Comprehensive Evaluation of Quality Characteristics of Four Oil-Tea Camellia Species with Red Flowers and Large Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals and Standards
2.3. Determination of Morphological Index of ROC
2.4. Analysis of Macronutrients in ROC Fruit
2.4.1. Moisture Content Analysis
2.4.2. Oil Content Analysis
2.4.3. Soluble-Sugar and Starch Content Analysis
2.4.4. Protein Content Analysis
2.4.5. FCR-Reducing Capacity and Total Flavonoid Content Analysis
2.5. Analysis of Lipid Concomitant Content of ROC Oil
2.5.1. Oil Extraction
2.5.2. Determination of α-Tocopherol
2.5.3. Determination of Sterols and Squalene
2.6. Determination of Fatty Acid Composition
2.7. Triglyceride Analysis
2.8. Gray Correlation Coefficient Method
2.9. Statistical Analysis
3. Results and Discussion
3.1. Phenotype, Fresh Seed Rate and Kernel Rate of Four ROC Fruits
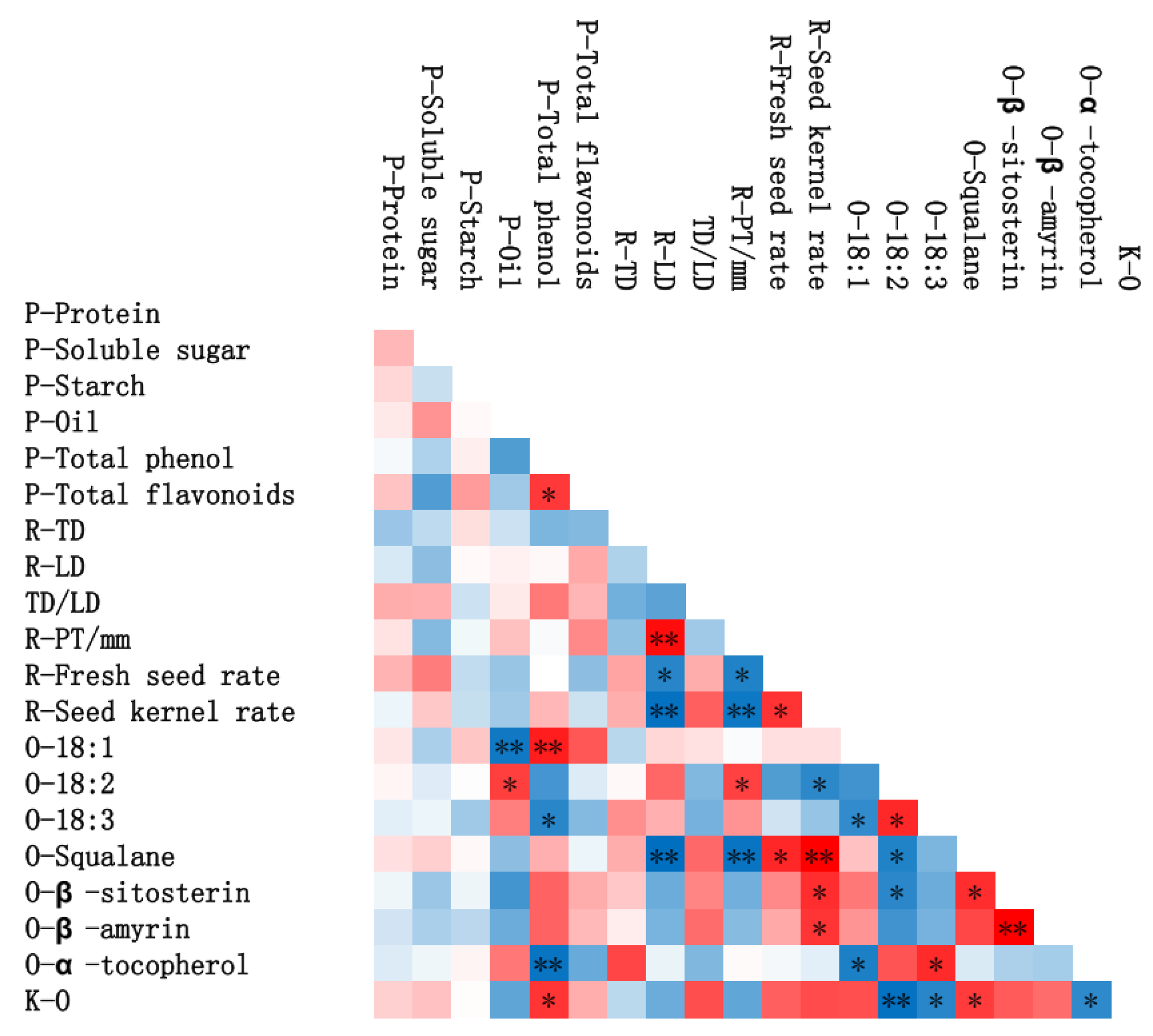
3.2. Chemical Compositions of ROC Kernel/Peel Depending on Different Species
3.3. Lipid Concomitants of the Oil of Four ROC Oil
3.4. Fatty Acid Composition
3.5. Evaluation of the Quality of Four ROC Oils by Gray Correlation Coefficient Method
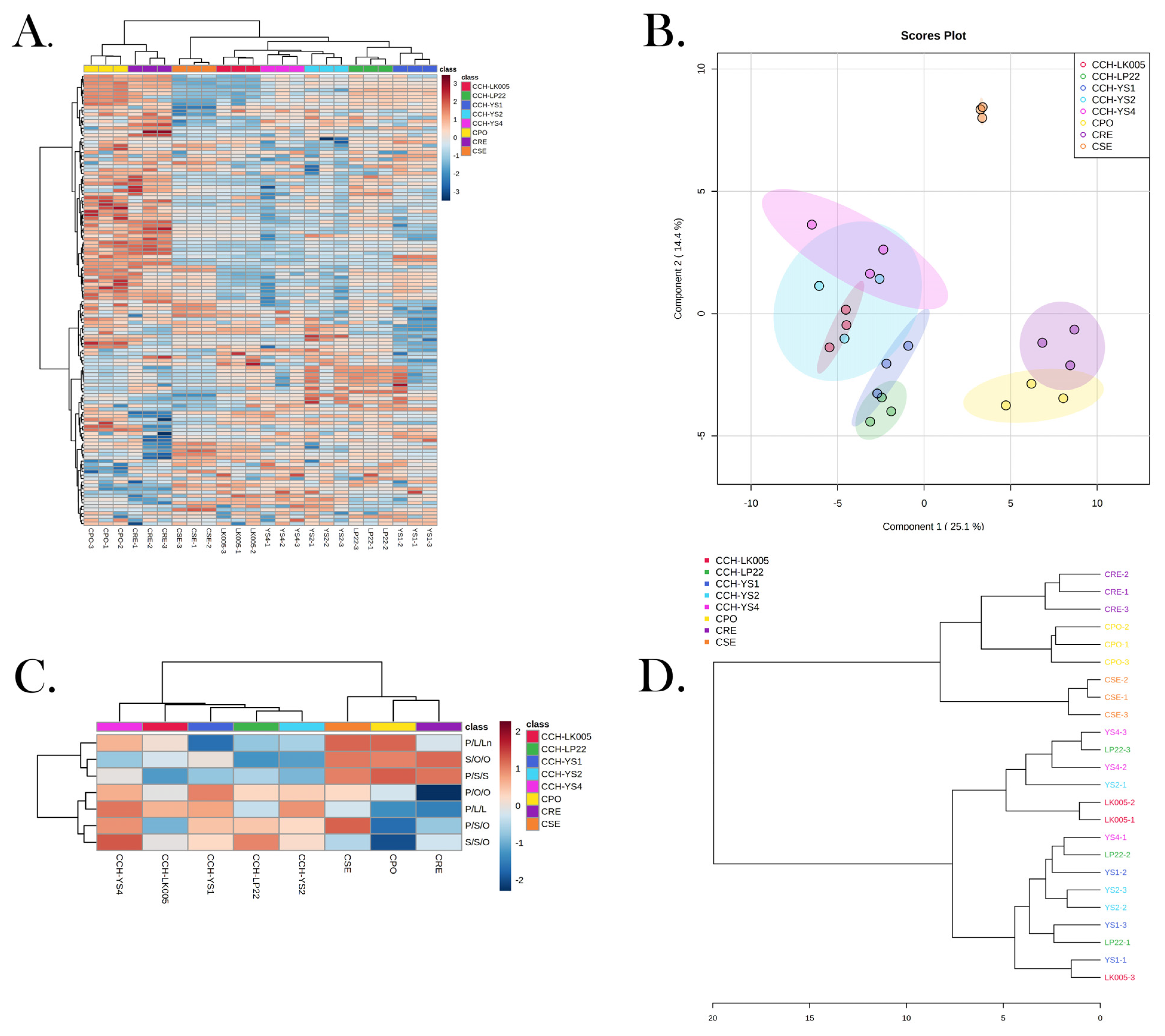
3.6. Triglyceride Composition Could Be Used to Identify Four ROC Oils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, J.; Yan, H.; Wu, Y.; Wang, Y.; Xia, P. Quality Evaluation of the Oil of Camellia spp. Foods 2022, 11, 2221. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yang, T.; Wang, Y.; Zhou, B.; Yan, L.; Teng, L.; Wang, F.; Chen, L.; He, Y.; Guo, K.; et al. New method for effective identification of adulterated Camellia oil basing on Camellia oleifera-specific DNA. Arab. J. Chem. 2018, 11, 815–826. [Google Scholar] [CrossRef]
- Su, M.H.; Shih, M.C.; Lin, K.H. Chemical composition of seed oils in native Taiwanese Camellia species. Food Chem. 2014, 156, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, B.; Ye, J.; He, Y.; Tang, S.; Wang, H.; Wen, Q. Comparative transcriptomic analysis reveals genes related to the rapid accumulation of oleic acid in Camellia chekiangoleosa, an oil tea plant with early maturity and large fruit. Plant Physiol. Biochem. 2022, 171, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Tan, H.Y.; Zhou, J.P. Proximate composition of Camellia chekiangoleosa Hu fruit and fatty acid constituents of its seed oil. J. Zhejiang Univ. 2010, 36, 662–669. [Google Scholar]
