Lid Films of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Microfibrillated Cellulose Composites for Fatty Food Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PHBV-Based Green Composites
2.3. Evaluation of Packaged Foods
2.3.1. Minced Pork Meat
2.3.2. Sunflower Oil
2.4. Migration Tests
2.5. Statistical Analysis
3. Results and Discussion
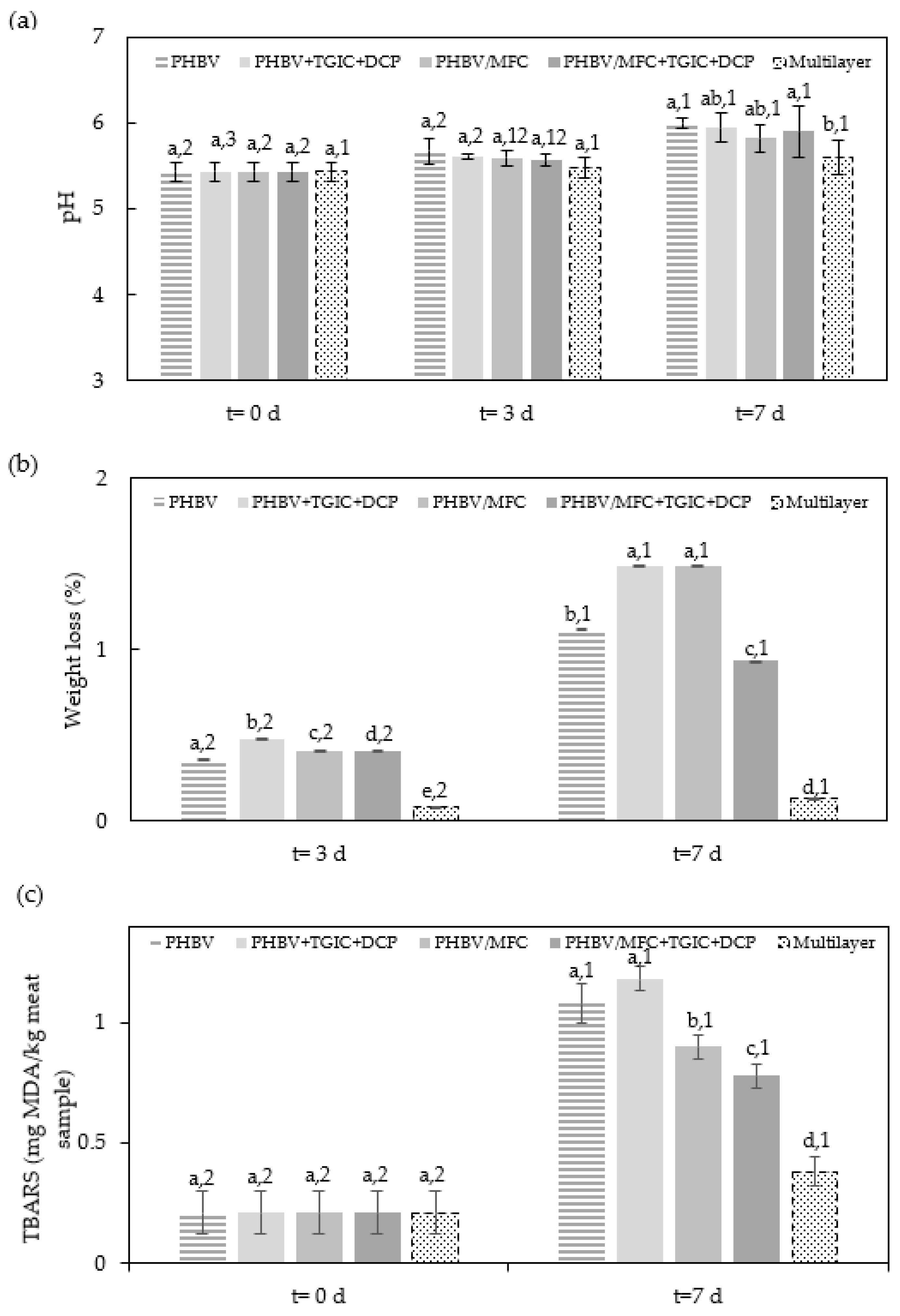
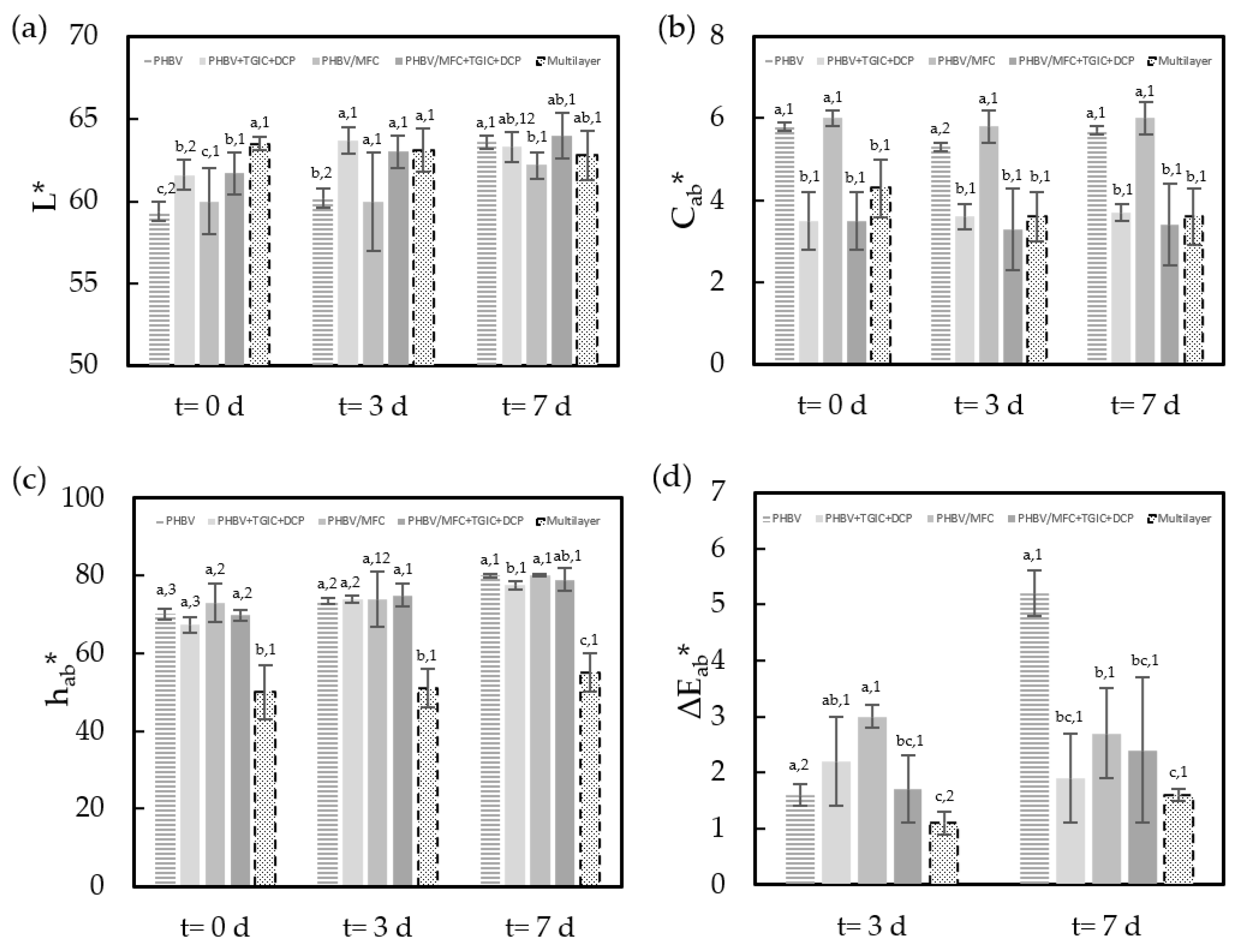
3.1. Physicochemical Properties of Packaged Minced Pork Meat
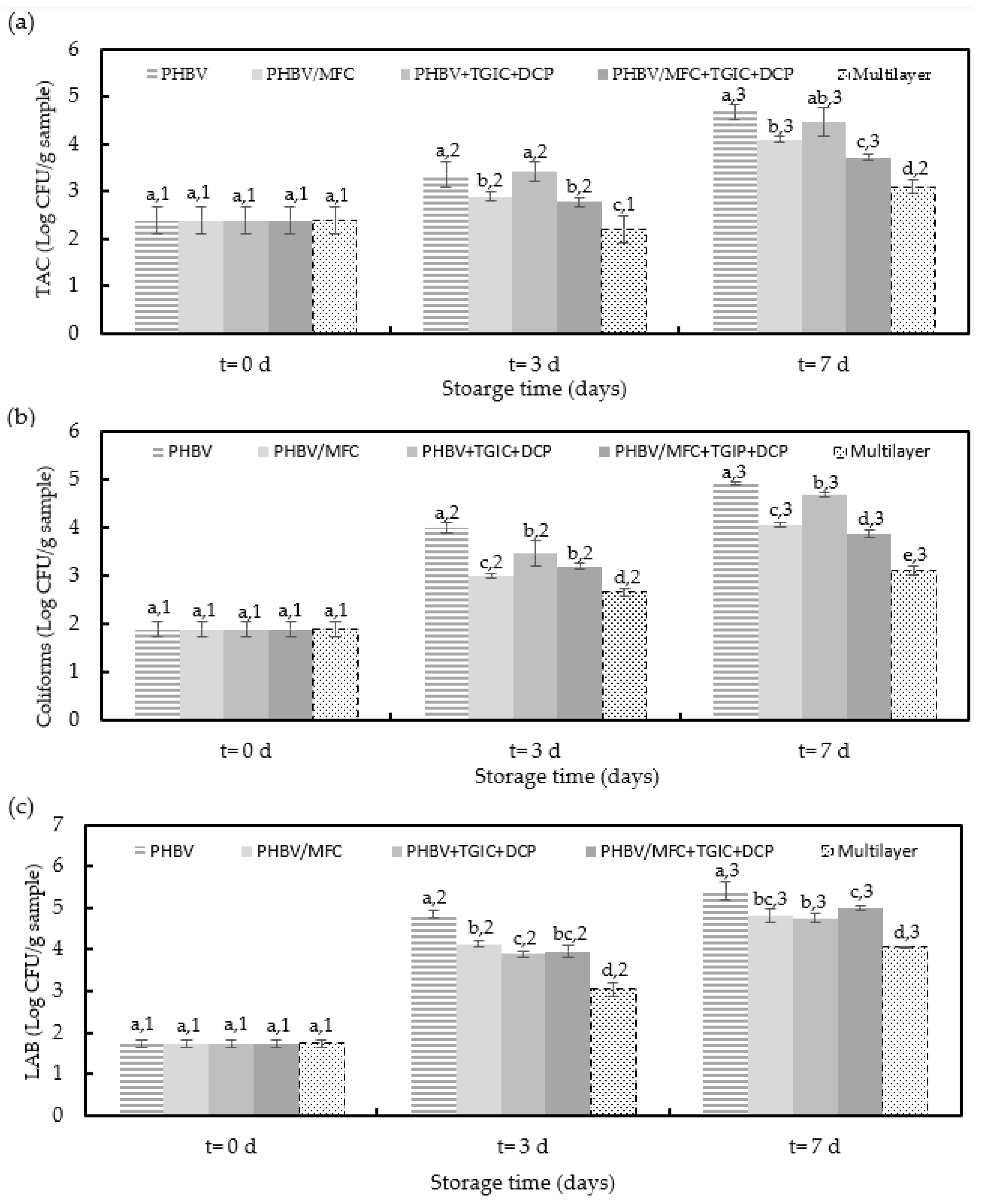
3.2. Microbial Characteristics of Packaged Minced Pork Meat
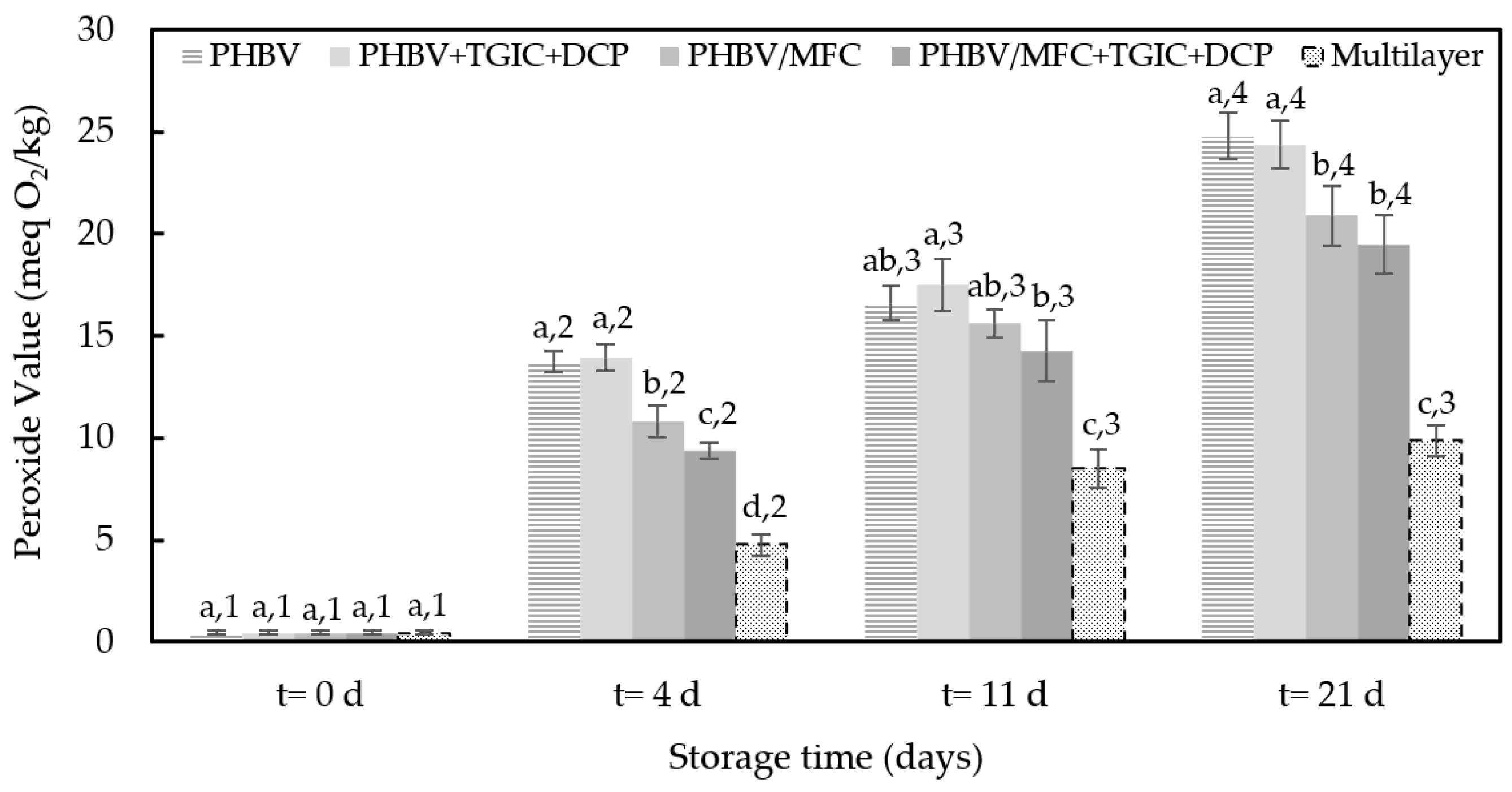
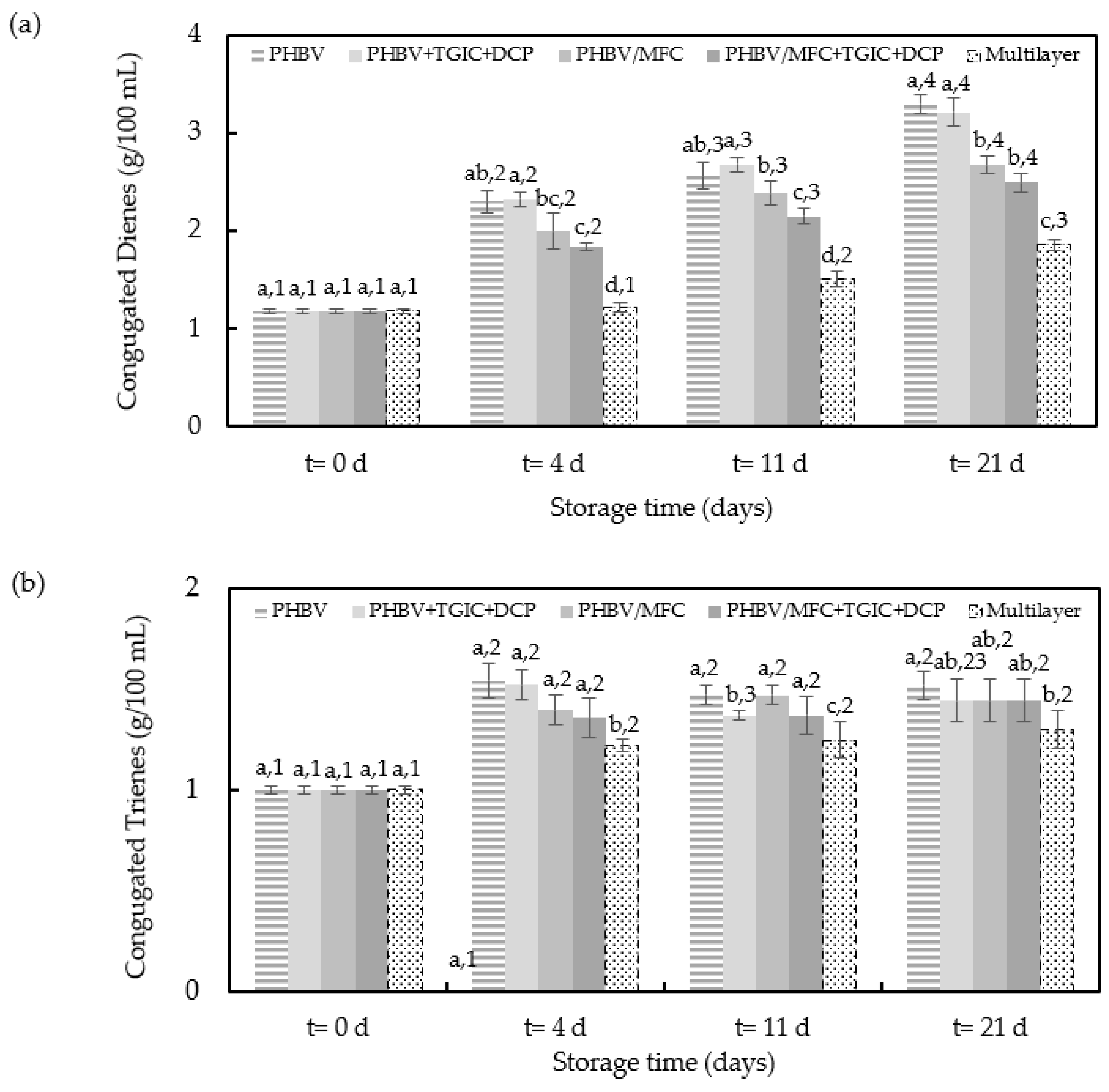
3.3. Oxidative Stability of Packaged Sunflower Oil
3.4. Overall Migration in Food Simulants
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robertson, G.L. Packaging and Food and Beverage Shelf Life. In The Stability and Shelf Life of Food, 2nd ed.; Subramaniam, P., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 77–106. [Google Scholar]
- Torres-Giner, S.; Gil, L.; Pascual-Ramírez, L.; Garde-Belza, J.A. Packaging: Food waste reduction. In Encyclopedia of Polymer Applications; Mishra, M., Ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Radusin, T.; Torres-Giner, S.; Stupar, A.; Ristic, I.; Miletic, A.; Novakovic, A.; Lagaron, J.M. Preparation, characterization