A Mixture of Full-Fat and Defatted Hermetia illucens Larvae and Poultry By-Products as Sustainable Protein Sources Improved Fillet Quality Traits in Farmed Barramundi, Lates calcarifer
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement, Diets, Animal Husbandry, and Fish Sampling
2.2. Proximate Composition
2.2.1. Crude Protein
2.2.2. Crude Fat
2.2.3. Ash
2.2.4. Moisture
2.3. Amino Acid Analysis
2.4. Sensory Quality
2.5. Simulated Retail Display Protocols
2.6. Physical Parameters
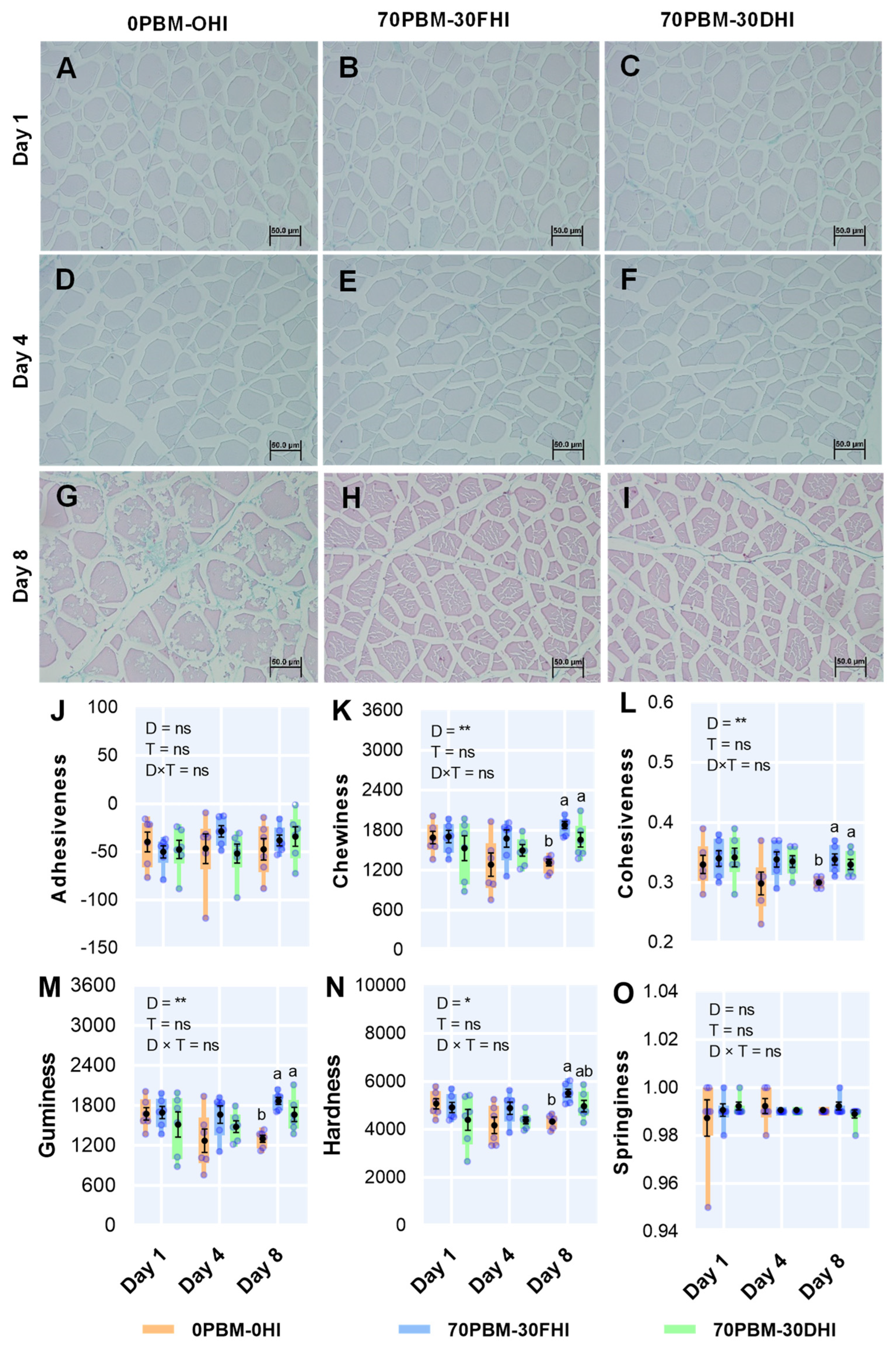
2.6.1. Texture Profile Analysis (TPA)
2.6.2. Microscopic Observation of Fillet Tissues
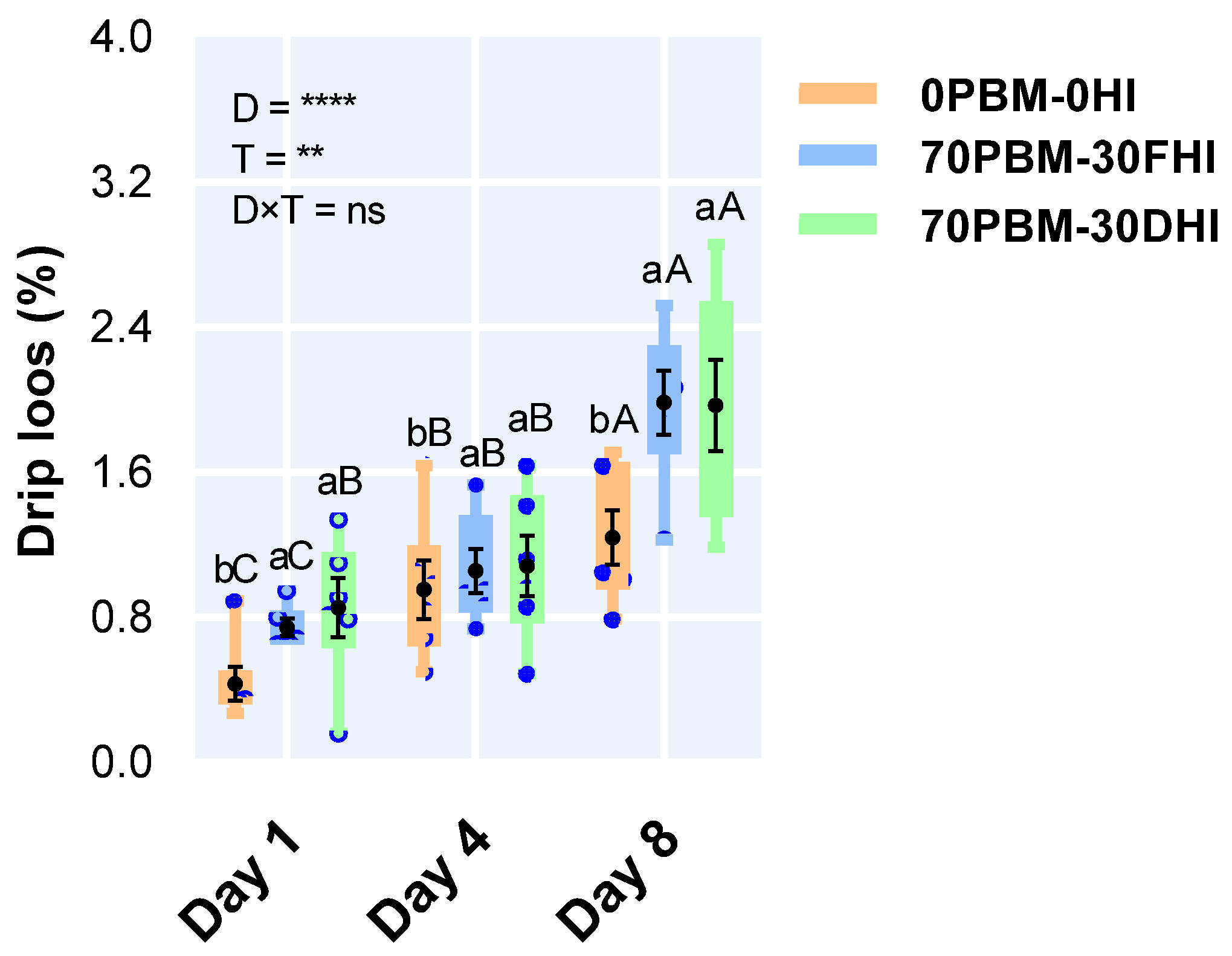
2.6.3. Drip Loss
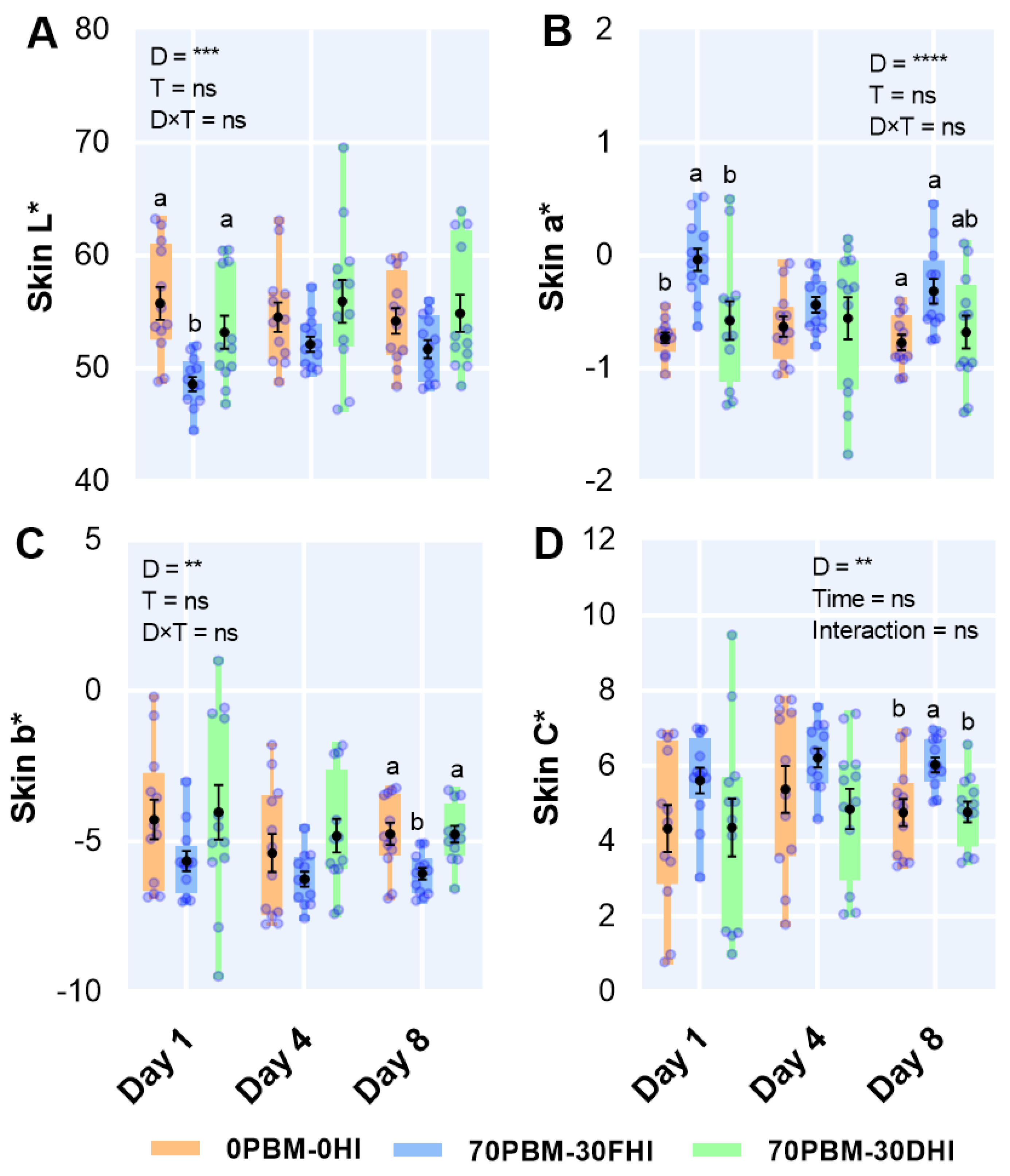
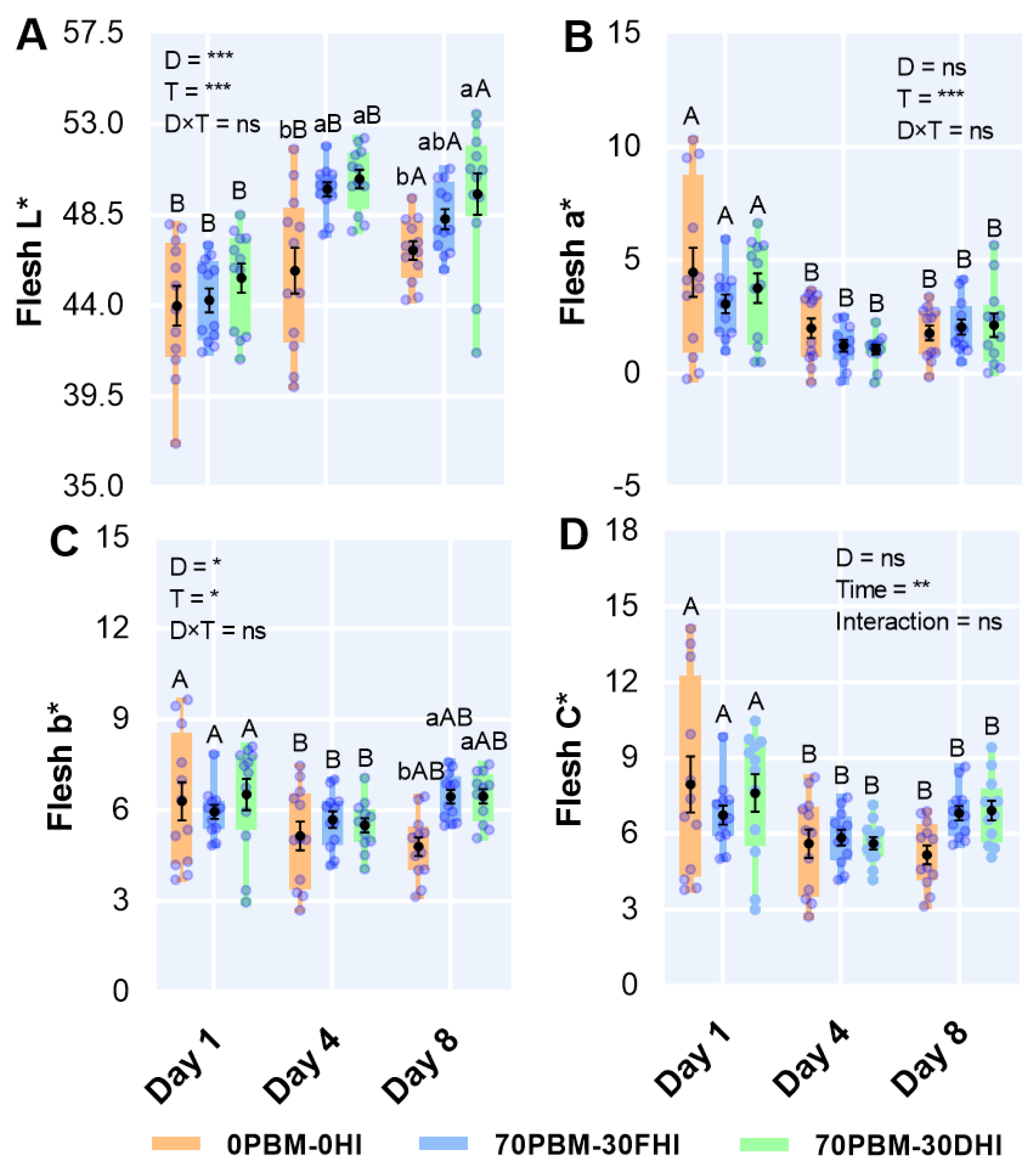
2.6.4. Color
2.6.5. Quality Index (QI)
2.7. Chemical Parameters
2.7.1. pH
2.7.2. Lipid Oxidation
2.8. Statistical Analysis
3. Results
3.1. Proximate and Amino Acid Muscle Compositions
3.2. Muscle Fatty Acid Composition
3.3. Sensory Evaluation
3.4. Microstructure and Texture Profiles of Fillets
3.5. Drip Loss
3.6. Skin Color
3.7. Flesh Color
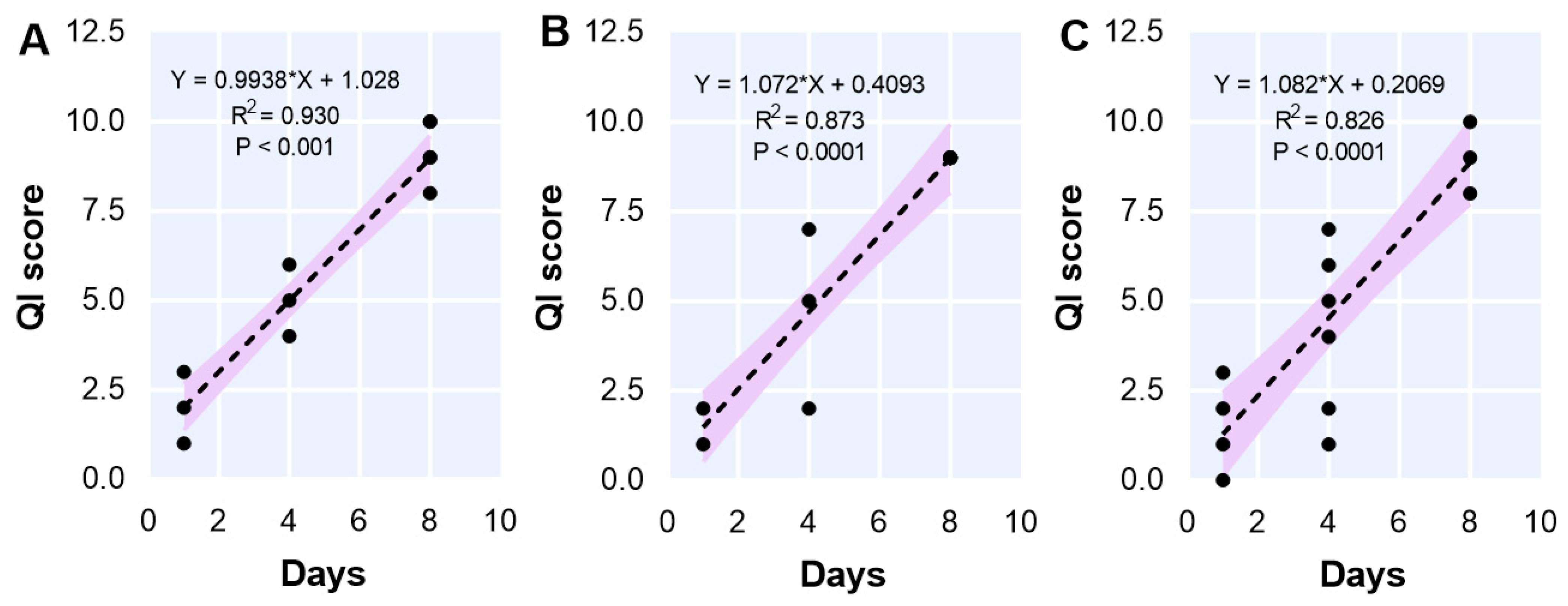
3.8. Quality Index (QI) Method
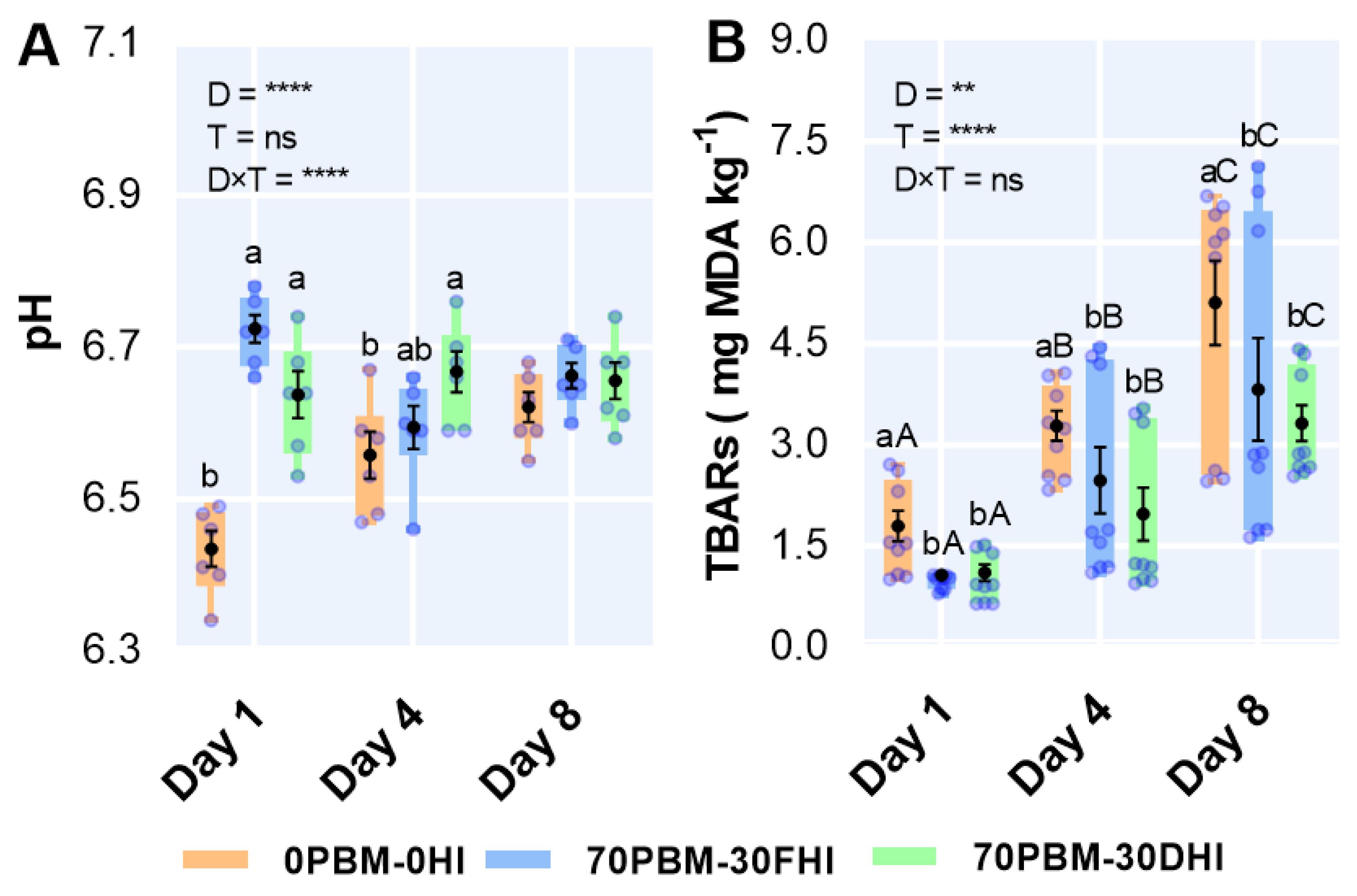
3.9. pH and Lipid Oxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Codabaccus, M.B.; Ng, W.-K.; Nichols, P.D.; Carter, C.G. Restoration of EPA and DHA in rainbow trout (Oncorhynchus mykiss) using a finishing fish oil diet at two different water temperatures. Food Chem. 2013, 141, 236–244. [Google Scholar] [CrossRef]
- Haliloǧlu, H.İ.; Bayır, A.; Sirkecioǧlu, A.N.; Aras, N.M.; Atamanalp, M. Comparison of fatty acid composition in some tissues of rainbow trout (Oncorhynchus mykiss) living in seawater and freshwater. Food Chem. 2004, 86, 55–59. [Google Scholar] [CrossRef]
- Han, D.; Shan, X.; Zhang, W.; Chen, Y.; Wang, Q.; Li, Z.; Zhang, G.; Xu, P.; Li, J.; Xie, S. A revisit to fishmeal usage and associated consequences in Chinese aquaculture. Rev. Aquac. 2018, 10, 493–507. [Google Scholar] [CrossRef]
- Quiñones, J.; Díaz, R.; Dantagnan, P.; Hernández, A.; Valdes, M.; Lorenzo, J.M.; Cancino, D.; Sepúlveda, N.; Farías, J.G. Dietary inclusion of Durvillaea antarctica meal and rapeseed (Brassica napus) oil on growth, feed utilization and fillet quality of rainbow trout (Oncorhynchus mykiss). Aquaculture 2021, 530, 735882. [Google Scholar] [CrossRef]
- Xu, H.; Bi, Q.; Liao, Z.; Sun, B.; Jia, L.; Wei, Y.; Liang, M. Long-term alternate feeding between fish oil-and terrestrially sourced oil-based diets mitigated the adverse effects of terrestrially sourced oils on turbot fillet quality. Aquaculture 2021, 531, 735974. [Google Scholar] [CrossRef]
- FAO. Sustainability in action. State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2020; p. 200. [Google Scholar]
- Chaklader, M.R.; Siddik, M.A.; Fotedar, R. Total replacement of fishmeal with poultry by-product meal affected the growth, muscle quality, histological structure, antioxidant capacity and immune response of juvenile barramundi, Lates calcarifer. PLoS ONE 2020, 15, e0242079. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.J.; Salini, M.J.; Irvin, S.; Blyth, D.; Bourne, N.; Smullen, R. The effect of poultry protein concentrate and phosphorus supplementation on growth, digestibility and nutrient retention efficiency in barramundi Lates calcarifer. Aquaculture 2019, 498, 305–314. [Google Scholar] [CrossRef]
