Sulforaphane Bioavailability in Healthy Subjects Fed a Single Serving of Fresh Broccoli Microgreens
Abstract
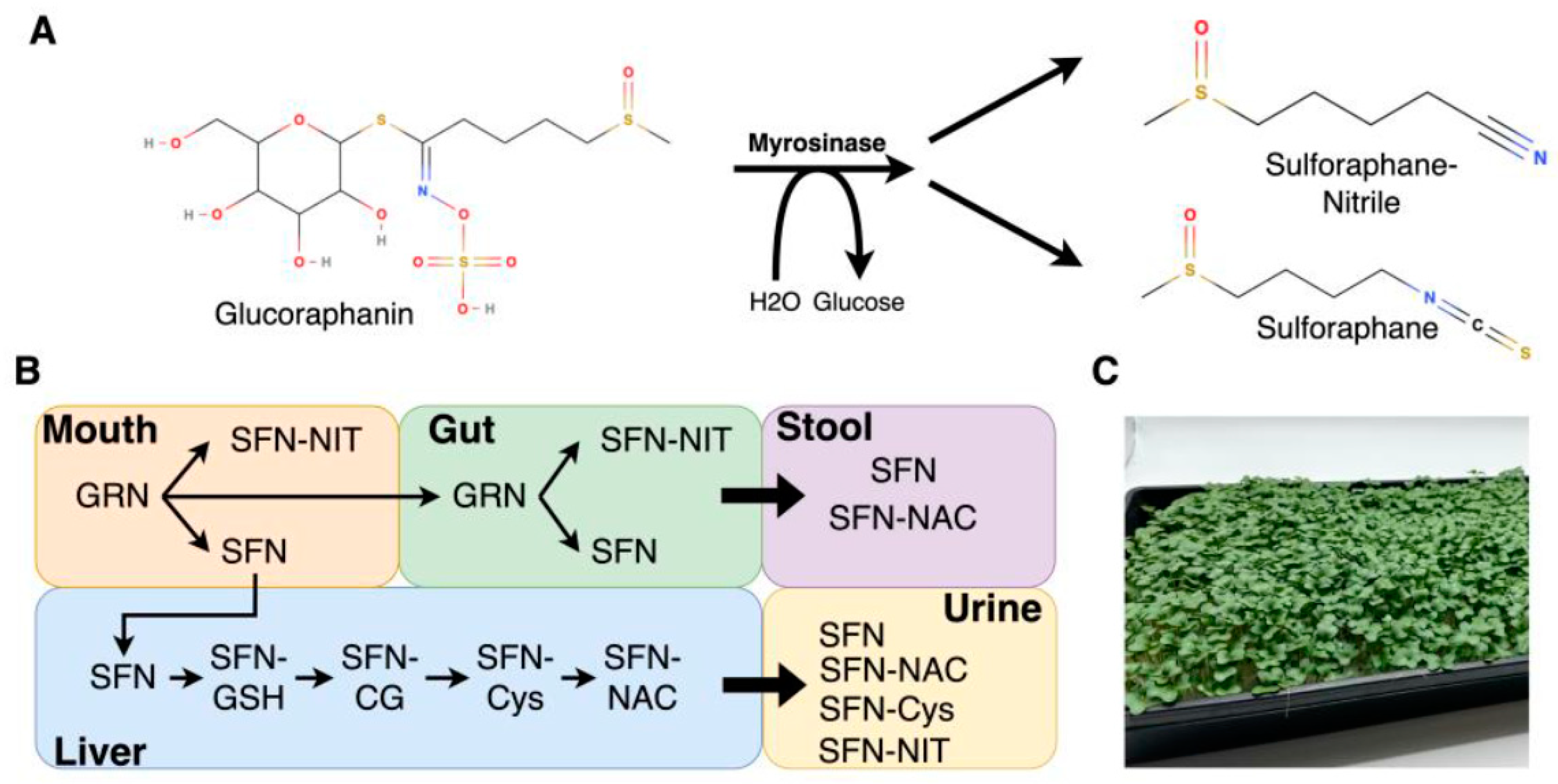
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Sample Collection
2.4. Sample Processing for Mass Spectrometry
2.5. Mass Spectrometry Analyses
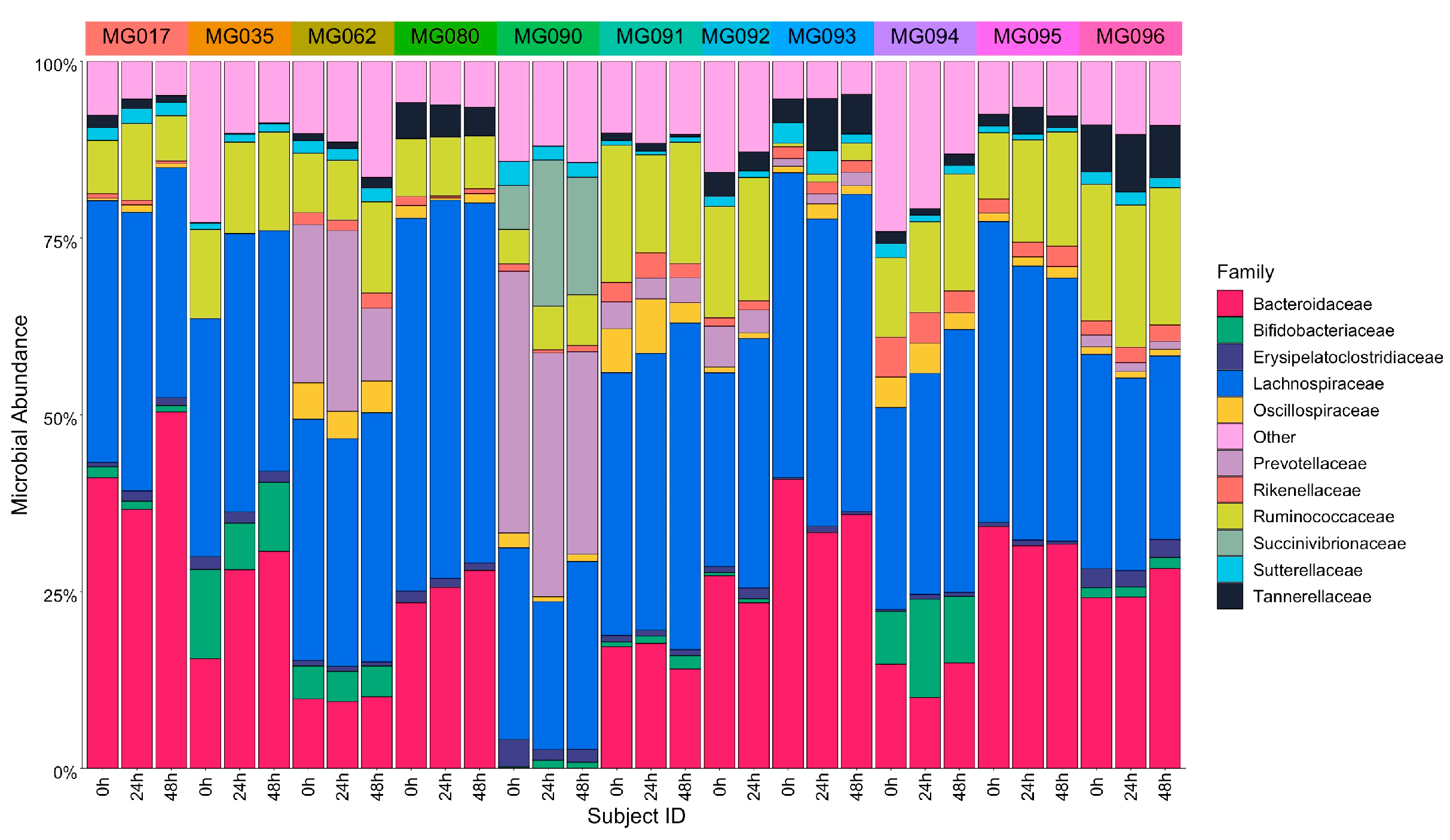
2.6. Stool Sample Processing for 16S Amplicon Sequencing
2.7. Microbiome Data Management and Quantification of ASVs
2.8. Microbiome Analysis
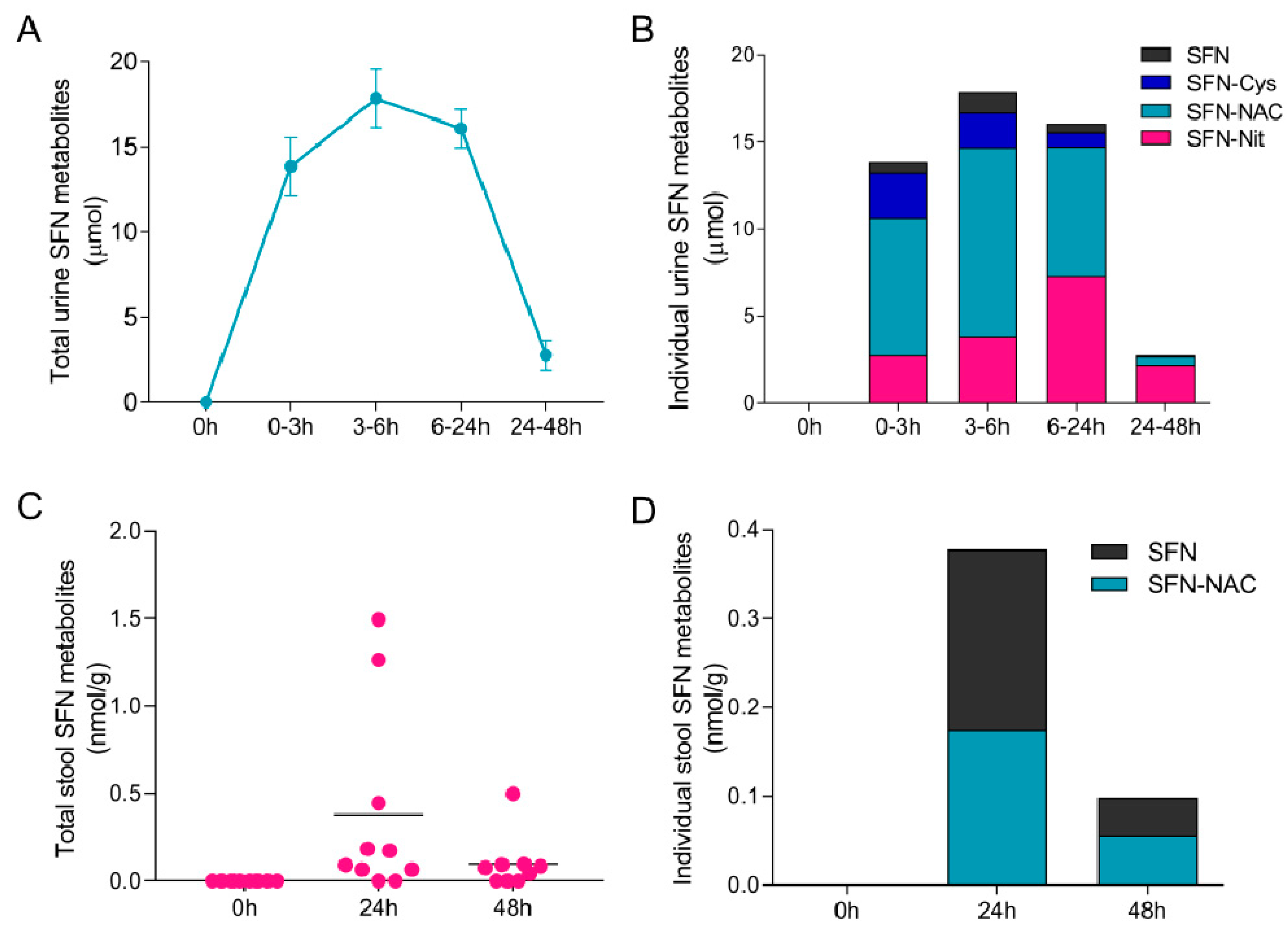
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.L.; Sim, M.; Travica, N.; Marx, W.; Beasy, G.; Lynch, G.S.; Bondonno, C.P.; Lewis, J.R.; Hodgson, J.M.; Blekkenhorst, L.C. Glucosinolates From Cruciferous Vegetables and Their Potential Role in Chronic Disease: Investigating the Preclinical and Clinical Evidence. Front. Pharmacol. 2021, 12, 767975. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Wu, C.; Rao, Z.; Du, L.; Zhou, Y. Cruciferous vegetable and isothiocyanate intake and multiple health outcomes. Food Chem. 2022, 375, 131816. [Google Scholar] [CrossRef]
- Schepici, G.; Bramanti, P.; Mazzon, E. Efficacy of Sulforaphane in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8637. [Google Scholar] [CrossRef]
- Kushad, M.M.; Brown, A.F.; Kurilich, A.C.; Juvik, J.A.; Klein, B.P.; Wallig, M.A.; Jeffery, E.H. Variation of glucosinolates in vegetable crops of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1541–1548. [Google Scholar] [CrossRef]