and antimicrobial properties of electrospun polylactide films containing Allium ursinum L. extract. Food Packag. Shelf Life 2019, 21, 100357. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Quiles-Carrillo, L.; Balart, R.; Torres-Giner, S.; Arrieta, M.P. Innovative solutions and challenges to increase the use of Poly(3-hydroxybutyrate) in food packaging and disposables. Eur. Polym. J. 2022, 178, 111505. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Active Starch-Polyester Bilayer Films with Surface-Incorporated Ferulic Acid. Membranes 2022, 12, 976. [Google Scholar] [CrossRef]
- Hottle, T.A.; Bilec, M.M.; Landis, A.E. Biopolymer production and end of life comparisons using life cycle assessment. Resour. Conserv. Recycl. 2017, 122, 295–306. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Thermoprocessed starch-polyester bilayer films as affected by the addition of gellan or xanthan gum. Food Hydrocoll. 2021, 113, 106509. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Chiralt, A.; González-Martínez, C. Biodegradation of PLA-PHBV Blend Films as Affected by the Incorporation of Different Phenolic Acids. Foods 2022, 11, 243. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Starch-polyester bilayer films with phenolic acids for pork meat preservation. Food Chem. 2022, 385, 132650. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Effect of active phenolic acids on properties of PLA-PHBV blend films. Food Packag. Shelf Life 2022, 33, 100824. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Figueroa-Lopez, K.J.; Melendez-Rodriguez, B.; Prieto, C.; Pardo-Figuerez, M.; Lagaron, J.M. Emerging Trends in Biopolymers for Food Packaging. In Sustainable Food Packaging Technology; Athanassiou, A., Ed.; WILEY-VCH GmbH: Weinheim, Germany, 2021; pp. 1–33. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; González-Martínez, C.; Chiralt, A. Biodegradable Antimicrobial Films for Food Packaging: Effect of Antimicrobials on Degradation. Foods 2021, 10, 1256. [Google Scholar] [CrossRef]
- Arcos-Hernandez, M.V.; Laycock, B.; Pratt, S.; Donose, B.C.; Nikolić, M.A.L.; Luckman, P.; Werker, A.; Lant, P.A. Biodegradation in a soil environment of activated sludge derived polyhydroxyalkanoate (PHBV). Polym. Degrad. Stat. 2012, 97, 2301–2312. [Google Scholar] [CrossRef]
