whey and Evaluation of Its Quality. LWT Food Sci. Technol. 2017, 82, 227–234. [Google Scholar] [CrossRef]
- Hickisch, A.; Beer, R.; Vogel, R.F.; Toelstede, S. Influence of Lupin-Based Milk Alternative Heat Treatment and Exopolysaccharide-Producing Lactic Acid Bacteria on the Physical Characteristics of Lupin-Based Yogurt Alternatives. Food Res. Int. 2016, 84, 180–188. [Google Scholar] [CrossRef]
- Kumar, P.A.; Pushpadass, H.A.; Franklin, M.E.E.; Simha, H.V.V.; Nath, B.S. Effect of Enzymatic Hydrolysis of Starch on Pasting, Rheological and Viscoelastic Properties of Milk-Barnyard Millet (Echinochloa Frumentacea) Blends Meant for Spray Drying. Int. J. Biol. Macromol. 2016, 91, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Langó, B.; Jaiswal, S.; Bóna, L.; Tömösközi, S.; Ács, E.; Chibbar, R.N. Grain Constituents and Starch Characteristics Influencing in Vitro Enzymatic Starch Hydrolysis in Hungarian Triticale Genotypes Developed for Food Consumption. Cereal Chem. 2018, 95, 861–871. [Google Scholar] [CrossRef]
- Abebe, W.; Collar, C.; Ronda, F. Impact of Variety Type and Particle Size Distribution on Starch Enzymatic Hydrolysis and Functional Properties of Tef Flours. Carbohydr. Polym. 2015, 115, 260–268. [Google Scholar] [CrossRef]
- Zhou, Y.; Jin, Y.; Yang, N.; Xie, Z.; Xu, X. Electrofluid Enhanced Hydrolysis of Maize Starch and Its Impacts on Physical Properties. RSC Adv. 2017, 7, 19145–19152. [Google Scholar] [CrossRef]
- Soria-Hernández, C.; Serna-Saldívar, S.; Chuck-Hernández, C. Physicochemical and Functional Properties of Vegetable and Cereal Proteins as Potential Sources of Novel Food Ingredients. Food Technol. Biotechnol. 2015, 53, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Masiá, C.; Geppel, A.; Jensen, P.E.; Buldo, P. Effect of Lactobacillus Rhamnosus on Physicochemical Properties of Fermented Plant-Based Raw Materials. Foods 2021, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Cox, S.; Abu-Ghannam, N. Process Optimization for the Development of a Functional Beverage Based on Lactic Acid Fermentation of Oats. Biochem. Eng. J. 2010, 52, 199–204. [Google Scholar] [CrossRef]
- Gallo, M.; Passannanti, F.; Cante, R.C.; Nigro, F.; Salameh, D.; Schiattarella, P.; Schioppa, C.; Budelli, A.; Nigro, R. Effects of the Glucose Addition during Lactic Fermentation of Rice, Oat and Wheat Flours. Appl. Food Biotechnol. 2020, 7, 21–30. [Google Scholar]
- Almeida Neta, M.C.; Rocha de Queiroga, A.P.; Almeida, R.L.J.; Caetano Soares, A.; Marinho Gonçalves, J.; Soares Fernandes, S.; De Sousa, M.C.; Olbrich dos Santos, K.M.; Alonso Buriti, F.C.; Rolim Florentino, E. Fermented Dessert with Whey, Ingredients from the Peel of Jabuticaba (Myrciaria Cauliflora) and an Indigenous Culture of Lactobacillus Plantarum: Composition, Microbial Viability, Antioxidant Capacity and Sensory Features. Nutrients 2018, 10, 1214. [Google Scholar] [CrossRef]
- Genevois, C.E.; Castellanos Fuentes, A.P.; Flores, S.K.; de Escalada Pla, M.F. The Functional and Organoleptic Characterization of a Dairy-Free Dessert Containing a Novel Probiotic Food Ingredient. Food Funct. 2018, 9, 5697–5706. [Google Scholar] [CrossRef]
- Grasso, N.; Alonso-Miravalles, L.; O’Mahony, J.A. Composition, Physicochemical and Sensorial Properties of Commercial Plant-Based Yogurts. Foods 2020, 9, 252. [Google Scholar] [CrossRef]
