Recent Advances in Cellulose-Based Hydrogels: Food Applications
Abstract
1. Introduction
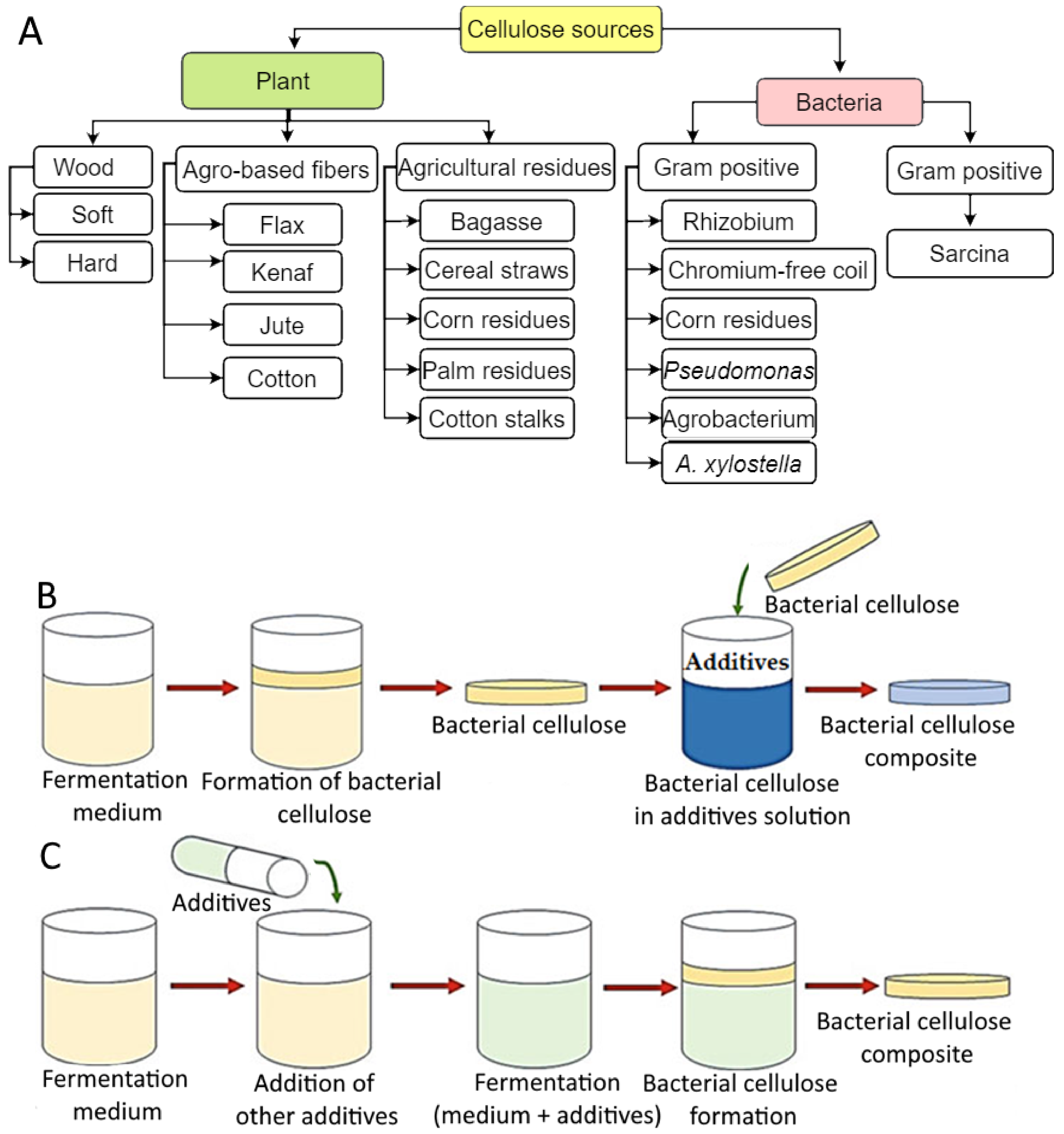
2. Sources of Cellulose-Based Hydrogels Production
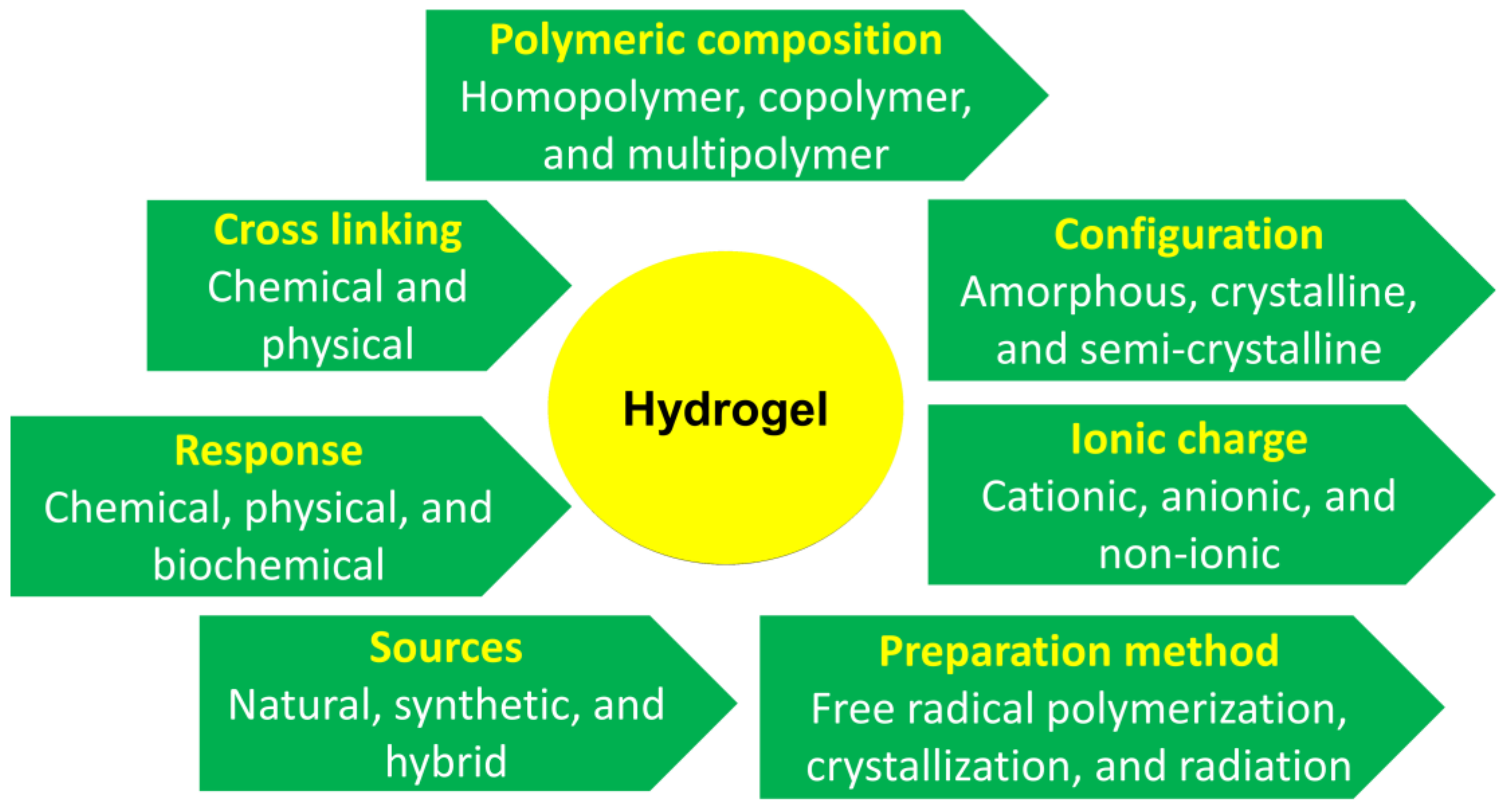
3. Different Types of Hydrogels
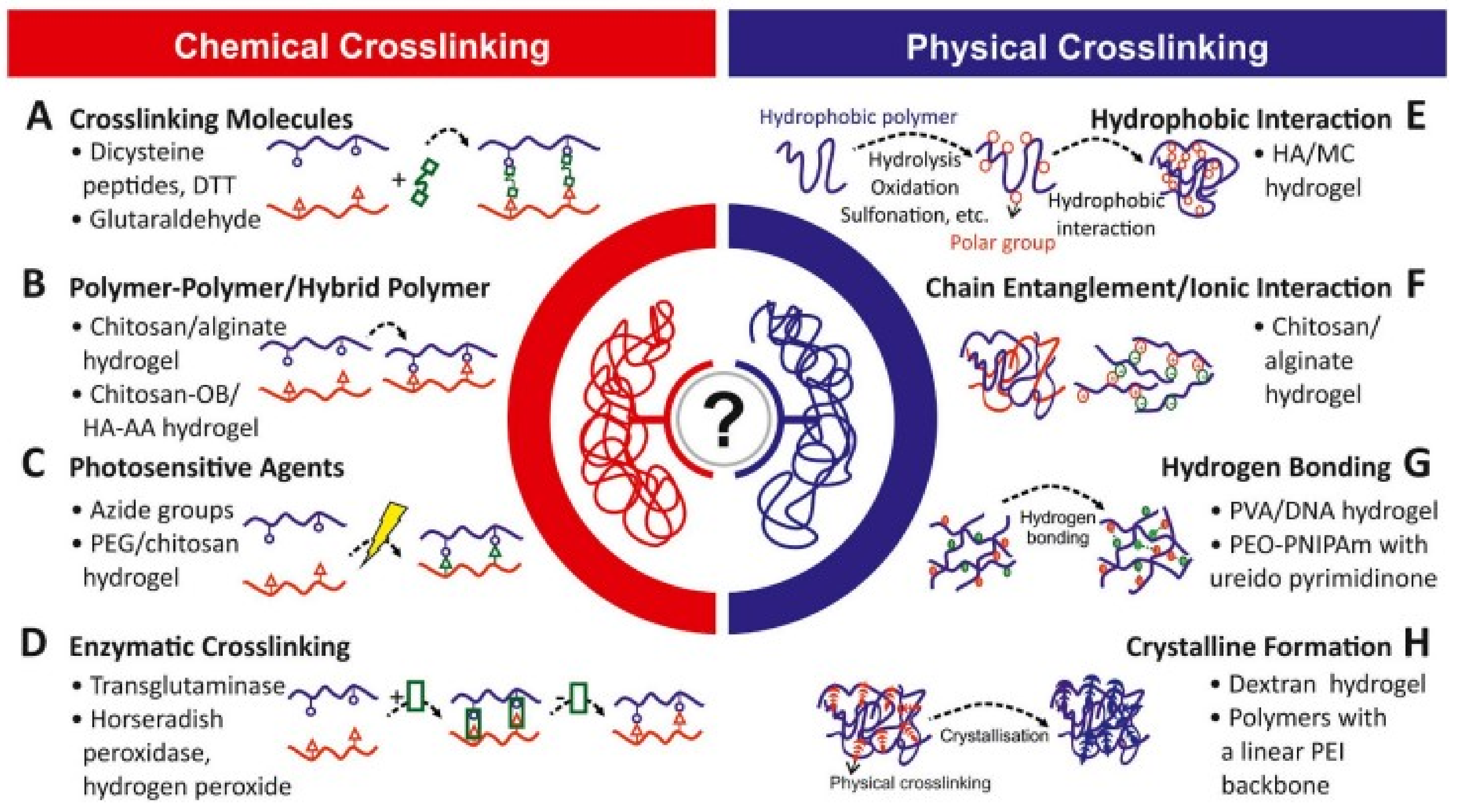
4. Crosslinking in Hydrogels
4.1. Physical Crosslinking
4.1.1. Crosslinking by Radical Polymerization
4.1.2. Crosslinking by Ionic Interactions
4.1.3. Crosslinking by Host-Guest Interactions
4.1.4. Crosslinking by Crystallization
4.1.5. Freeze–thaw Process, Hydrogen-Bonding, and Complex Coacervation
4.1.6. Maturation
4.2. Chemical Crosslinking
4.2.1. Citric Acid (CA)
4.2.2. Epichlorohydrin
4.2.3. Glutaraldehyde
4.2.4. Chemical Reaction of Complementary Groups
4.2.5. Enzyme Mediated Crosslinking
4.2.6. Disulfide Bonds
4.3. Polymerization Method
5. Cellulose Derivatives
5.1. Hydroxypropyl Methylcellulose (HPMC)
5.2. Ethyl Cellulose (EC)
5.3. Carboxymethyl Cellulose (CMC)
5.4. Nanocellulose (NC)
5.5. Cellulose Nitrate (CN)
5.6. Cellulose Sulphate (CS)
5.7. Cellulose Acetate (CA)
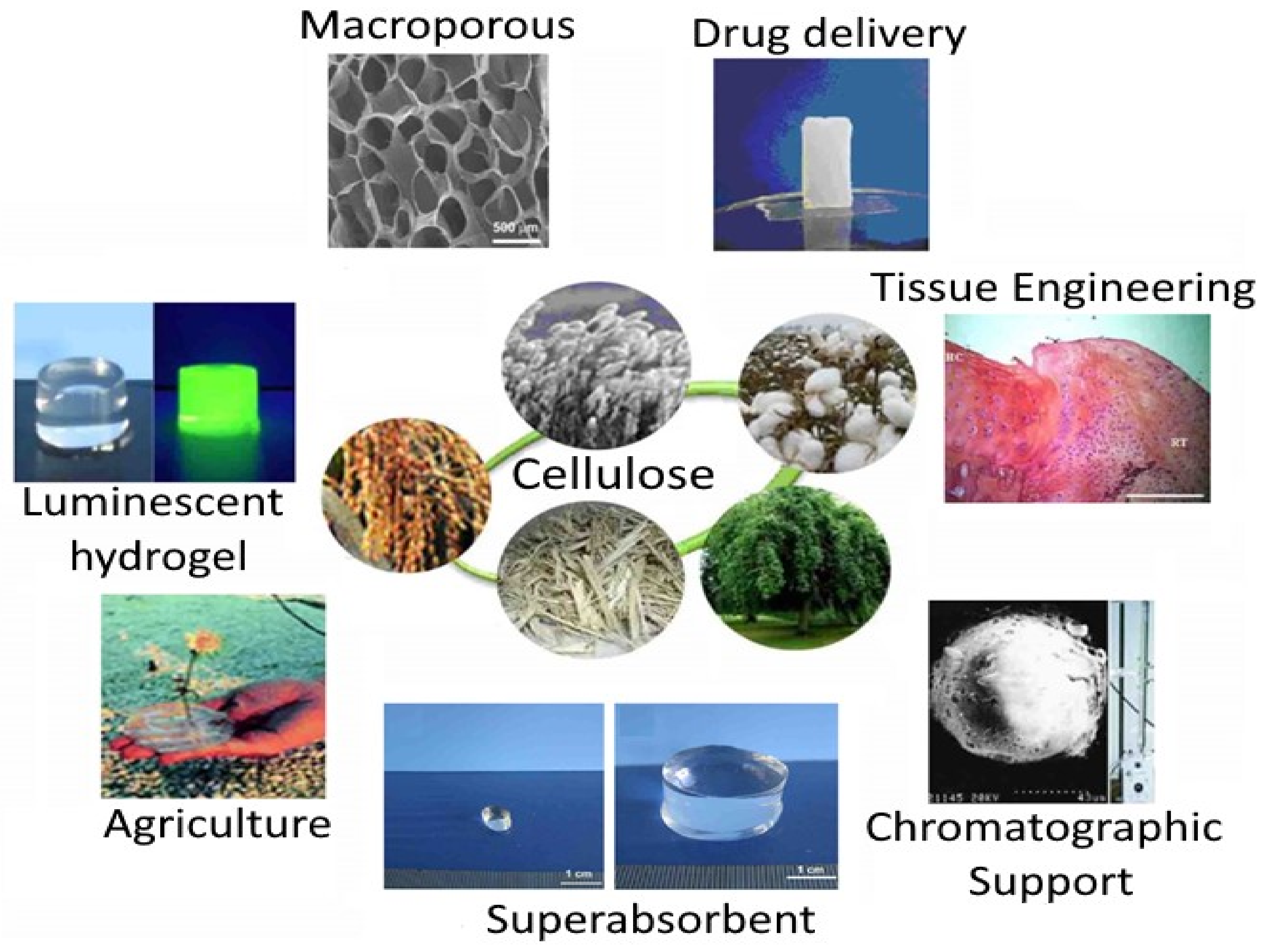
6. Potential Applications in the Food Industry
6.1. Food Biosensors
6.2. Hydrogels Based on Cellulose for the Industry of Food Processing
6.3. Food Packaging Industry
6.4. Hydrogels Derived from Cellulose for Use in Healthy Foods
