Comparative Analysis of Saccharification Characteristics of Different Type Sweetpotato Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Experimental Design
2.3. Physicochemical Index Determination
2.3.1. Determination of Dry Matter Content, Starch Content, Amylose Content, and Amylase Activity
2.3.2. Analysis of Gelatinization Characteristics of Starch
2.3.3. Sensory Evaluation, Physiological Sweetness, and Electronic Tongue Analysis of Sweetpotato
2.3.4. Observation on Tissue Structure of Storage Root in Sweetpotato
2.3.5. Expression Analysis of Saccharification-Related Genes in Sweetpotato
2.4. Data Analysis
3. Results and Discussion
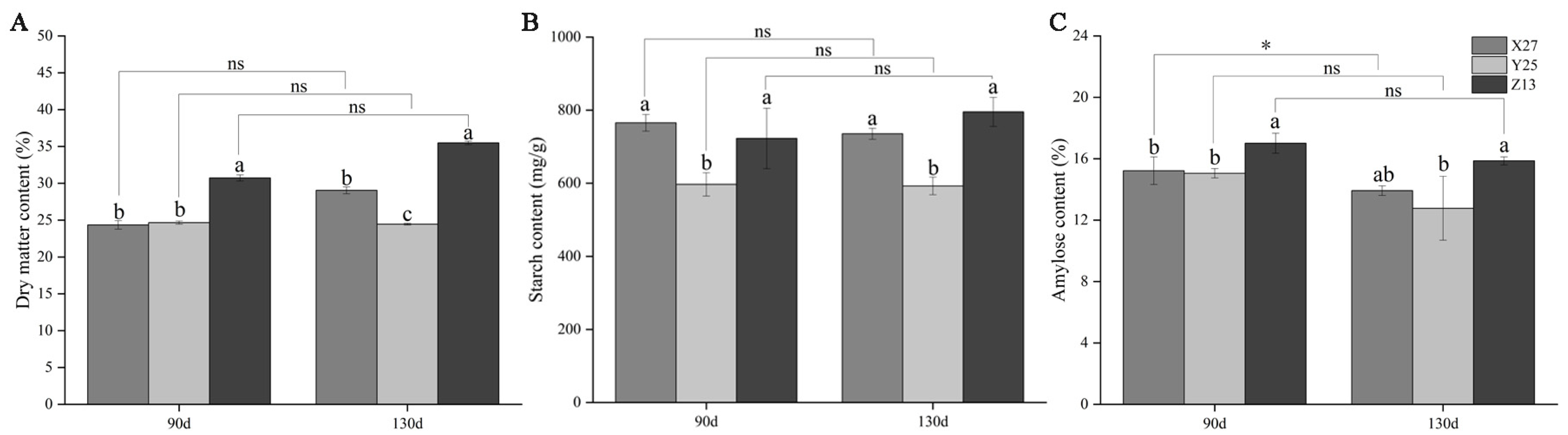
3.1. Analysis of Dry Matter Content, Starch, and Amylose Content
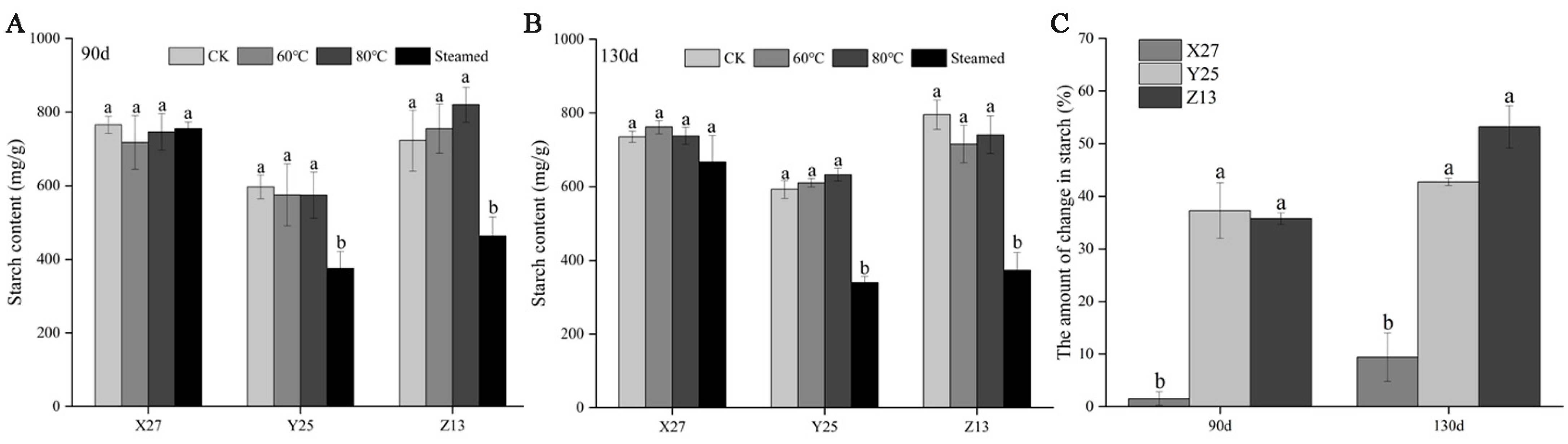
3.2. Analysis of Starch Content and Decomposition Rate
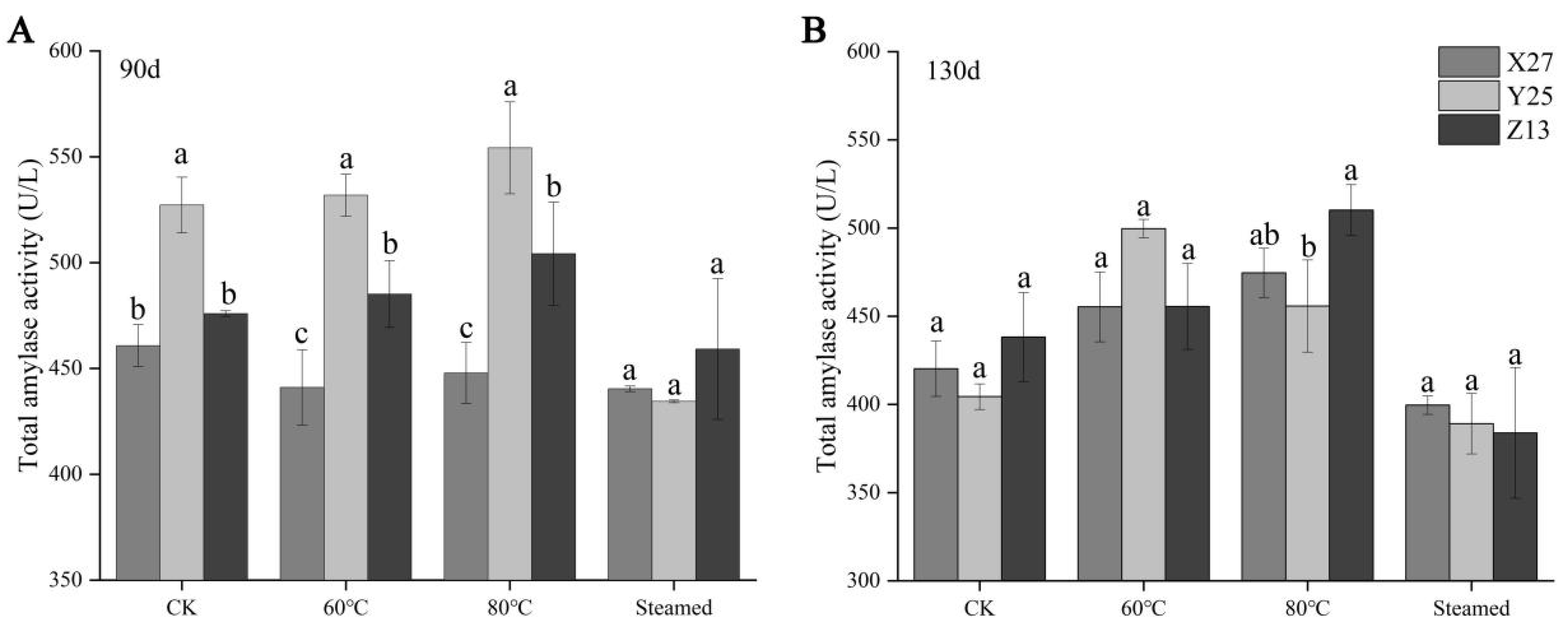
3.3. Analysis of Total Amylase Activity
3.4. Analysis of Maltose, Sucrose, Fructose, Glucose, Soluble Sugar Contents, and Physiological Sweetness
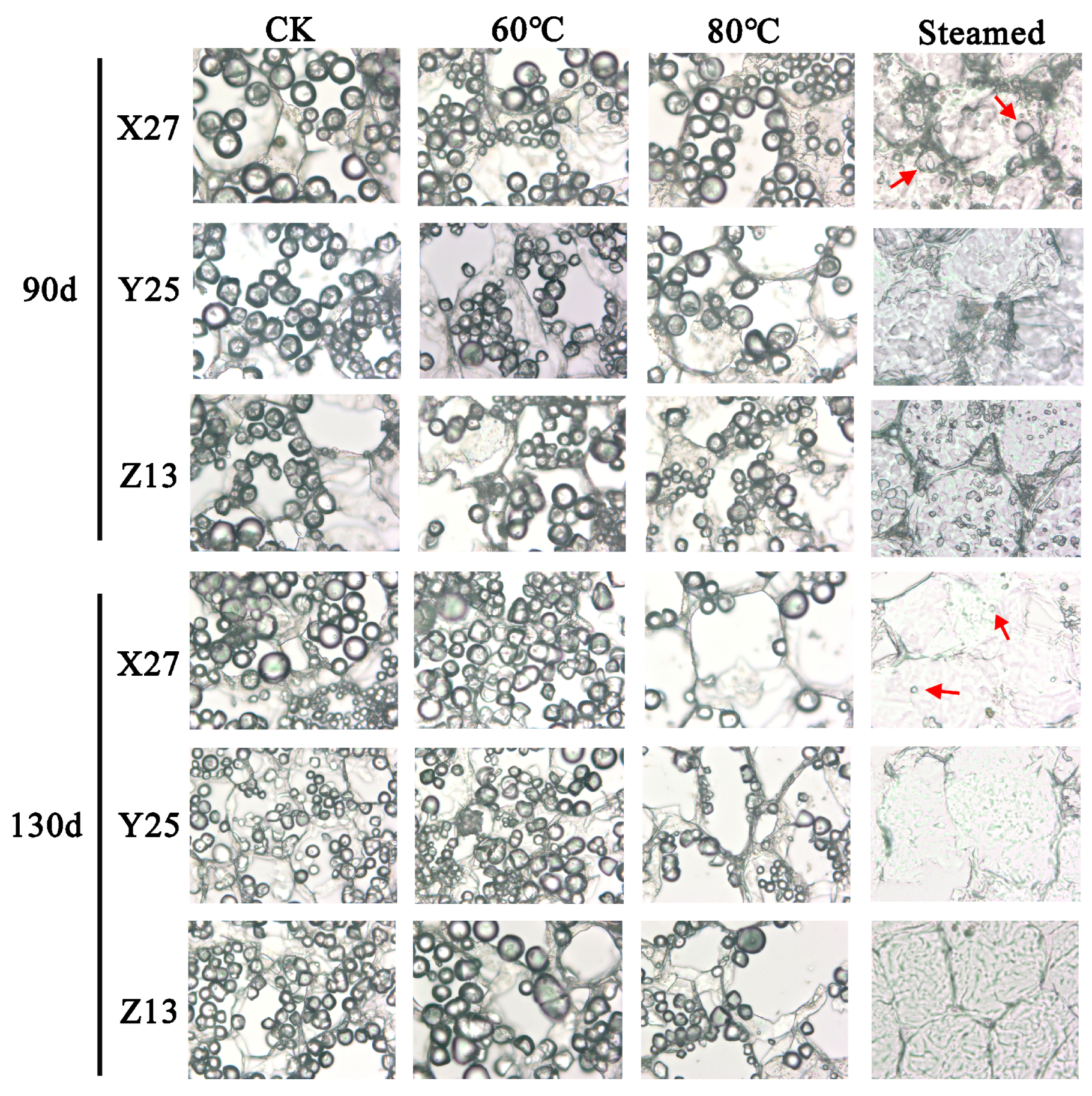
3.5. Changes of Starch Granules in Sweetpotato Storage Root during Heating
3.6. Analysis of Starch Gelatinization Characteristics
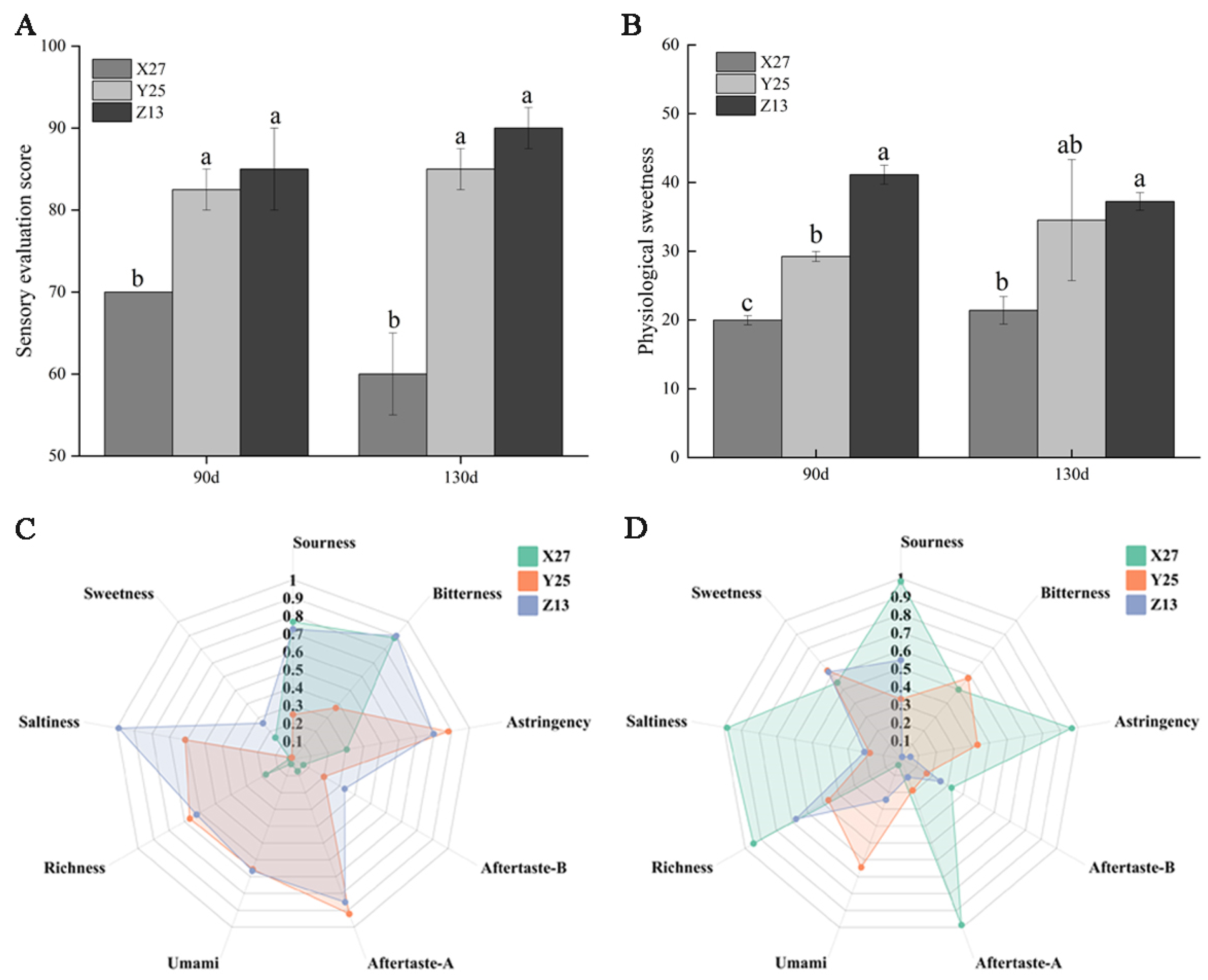
3.7. Analysis of Sensory Evaluation, Physiological Sweetness, and Electronic Tongue
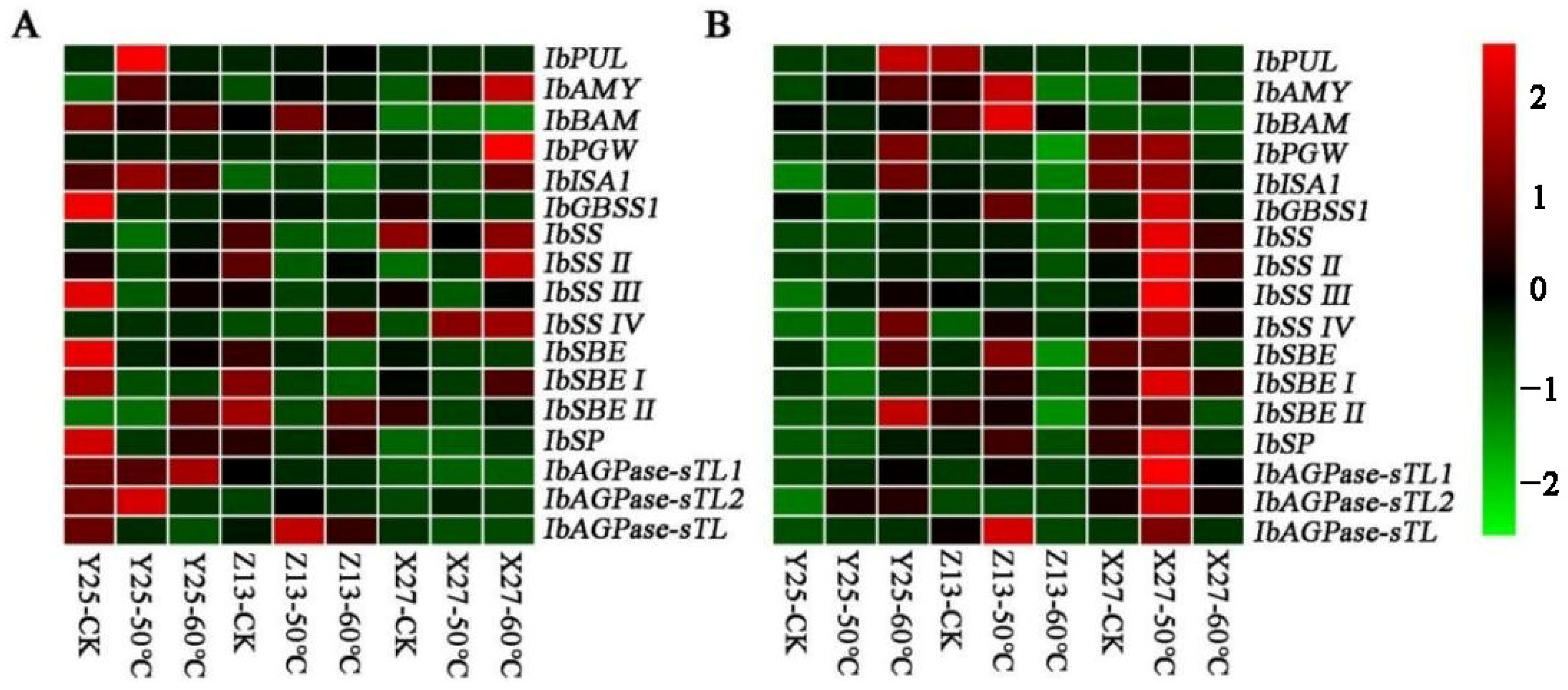