- Raikos, V.; Juskaite, L.; Vas, F.; Hayes, H.E. Physicochemical Properties, Texture, and Probiotic Survivability of Oat-Based Yogurt Using Aquafaba as a Gelling Agent. Food Sci. Nutr. 2020, 8, 6426–6432. [Google Scholar] [CrossRef] [PubMed]
- Gularte, M.A.; Rosell, C.M. Physicochemical Properties and Enzymatic Hydrolysis of Different Starches in the Presence of Hydrocolloids. Carbohydr. Polym. 2011, 85, 237–244. [Google Scholar] [CrossRef]
- de Souza, E.L.; de Oliveira, K.Á.; de Oliveira, M.E. Influence of Lactic Acid Bacteria Metabolites on Physical and Chemical Food Properties. Curr. Opin. Food Sci. 2023, 49, 100981. [Google Scholar] [CrossRef]
- Demirci, T. Enumeration Of Bifidobacterium Spp., Lactobacillus Acidophilus and Starter Cultures from Commercial Probiotic Yogurts and Freeze-Dried Yogurt Starter Mixes. SJAFS 2022, 36, 421–427. [Google Scholar] [CrossRef]
- Jaster, H.; Arend, G.D.; Rezzadori, K.; Chaves, V.C.; Reginatto, F.H.; Petrus, J.C.C. Enhancement of Antioxidant Activity and Physicochemical Properties of Yogurt Enriched with Concentrated Strawberry Pulp Obtained by Block Freeze Concentration. Food Res. Int. 2018, 104, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Szydłowska, A.; Siwińska, J.; Kołożyn-Krajewska, D. Cereal-Based Vegan Desserts as Container of Potentially Probiotic Bacteria Isolated from Fermented Plant-Origin Food. CyTA J. Food 2021, 19, 691–700. [Google Scholar] [CrossRef]
- Uriot, O.; Denis, S.; Junjua, M.; Roussel, Y.; Dary-Mourot, A.; Blanquet-Diot, S. Streptococcus Thermophilus: From Yogurt Starter to a New Promising Probiotic Candidate. J. Funct. Foods 2017, 37, 74–89. [Google Scholar] [CrossRef]
- Ale, E.C.; Ibáñez, R.A.; Wilbanks, D.J.; Peralta, G.H.; Ceylan, F.D.; Binetti, A.G.; Bolling, B.W.; Lucey, J.A. Technological Role and Metabolic Profile of Two Probiotic EPS-Producing Strains with Potential Application in Yoghurt: Impact on Rheology and Release of Bioactive Peptides. Int. Dairy J. 2023, 137, 105533. [Google Scholar] [CrossRef]
- Zannini, E.; Jeske, S.; Lynch, K.M.; Arendt, E.K. Development of Novel Quinoa-Based Yoghurt Fermented with Dextran Producer Weissella Cibaria MG1. Int. J. Food Microbiol. 2018, 268, 19–26. [Google Scholar] [CrossRef]
- Yalmanci, D.; İspirli, H.; Dertli, E. Identification of Lactic Acid Bacteria (LAB) from Pre-Fermented Liquids of Selected Cereals and Legumes and Characterization of Their Exopolysaccharides (EPS). Food Biosci. 2022, 50, 102014. [Google Scholar] [CrossRef]
- Comte, S.; Guibaud, G.; Baudu, M. Relations between Extraction Protocols for Activated Sludge Extracellular Polymeric Substances (EPS) and EPS Complexation Properties Part I. Comparison of the Efficiency of Eight EPS Extraction Methods. Enzym. Microb. Technol. 2006, 38, 237–245. [Google Scholar] [CrossRef]
- Luana, N.; Rossana, C.; Curiel, J.A.; Kaisa, P.; Marco, G.; Rizzello, C.G. Manufacture and Characterization of a Yogurt-like Beverage Made with Oat Flakes Fermented by Selected Lactic Acid Bacteria. Int. J. Food Microbiol. 2014, 185, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, W.; Chen, X.; Feng, M.; Rui, X.; Jiang, M.; Dong, M. Microbiological, Physicochemical and Rheological Properties of Fermented Soymilk Produced with Exopolysaccharide (EPS) Producing Lactic Acid Bacteria Strains. LWT Food Sci. Technol. 2014, 57, 477–485. [Google Scholar] [CrossRef]