6.4.1. Enzyme Immobilization
6.4.2. Encapsulation
6.5. Texture and Disease Control
6.6. Food Preservation
6.6.1. Fruits Preservation
6.6.2. Vegetable’s Preservation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nath, P.C.; Debnath, S.; Sridhar, K.; Inbaraj, B.S.; Nayak, P.K.; Sharma, M. A Comprehensive Review of Food Hydrogels: Principles, Formation Mechanisms, Microstructure, and Its Applications. Gels 2022, 9, 1. [Google Scholar] [CrossRef]
- Krop, E.M.; Hetherington, M.M.; Holmes, M.; Miquel, S.; Sarkar, A. On relating rheology and oral tribology to sensory properties in hydrogels. Food Hydrocoll. 2019, 88, 101–113. [Google Scholar] [CrossRef]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Wu, D.-Q.; Wang, T.; Lu, B.; Xu, X.-D.; Cheng, S.-X.; Jiang, X.-J.; Zhang, X.-Z.; Zhuo, R.-X. Fabrication of supramolecular hydrogels for drug delivery and stem cell encapsulation. Langmuir 2008, 24, 10306–10312. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, S.; Ren, X.; Zhang, J.; Lin, Q.; Zhao, Y. Supramolecular Adhesive Hydrogels for Tissue Engineering Applications. Chem. Rev. 2022, 122, 5604–5640. [Google Scholar] [CrossRef]
- Radulescu, D.-M.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. New insights of scaffolds based on hydrogels in tissue engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef]