- Bucci, D.Z.; Tavares, L.B.B.; Sell, I. Biodegradation and physical evaluation of PHB packaging. Polym. Test. 2007, 26, 908–915. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Montanes, N.; Fombuena, V.; Boronat, T.; Sanchez-Nacher, L. Preparation and characterization of compression-molded green composite sheets made of poly(3-hydroxybutyrate) reinforced with long pita fibers. Adv. Polym. Technol. 2018, 37, 1305–1315. [Google Scholar] [CrossRef]
- Laycock, B.; Arcos-Hernandez, M.V.; Langford, A.; Buchanan, J.; Halley, P.J.; Werker, A.; Lant, S.; Pratt, P.A. Thermal properties and crystallization behavior of fractionated blocky and random polyhydroxyalkanoate copolymers from mixed microbial cultures. J. Appl. Polym. Sci. 2014, 131, 40836. [Google Scholar] [CrossRef]
- Tajeddin, B. Cellulose-Based Polymers for Packaging Applications. In Lignocellulosic Polymer Composites; Wiley: Hoboken, NJ, USA, 2014; pp. 477–498. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Barrrasa, H.; Vargas, F.; Rivera, D.; Vargas, M.; Torres-Giner, S. Atomization of Microfibrillated Cellulose and Its Incorporation into Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Reactive Extrusion. Appl. Sci. 2022, 12, 2111. [Google Scholar] [CrossRef]
- Cormier, A.; Vautour, G.; Allard, J. Canada-Wide Survey of the Nutritional Composition of Six Retail Pork Cuts. J. Food Compost. Anal. 1996, 9, 255–268. [Google Scholar] [CrossRef]