3.8. Analysis of Expression of Glycation Related Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sapakhova, Z.; Raissova, N.; Daurov, D.; Zhapar, K.; Daurova, A.; Zhigailov, A.; Zhambakin, K.; Shamekova, M. sweetpotato as a Key Crop for Food Security under the Conditions of Global Climate Change: A Review. Plants 2023, 12, 2516. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, H.; Jin, Y.L.; Zhu, J.C.; Ma, D.F. Analysis and perspectives of sweetpotato industry contributing to national food security in China. Jiangsu J. Agric. Sci. 2022, 38, 1484–1491. [Google Scholar]
- Xiao, Y.; Zhu, M.; Gao, S. Genetic and Genomic Research on sweetpotato for Sustainable Food and Nutritional Security. Genes 2022, 13, 1833. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.K. A comprehensive review of sweetpotato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- Baafi, E.; Gracen, V.E.; Manu-Aduening, J.; Blay, E.T.; Ofori, K.; Carey, E.E. Genetic control of dry matter, starch and sugar content in sweetpotato. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2016, 67, 110–118. [Google Scholar] [CrossRef]
- Kim, J.-M.; Park, S.-J.; Lee, C.-S.; Ren, C.; Kim, S.-S.; Shin, M. Functional properties of different Korean sweetpotato varieties. Food Sci. Biotechnol. 2011, 20, 1501–1507. [Google Scholar] [CrossRef]