- Zhou, X.U.; Li, Q.; Zhou, Q.; Li, X.; Ding, C. Oil content and fatty acid composition of 15 Camellia polyodonta in ya’an. Sci. Technol. Food Ind. 2014, 35, 305–308. [Google Scholar]
- Wang, X.; Zeng, Q.; Verardo, V.; del Mar Contreras, M. Fatty acid and sterol composition of tea seed oils: Their comparison by the “FancyTiles” approach. Food Chem. 2017, 233, 302–310. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Arch. Med. Sci. AMS 2017, 13, 965–1005. [Google Scholar] [CrossRef]
- Zeb, A. Triacylglycerols composition, oxidation and oxidation compounds in camellia oil using liquid chromatography–mass spectrometry. Chem. Phys. Lipids 2012, 165, 608–614. [Google Scholar] [CrossRef]
- Wang, X.N.; Chen, Y.Z.; Wu, L.Q.; Liu, R.K.; Yang, X.H.; Wang, R.; Yu, K.W. Oil Content and Fatty Acid Composition of Camellia oleifera Seed. J. Cent. S. Univ. For. Technol. 2008, 28, 11–17. [Google Scholar]
- Huang, J.; Kan, H.; Liu, Y. Free radicals scavenging effects of polyphenols in Camellia reticulata Lindl. seed oil. China Oils Fats 2011, 32, 54–57. [Google Scholar]
- Lu, S.Y.; Hu, D.N.; Guo, X.M.; Liu, X.; Yi, S.; Tu, S.; Yu, S. Comparison of fruit growth dynamics and economic characteristics of four Camellia oleifera clones. Non-Wood For. Res. 2020, 38, 46–52. [Google Scholar]
- Wei, T.; Dong, L.; Zhong, S.; Jing, H.; Deng, Z.; Wen, Q.; Li, J. Chemical composition of Camellia chekiangoleosa H. seeds during ripening and evaluations of seed oils quality. Ind. Crops Prod. 2022, 177, 114499. [Google Scholar] [CrossRef]
- Bouali, I.; Trabelsi, H.; Abdallah, I.B.; Albouchi, A.; Martine, L.; Grégoire, S.; Bouzaien, G.; Gandour, M.; Boukhchina, S.; Berdeaux, O. Changes in Fatty Acid, Tocopherol and Xanthophyll Contents During the Development of Tunisian-Grown Pecan Nuts. J. Am. Oil Chem. Soc. 2013, 90, 1869–1876. [Google Scholar] [CrossRef]
- Luo, X.; Huang, Q. Relationships between Leaf and Stem Soluble Sugar Content and Tuberous Root Starch Accumulation in Cassava. J. Agric. Sci. 2011, 3, 64. [Google Scholar] [CrossRef]
- Wang, H.; Pampati, N.; McCormick, W.M.; Bhattacharyya, L. Protein Nitrogen Determination by Kjeldahl Digestion and Ion Chromatography. J. Pharm. Sci. 2016, 105, 1851–1857. [Google Scholar] [CrossRef]