- Yang, J.; Yu, H.; Wang, L.; Liu, J.; Liu, X.; Hong, Y.; Huang, Y.; Ren, S. Advances in adhesive hydrogels for tissue engineering. Eur. Polym. J. 2022, 172, 111241. [Google Scholar] [CrossRef]
- Li, M.; Chen, D.; Sun, X.; Xu, Z.; Yang, Y.; Song, Y.; Jiang, F. An environmentally tolerant, highly stable, cellulose nanofiber-reinforced, conductive hydrogel multifunctional sensor. Carbohydr. Polym. 2022, 284, 119199. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, J.; Xie, Y.; Gao, S.; Ling, Z.; Lai, C.; Wang, J.; Wang, C.; Chu, F.; Dumont, M.-J. Mimicking skin cellulose hydrogels for sensor applications. Chem. Eng. J. 2022, 427, 130921. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ihara, H.; Takafuji, M. Nanomaterial Hybridized Hydrogels as a Potential Adsorbent for Toxic Remediation of Substances from Wastewater. In Recent Trends in Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2022; pp. 365–393. [Google Scholar]
- Desai, D.T.; Maulvi, F.A.; Desai, A.R.; Shukla, M.R.; Desai, B.V.; Khadela, A.D.; Shetty, K.H.; Shah, D.O.; Willcox, M.D. In vitro and in vivo evaluation of cyclosporine-graphene oxide laden hydrogel contact lenses. Int. J. Pharm. 2022, 613, 121414. [Google Scholar] [CrossRef]