- Tao, X.; Zhang, Y.W.; Jiang, Y.S.; Wang, H.Y.; Zhang, Y.Z. Changes in root starch contents of sweetpotato cultivars during storage. Chin. J. Appl. Environ. Biol. 2010, 16, 741–744. [Google Scholar]
- Lai, Y.-C.; Huang, C.-L.; Chan, C.-F.; Lien, C.-Y.; Liao, W.C. Studies of sugar composition and starch morphology of baked sweetpotatoes (Ipomoea batatas (L.) Lam.). J. Food Sci. Technol. 2011, 50, 1193–1199. [Google Scholar] [CrossRef]
- Kitahara, K.; Nakamura, Y.; Otani, M.; Hamada, T.; Nakayachi, O.; Takahata, Y. Carbohydrate components in sweetpotato storage roots: Their diversities and genetic improvement. Breed. Sci. 2017, 67, 62–72. [Google Scholar] [CrossRef]
- Wu, L.H.; Shen, S.F.; Li, B. Study on the correlation between sweetness and sugar of sweetpotato before and after steaming. J. Chin. Cereals Oils Assoc. 2012, 27, 25–29. [Google Scholar]
- Nabubuya, A.; Namutebi, A.; Byaruhanga, Y.; Narvhus, J.; Stenstrøm, Y.; Wicklund, T. Amylolytic Activity in Selected Sweetpotato (Ipomoea batatas Lam.) Varieties during Development and in Storage. Food Nutr. Sci. 2012, 3, 660–668. [Google Scholar] [CrossRef][Green Version]
- Kukimura, H.; Yoshida, T.; Komaki, K. New sweetpotato cultivars, benihayato and satsumahikari, making a new turn for processing. Jpn. Agric. Res. Q. 1988, 22, 7–13. [Google Scholar]
- Kumagai, T.; Umemura, Y.; Baba, T.; Iwanaga, M. The inheritance of β-amylase null in storage roots of sweetpotato, Ipomoea batatas (L.) Lam. Theor. Appl. Genet. 1990, 79, 369–376. [Google Scholar] [CrossRef]