- Dawson, M.R.; Alam, M.S.; Watanabe, W.O.; Carroll, P.M.; Seaton, P.J. Evaluation of poultry by-product meal as an alternative to fish meal in the diet of juvenile Black Sea Bass reared in a recirculating aquaculture system. N. Am. J. Aquac. 2018, 80, 74–87. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture. In Opportunities and Challenges; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Williams, K.C.; Paterson, B.D.; Barlow, C.G.; Ford, A.; Roberts, R. Potential of meat meal to replace fish meal in extruded dry diets for barramundi, Lates calcarifer (Bloch). II. Organoleptic characteristics and fatty acid composition. Aquac. Res. 2003, 34, 33–42. [Google Scholar] [CrossRef]
- Glencross, B. The nutritional management of barramundi, Lates calcarifer—A review. Aquac. Nutr. 2006, 12, 291–309. [Google Scholar] [CrossRef]
- Badillo, D.; Herzka, S.; Viana, M. Protein Retention Assessment of Four Levels of Poultry By-Product Substitution of Fishmeal in Rainbow Trout (Oncorhynchus mykiss) Diets Using Stable Isotopes of Nitrogen (δ15N) as Natural Tracers. PLoS ONE 2014, 9, e107523. [Google Scholar] [CrossRef] [PubMed]
- Chaklader, M.R.; Fotedar, R.; Howieson, J.; Siddik, M.A.; Foysal, J. The ameliorative effects of various fish protein hydrolysates in poultry by-product meal based diets on muscle quality, serum biochemistry and immunity in juvenile barramundi, Lates calcarifer. Fish Shellfish. Immunol. 2020, 104, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Chaklader, M.R.; Howieson, J.; Fotedar, R. Growth, hepatic health, mucosal barrier status and immunity of juvenile barramundi, Lates calcarifer fed poultry by-product meal supplemented with full-fat or defatted Hermetia illucens larval meal. Aquaculture 2021, 543, 737026. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Hardy, R.W. Apparent Digestibility Coefficients of Nutrients and Nutritional Value of Poultry By-product Meals for Rainbow Trout Oncorhynchus mykiss Measured in vivo Using Settlement. J. World Aquac. Soc. 2002, 33, 458–465. [Google Scholar] [CrossRef]
- Galkanda-Arachchige, H.S.; Wilson, A.E.; Davis, D.A. Success of fishmeal replacement through poultry by-product meal in aquaculture feed formulations: A meta-analysis. Rev. Aquac. 2020, 12, 1624–1636. [Google Scholar] [CrossRef]
- Chaklader, M.R.; Siddik, M.A.B.; Fotedar, R.; Howieson, J. Insect larvae, Hermetia illucens in poultry by-product meal for barramundi, Lates calcarifer modulates histomorphology, immunity and resistance to Vibrio harveyi. Sci. Rep. 2019, 9, 16703. [Google Scholar] [CrossRef] [PubMed]
- Chaklader, M.R.; Howieson, J.; Siddik, M.A.B.; Foysal, M.J.; Fotedar, R. Supplementation of tuna hydrolysate and insect larvae improves fishmeal replacement efficacy of poultry by-product in Lates calcarifer (Bloch, 1790) juveniles. Sci. Rep. 2021, 11, 4997. [Google Scholar] [CrossRef]
- Chaklader, M.R.; Howieson, J.; Foysal, M.J.; Fotedar, R. Transformation of fish waste protein to Hermetia illucens protein improves the efficacy of poultry by-products in the culture of juvenile barramundi, Lates calcarifer. Sci. Total Environ. 2021, 796, 149045. [Google Scholar] [CrossRef]
- Chaklader, M.R.; Howieson, J.; Fotedar, R.; Siddik, M.A. Supplementation of Hermetia illucens larvae in poultry by-product meal based barramundi, Lates calcarifer diets improves adipocyte cell size, skin barrier functions, and immune responses. Front. Nutr. 2020, 7, 61358. [Google Scholar] [CrossRef]