- Elkashty, O.A.; Tran, S.D. Sulforaphane as a Promising Natural Molecule for Cancer Prevention and Treatment. Curr. Med. Sci. 2021, 41, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Ruhee, R.T.; Suzuki, K. The Integrative Role of Sulforaphane in Preventing Inflammation, Oxidative Stress and Fatigue: A Review of a Potential Protective Phytochemical. Antioxidants 2020, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Khor, T.O.; Kong, A.N. Synergistic effect of combination of phenethyl isothiocyanate and sulforaphane or curcumin and sulforaphane in the inhibition of inflammation. Pharm. Res. 2009, 26, 224–231. [Google Scholar] [CrossRef]
- Li, Y.; Meeran, S.M.; Tollefsbol, T.O. Combinatorial bioactive botanicals re-sensitize tamoxifen treatment in ER-negative breast cancer via epigenetic reactivation of ERalpha expression. Sci. Rep. 2017, 7, 9345. [Google Scholar] [CrossRef]
- Paul, B.; Li, Y.; Tollefsbol, T.O. The Effects of Combinatorial Genistein and Sulforaphane in Breast Tumor Inhibition: Role in Epigenetic Regulation. Int. J. Mol. Sci. 2018, 19, 1754. [Google Scholar] [CrossRef]
- Rakariyatham, K.; Wu, X.; Tang, Z.; Han, Y.; Wang, Q.; Xiao, H. Synergism between luteolin and sulforaphane in anti-inflammation. Food Funct. 2018, 9, 5115–5123. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Tang, S.N.; Zhu, W.; Meeker, D.; Shankar, S. Sulforaphane synergizes with quercetin to inhibit self-renewal capacity of pancreatic cancer stem cells. Front. Biosci. 2011, 3, 515–528. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef]