- Górnaś, P.; Dwiecki, K.; Siger, A.; Tomaszewska-Gras, J.; Michalak, M.; Polewski, K. Contribution of phenolic acids isolated from green and roasted boiled-type coffee brews to total coffee antioxidant capacity. Eur. Food Res. Technol. 2015, 242, 641–653. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Rover, M.R.; Brown, R.C. Quantification of total phenols in bio-oil using the Folin–Ciocalteu method—ScienceDirect. J. Anal. Appl. Pyrolysis 2013, 104, 366–371. [Google Scholar] [CrossRef]
- Yu, C.; Hu, Q.; Wang, H.; Deng, Z. Comparison of 11 rice bran stabilization methods by analyzing lipase activities. J. Food Process. Preserv. 2020, 44, e14370. [Google Scholar] [CrossRef]
- Li, C.; Yao, Y.; Zhao, G.; Cheng, W.; Liu, H.; Liu, C.; Shi, Z.; Chen, Y.; Wang, S. Comparison and analysis of fatty acids, sterols, and tocopherols in eight vegetable oils. J. Agric. Food Chem. 2011, 59, 12493–12498. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Wu, G.; Jin, Q.; Wang, X. Detection of camellia oil adulteration using chemometrics based on fatty acids GC fingerprints and phytosterols GC–MS fingerprints. Food Chem. 2021, 352, 129422. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.; Deng, Z.-Y.; Zhang, N.; Zou, Q.; Fan, Y.-W.; Liu, R.; Zhen, L.-F.; Li, J. Lipid profiles of Chinese soft-shell turtle eggs (Pelodiscus sinensis). J. Food Compos. Anal. 2020, 94, 103627. [Google Scholar] [CrossRef]
- Jia, W.; Liu, Y.; Shi, L. Integrated metabolomics and lipidomics profiling reveals beneficial changes in sensory quality of brown fermented goat milk. Food Chem. 2021, 364, 130378. [Google Scholar] [CrossRef]
- Sheng, L.X.; Liang, L.; Zhang, W.; Wang, Q.; Duan, C.; Chen, X. Analysis of the Fruit Characteristics of Camellia reticulata f. simplex from Tengchong County. J. West China For. Sci. 2009, 38, 9–15. [Google Scholar]
- Shi, T.; Zhu, M.; Zhou, X.; Huo, X.; Long, Y.; Zeng, X.; Chen, Y. 1H NMR combined with PLS for the rapid determination of squalene and sterols in vegetable oils. Food Chem. 2019, 287, 46–54. [Google Scholar] [CrossRef]
- Shi, T.; Wu, G.; Jin, Q.; Wang, X. Camellia oil authentication: A comparative analysis and recent analytical techniques developed for its assessment. A review. Trends Food Sci. Technol. 2020, 97, 88–99. [Google Scholar] [CrossRef]
- Zaunschirm, M.; Pignitter, M.; Kienesberger, J.; Hernler, N.; Riegger, C.; Eggersdorfer, M.; Somoza, V. Contribution of the Ratio of Tocopherol Homologs to the Oxidative Stability of Commercial Vegetable Oils. Molecules 2018, 23, 206. [Google Scholar] [CrossRef]