- Moutinho, S.; Pedrosa, R.; Magalhães, R.; Oliva-Teles, A.; Parisi, G.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae larvae meal in diets for European seabass (Dicentrarchus labrax) juveniles: Effects on liver oxidative status and fillet quality traits during shelf-life. Aquaculture 2020, 533, 736080. [Google Scholar] [CrossRef]
- Barroso, F.G.; de Haro, C.; Sánchez-Muros, M.-J.; Venegas, E.; Martínez-Sánchez, A.; Pérez-Bañón, C. The potential of various insect species for use as food for fish. Aquaculture 2014, 422–423, 193–201. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed. Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Nogales-Mérida, S.; Gobbi, P.; Józefiak, D.; Mazurkiewicz, J.; Dudek, K.; Rawski, M.; Kierończyk, B.; Józefiak, A. Insect meals in fish nutrition. Rev. Aquac. 2018, 11, 1080–1103. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Khalil, R.H.; Metwally, A.A.; Shakweer, M.S.; Khallaf, M.A.; Abdel-Latif, H.M. Effects of black soldier fly (Hermetia illucens L.) larvae meal on growth performance, organs-somatic indices, body composition, and hematological and biochemical variables of European sea bass, Dicentrarchus labrax. Aquaculture 2020, 522, 735136. [Google Scholar] [CrossRef]
- Wang, G.; Peng, K.; Hu, J.; Yi, C.; Chen, X.; Wu, H.; Huang, Y. Evaluation of defatted black soldier fly (Hermetia illucens L.) larvae meal as an alternative protein ingredient for juvenile Japanese seabass (Lateolabrax japonicus) diets. Aquaculture 2019, 507, 144–154. [Google Scholar] [CrossRef]
- Sealey, W.M.; Gaylord, T.G.; Barrows, F.T.; Tomberlin, J.K.; McGuire, M.A.; Ross, C.; St-Hilaire, S. Sensory Analysis of Rainbow Trout, Oncorhynchus mykiss, Fed Enriched Black Soldier Fly Prepupae, Hermetia illucens. J. World Aquac. Soc. 2011, 42, 34–45. [Google Scholar] [CrossRef]
- Borgogno, M.; Dinnella, C.; Iaconisi, V.; Fusi, R.; Scarpaleggia, C.; Schiavone, A.; Monteleone, E.; Gasco, L.; Parisi, G. Inclusion of Hermetia illucens larvae meal on rainbow trout (Oncorhynchus mykiss) feed: Effect on sensory profile according to static and dynamic evaluations. J. Sci. Food Agric. 2017, 97, 3402–3411. [Google Scholar] [CrossRef] [PubMed]
- Iaconisi, V.; Marono, S.; Parisi, G.; Gasco, L.; Genovese, L.; Maricchiolo, G.; Bovera, F.; Piccolo, G. Dietary inclusion of Tenebrio molitor larvae meal: Effects on growth performance and final quality treats of blackspot sea bream (Pagellus bogaraveo). Aquaculture 2017, 476, 49–58. [Google Scholar] [CrossRef]
- Secci, G.; Mancini, S.; Iaconisi, V.; Gasco, L.; Basto, A.; Parisi, G. Can the inclusion of black soldier fly (Hermetia illucens) in diet affect the flesh quality/nutritional traits of rainbow trout (Oncorhynchus mykiss) after freezing and cooking? Int. J. Food Sci. Nutr. 2019, 70, 161–171. [Google Scholar] [CrossRef]
- Cao, H.; Yu, R.; Zhang, Y.; Hu, B.; Jian, S.; Wen, C.; Kajbaf, K.; Kumar, V.; Yang, G. Effects of dietary supplementation with β-glucan and Bacillus subtilis on growth, fillet quality, immune capacity, and antioxidant status of Pengze crucian carp (Carassius auratus var. Pengze). Aquaculture 2019, 508, 106–112. [Google Scholar] [CrossRef]
- Llagostera, P.F.; Kallas, Z.; Reig, L.; De Gea, D.A. The use of insect meal as a sustainable feeding alternative in aquaculture: Current situation, Spanish consumers’ perceptions and willingness to pay. J. Clean. Prod. 2019, 229, 10–21. [Google Scholar] [CrossRef]
- Carton, A.G.; Jones, B. Post-harvest quality in farmed Lates calcarifer. In Biology and Culture of Asian Seabass Lates Calcarifer; Jerry, D.R., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 229–257. [Google Scholar]