- Wojdylo, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. Microgreens as Novel Functional Foods: Variation of Nutritional and Phytochemical Profiles and Their In Vitro Bioactive Properties. Molecules 2020, 25, 4648. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.W. Sprouts and Microgreens-Novel Food Sources for Healthy Diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef]
- de la Fuente, B.; Lopez-Garcia, G.; Manez, V.; Alegria, A.; Barbera, R.; Cilla, A. Evaluation of the Bioaccessibility of Antioxidant Bioactive Compounds and Minerals of Four Genotypes of Brassicaceae Microgreens. Foods 2019, 8, 250. [Google Scholar] [CrossRef]
- Marchioni, I.; Martinelli, M.; Ascrizzi, R.; Gabbrielli, C.; Flamini, G.; Pistelli, L.; Pistelli, L. Small Functional Foods: Comparative Phytochemical and Nutritional Analyses of Five Microgreens of the Brassicaceae Family. Foods 2021, 10, 427. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Kaczmarek, J.L.; Liu, X.; Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Seifried, H.E.; Ross, S.A.; Miller, M.J.; Swanson, K.S.; Holscher, H.D. Broccoli consumption affects the human gastrointestinal microbiota. J. Nutr. Biochem. 2019, 63, 27–34. [Google Scholar] [CrossRef]
- Li, F.; Hullar, M.A.; Schwarz, Y.; Lampe, J.W. Human gut bacterial communities are altered by addition of cruciferous vegetables to a controlled fruit- and vegetable-free diet. J. Nutr. 2009, 139, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Eve, A.A.; Liu, X.; Wang, Y.; Miller, M.J.; Jeffery, E.H.; Madak-Erdogan, Z. Biomarkers of Broccoli Consumption: Implications for Glutathione Metabolism and Liver Health. Nutrients 2020, 12, 2514. [Google Scholar] [CrossRef] [PubMed]