- Mazzola, N.; Sarantópoulos, C.I.G.L. Packaging Design Alternatives for Meat Products. In Food Processing; Marc, R.A., Díaz, A.V., Izquierdo, G.D.P., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Torres-Giner, S. Quality and Shelf-Life Stability of Pork Meat Fillets Packaged in Multilayer Polylactide Films. Foods 2022, 11, 426. [Google Scholar] [CrossRef]
- Khan, S.; Choudhary, S.; Pandey, A.; Khan, M.K.; Thomas, G. Sunflower oil: Efficient oil source for human consumption. Emergent Life Sci. Res. 2015, 1, 1–3. [Google Scholar]
- Kucuk, M.; Caner, C. Effect of packaging materials and storage conditions on sunflower oil quality. J. Food Lipids 2005, 12, 222–231. [Google Scholar] [CrossRef]
- Cenci-goga, B.T.; Lulietto, M.F.; Sechi, P.; Borgogni, E.; Karama, M.; Grispoldi, L. New Trends in Meat Packaging. Microbiol. Res. 2020, 11, 56–67. [Google Scholar] [CrossRef]
- Siu, G.; Draper, H.H. A survey of malonaldehyde content of retail meats and fish. J. Food Sci. 1978, 43, 1147–1149. [Google Scholar] [CrossRef]
- Talon, E.; Vargas, M.; Chiralt, A.; Gonzalez-Martínez, C. Antioxidant starchbased films with encapsulated eugenol. Appl. Sunflower Oil Preserv. Lwt. 2019, 113, 108290. [Google Scholar] [CrossRef]