- Kritchevsky, D.; Chen, S.C. Phytosterols—Health benefits and potential concerns: A review. Nutr. Res. 2005, 25, 413–428. [Google Scholar] [CrossRef]
- Jones, P.J.H.; Shamloo, M.; MacKay, D.S.; Rideout, T.C.; Myrie, S.B.; Plat, J.; Roullet, J.-B.; Baer, D.J.; Calkins, K.L.; Davis, H.R.; et al. Progress and perspectives in plant sterol and plant stanol research. Nutr. Rev. 2018, 76, 725–746. [Google Scholar] [CrossRef]
- Warleta, F.; Campos, M.; Allouche, Y.; Sánchez-Quesada, C.; Ruiz-Mora, J.; Beltrán, G.; Gaforio, J.J. Squalene protects against oxidative DNA damage in MCF10A human mammary epithelial cells but not in MCF7 and MDA-MB-231 human breast cancer cells. Food Chem. Toxicol. 2010, 48, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, X.; Chen, Z.; Lin, Y.; Wang, S. Comparison of Oil Content and Fatty Acid Profile of Ten New Camellia oleifera Cultivars. J. Lipids 2016, 2016, 3982486. [Google Scholar] [CrossRef] [PubMed]
- Sahari, M.A.; Ataii, D.; Hamedi, M. Characteristics of tea seed oil in comparison with sunflower and olive oils and its effect as a natural antioxidant. J. Am. Oil Chem. Soc. 2004, 81, 585–588. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, C.; Chen, H.; Zhou, H.; Ye, J. Prediction of fatty acid composition in Camellia oleifera oil by near infrared transmittance spectroscopy (NITS). Food Chem. 2013, 138, 1657–1662. [Google Scholar] [CrossRef]
- 35. Wei, Z.; Guo, M.; Wang, Y.; Duan, Z.; Yang, K.; Luan, X. Recent Advances in Research on Polyphenol Compounds in Camellia oleifera Seed Oil. Food Sci. 2021, 42, 311–320. [Google Scholar]
- Gago-Dominguez, M.; Yuan, J.-M.; Sun, C.-L.; Lee, H.-P.; Yu, M.C. Opposing effects of dietary n-3 and n-6 fatty acids on mammary carcinogenesis: The Singapore Chinese Health Study. Br. J. Cancer 2003, 89, 1686–1692. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-6/Omega-3 Essential Fatty Acid Ratio and Chronic Diseases. Food Rev. Int. 2004, 20, 77–90. [Google Scholar] [CrossRef]
- Zhu, X.Y. Optimization of ultrasound-assisted extraction of phenolic compounds from camellia oleifera seeds using response surface method. Food Mach. 2012, 28, 166–170. [Google Scholar]
- Harkat, H.; Bousba, R.; Benincasa, C.; Atrouz, K.; Gültekin-Özgüven, M.; Altuntaş, Ü.; Demircan, E.; Zahran, H.A.; Özçelik, B. Assessment of Biochemical Composition and Antioxidant Properties of Algerian Date Palm (Phoenix dactylifera L.) Seed Oil. Plants 2022, 11, 381. [Google Scholar] [CrossRef]