- Bouranis, J.A.; Beaver, L.M.; Choi, J.; Wong, C.P.; Jiang, D.; Sharpton, T.J.; Stevens, J.F.; Ho, E. Composition of the Gut Microbiome Influences Production of Sulforaphane-Nitrile and Iberin-Nitrile from Glucosinolates in Broccoli Sprouts. Nutrients 2021, 13, 3013. [Google Scholar] [CrossRef] [PubMed]
- Matusheski, N.V.; Juvik, J.A.; Jeffery, E.H. Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry 2004, 65, 1273–1281. [Google Scholar] [CrossRef]
- Zhang, Z.; Garzotto, M.; Davis, E.W., 2nd; Mori, M.; Stoller, W.A.; Farris, P.E.; Wong, C.P.; Beaver, L.M.; Thomas, G.V.; Williams, D.E.; et al. Sulforaphane Bioavailability and Chemopreventive Activity in Men Presenting for Biopsy of the Prostate Gland: A Randomized Controlled Trial. Nutr. Cancer 2020, 72, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Kaehler, B.D.; Bokulich, N.A.; McDonald, D.; Knight, R.; Caporaso, J.G.; Huttley, G.A. Species abundance information improves sequence taxonomy classification accuracy. Nat. Commun. 2019, 10, 4643. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; Hara, B.; Simpson, G.; Solymos, P.; Henry, M.; Stevens, H.; Maintainer, H. The vegan package. Commun. Ecol. Package 2009, 10, 631–637. [Google Scholar]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Bouranis, J.A.; Beaver, L.M.; Wong, C.P.; Choi, J.; Hamer, S.; Davis, E.W.; Brown, K.S.; Jaing, D.; Sharpton, T.J.; Stevens, J.F.; et al. Sulforaphane and sulforaphane-nitrile metabolism in humans following broccoli sprout consumption: Inter-individual variation, association with gut microbiome composition, and differential bioactivity. Mol. Nutr. Food Res. (no publish).