- European Commission. Regulation EEC/2568/91 on the characteristics of olive and olive pomace oils and their analytical methods. Off. J. Eur. Communities L 1991, 248, 1–83. [Google Scholar]
- UNE-EN-ISO 1186-2; Asociación Española de Normalización. Materiales y Artículos en Contacto con Productos Alimenticios. Plásticos. Parte 2: Métodos de Ensayo para la Migración Global en Aceite de Oliva por Inmersión Total. Asociación Española de Normalización: Madrid, Spain, 2002.
- UNE-EN-ISO 1186-3; Asociación Española de Normalización. Materiales y Artículos en Contacto con Productos Alimenticios. Plásticos. Parte 3: Métodos de Ensayo para la Migración Global en Simuladores de Alimentos Acuosos por Inmersión Total. Asociación Española de Normalización: Madrid, Spain, 2002.
- Wang, G.; Liu, Y.; Yong, H.; Zong, S.; Jin, C.; Liu, J. Effect of ferulic acid-grafted-chitosan coating on the quality of pork during refrigerated storage. Foods 2021, 10, 1374. [Google Scholar] [CrossRef] [PubMed]
- Daniloski, D.; Petkoska, A.T.; Galić, K.; Ščetar, M.; Kurek, M.; Vaskoska, R.; Nedelkoska, D.N. The effect of barrier properties of polymeric films on the shelf-life of vacuum packaged fresh pork meat. Meat Sci. 2019, 158, 107880. [Google Scholar] [CrossRef] [PubMed]