- Ramchandran, L.; Shah, N.P. Effect of Exopolysaccharides on the Proteolytic and Angiotensin-I Converting Enzyme-Inhibitory Activities and Textural and Rheological Properties of Low-Fat Yogurt during Refrigerated Storage. J. Dairy Sci. 2009, 92, 895–906. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Q. EPS-Producing Lactobacillus Plantarum MC5 as a Compound Starter Improves Rheology, Texture, and Antioxidant Activity of Yogurt during Storage. Foods 2022, 11, 1660. [Google Scholar] [CrossRef]
| Ingredients | Product 1 | Product 2 | Product 3 |
|---|---|---|---|
| Oat flour | 3.0% | 1.5% | 0.9% |
| Oat hydrolysate | - | 1.5% | 2.1% |
| Chickpea flour | 9.0% | 9.0% | 9.0% |
| Coconut flour | 3.0% | 3.0% | 3.0% |
| Storage Time (Days) | pH | Acidity | WHC (%) | Hardness (N) | Cohesion | Elasticity (mm) | |
|---|---|---|---|---|---|---|---|
| P1 | 1 | 4.45 ± 0.01 a | 4.77 ± 0.15 ab | 100.00 ± 0.00 a | 0.19 ± 0.004 a | 0.50 ± 0.02 a | 17.58 ± 0.53 a |
| 7 | 4.36 ± 0.01 ab | 4.47 ± 0.06 ab | 83.07 ± 1.29 b | 0.26 ± 0.01 b | 0.32 ± 0.10 ab | 14.27 ± 0.84 ab | |
| 15 | 4.43 ± 0.03 bc | 5.37 ± 0.21 cd | 80.13 ± 0.11 b | 0.35 ± 0.02 b | 0.33 ± 0.05 abc | 15.89 ± 0.39 abc | |
| 23 | 4.41 ± 0.01 cd | 5.53 ± 0.06 c | 72.57 ± 0.21 c | 0.55 ± 0.04 b | 0.36 ± 0.02 abcd | 18.03 ± 0.70 abcd | |
| P2 | 1 | 4.40 ± 0.02 cde | 5.03 ± 0.06 ad | 72.22 ± 0.50 c | 0.09 ± 0.004 c | 0.46 ± 0.07 bcd | 13.19 ± 0.48 abcde |
| 7 | 4.28 ± 0.02 cde | 4.60 ± 0.10 ad | 65.99 ± 0.93 d | 0.18 ± 0.01 cd | 0.33 ± 0.06 bcd | 13.90 ± 1.02 bcdef | |
| 15 | 4.38 ± 0.01 de | 6.00 ± 0.10 e | 68.44 ± 2.43 d | 0.23 ± 0.02 cd | 0.27 ± 0.04 cd | 12.52 ± 1.07 cdef | |
| 23 | 4.27 ± 0.01 e | 6.37 ± 0.15 b | 63.28 ± 1.23 de | 0.31 ± 0.01 cd | 0.26 ± 0.10 d | 11.21 ± 0.63 cdef | |
| P3 | 1 | 4.47 ± 0.02 f | 4.73 ± 0.06 ef | 68.67 ± 1.25 def | 0.09 ± 0.002 d | 0.53 ± 0.08 d | 16.82 ± 1.48 def |
| 7 | 4.39 ± 0.01 fg | 5.03 ± 0.12 b | 65.26 ± 0.81 ef | 0.19 ± 0.004 d | 0.26 ± 0.01 d | 13.54 ± 0.72 ef | |
| 15 | 4.30 ± 0.01 fg | 6.30 ± 0.17 fg | 68.27 ± 0.38 fg | 0.20 ± 0.01 e | 0.23 ± 0.01 d | 11.10 ± 1.27 f | |
| 23 | 4.25 ± 0.01 g | 6.70 ± 0.10 g | 62.12 ± 0.49 g | 0.33 ± 0.005 e | 0.24 ± 0.02 d | 15.42 ± 2.61 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ait Chekdid, A.; Kahn, C.J.F.; Lemois, B.; Linder, M. Impact of a Starch Hydrolysate on the Production of Exopolysaccharides in a Fermented Plant-Based Dessert Formulation. Foods 2023, 12, 3868. https://doi.org/10.3390/foods12203868
Ait Chekdid A, Kahn CJF, Lemois B, Linder M. Impact of a Starch Hydrolysate on the Production of Exopolysaccharides in a Fermented Plant-Based Dessert Formulation. Foods. 2023; 12(20):3868. https://doi.org/10.3390/foods12203868
Chicago/Turabian StyleAit Chekdid, Aldjia, Cyril J. F. Kahn, Béatrice Lemois, and Michel Linder. 2023. "Impact of a Starch Hydrolysate on the Production of Exopolysaccharides in a Fermented Plant-Based Dessert Formulation" Foods 12, no. 20: 3868. https://doi.org/10.3390/foods12203868
APA StyleAit Chekdid, A., Kahn, C. J. F., Lemois, B., & Linder, M. (2023). Impact of a Starch Hydrolysate on the Production of Exopolysaccharides in a Fermented Plant-Based Dessert Formulation. Foods, 12(20), 3868. https://doi.org/10.3390/foods12203868