- Erikson, U.; Lambooij, B.; Digre, H.; Reimert, H.; Bondø, M.; Van der Vis, H. Conditions for instant electrical stunning of farmed Atlantic cod after de-watering, maintenance of unconsciousness, effects of stress, and fillet quality—A comparison with AQUI-S™. Aquaculture 2012, 324, 135–144. [Google Scholar] [CrossRef]
- Jones, B.C.; Carton, A.G. Effects of dietary enrichment with alpha-tocopherol acetate and post-harvest filleting on lipid oxidation and flesh quality of tropical farmed barramundi (Lates calcarifer). Aquaculture 2015, 448, 280–287. [Google Scholar] [CrossRef]
- Wilkinson, R.J.; Paton, N.; Porter, M.J. The effects of pre-harvest stress and harvest method on the stress response, rigor onset, muscle pH and drip loss in barramundi (Lates calcarifer). Aquaculture 2008, 282, 26–32. [Google Scholar] [CrossRef]
- Bonilla, A.C.; Sveinsdottir, K.; Martinsdottir, E. Development of Quality Index Method (QIM) scheme for fresh cod (Gadus morhua) fillets and application in shelf life study. Food Control 2007, 18, 352–358. [Google Scholar] [CrossRef]
- Gram, L. Evaluation of the bacteriological quality of seafood. Int. J. Food Microbiol. 1992, 16, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef]
- Olafsdottir, G.; Martinsdóttir, E.; Oehlenschläger, J.; Dalgaard, P.; Jensen, B.; Undeland, I.; Mackie, I.; Henehan, G.; Nielsen, J.; Nilsen, H. Methods to evaluate fish freshness in research and industry. Trends Food Sci. Technol. 1997, 8, 258–265. [Google Scholar] [CrossRef]
- Fuentes-Amaya, L.F.; Munyard, S.; Fernandez-Piquer, J.; Howieson, J. Sensory, microbiological and chemical changes in vacuum-packaged blue spotted emperor (Lethrinus sp), saddletail snapper (Lutjanus malabaricus), crimson snapper (Lutjanus erythropterus), barramundi (Lates calcarifer) and Atlantic salmon (Salmo salar) fillets stored at 4 °C. Food Sci. Nutr. 2016, 4, 479–489. [Google Scholar]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Cohen, S.A.; De Antonis, K.M. Applications of amino acid derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate: Analysis of feed grains, intravenous solutions and glycoproteins. J. Chromatogr. A 1994, 661, 25–34. [Google Scholar] [CrossRef]
- Cohen, S.A.; Michaud, D.P. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal. Biochem. 1993, 211, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Wheat, T.E.; Grumbach, E.S.; Mazzeo, J.R. UPLC amino acid analysis solution. Appl. Note 2008. [Google Scholar]
- Bosch, L.; Alegría, A.; Farré, R. Application of the 6-aminoquinolyl-N-hydroxysccinimidyl carbamate (AQC) reagent to the RP-HPLC determination of amino acids in infant foods. J. Chromatogr. B 2006, 831, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Gedarawatte, S.T.; Ravensdale, J.T.; Johns, M.L.; Azizi, A.; Al-Salami, H.; Dykes, G.A.; Coorey, R. Effectiveness of bacterial cellulose in controlling purge accumulation and improving physicochemical, microbiological, and sensorial properties of vacuum-packaged beef. J. Food Sci. 2020, 85, 2153–2163. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Australia, S. Sensory Analysis Part 1.3: Methodology-General Guidance (AS 2542.1.3:2014). 2014. Available online: https://www.saiglobal.com (accessed on 5 July 2021).
- Alimentarius, C. Codex Alimentarius Volume 9: Codex Guidelines for the Sensory Evaluation of Fish and Shellfish in Laboratories (CAC-GL 31–1999). 1991. Available online: http://www.fao.org (accessed on 5 July 2021).