- Bouranis, J.A.; Beaver, L.M.; Ho, E. Metabolic Fate of Dietary Glucosinolates and Their Metabolites: A Role for the Microbiome. Front. Nutr. 2021, 8, 748433. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Swarup, R.; Juvik, J.A.; Mithen, R.; Bennett, M.; Jeffery, E.H. Epithiospecifier protein from broccoli (Brassica oleracea L. ssp. italica) inhibits formation of the anticancer agent sulforaphane. J. Agric. Food Chem. 2006, 54, 2069–2076. [Google Scholar] [CrossRef]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; De Schrijver, R.; Hansen, M.; Gerhauser, C.; Mithen, R.; et al. Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53, S219. [Google Scholar] [CrossRef]
- Mullaney, J.A.; Kelly, W.J.; McGhie, T.K.; Ansell, J.; Heyes, J.A. Lactic acid bacteria convert glucosinolates to nitriles efficiently yet differently from enterobacteriaceae. J. Agric. Food Chem. 2013, 61, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Luang-In, V.; Narbad, A.; Nueno-Palop, C.; Mithen, R.; Bennett, M.; Rossiter, J.T. The metabolism of methylsulfinylalkyl- and methylthioalkyl-glucosinolates by a selection of human gut bacteria. Mol. Nutr. Food Res. 2014, 58, 875–883. [Google Scholar] [CrossRef]
- Luang-In, V.; Albaser, A.A.; Nueno-Palop, C.; Bennett, M.H.; Narbad, A.; Rossiter, J.T. Glucosinolate and Desulfo-glucosinolate Metabolism by a Selection of Human Gut Bacteria. Curr. Microbiol. 2016, 73, 442–451. [Google Scholar] [CrossRef]
- Luang-In, V.; Deeseenthum, S.; Udomwong, P.; Saengha, W.; Gregori, M. Formation of Sulforaphane and Iberin Products from Thai Cabbage Fermented by Myrosinase-Positive Bacteria. Molecules 2018, 23, 955. [Google Scholar] [CrossRef]
- Clarke, J.D.; Hsu, A.; Riedl, K.; Bella, D.; Schwartz, S.J.; Stevens, J.F.; Ho, E. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol. Res. 2011, 64, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Grady, R.S.; Traustadottir, T.; Lagalante, A.F.; Eggler, A.L. Bioavailable Sulforaphane Quantitation in Plasma by LC-MS/MS Is Enhanced by Blocking Thiols. J. Agric. Food Chem. 2023, 71, 12875–12882. [Google Scholar] [CrossRef]
- Kellingray, L.; Tapp, H.S.; Saha, S.; Doleman, J.F.; Narbad, A.; Mithen, R.F. Consumption of a diet rich in Brassica vegetables is associated with a reduced abundance of sulphate-reducing bacteria: A randomised crossover study. Mol. Nutr. Food Res. 2017, 61, 992. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, Y.; Zhu, Y.; Mupunga, J.; Zou, L.; Liu, C.; Liu, S.; Mao, J. Broccoli ingestion increases the glucosinolate hydrolysis activity of microbiota in the mouse gut. Int. J. Food Sci. Nutr. 2019, 70, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Hoeflinger, J.L.; Neme, B.P.; Jeffery, E.H.; Miller, M.J. Dietary Broccoli Alters Rat Cecal Microbiota to Improve Glucoraphanin Hydrolysis to Bioactive Isothiocyanates. Nutrients 2017, 9, 262. [Google Scholar] [CrossRef]