- Zhang, B.; Wong, P.W.; An, A.K. Photothermally enabled MXene hydrogel membrane with integrated solar-driven evaporation and photodegradation for efficient water purification. Chem. Eng. J. 2022, 430, 133054. [Google Scholar] [CrossRef]
- Nagahama, K.; Ouchi, T.; Ohya, Y. Temperature-induced hydrogels through self-assembly of cholesterol-substituted star PEG-b-PLLA copolymers: An injectable scaffold for tissue engineering. Adv. Funct. Mater. 2008, 18, 1220–1231. [Google Scholar] [CrossRef]
- Martens, P.J.; Bryant, S.J.; Anseth, K.S. Tailoring the degradation of hydrogels formed from multivinyl poly (ethylene glycol) and poly (vinyl alcohol) macromers for cartilage tissue engineering. Biomacromolecules 2003, 4, 283–292. [Google Scholar] [CrossRef]
- Ferruti, P.; Bianchi, S.; Ranucci, E.; Chiellini, F.; Piras, A.M. Novel agmatine-containing poly (amidoamine) hydrogels as scaffolds for tissue engineering. Biomacromolecules 2005, 6, 2229–2235. [Google Scholar] [CrossRef]
- Nayak, S.; Lee, H.; Chmielewski, J.; Lyon, L.A. Folate-mediated cell targeting and cytotoxicity using thermoresponsive microgels. J. Am. Chem. Soc. 2004, 126, 10258–10259. [Google Scholar] [CrossRef]
- Gao, D.; Xu, H.; Philbert, M.A.; Kopelman, R. Ultrafine hydrogel nanoparticles: Synthetic approach and therapeutic application in living cells. Angew. Chem. 2007, 119, 2274–2277. [Google Scholar] [CrossRef]