- Kalva, J.J.; Sims, C.A.; Puentes, L.A.; Snyder, D.J.; Bartoshuk, L.M. Comparison of the hedonic general labeled magnitude scale with the hedonic 9-point scale. J. Food Sci. 2014, 79, S238–S245. [Google Scholar] [CrossRef]
- Kathuria, D.; Dhiman, A.K.; Attri, S. Sous vide, a culinary technique for improving quality of food products: A review. Trends Food Sci. Technol. 2022, 119, 57–68. [Google Scholar] [CrossRef]
- Bourne, M.C. Texture Profile Analysis. Food Technol. 1978, 41, 163–178. [Google Scholar]
- Raharjo, S.; Sofos, J.N.; Schmidt, G.R. Improved speed, specificity, and limit of determination of an aqueous acid extraction thiobarbituric acid-C18 method for measuring lipid peroxidation in beef. J. Agric. Food Chem. 1992, 40, 2182–2185. [Google Scholar] [CrossRef]
- González-Rodríguez, Á.; Celada, J.D.; Carral, J.M.; Sáez-Royuela, M.; García, V.; Fuertes, J.B. Evaluation of poultry by-product meal as partial replacement of fish meal in practical diets for juvenile tench (Tinca tinca L). Aquac. Res. 2016, 47, 1612–1621. [Google Scholar] [CrossRef]
- Rodehutscord, M.; Mandel, S.; Pack, M.; Jacobs, S.; Pfeffer, E. Free amino acids can replace protein-bound amino acids in test diets for studies in rainbow trout (Oncorhynchus mykiss). J. Nutr. 1995, 125, 956–963. [Google Scholar]
- Siddik, M.; Chungu, P.; Fotedar, R.; Howieson, J. Bioprocessed poultry by-product meals on growth, gut health and fatty acid synthesis of juvenile barramundi, Lates calcarifer (Bloch). PLoS ONE 2019, 14, e0215025. [Google Scholar] [CrossRef]
- Irm, M.; Taj, S.; Jin, M.; Luo, J.; Andriamialinirina, H.J.T.; Zhou, Q. Effects of replacement of fish meal by poultry by-product meal on growth performance and gene expression involved in protein metabolism for juvenile black sea bream (Acanthoparus schlegelii). Aquaculture 2020, 528, 735544. [Google Scholar] [CrossRef]
- Sabbagh, M.; Schiavone, R.; Brizzi, G.; Sicuro, B.; Zilli, L.; Vilella, S. Poultry by-product meal as an alternative to fish meal in the juvenile gilthead seabream (Sparus aurata) diet. Aquaculture 2019, 511, 734220. [Google Scholar] [CrossRef]
- Hernández, C.; Osuna-Osuna, L.; Hernandez, A.B.; Sanchez-Gutierrez, Y.; González-Rodríguez, B.; Dominguez-Jimenez, P. Replacement of fish meal by poultry by-product meal, food grade, in diets for juvenile spotted rose snapper (Lutjanus guttatus). Lat. Am. J. Aquat. Res. 2014, 42, 111–120. [Google Scholar] [CrossRef]
- Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. Black Soldier Fly Full-Fat Larvae Meal as an Alternative to Fish Meal and Fish Oil in Siberian Sturgeon Nutrition: The Effects on Physical Properties of the Feed, Animal Growth Performance, and Feed Acceptance and Utilization. Animals 2020, 10, 2119. [Google Scholar] [CrossRef] [PubMed]
- Mouithys-Mickalad, A.; Schmitt, E.; Dalim, M.; Franck, T.; Tome, N.M.; van Spankeren, M.; Serteyn, D.; Paul, A. Black soldier fly (Hermetia illucens) larvae protein derivatives: Potential to promote animal health. Animals 2020, 10, 941. [Google Scholar] [CrossRef]
- Ganapathy, V. Intestinal transport of amino acids and peptides. Physiol. Gastrointest. Tract 1994, 1773, 1794. [Google Scholar]
- Ha, N.; Jesus, G.F.A.; Gonçalves, A.F.N.; de Oliveira, N.S.; Sugai, J.K.; Pessatti, M.L.; Mouriño, J.L.P.; El Hadi Perez Fabregat, T. Sardine (Sardinella spp.) protein hydrolysate as growth promoter in South American catfish (Rhamdia quelen) feeding: Productive performance, digestive enzymes activity, morphometry and intestinal microbiology. Aquaculture 2019, 500, 99–106. [Google Scholar] [CrossRef]
- Chaklader, M.R.; Chung, W.H.; Howieson, J.; Fotedar, R. A Combination of Hermetia illucens Reared on Fish Waste and Poultry By-Product Meal Improves Sensory and Physicochemical Quality of Farmed Barramundi Filets. Front. Nutr. 2021, 8, 788064. [Google Scholar] [PubMed]
- Panicz, R.; Żochowska-Kujawska, J.; Sadowski, J.; Sobczak, M. Effect of feeding various levels of poultry by-product meal on the blood parameters, filet composition and structure of female tenches (Tinca tinca). Aquac. Res. 2017, 48, 5373–5384. [Google Scholar] [CrossRef]
- Fan, W.; Sun, J.; Chen, Y.; Qiu, J.; Zhang, Y.; Chi, Y. Effects of chitosan coating on quality and shelf life of silver carp during frozen storage. Food Chem. 2009, 115, 66–70. [Google Scholar] [CrossRef]
- Farajzadeh, F.; Motamedzadegan, A.; Shahidi, S.-A.; Hamzeh, S. The effect of chitosan-gelatin coating on the quality of shrimp (Litopenaeus vannamei) under refrigerated condition. Food Control 2016, 67, 163–170. [Google Scholar] [CrossRef]
- Mohan, C.; Ravishankar, C.; Lalitha, K.; Gopal, T.S. Effect of chitosan edible coating on the quality of double filleted Indian oil sardine (Sardinella longiceps) during chilled storage. Food Hydrocoll. 2012, 26, 167–174. [Google Scholar] [CrossRef]
- Bruni, L.; Belghit, I.; Lock, E.J.; Secci, G.; Taiti, C.; Parisi, G. Total replacement of dietary fish meal with black soldier fly (Hermetia illucens) larvae does not impair physical, chemical or volatile composition of farmed Atlantic salmon (Salmo salar L.). J. Sci. Food Agric. 2020, 100, 1038–1047. [Google Scholar] [CrossRef]
- Johnsen, C.A.; Hagen, Ø.; Adler, M.; Jönsson, E.; Kling, P.; Bickerdike, R.; Solberg, C.; Björnsson, B.T.; Bendiksen, E.Å. Effects of feed, feeding regime and growth rate on flesh quality, connective tissue and plasma hormones in farmed Atlantic salmon (Salmo salar L.). Aquaculture 2011, 318, 343–354. [Google Scholar] [CrossRef]
- Rasmussen, R.S. Quality of farmed salmonids with emphasis on proximate composition, yield and sensory characteristics. Aquac. Res. 2001, 32, 767–786. [Google Scholar] [CrossRef]
- Cai, W.C.; Jiang, G.Z.; Li, X.F.; Sun, C.X.; Mi, H.F.; Liu, S.Q.; Liu, W.B. Effects of complete fish meal replacement by rice protein concentrate with or without lysine supplement on growth performance, muscle development and flesh quality of blunt snout bream (Megalobrama amblycephala). Aquac. Nutr. 2018, 24, 481–491. [Google Scholar] [CrossRef]
- Moreno, H.; Montero, M.; Gómez-Guillén, M.; Fernández-Martín, F.; Mørkøre, T.; Borderías, J. Collagen characteristics of farmed Atlantic salmon with firm and soft fillet texture. Food Chem. 2012, 134, 678–685. [Google Scholar] [CrossRef]
- Wu, F.; Wen, H.; Tian, J.; Jiang, M.; Liu, W.; Yang, C.; Yu, L.; Lu, X. Effect of stocking density on growth performance, serum biochemical parameters, and muscle texture properties of genetically improved farm tilapia, Oreochromis niloticus. Aquac. Int. 2018, 26, 1247–1259. [Google Scholar] [CrossRef]
- Andersen, U.B.; Thomassen, M.S.; Rørå, A.M.B. Texture properties of farmed rainbow trout (Oncorhynchus mykiss): Effects of diet, muscle fat content and time of storage on ice. J. Sci. Food Agric. 1997, 74, 347–353. [Google Scholar] [CrossRef]
- Carbonell, I.; Duran, L.; Izquierdo, L.; Costell, E. Texture of cultured gilthead sea bream (Sparus aurata): Instrumental and sensory measurement. J. Texture Stud. 2003, 34, 203–217. [Google Scholar] [CrossRef]
- Mørkøre, T.; Hansen, A.; Unander, E.; Einen, O. Composition, liquid leakage, and mechanical properties of farmed rainbow trout: Variation between fillet sections and the impact of ice and frozen storage. J. Food Sci. 2002, 67, 1933–1938. [Google Scholar] [CrossRef]
- Trullàs, C.; Tres, A.; Saldo, J.; Fontanillas, R.; Sala, R. Quality characteristics of fillets of rainbow trout fed acid or re-esterified rapeseed oils as dietary fat sources. Aquaculture 2017, 480, 22–31. [Google Scholar] [CrossRef]
- Ando, M.; Yoshimoto, Y.; Inabu, K.; Nakagawa, T.; Makinodan, Y. Post-mortem change of three-dimensional structure of collagen fibrillar network in fish muscle pericellular connective tissues corresponding to post-mortem tenderization. Fish. Sci. 1995, 61, 327–330. [Google Scholar] [CrossRef]
- Taylor, R.; Fjaera, S.; Skjervold, P. Salmon fillet texture is determined by myofiber-myofiber and myofiber-myocommata attachment. J. Food Sci. 2002, 67, 2067–2071. [Google Scholar] [CrossRef]
- Taylor, R.G.; Geesink, G.H.; Thompson, V.F.; Koohmaraie, M.; Goll, D.E. Is Z-disk degradation responsible for postmortem tenderization? J. Anim. Sci. 1995, 73, 1351–1367. [Google Scholar] [CrossRef]
- Ayala, M.D.; Abdel, I.; Santaella, M.; Martínez, C.; Periago, M.J.; Gil, F.; Blanco, A.; Albors, O.L. Muscle tissue structural changes and texture development in sea bream, Sparus aurata L., during post-mortem storage. LWT-Food Sci. Technol. 2010, 43, 465–475. [Google Scholar] [CrossRef]