- Egner, P.A.; Chen, J.G.; Zarth, A.T.; Ng, D.K.; Wang, J.B.; Kensler, K.H.; Jacobson, L.P.; Munoz, A.; Johnson, J.L.; Groopman, J.D.; et al. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: Results of a randomized clinical trial in China. Cancer Prev. Res. 2014, 7, 813–823. [Google Scholar] [CrossRef]
- Kensler, T.W.; Chen, J.G.; Egner, P.A.; Fahey, J.W.; Jacobson, L.P.; Stephenson, K.K.; Ye, L.; Coady, J.L.; Wang, J.B.; Wu, Y.; et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.; Schwarz, Y.; Li, S.S.; Li, L.; King, I.B.; Chen, C.; Eaton, D.L.; Potter, J.D.; Lampe, J.W. CYP1A2, GSTM1, and GSTT1 polymorphisms and diet effects on CYP1A2 activity in a crossover feeding trial. Cancer Epidemiol. Biomark. Prev. 2009, 18, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- Triska, J.; Balik, J.; Houska, M.; Novotna, P.; Magner, M.; Vrchotova, N.; Hic, P.; Jilek, L.; Thorova, K.; Snurkovic, P.; et al. Factors Influencing Sulforaphane Content in Broccoli Sprouts and Subsequent Sulforaphane Extraction. Foods 2021, 10, 1927. [Google Scholar] [CrossRef] [PubMed]

| Demographics a | |
|---|---|
| Biological Sex | Male (n = 4) |
| Female (n = 7) | |
| Age (years) | 35.2 ± 3.2 |
| BMI (kg/m2) | 23.6 ± 0.7 |
| Race | n (%) |
| White | 7 (63) |
| Non-White | 4 (36) |
| Average caloric and macronutrient intake a.b | |
| Calories (kcal) | 1930.1 ± 249 |
| Protein (g) | 78.9 ± 12.8 |
| Carbohydrate (g) | 232.7 ± 30.7 |
| Fat (g) | 76.8 ± 10.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouranis, J.A.; Wong, C.P.; Beaver, L.M.; Uesugi, S.L.; Papenhausen, E.M.; Choi, J.; Davis, E.W., 2nd; Da Silva, A.N.; Kalengamaliro, N.; Chaudhary, R.; et al. Sulforaphane Bioavailability in Healthy Subjects Fed a Single Serving of Fresh Broccoli Microgreens. Foods 2023, 12, 3784. https://doi.org/10.3390/foods12203784
Bouranis JA, Wong CP, Beaver LM, Uesugi SL, Papenhausen EM, Choi J, Davis EW 2nd, Da Silva AN, Kalengamaliro N, Chaudhary R, et al. Sulforaphane Bioavailability in Healthy Subjects Fed a Single Serving of Fresh Broccoli Microgreens. Foods. 2023; 12(20):3784. https://doi.org/10.3390/foods12203784
Chicago/Turabian StyleBouranis, John A., Carmen P. Wong, Laura M. Beaver, Sandra L. Uesugi, Ethan M. Papenhausen, Jaewoo Choi, Edward W. Davis, 2nd, Adilson Nunes Da Silva, Newton Kalengamaliro, Rekha Chaudhary, and et al. 2023. "Sulforaphane Bioavailability in Healthy Subjects Fed a Single Serving of Fresh Broccoli Microgreens" Foods 12, no. 20: 3784. https://doi.org/10.3390/foods12203784
APA StyleBouranis, J. A., Wong, C. P., Beaver, L. M., Uesugi, S. L., Papenhausen, E. M., Choi, J., Davis, E. W., 2nd, Da Silva, A. N., Kalengamaliro, N., Chaudhary, R., Kharofa, J., Takiar, V., Herzog, T. J., Barrett, W., & Ho, E. (2023). Sulforaphane Bioavailability in Healthy Subjects Fed a Single Serving of Fresh Broccoli Microgreens. Foods, 12(20), 3784. https://doi.org/10.3390/foods12203784