- Stella, S.; Garavaglia, D.; Francini, S.; Viganò, V.; Bernardi, C.; Tirloni, E.A. Evaluation of the weight loss of raw beef cuts vacuum packaged with two different techniques. Ital. J. Food Saf. 2019, 8, 8111. [Google Scholar] [PubMed]
- Konuk Takma, D.; Korel, F. Active packaging films as a carrier of black cumin essential oil: Development and effect on quality and shelf-life of chicken breast meat. Food Packag. Shelf Life 2019, 19, 210–217. [Google Scholar] [CrossRef]
- Sheard, P.R.; Enser, M.; Wood, J.D.; Nute, G.R.; Gill, B.P.; Richardson, R.I. Shelf life and quality of pork and pork products with raised n-3 PUFA. Meat Sci. 2000, 55, 213–221. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Karamucki, T.; Jakubowska, M.; Rybarczyk, A.; Gardzielewska, J. The influence of myoglobin on the colour of minced pork loin. Meat Sci. 2013, 94, 234–238. [Google Scholar] [CrossRef]
- Hutchings, J.B. Instrumental specification. In Food Colour and Appearance; Springer: New York, NY, USA, 1999; pp. 199–237. [Google Scholar]
- Huang, L.; Zhao, J.; Chen, Q.; Zhang, Y. Rapid detection of total viable count (TVC) in pork meat by hyperspectral imaging. Food Res. Int. 2013, 54, 821–828. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union. 2005, 50, 1–26. Available online: http://data.europa.eu/eli/reg/2005/2073/oj (accessed on 1 September 2022).