- Caballero, M.; Betancor, M.; Escrig, J.; Montero, D.; De Los Monteros, A.E.; Castro, P.; Ginés, R.; Izquierdo, M. Post mortem changes produced in the muscle of sea bream (Sparus aurata) during ice storage. Aquaculture 2009, 291, 210–216. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, Y.; Tang, T.; Zhong, L.; Chu, W.; Dai, Z.; Chen, K.; Hu, Y. Effect of partial black soldier fly (Hermetia illucens L.) larvae meal replacement of fish meal in practical diets on the growth, digestive enzyme and related gene expression for rice field eel (Monopterus albus). Aquac. Rep. 2020, 17, 100345. [Google Scholar] [CrossRef]
- Pati, S.; Chatterji, A.; Dash, B.P.; Raveen Nelson, B.; Sarkar, T.; Shahimi, S.; Atan Edinur, H.; Binti Abd Manan, T.S.; Jena, P.; Mohanta, Y.K. Structural Characterization and Antioxidant Potential of Chitosan by γ-Irradiation from the Carapace of Horseshoe Crab. Polymers 2020, 12, 2361. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as a preservative for fruits and vegetables: A review on chemistry and antimicrobial properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Hafsa, J.; Smach, M.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Antioxidant and antimicrobial proprieties of chitin and chitosan extracted from Parapenaeus Longirostris shrimp shell waste. Ann. Pharm. Fr. 2016, 74, 27–33. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Samar, M.M.; El-Kalyoubi, M.; Khalaf, M.; Abd El-Razik, M. Physicochemical, functional, antioxidant and antibacterial properties of chitosan extracted from shrimp wastes by microwave technique. Ann. Agric. Sci. 2013, 58, 33–41. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Yao, H. Comprehensive resource utilization of waste using the black soldier fly (Hermetia illucens (L.))(Diptera: Stratiomyidae). Animals 2019, 9, 349. [Google Scholar] [CrossRef]
- No, H.; Meyers, S.P.; Prinyawiwatkul, W.; Xu, Z. Applications of chitosan for improvement of quality and shelf life of foods: A review. J. Food Sci. 2007, 72, R87–R100. [Google Scholar] [CrossRef]
- Riaz Rajoka, M.S.; Mehwish, H.M.; Wu, Y.; Zhao, L.; Arfat, Y.; Majeed, K.; Anwaar, S. Chitin/chitosan derivatives and their interactions with microorganisms: A comprehensive review and future perspectives. Crit. Rev. Biotechnol. 2020, 40, 365–379. [Google Scholar] [CrossRef]
- Sahariah, P.; Masson, M. Antimicrobial chitosan and chitosan derivatives: A review of the structure–activity relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Dabbou, S.; Ferrocino, I.; Gasco, L.; Schiavone, A.; Trocino, A.; Xiccato, G.; Barroeta, A.C.; Maione, S.; Soglia, D.; Biasato, I. Antimicrobial effects of black soldier fly and yellow mealworm fats and their impact on gut microbiota of growing rabbits. Animals 2020, 10, 1292. [Google Scholar] [CrossRef]
- Saviane, A.; Tassoni, L.; Naviglio, D.; Lupi, D.; Savoldelli, S.; Bianchi, G.; Cortellino, G.; Bondioli, P.; Folegatti, L.; Casartelli, M. Mechanical Processing of Hermetia illucens Larvae and Bombyx mori Pupae Produces Oils with Antimicrobial Activity. Animals 2021, 11, 783. [Google Scholar] [CrossRef]
- Kotzamanis, Y.; Tsironi, T.; Brezas, A.; Grigorakis, K.; Ilia, V.; Vatsos, I.; Romano, N.; van Eys, J.; Kumar, V. High taurine supplementation in plant protein-based diets improves growth and organoleptic characteristics of European seabass (Dicentrarchus labrax). Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Østbye, T.K.; Ruyter, B.; Standal, I.B.; Stien, L.H.; Bahuaud, D.; Dessen, J.E.; Latif, M.S.; Fyhn-Terjesen, B.; Rørvik, K.A.; Mørkøre, T. Functional amino acids stimulate muscle development and improve fillet texture of Atlantic salmon. Aquac. Nutr. 2018, 24, 14–26. [Google Scholar] [CrossRef]
- Kragten, S.A.; Bee, G. Drip loss determination in pork chops with NIR. In Proceedings of the NIR on the GO 2010, Padova, Italy, 27–28 May 2010; p. 56. [Google Scholar]
- Truong, B.Q. High Pressure Processing of Barramundi Fish (Lates calcarifer). PhD Thesis, The University of Newcastle, Callaghan, Australia, 2017. [Google Scholar]
- Halliwell, B.; Gutteridge, J. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Morrissey, P.; Kerry, J. Lipid oxidation and the shelf-life of muscle foods. In Understanding and Measuring the Shelf-Life of Food; 2004; p. 357. Available online: https://books.google.co.uk/books?hl=en&lr=lang_en&id=bdnfe_Q5UAMC&oi=fnd&pg=PA357&dq=Lipid+oxidation+and+the+shelf-life+of.+&ots=RwJL7Uy-nR&sig=0FmECkhbxk7GpgxbaxuSRqN6rOk#v=onepage&q=Lipid%20oxidation%20and%20the%20shelf-life%20of.&f=false (accessed on 23 November 2022).
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Einen, O.; Guerin, T.; Fjæra, S.O.; Skjervold, P.O. Freezing of pre-rigor fillets of Atlantic salmon. Aquaculture 2002, 212, 129–140. [Google Scholar] [CrossRef]
- Kaale, L.D.; Eikevik, T.M. The influence of superchilling storage methods on the location/distribution of ice crystals during storage of Atlantic salmon (Salmo salar). Food Control 2015, 52, 19–26. [Google Scholar] [CrossRef]
- Duun, A.; Rustad, T. Quality of superchilled vacuum packed Atlantic salmon (Salmo salar) fillets stored at− 1.4 and− 3.6 C. Food Chem. 2008, 106, 122–131. [Google Scholar] [CrossRef]
- Cooper, M.; Midling, K.Ø. Blood vessel melanosis: Howieson, J.; Glencross, B.; Little, S.; Bourne, N.; Aris, A.; Partridge, G.J.; Paton, N.; Tonkin, R.; Allan, D.; Wilkinson, R.; et al. Understanding and Minimising “Greying” of Farmed Barramundi Fillets. Bedford park: The Australian Seafood Cooperative Research Centre. 2013. Available online: https://www.seafoodcrc.com/barramundi/finfish/barramundi/2011-721-understanding-and-minimising-greying-of-farmed-barramundi-fillets.html (accessed on 5 July 2021).
- A physiological detoxification mechanism in Atlantic cod (Gadus morhua). Aquac. Int. 2007, 15, 43–54. [CrossRef]
- Cooper, M.; Olsen, R.L.; Seliussen, J.; Gannefors, C. Dietary Trace Metal Supplements Promote Blood Vessel Melanosis in Fillets of Juvenile Farmed Atlantic Cod, Gadus morhua L. J. World Aquac. Soc. 2011, 42, 222–229. [Google Scholar] [CrossRef]
- Valente, L.; Cornet, J.; Donnay-Moreno, C.; Gouygou, J.-P.; Bergé, J.-P.; Bacelar, M.; Escórcio, C.; Rocha, E.; Malhão, F.; Cardinal, M. Quality differences of gilthead sea bream from distinct production systems in Southern Europe: Intensive, integrated, semi-intensive or extensive systems. Food Control 2011, 22, 708–717. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biol. 2002, 21, 269–285. [Google Scholar] [CrossRef]
- Iaconisi, V.; Bonelli, A.; Pupino, R.; Gai, F.; Parisi, G. Mealworm as dietary protein source for rainbow trout: Body and fillet quality traits. Aquaculture 2018, 484, 197–204. [Google Scholar] [CrossRef]
- Bruni, L.; Randazzo, B.; Cardinaletti, G.; Zarantoniello, M.; Mina, F.; Secci, G.; Tulli, F.; Olivotto, I.; Parisi, G. Dietary inclusion of full-fat Hermetia illucens prepupae meal in practical diets for rainbow trout (Oncorhynchus mykiss): Lipid metabolism and fillet quality investigations. Aquaculture 2020, 529, 735678. [Google Scholar] [CrossRef]
- Nyakeri, E.; Ogola, H.; Ayieko, M.; Amimo, F. An open system for farming black soldier fly larvae as a source of proteins for smallscale poultry and fish production. J. Insects Food Feed 2017, 3, 51–56. [Google Scholar] [CrossRef]
- Alexi, N.; Hvam, J.; Lund, B.W.; Nsubuga, L.; de Oliveira Hansen, R.M.; Thamsborg, K.; Lofink, F.; Byrne, D.V.; Leisner, J.J. Potential of novel cadaverine biosensor technology to predict shelf life of chilled yellowfin tuna (Thunnus albacares). Food Control 2021, 119, 107458. [Google Scholar] [CrossRef]
- Boziaris, I.S. Seafood processing: Technology, Quality and Safety; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Calanche, J.; Tomas, A.; Martinez, S.; Jover, M.; Alonso, V.; Roncalés, P.; Beltrán, J.A. Relation of quality and sensory perception with changes in free amino acids of thawed seabream (Sparus aurata). Food Res. Int. 2019, 119, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Luten, J.; Martinsdottir, E. QIM: A European tool for fish freshness evaluation in the fishery chain. In Proceedings of the Methods to Determine the Freshness of Fish in Research and Industry: Proceedings of the Final Meeting of the Concerted Action’Evaluation of Fish Freshness’ AIR3CT94 2283, Nantes, France, 12–14 November l997.