- Katayama, K. New sweetpotato cultivars bred for recently in Japan. In Proceedings of the Japan-China-Korea Sweetpotato Workshop, Kagoshima, Japan, 28–30 November 2014; pp. 14–15. [Google Scholar]
- Nakamura, Y.; Kuranouchi, T.; Ohara-Takada, A.; Katayama, K. The Effects of β-Amylase Activity and Starch Pasting Temperature on Maltose Generation in Steamed Storage Roots of sweetpotato. Nippon Shokuhin Kagaku Kogaku Kaishi 2014, 61, 577–585. [Google Scholar] [CrossRef]
- Shen, S.F.; Xiang, C.; Wu, L.H.; Li, B.; Liu, Z.G. Analysis on the Characteristics of Soluble Sugar Components in sweetpotato storage root and its relationship with taste. Sci. Agric. Sin. 2021, 54, 34–45. [Google Scholar] [CrossRef]
- Guo, K.; Liu, T.; Xu, A.; Zhang, L.; Bian, X.; Wei, C. Structural and functional properties of starches from root tubers of white, yellow, and purple sweetpotatoes. Food Hydrocoll. 2018, 89, 829–836. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Fang, B.P.; Tang, J. Description Specification and Data Standard of Sweetpotato Germplasm Resources; China Agriculture Press: Beijing, China, 2006; pp. 1–50. [Google Scholar]
- Soukup, A.; Tylová, E. Essential Methods of Plant Sample Preparation for Light Microscopy; Humana Press: Totowa, NJ, USA, 2014; pp. 1–23. [Google Scholar]