- Shen, B.; Wu, X.; Li, Y.; Duan, Z. Study on the quality of three varieties of Camellia chekiangoleosa seed oils. Sci. Technol. Food Ind. 2016, 37, 97–104. [Google Scholar]
- Ou, M.; Wu, Y.; Xu, H.; Lv, H.; Lv, D.; Wang, F. Grey System Analysis on Inner Quality of Tobacco Blending Module. J. Food Sci. Biotechnol. 2009, 28, 509–512. [Google Scholar]
- Wei, W.; Sun, C.; Jiang, W.; Zhang, X.; Hong, Y.; Jin, Q.; Tao, G.; Wang, X.; Yang, Z. Triacylglycerols fingerprint of edible vegetable oils by ultra-performance liquid chromatography-Q-ToF-MS. LWT 2019, 112, 108261. [Google Scholar] [CrossRef]

| ROC | TD/mm | LD/mm | PT/mm | TD/LD | Fresh Seed Rate (%) | Seed Kernel Rate (%) | |
|---|---|---|---|---|---|---|---|
| C.chekiangoleosa Hu. | YS-1 | 51 ± 0.24 a | 55 ± 0.90 b | 9 ± 0.42 b | 0.927 | 32.68 ± 0.10 d | 61.52 ± 0.31 b |
| YS-2 | 58 ± 0.36 e | 63 ± 0.54 d | 9 ± 0.72 b | 0.921 | 34.40 ± 0.16 e | 63.57 ± 0.41 d | |
| YS-4 | 52 ± 0.92 b | 59 ± 0.62 c | 10 ± 0.50 c | 0.881 | 29.03 ± 0.17 c | 60.3 ± 0.56 a | |
| Lk005 | 56 ± 0.72 d | 5 ± 0.40 c | 7 ± 0.46 a | 0.949 | 20.75 ± 0.49 a | 65.62 ± 0.68 e | |
| LP22 | 52 ± 0.46 b,c | 53 ± 0.64 a | 9 ± 0.44 b | 0.981 | 22.86 ± 0.52 b | 62.19 ± 0.13 c | |
| CCH | mean | 54 ± 0.54 B | 58 ± 0.62 B | 9 ± 0.51 A | 0.932 | 27.94 ± 0.29 D | 62.64 ± 0.42 D |
| C. semiserrata Chi. | CSE | 108 ± 0.42 D | 117 ± 0.40 D | 28 ± 0.14 D | 0.923 | 9.32 ± 0.34 A | 49.13 ± 0.25 A |
| C. polyodonta How ex. Hu. | CPO | 79 ± 0.52 C | 107 ± 0.82 C | 21 ± 0.98 C | 0.738 | 14.12 ± 0.26 B | 50.41 ± 0.45 B |
| C. reticulata Lindl. | CRE | 50 ± 0.10 A | 53 ± 0.74 A | 11 ± 0.30 B | 0.943 | 22.5 ± 0.33 C | 58.50 ± 0.74 C |
| C. chekiangoleosa Hu. | CCH | C. semiserrata Chi. | C. polyodonta How ex. Hu. | C. reticulata Lindl | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peel | YS-1 | YS-2 | YS-4 | LK005 | LP22 | Mean | CSE | CPO | CRE | |
| Fresh | Moisture (%) | 71.60 ± 0.56 a | 76.07 ± 0.24 c | 79.86 ± 0.25 e | 77.45 ± 0.29 d | 73.60 ± 0.53 b | 75.72 ± 2.89 A | 80.59 ± 0.81 B | 82.10 ± 0.12 C | 74.87 ± 0.18 A |
| Dried | Protein (mg/g) | 47.24 ± 0.56 b | 27.41 ± 0.56 a,b | 25.53 ± 0.52 a | 17.58 ± 1.65 a | 30.61 ± 0.45 b | 29.67 ± 9.78 A,B | 33.6 ± 0.66 B | 22.6 ± 1.82 A | 34.3 ± 2.93 B |