- Martinsdóttir, E.; Sveinsdottir, K.; Luten, J.; Schelvis-Smit, R.; Hyldig, G. Reference Manual for the Fish Sector: Sensory Evaluation of Fish Freshness; QIM Eurofish: Jmuiden, The Netherlands, 2001. [Google Scholar]
- Mausse, E.C.J.; Valdimarsdottir, T.; Sveinsdottir, K. Shelf life of red fish stored in ice and modified atmosphere (MA) and some aspects on the development of a Quality Index Method (QIM) scheme for red fish stored in MA. In AD-CACS, Training Programme; The United Nations University: Reykjavik, Iceland, 2000. [Google Scholar]
- Chow, C.-J.; Yang, J.-I.; Lee, P.-F.; Ochiai, Y. Effects of acid and alkaline pretreatment on the discoloration rates of dark muscle and myoglobin extract of skinned tilapia fillet during iced storage. Fish. Sci. 2009, 75, 1481–1488. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Gao, Y.; Ye, C.; Wang, H. Effect of collagen-lysozyme coating on fresh-salmon fillets preservation. LWT 2017, 75, 59–64. [Google Scholar] [CrossRef]
- do Vale, D.A.; Vieira, C.B.; de Oliveria, J.M.; Vidal, M.F.; de Alcântara, L.O.; da Silva, A.I.M.; de Lima Silva, J.M.; Andrade, F.K.; Sousa, J.R.; Souza Filho, M.d.S.M. Determining the wetting capacity of the chitosan coatings from Ucides cordatus and evaluating the shelf-life quality of Scomberomorus brasiliensis fillets. Food Control 2020, 116, 107329. [Google Scholar] [CrossRef]
- Morachis-Valdez, A.G.; Gómez-Oliván, L.M.; García-Argueta, I.; Hernández-Navarro, M.D.; Díaz-Bandera, D.; Dublán-García, O. Effect of chitosan edible coating on the biochemical and physical characteristics of carp fillet (Cyprinus carpio) stored at− 18 °C. Int. J. Food Sci. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Fernández-Segovia, I.; Fuentes, A.; Aliño, M.; Masot, R.; Alcañiz, M.; Barat, J.M. Detection of frozen-thawed salmon (Salmo salar) by a rapid low-cost method. J. Food Eng. 2012, 113, 210–216. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free. Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.; Newsham, P. Lipids, Oils, Fats, and Extracts. In Food Science and the Culinary Arts; Academic Press: London, UK, 2018; pp. 323–340. [Google Scholar]
- Alak, G. The effect of chitosan prepared in different solvents on the quality parameters of brown trout fillets (Salmo trutta fario). Food Nutr. Sci. 2012, 3, 1303. [Google Scholar]
- Baptista, R.C.; Horita, C.N.; Sant’Ana, A.S. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: A review. Food Res. Int. 2020, 127, 108762. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Kamil, J.; Jeon, Y.J.; Kim, S.K. Antioxidant role of chitosan in a cooked cod (Gadus morhua) model system. J. Food Lipids 2002, 9, 57–64. [Google Scholar] [CrossRef]
- Öz, M. Effects of garlic (Allium sativum) supplemented fish diet on sensory, chemical and microbiological properties of rainbow trout during storage at −18 °C. LWT 2018, 92, 155–160. [Google Scholar] [CrossRef]
- Varlik, C. Su urunlerinde kalite kontrol ilke ve yontemleri. Gida Teknol. Dern. 1993, 17, 16–17. [Google Scholar]
| Ingredients (g/100 g) | 0PBM-0HI | 70PBM-30HI | 70PBM-30DHI | PBM * | FHI * | DHI |
|---|---|---|---|---|---|---|
| FM | 72.00 | 0.00 | 0.00 | - | - | - |
| PBM | 0.00 | 50.50 | 50.50 | - | - | - |
| Canola oil | 1.00 | 0.50 | 0.50 | - | - | - |
| Full-fat HI | 0.00 | 35.00 | 0.00 | - | - | - |
| Defatted HI | 0.00 | 0.00 | 27.83 | - | - | - |
| Corn/wheat starch | 7.00 | 5.90 | 11.00 | - | - | - |
| Lecithin-Soy (70%) | 1.00 | 2.00 | 1.00 | - | - | - |
| Vitamin C | 0.05 | 0.05 | 0.05 | - | - | - |
| Dicalcium Phosphate | 0.05 | 0.05 | 0.05 | - | - | - |
| Wheat (10 CP) | 16.90 | 4.00 | 7.07 | - | - | - |
| Vitamin and mineral premix | 0.50 | 0.50 | 0.50 | - | - | - |
| Salt (NaCl) | 1.00 | 1.00 | 1.00 | - | - | - |
| Cod liver oil | 0.50 | 0.50 | 0.50 | - | - | - |
| Nutritional Composition (%) | ||||||
| Dry matter | 89.95 | 90.21 | 90.16 | - | - | - |
| Crude protein | 47.88 | 47.94 | 48.06 | - | - | - |
| Crude lipid | 12.59 | 13.61 | 13.96 | - | - | - |
| Ash | 11.23 | 11.69 | 11.51 | - | - | - |
| Essential amino acid (% of total amino acid) | ||||||
| Arginine | 6.40 | 6.66 | 6.64 | 7.32 | 5.45 | 5.31 |
| Histidine | 3.24 | 2.52 | 2.52 | 2.96 | 3.27 | 3.17 |
| Threonine | 4.71 | 4.16 | 4.18 | 4.19 | 4.35 | 4.39 |
| Lysine | 7.50 | 6.64 | 6.57 | 6.58 | 7.10 | 6.78 |
| Methionine | 2.88 | 2.10 | 2.07 | 2.23 | 2.02 | 1.97 |
| Valine | 5.64 | 5.63 | 5.62 | 4.92 | 6.40 | 6.70 |
| Isoleucine | 4.91 | 4.51 | 4.47 | 4.07 | 4.86 | 5.03 |
| Leucine | 8.16 | 7.52 | 7.44 | 7.37 | 7.59 | 7.63 |
| Phenylalanine | 4.55 | 4.36 | 4.33 | 4.09 | 4.67 | 4.88 |
| Non-Essential amino acid (% of total amino acid) | ||||||
| Serine | 4.39 | 4.27 | 4.30 | 4.34 | 4.48 | 4.43 |
| Glycine | 7.96 | 9.62 | 9.79 | 10.13 | 5.86 | 6.01 |
| Aspartic acid | 9.57 | 9.41 | 9.21 | 8.50 | 10.42 | 10.93 |
| Glutamic acid | 14.42 | 14.73 | 14.94 | 13.83 | 13.42 | 13.46 |
| Alanine | 7.10 | 7.28 | 7.36 | 6.61 | 6.59 | 7.06 |
| Proline | 5.65 | 6.87 | 7.01 | 6.63 | 6.24 | 6.22 |
| Tyrosine | 2.92 | 3.71 | 3.55 | 2.97 | 5.97 | 6.05 |
| 0PBM-0HI | 70PBM-30HI | 70PBM-30DHI | p-Value | |
|---|---|---|---|---|
| Proximate composition (%, Wet basis) | ||||
| Moisture | 76.10 ± 0.25 | 76.71 ± 0.42 | 76.70 ± 0.41 | 0.46 |
| Crude protein | 20.58 ± 0.56 | 19.82 ± 0.03 | 20.33 ± 0.30 | 0.38 |
| Crude lipid | 1.63 ± 0.15 | 1.90 ± 0.30 | 1.69 ± 0.36 | 0.79 |
| Ash | 1.10 ± 0.07 | 1.10 ± 0.02 | 1.19 ± 0.03 | 0.35 |
| Essential amino acids (% of total amino acids) | ||||
| Arginine | 6.30 ± 0.02 | 6.32 ± 0.03 | 6.33 ± 0.03 | 0.48 |
| Histidine | 2.40 ± 0.04 a | 2.28 ± 0.02 b | 2.28 ± 0.02 b | 0.00 |
| Threonine | 4.65 ± 0.01 | 4.61 ± 0.03 | 4.61 ± 0.02 | 0.11 |
| Lysine | 9.71 ± 0.02 a | 9.71 ± 0.06 a | 9.49 ± 0.13 b | 0.03 |
| Methionine | 3.22 ± 0.03 | 3.19 ± 0.03 | 3.17 ± 0.02 | 0.08 |