- Park, S.-C.; Kim, Y.-H.; Ji, C.Y.; Park, S.; Jeong, J.C.; Lee, H.-S.; Kwak, S.-S. Stable Internal Reference Genes for the Normalization of Real-Time PCR in Different Sweetpotato Cultivars Subjected to Abiotic Stress Conditions. PLoS ONE 2012, 7, e51502. [Google Scholar] [CrossRef]
- Kou, M.; Liu, Y.-J.; Li, Z.-Y.; Zhang, Y.-G.; Tang, W.; Yan, H.; Wang, X.; Chen, X.-G.; Su, Z.-X.; Arisha, M.H.; et al. A novel glutathione S-transferase gene from sweetpotato, IbGSTF4, is involved in anthocyanin sequestration. Plant Physiol. Biochem. 2019, 135, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, W.M. A new sweetpotato variety zheshu 13 and its main cultivation techniques. CROPS 2005, 4, 51. [Google Scholar] [CrossRef]
- Xin, G.S.; Lin, Z.J.; Han, J.J.; Shang, L.L.; Qiu, P.F.; Liu, Q.C. Study on the selective breeding and high yield physiology of a new high-yield superior sweetpotato variety ‘yanshu 25’. Acta Agric. Shanghai 2015, 31, 119–124. [Google Scholar] [CrossRef]
- Tang, Z.H.; Zhang, A.J.; Shi, X.M.; Wei, M.; Li, H.M. Breeding and quality characteristics of a new sweetpotato cultivar xushu 27 with high yield and high starch content. J. Plant Genet. Resour. 2012, 13, 502–506. [Google Scholar] [CrossRef]
- Noda, T.; Takahata, Y.; Sato, T.; Suda, I.; Morishita, T.; Ishiguro, K.; Yamakawa, O. Relationships between chain length distribution of amylopectin and gelatinization properties within the same botanical origin for sweetpotato and buckwheat. Carbohydr. Polym. 1998, 37, 153–158. [Google Scholar] [CrossRef]