| Soluble sugars (mg/g) | 86.92 ± 1.71 c | 106.62 ± 2.82 d | 136.12 ± 3.39 f | 60.18 ± 5.04 a | 71.33 ± 1.99 b | 92.23 ± 26.93 C | 85.30 ± 1.45 B | 48.20 ± 4.06 A | 128.08 ± 3.24 D | |
| Starch (mg/g) | 125.71 ± 3.0 c,d | 104.85 ± 2.39 a | 128.95 ± 4.5 d,e | 117.54 ± 3.28 b | 132.41 ± 2.77 e | 121.89 ± 9.84 B | 119.62 ± 2.5 A | 127.59 ± 3.1 C | 118.61 ± 2.24 A | |
| Oil (mg/g) | 2.46 ± 0.051 a | 2.58 ± 0.066 a | 2.83 ± 0.044 a | 2.63 ± 0.079 a | 2.89 ± 0.032 a | 2.68 ± 0.16 A | 3.10 ± 0.042 A | 2.92 ± 0.073 A | 4.03 ± 0.044 B | |
| FCR-reducing capacity | 8.69 ± 0.49 a | 8.47 ± 0.08 a | 8.36 ± 0.49 a | 10.11 ± 0.22 b | 10.36 ± 0.24 b | 9.20 ± 0.86 C | 10.31 ± 0.15 C | 5.78 ± 0.21 B | 4.46 ± 0.09 A | |
| Total flavonoids (mg/g) | 4.32 ± 0.09 c | 2.61 ± 0.19 a | 2.39 ± 0.31 a | 3.92 ± 0.16 b,c | 5.47 ± 0.14 d | 3.74 ± 1.14 C | 5.32 ± 0.44 D | 3.40 ± 0.07 B | 1.94 ± 0.03 A | |
| Seed | ||||||||||
| Fresh | Moisture (%) | 21.97 ± 0.33 b | 20.84 ± 0.16 a | 20.66 ± 0.39 a | 23.32 ± 0.19 d | 22.57 ± 0.65 c | 21.87 ± 1.01 A | 37.71 ± 0.51 C | 45.33 ± 0.12 D | 34.80 ± 0.29 B |
| Dried | Protein (mg/g) | 95.56 ± 0.19 d | 92.51 ± 0.11 c,d | 90.06 ± 0.36 b,c,d | 75.35 ± 0.43 a | 105.86 ± 0.39 d | 91.87 ± 9.86 C | 76.81 ± 0.21 B,C | 71.03 ± 0.018 A | 75.92 ± 0.12 A,B |
| Soluble sugars (mg/g) | 148.34 ± 9.50 b,c | 146.44 ± 4.55 b,c | 118.42 ± 3.07 a | 153.28 ± 4.03 c | 141.27 ± 2.59 b | 141.55 ± 12.19 B | 125.22 ± 2.32 A | 178.84 ± 6.15 d | 147.51 ± 5.88 b,c | |
| Starch (mg/g) | 97.33 ± 4.34 d | 74.56 ± 3.57 b,c | 61.86 ± 2.33 a | 73.12 ± 7.32 a,b | 67.77 ± 1.09 a,b | 74.93 ± 12.06 A | 75.37 ± 3.15 A | 84.37 ± 3.92 B | 105.41 ± 6.16 C | |
| Oil (%) | 56.12 ± 0.23 b,c | 56.44 ± 0.12 b,c | 57.19 ± 0.38 b,c | 53.61 ± 0.44 a,b | 58.84 ± 0.53 c | 56.44 ± 1.70 D | 50.49 ± 0.26 C | 39.13 ± 0.08 A | 43.69 ± 0.02 B | |
| FCR-reducing capacity | 8.59 ± 0.33 a,b | 9.21 ± 0.14 b,c | 8.82 ± 0.11 a,b,c | 9.13 ± 0.18 a,b,c | 9.35 ± 0.27 c | 9.02 ± 0.28 C | 7.37 ± 0.13 A | 7.02 ± 0.40 A | 8.46 ± 0.13 B | |
| Total flavonoids (mg/g) | 10.52 ± 0.49 a,b | 9.43 ± 0.38 a | 10.63 ± 0.14 a,b | 11.28 ± 0.42 b,c | 10.87 ± 0.73 a,b | 10.55 ± 0.62 A | 11.51 ± 0.85 B | 12.58 ± 0.57 C | 13.09 ± 0.14 D | |
| C. chekiangoleosa Hu. | CCH | C. semiserrata Chi. | C. polyodonta How ex.Hu. | C. reticulata Lindl | |||||
|---|---|---|---|---|---|---|---|---|---|
| YS-1 | YS-2 | YS-4 | LK005 | LP22 | Mean | CSE | CPO | CRE | |