- Tomatsu, I.; Hashidzume, A.; Harada, A. Contrast viscosity changes upon photoirradiation for mixtures of poly (acrylic acid)-based α-cyclodextrin and azobenzene polymers. J. Am. Chem. Soc. 2006, 128, 2226–2227. [Google Scholar] [CrossRef]
- Kim, J.; Singh, N.; Lyon, L.A. Label-free biosensing with hydrogel microlenses. Angew. Chem. Int. Ed. 2006, 45, 1446–1449. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef]
- Shoichet, M.S. Polymer scaffolds for biomaterials applications. Macromolecules 2010, 43, 581–591. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Guillot, S.; Dabboue, H.; Tranchant, J.-F.; Salvetat, J.-P. Carbon nanotubes as structural nanofibers for hyaluronic acid hydrogel scaffolds. Biomacromolecules 2008, 9, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.; Whitney, R.A.; Neufeld, R.J. Semisynthesis of a controlled stimuli-responsive alginate hydrogel. Biomacromolecules 2009, 10, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Gattás-Asfura, K.M.; Weisman, E.; Andreopoulos, F.M.; Micic, M.; Muller, B.; Sirpal, S.; Pham, S.M.; Leblanc, R.M. Nitrocinnamate-functionalized gelatin: Synthesis and “smart” hydrogel formation via photo-cross-linking. Biomacromolecules 2005, 6, 1503–1509. [Google Scholar] [CrossRef]