- Noda, T.; Kobayashi, T.; Suda, I. Effect of soil temperature on starch properties of sweetpotatoes. Carbohydr. Polym. 2001, 44, 239–246. [Google Scholar] [CrossRef]
- Tang, Z.H.; Zhu, X.Q.; Li, Q.; Li, H.M.; Xu, F. Genotype variation in amylose content of sweetpotato. Sci. Technol. Food Ind. 2011, 32, 108–110. [Google Scholar] [CrossRef]
- Mikami, B.; Degano, M.; Hehre, E.J.; Sacchettini, J.C. Crystal Structures of Soybean .beta.-Amylase Reacted with .beta.-Maltose and Maltal: Active Site Components and Their Apparent Roles in Catalysis. Biochemistry 1994, 33, 7779–7787. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ohara-Takada, A.; Kuranouchi, T.; Masuda, R.; Katayama, K. Mechanism for maltose generation by heating in the storage roots of sweetpotato cultivar “Quick Sweet” containing starch with a low pasting temperature. Nippon Shokuhin Kagaku Kogaku Kaishi 2014, 61, 577–585. [Google Scholar] [CrossRef]
- Grabowski, J.; Truong, V.D.; Daubert, C. Nutritional and rheological characterization of spray dried sweetpotato powder. LWT 2008, 41, 206–216. [Google Scholar] [CrossRef]
- Tang, H.; Mitsunaga, T.; Kawamura, Y. Molecular arrangement in blocklets and starch granule architecture. Carbohydr. Polym. 2006, 63, 555–560. [Google Scholar] [CrossRef]