| Squalane (mg/g) | 0.72 ± 0.031 b | 0.67 ± 0.074 a | 0.66 ± 0.042 a | 0.70 ± 0.024 b | 0.71 ± 0.063 b | 0.69 ± 0.02 C | 0.43 ± 0.032 A | 0.47 ± 0.051 A | 0.59 ± 0.033 B |
| β-Sitosterin (mg/g) | 0.67 ± 0.064 b,c | 0.60 ± 0.10 a,b,c | 0.53 ± 0.073 a,b | 0.74 ± 0.057 c | 0.63 ± 0.082 a,b,c | 0.63 ± 0.07 B | 0.48 ± 0.012 A | 0.49 ± 0.066 A | 0.47 ± 0.048 A |
| β-Amyrin (mg/g) | 1.42 ± 0.41 b | 1.34 ± 1.43 b | 1.04 ± 1.88 a | 1.91 ± 3.30 c | 1.33 ± 3.05 b | 1.41 ± 0.28 C | 1.03 ± 0.80 B | 0.87 ± 0.67 A | 0.99 ± 0.97 B |
| α-Tocopherol (mg/kg) | 243.12 ± 1.11 b | 251.25 ± 1.24 c | 223.08 ± 0.38 a | 245.79 ± 0.39 b,c | 243.80 ± 0.48 b | 241.41 ± 9.60 B | 177.52 ± 0.97 A | 350.71 ± 0.63 C | 352.27 ± 0.31 C |
| FCR-reducing capacity (ug/g) | 162.79 ± 4.34 a,b,c | 158.25 ± 4.49 a,b | 156.46 ± 3.64 a | 168.34 ± 3.41 b,c | 171.65 ± 2.65 c | 163.50 ± 5.79 C | 130.72 ± 5.32 A | 164.82 ± 4.10 C | 157.41 ± 3.24 B |
| Total flavonoids (ug/g) | 428.61 ± 4.96 a | 437.38 ± 5.34 a,b | 435.26 ± 4.56 a | 440.37 ± 3.68 a,b | 449.27 ± 4.23 b | 438.18 ± 6.76 B,C | 353.49 ± 4.23 A | 437.65 ± 4.21 B,C | 429.04 ± 4.39 B |
| C. chekiangoleosa Hu. | CCH | C. semiserrata Chi. | C. polyodonta How ex. Hu. | C. reticulata Lindl | |||||
|---|---|---|---|---|---|---|---|---|---|
| YS-1 | YS-2 | YS-4 | LK005 | LP22 | Mean | CSE | CPO | CRE | |
| C14:0 | 0.049 ± 0.0061 a | 0.095 ± 0.019 b | 0.080 ± 0.0051 b | 0.087 ± 0.010 b | 0.081 ± 0.0076 b | 0.078 ± 0.016 B | 0.041 ± 0.0064 A | 0.084 ± 0.0076 B | 0.050 ± 0.0071 A |
| C16:0 | 8.48 ± 0.092 a | 9.85 ± 0.013 c | 9.28 ± 0.069 b | 9.41 ± 0.12 b | 9.31 ± 0.040 b | 9.27 ± 0.44 C | 7.51 ± 0.036 A | 8.51 ± 0.0394 B | 10.88 ± 0.052 D |
| C17:0 | 0.11 ± 0.013 a,b | 0.13 ± 0.002 b,c | 0.11 ± 0.0084 a,b | 0.16 ± 0.011 c | 0.11 ± 0.031 a,b | 0.12 ± 0.02 B | 0.058 ± 0.013 A | 0.11 ± 0.024 B | 0.057 ± 0.0047 A |
| C18:0 | 3.96 ± 0.18 c,d | 3.22 ± 0.083 a | 4.37 ± 0.15 d | 4.05 ± 0.14 d | 3.49 ± 0.12 b,c | 3.82 ± 0.41 C | 3.25 ± 0.11 B | 2.47 ± 0.089 A | 3.40 ± 0.22 B |
| SFA | 12.60 ± 0.29 a | 13.30 ± 0.12 a,b | 13.84 ± 0.23 b | 13.71 ± 0.28 b | 12.99 ± 0.20 a | 13.29 ± 0.46 B | 10.86 ± 0.17 A | 11.17 ± 0.16 A | 14.39 ± 0.28 C |
| 10c C17:1 | 0.066 ± 0.014 a | 0.075 ± 0.017 a | 0.067 ± 0.0071 a | 0.10 ± 0.017 a | 0.088 ± 0.0037 a | 0.079 ± 0.013 A | 0.062 ± 0.0068 A | 0.083 ± 0.010 A | 0.064 ± 0.016 A |