- Andrade, L.; González-Martínez, C.; Chiralt, A. Antimicrobial PLA-PVA multilayer films containing phenolic compounds. Food Chem 2021, 375, 131861. [Google Scholar] [CrossRef]
- Qin, Y.-Y.; Yang, J.-Y.; Lu, H.-B.; Wang, S.-S.; Yang, J.; Yang, X.-C.; Cao, J.-X. Effect of chitosan film incorporated with tea polyphenol on quality and shelf life of pork meat patties. Int. J. Biol. Macromol. 2013, 61, 312–316. [Google Scholar] [CrossRef]
- Sadeghi, E.; Karami, F.; Etminan, A. The effect of Ferulago angulata (Schlecht) Boiss essential oil on stabilization of sunflower oil during accelerated storage. J. Food Process. Preserv. 2017, 41, e12745. [Google Scholar] [CrossRef]
- Codex-Alimentarius. Codex-Standards for fats and oils from vegetable sources. Codex-Stan 1999, 210, 210–1999. [Google Scholar]
- Godswill, A.C.; Amagwula, I.O.; Victory, I.S.; Gonzaga, A.I. Effects of repeated deep frying on refractive index and peroxide value of selected vegetable oils. Int. J. Adv. Acad. 2018, 4, 106–119. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Antioxidant starch composite films containing rice straw extract and cellulose fibres. Food Chem. 2023, 400, 134073. [Google Scholar] [CrossRef] [PubMed]
- Mohdaly, A.A.A.; Sarhan, M.A.; Mahmoud, A.; Ramadan, M.F.; Smetanska, I. Antioxidant efficacy of potato peels and sugar beet pulp extracts in vegetable oils protection. Food Chem. 2010, 123, 1019–1026. [Google Scholar] [CrossRef]
- Li, F.; Abdalkarim, S.Y.H.; Yu, H.Y.; Zhu, J.; Zhou, Y.; Guan, Y. Bifunctional reinforcement of green biopolymer packaging nanocomposites with natural cellulose nanocrystral-rosin hybrids. Appl. Bio. Mater 2020, 3, 1944–1954. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, H.Y.; Wang, C.; Yao, J. Effect of silver contents in cellulose nanocrystal/silver nanohybrids on PHBV crystallization and property improvements. Carbohydr. Polym. 2022, 173, 7–16. [Google Scholar] [CrossRef] [PubMed]
| Sample | PHBV (wt%) | MFC (wt%) | TGIC (phr) | DCP (phr) |
|---|---|---|---|---|
| PHBV | 100 | 0 | 0 | 0 |
| PHBV/MFC | 95 | 5 | 0 | 0 |
| PHBV + TGIC + DCP | 100 | 0 | 1 | 0.25 |
| PHBV/MFC + TGIC + DCP | 95 | 5 | 1 | 0.25 |
| Film | Ethanol 10% v/v (mg/dm2) | Vegetable Oil (mg/dm2) |
|---|---|---|
| PHBV | 1.7 ± 0.2 a | 2.2 ± 0.1 a |
| PHBV/MFC | 1.9 ± 0.5 a | 2.6 ± 0.2 b |
| PHBV/MFC + TGIC + DCP | 2.8 ± 0.4 b | 2.7 ± 0.3 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-García, E.; Chiralt, A.; Vargas, M.; Torres-Giner, S. Lid Films of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Microfibrillated Cellulose Composites for Fatty Food Preservation. Foods 2023, 12, 375. https://doi.org/10.3390/foods12020375
Hernández-García E, Chiralt A, Vargas M, Torres-Giner S. Lid Films of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Microfibrillated Cellulose Composites for Fatty Food Preservation. Foods. 2023; 12(2):375. https://doi.org/10.3390/foods12020375
Chicago/Turabian StyleHernández-García, Eva, Amparo Chiralt, Maria Vargas, and Sergio Torres-Giner. 2023. "Lid Films of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Microfibrillated Cellulose Composites for Fatty Food Preservation" Foods 12, no. 2: 375. https://doi.org/10.3390/foods12020375
APA StyleHernández-García, E., Chiralt, A., Vargas, M., & Torres-Giner, S. (2023). Lid Films of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Microfibrillated Cellulose Composites for Fatty Food Preservation. Foods, 12(2), 375. https://doi.org/10.3390/foods12020375