| Valine | 5.37 ± 0.02 | 5.31 ± 0.02 | 5.34 ± 0.02 | 0.19 |
| Isoleucine | 5.12 ± 0.03 | 5.06 ± 0.04 | 5.06 ± 0.06 | 0.25 |
| Leucine | 8.49 ± 0.03 | 8.46 ± 0.04 | 8.43 ± 0.06 | 0.68 |
| Phenylalanine | 4.62 ± 0.03 | 4.64 ± 0.02 | 4.64 ± 0.03 | 0.79 |
| Non-essential amino acids (% of total amino acids) | ||||
| Serine | 4.28 ± 0.07 a | 4.15 ± 0.03 b | 4.13 ± 0.02 b | 0.01 |
| Glycine | 6.12 ± 0.15 | 6.22 ± 0.30 | 6.46 ± 0.21 | 0.24 |
| Aspartic acid | 10.44 ± 0.08 | 10.55 ± 0.05 | 10.52 ± 0.17 | 0.45 |
| Glutamic acid | 15.97 ± 0.07 | 16.12 ± 0.08 | 15.98 ± 0.10 | 0.14 |
| Alanine | 6.40 ± 0.05 | 6.45 ± 0.07 | 6.52 ± 0.06 | 0.09 |
| Proline | 3.58 ± 0.05 | 3.60 ± 0.10 | 3.72 ± 0.08 | 0.15 |
| Tyrosine | 3.35 ± 0.01 | 3.33 ± 0.06 | 3.33 ± 0.02 | 0.80 |
| 0PBM-0HI | 70PBM-30FHI | 70PBM-30DHI | p-Value | |
|---|---|---|---|---|
| C12:0 | 1.39 ± 0.85 c | 10.29 ± 0.15 a | 6.52 ± 0.35 b | 0.00 |
| C14:0 | 2.30 ± 0.15 c | 3.76 ± 0.03 a | 3.00 ± 0.15 b | 0.00 |
| C16:0 | 19.26 ± 0.24 a | 18.09 ± 0.07 b | 18.33 ± 0.18 b | 0.01 |
| C16:1 n7 | 3.35 ± 0.15 b | 4.17 ± 0.03 a | 4.13 ± 0.17 a | 0.01 |
| C18:1 cis+trans | 25.53 ± 1.56 b | 31.01 ± 0.06 a | 34.57 ± 0.63 a | 0.00 |
| C18:2 cis | 9.59 ± 0.80 b | 14.75 ± 0.07 a | 14.40 ± 0.25 a | 0.00 |
| C18:3 n6 | 0.42 ± 0.09 b | 0.76 ± 0.03 a | 0.99 ± 0.03 a | 0.00 |
| C18:3 n3 | 2.00 ± 0.10 b | 2.90 ± 0.00 a | 2.69 ± 0.06 a | 0.00 |
| C18:4 n3 | 0.41 ± 0.00 b | 0.58 ± 0.00 a | 0.49 ± 0.03 b | 0.00 |
| C20:3 n6 | 0.36 ± 0.03 b | 0.42 ± 0.00 b | 0.66 ± 0.09 a | 0.01 |
| C20:4 n6 | 1.93 ± 0.07 a | 1.47 ± 0.03 a | 1.57 ± 0.23 a | 0.00 |
| C20:5 n3 | 2.51 ± 0.20 a | 1.37 ± 0.00 b | 1.31 ± 0.10 b | 0.00 |
| C22:4 n6 | 1.58 ± 0.25 a | 0.16 ± 0.00 b | 0.14 ± 0.03 b | 0.00 |
| C22:5 n3 | 1.71 ± 0.10 a | 0.94 ± 0.03 b | 1.07 ± 0.12 b | 0.00 |
| C22:6 n3 | 17.38 ± 2.65 a | 1.39 ± 0.00 b | 1.74 ± 0.22 b | 0.00 |
| ∑SFA | 31.20 ± 0.56 b | 38.67 ± 0.09 a | 34.77 ± 0.09 a | 0.00 |
| ∑MUFA | 30.57 ± 1.65 b | 36.27 ± 0.09 a | 39.83 ± 0.84 a | 0.00 |
| ∑PUFA | 38.23 ± 2.18 a | 25.07 ± 0.12 b | 25.33 ± 0.91 b | 0.00 |
| ∑n-3PUFA | 24.10 ± 2.81 a | 7.27 ± 0.07 b | 7.37 ± 0.37 b | 0.00 |
| ∑n-6PUFA | 13.88 ± 0.19 a | 17.56 ± 0.98 a | 17.76 ± 0.29 a | 0.06 |
| ∑n-3/n-6PUFA | 5.58 ± 0.43 a | 2.59 ± 2.58 b | 2.21 ± 2.20 b | 0.00 |
| Sensory Parameters | 0PBM-0HI | 70PBM-30FHI | 70PBM-30DHI | p-Value |
|---|---|---|---|---|
| Raw visual appearance | 3.64 ± 0.41 b | 5.88 ± 0.46 a | 6.20 ± 0.58 a | 0.00 |
| Raw odour | 3.56 ± 0.29 b | 5.90 ± 0.63 a | 5.89 ± 0.55 a | 0.00 |
| Raw overall quality | 3.67 ± 0.37 b | 5.57 ± 0.55 ab | 6.10 ± 0.67 a | 0.01 |
| Cooked visual appearance | 3.93 ± 0.58 | 4.80 ± 0.76 | 4.66 ± 0.70 | 0.65 |
| Cooked odour | 3.55 ± 0.36 b | 6.21 ± 0.68 a | 5.71 ± 0.39 a | 0.00 |
| Cooked texture | 4.90 ± 0.61 | 6.05 ± 0.60 | 6.33 ± 0.63 | 0.24 |
| Cooked taste | 3.99 ± 0.76 | 5.42 ± 0.80 | 6.33 ± 0.88 | 0.15 |
| Cooked overall quality | 3.48 ± 0.72 | 5.43 ± 0.79 | 6.02 ± 0.82 | 0.07 |
| Test Diets | Two-Way ANOVA | ||||||
|---|---|---|---|---|---|---|---|
| 0PBM-0HI | 70PBM-30FHI | 70PBM-30DHI | D | T | D × T | ||
| Skin brightness | Day 1 | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 0.00 ± 0.00 B | |||
| Day 4 | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 0.33 | 0.00 | 0.34 | |
| Day 8 | 1.33 ± 0.21 A | 1.00 ± 0.00 A | 1.17 ± 0.17 A | ||||
| Appearance transparency | Day 1 | 0.00 ± 0.00 C | 0.00 ± 0.00 C | 0.00 ± 0.00 C | |||
| Day 4 | 0.50 ± 0.22 B | 0.33 ± 0.21 B | 0.50 ± 0.22 B | 0.83 | 0.00 | 0.94 | |
| Day 8 | 1.00 ± 0.00 A | 1.00 ± 0.00 A | 1.00 ± 0.00 A | ||||
| Flesh texture | Day 1 | 0.17 ± 0.17 B | 0.00 ± 0.00 B | 0.17 ± 0.17 B | |||
| Day 4 | 0.83 ± 0.17 A | 0.83 ± 0.17 A | 0.67 ± 0.21 A | 0.83 | 0.00 | 0.77 | |
| Day 8 | 1.00 ± 0.00 A | 1.00 ± 0.00 A | 1.00 ± 0.00 A | ||||
| Flesh blood | Day 1 | 0.17 ± 0.16 C | 0.00 ± 0.00 C | 0.00 ± 0.00 C | |||
| Day 4 | 1.00 ± 0.00 B | 1.50 ± 0.10 B | 1.17 ± 0.13 B | 0.22 | 0.00 | 0.20 | |
| Day 8 | 1.83 ± 0.15 A | 2.00 ± 0.21 A | 1.83 ± 0.14 A | ||||
| Flesh odour | Day 1 | 0.00 ± 0.00 C | 0.00 ± 0.00 C | 0.00 ± 0.00 C | |||
| Day 4 | 1.00 ± 0.00 B | 1.00 ± 0.22 B | 1.00 ± 0.17 B | 0.38 | 0.00 | 0.42 | |
| Day 8 | 1.83 ± 0.17 A | 2.00 ± 0.00 A | 2.00 ± 0.00 A | ||||
| Flesh gaping | Day 1 | 1.50 ± 0.22 B | 1.67 ± 0.21 B | 1.17 ± 0.40 B | |||
| Day 4 | 2.00 ± 0.00 A | 1.67 ± 0.21 A | 1.83 ± 0.41 A | 0.56 | 0.00 | 0.43 | |
| Day 8 | 2.00 ± 0.00 A | 2.00 ± 0.00 A | 2.00 ± 0.00 A | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaklader, M.R.; Chung, W.H.; Howieson, J.; Fotedar, R. A Mixture of Full-Fat and Defatted Hermetia illucens Larvae and Poultry By-Products as Sustainable Protein Sources Improved Fillet Quality Traits in Farmed Barramundi, Lates calcarifer. Foods 2023, 12, 362. https://doi.org/10.3390/foods12020362
Chaklader MR, Chung WH, Howieson J, Fotedar R. A Mixture of Full-Fat and Defatted Hermetia illucens Larvae and Poultry By-Products as Sustainable Protein Sources Improved Fillet Quality Traits in Farmed Barramundi, Lates calcarifer. Foods. 2023; 12(2):362. https://doi.org/10.3390/foods12020362
Chicago/Turabian StyleChaklader, Md Reaz, Wing H. Chung, Janet Howieson, and Ravi Fotedar. 2023. "A Mixture of Full-Fat and Defatted Hermetia illucens Larvae and Poultry By-Products as Sustainable Protein Sources Improved Fillet Quality Traits in Farmed Barramundi, Lates calcarifer" Foods 12, no. 2: 362. https://doi.org/10.3390/foods12020362
APA StyleChaklader, M. R., Chung, W. H., Howieson, J., & Fotedar, R. (2023). A Mixture of Full-Fat and Defatted Hermetia illucens Larvae and Poultry By-Products as Sustainable Protein Sources Improved Fillet Quality Traits in Farmed Barramundi, Lates calcarifer. Foods, 12(2), 362. https://doi.org/10.3390/foods12020362