| 9t C16:1 | 0.047 ± 0.018 a | 0.056 ± 0.014 a | 0.041 ± 0.010 a | 0.059 ± 0.0052 a | 0.042 ± 0.013 a | 0.049 ± 0.007 A | 0.031 ± 0.0025 A | 0.071 ± 0.019 A | 0.035 ± 0.0081 A |
| 9c C16:1 | 0.088 ± 0.020 cd | 0.086 ± 0.013 c | 0.073 ± 0.0082 a,b | 0.12 ± 0.0015 e,f | 0.052 ± 0.0046 a | 0.084 ± 0.022 C | 0.075 ± 0.0067 B | 0.069 ± 0.0077 A | 0.13 ± 0.0057 D |
| 9c C18:1 | 80.97 ± 0.13 c | 79.45 ± 0.066 a | 80.26 ± 0.18 b | 80.03 ± 0.23 b | 80.05 ± 0.095 b | 80.15 ± 0.49 C | 80.58 ± 0.048 C | 78.70 ± 0.14 B | 75.65 ± 0.26 A |
| MUFA | 81.17 ± 0.18 c | 79.67 ± 0.11 a | 80.44 ± 0.21 b | 80.31 ± 0.25 b | 80.23 ± 0.12 b | 80.36 ± 0.48 C | 80.75 ± 0.064 C | 78.92 ± 0.18 B | 75.88 ± 0.29 A |
| 9c C18:1/MUFA | 99.75 a | 99.72 a | 99.78 a | 99.65 a | 99.78 a | 99.74 ± 0.048 A | 99.79 A | 99.72 A | 99.69 A |
| C18:2 n-6 | 5.71 ± 0.039 c | 6.16 ± 0.058 d | 5.30 ± 0.047 a | 5.47 ± 0.048 b | 6.20 ± 0.017 d | 5.77 ± 0.36 A | 7.79 ± 0.11 B | 8.86 ± 0.079 C | 8.77 ± 0.14 C |
| C18:3 n-3 | 0.52 ± 0.028 b | 0.88 ± 0.029 d | 0.42 ± 0.0075 a | 0.50 ± 0.024 b | 0.56 ± 0.036 b,c | 0.58 ± 0.16 A | 0.60 ± 0.028 B | 1.04 ± 0.029 C | 0.97 ± 0.032 C |
| n-6/n-3 | 10.98 b | 7.00 a | 12.62 c | 10.94 b | 11.07 b | 10.52 ± 1.87 C | 12.98 D | 8.52 A | 9.04 B |
| PUFA | 6.23 ± 0.067 a,b | 7.04 ± 0.087 c | 5.72 ± 0.055 a | 5.97 ± 0.072 a | 6.76 ± 0.053 b | 6.34 ± 0.49 A | 8.39 ± 0.14 B | 9.90 ± 0.11 C | 9.74 ± 0.17 C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, S.; Huang, B.; Wei, T.; Deng, Z.; Li, J.; Wen, Q. Comprehensive Evaluation of Quality Characteristics of Four Oil-Tea Camellia Species with Red Flowers and Large Fruit. Foods 2023, 12, 374. https://doi.org/10.3390/foods12020374
Zhong S, Huang B, Wei T, Deng Z, Li J, Wen Q. Comprehensive Evaluation of Quality Characteristics of Four Oil-Tea Camellia Species with Red Flowers and Large Fruit. Foods. 2023; 12(2):374. https://doi.org/10.3390/foods12020374
Chicago/Turabian StyleZhong, Shengyue, Bin Huang, Teng Wei, Zeyuan Deng, Jing Li, and Qiang Wen. 2023. "Comprehensive Evaluation of Quality Characteristics of Four Oil-Tea Camellia Species with Red Flowers and Large Fruit" Foods 12, no. 2: 374. https://doi.org/10.3390/foods12020374
APA StyleZhong, S., Huang, B., Wei, T., Deng, Z., Li, J., & Wen, Q. (2023). Comprehensive Evaluation of Quality Characteristics of Four Oil-Tea Camellia Species with Red Flowers and Large Fruit. Foods, 12(2), 374. https://doi.org/10.3390/foods12020374