- Morrison, T.A.; Pressey, R.; Kays, S.J. Changes in α- and β-amylase during storage of sweetpotato lines with varying starch hydrolysis potential. J. Am. Soc. Hortic. Sci. 1993, 118, 236–242. [Google Scholar] [CrossRef]
- Genkina, N.K.; Noda, T.; Koltisheva, G.I.; Wasserman, L.A.; Tester, R.F.; Yuryev, V.P. Effects of growth temperature on some structural properties of crystalline lamellae in starches extracted from sweetpotatoes (sunnyred and ayamurasaki). Starch-Stärke 2010, 55, 350–357. [Google Scholar] [CrossRef]
- Noda, T.; Takahata, Y.; Sato, T.; Hisamatsu, M.; Yamada, T. Physicochemical properties of starches extracted from sweetpotato roots differing in physiological age. J. Agric. Food Chem. 1995, 43, 3016–3020. [Google Scholar] [CrossRef]
- Noda, T.; Takahata, Y.; Nagata, T. Developmental Changes in Properties of sweetpotato Starches. Starch-Stärke 1992, 44, 405–409. [Google Scholar] [CrossRef]
- Shen, S.F.; Xiang, C.; Wu, L.H.; Li, B.; Lou, Z.G. Determination and difference analysis of soluble sugar content in 11 sweetpotato germplasm resources. Acta Agric. Zhejiangensis 2020, 32, 1934–1940. [Google Scholar] [CrossRef]
- Maeo, K.; Tomiya, T.; Hayashi, K.; Akaike, M.; Morikami, A.; Ishiguro, S.; Nakamura, K. Sugar-responsible elements in the promoter of a gene for β-amylase of sweetpotato. Plant Mol. Biol. 2001, 46, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Bustos, R.; Fahy, B.; Hylton, C.M.; Seale, R.; Nebane, N.M.; Edwards, A.; Martin, C.; Smith, A.M. Starch granule initiation is controlled by a heteromultimeric isoamylase in potato tubers. Proc. Natl. Acad. Sci. USA 2004, 101, 2215–2220. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Kou, M.; Song, W.; Arisha, M.H.; Gao, R.; Tang, W.; Yan, H.; Wang, X.; Zhang, Y.; Li, Q. Comparative Analysis of Saccharification Characteristics of Different Type Sweetpotato Cultivars. Foods 2023, 12, 3785. https://doi.org/10.3390/foods12203785
Li C, Kou M, Song W, Arisha MH, Gao R, Tang W, Yan H, Wang X, Zhang Y, Li Q. Comparative Analysis of Saccharification Characteristics of Different Type Sweetpotato Cultivars. Foods. 2023; 12(20):3785. https://doi.org/10.3390/foods12203785
Chicago/Turabian StyleLi, Chen, Meng Kou, Weihan Song, Mohamed Hamed Arisha, Runfei Gao, Wei Tang, Hui Yan, Xin Wang, Yungang Zhang, and Qiang Li. 2023. "Comparative Analysis of Saccharification Characteristics of Different Type Sweetpotato Cultivars" Foods 12, no. 20: 3785. https://doi.org/10.3390/foods12203785
APA StyleLi, C., Kou, M., Song, W., Arisha, M. H., Gao, R., Tang, W., Yan, H., Wang, X., Zhang, Y., & Li, Q. (2023). Comparative Analysis of Saccharification Characteristics of Different Type Sweetpotato Cultivars. Foods, 12(20), 3785. https://doi.org/10.3390/foods12203785