- Zhou, J.; Chang, C.; Zhang, R.; Zhang, L. Hydrogels prepared from unsubstituted cellulose in NaOH/urea aqueous solution. Macromol. Biosci. 2007, 7, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.J.; Figueiredo, M.M.; Gil, M.H. Rheological study of genipin cross-linked chitosan hydrogels. Biomacromolecules 2007, 8, 3823–3829. [Google Scholar] [CrossRef]
- Qu, X.; Wirsen, A.; Albertsson, A.-C. Novel pH-sensitive chitosan hydrogels: Swelling behavior and states of water. Polymer 2000, 41, 4589–4598. [Google Scholar] [CrossRef]
- Vrana, N.E.; Liu, Y.; McGuinness, G.B.; Cahill, P.A. Characterization of poly (vinyl alcohol)/chitosan hydrogels as vascular tissue engineering scaffolds. Macromol. Symp. 2008, 269, 106–110. [Google Scholar] [CrossRef]
- Hinterstoisser, B.; Salmén, L. Application of dynamic 2D FTIR to cellulose. Vib. Spectrosc. 2000, 22, 111–118. [Google Scholar] [CrossRef]
- Bochek, A. Effect of hydrogen bonding on cellulose solubility in aqueous and nonaqueous solvents. Russ. J. Appl. Chem. 2003, 76, 1711–1719. [Google Scholar] [CrossRef]
- Myasoedova, V.V. Physical Chemistry of Non-aqueous Solutions of Cellulose and Its Derivatives; J. Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Gross, R.A.; Scholz, C. Biopolymers from Polysaccharides and Agroproteins; ACS Publications: Washington, DC, USA. 2001. [Google Scholar]
- Baek, S.; Kim, D.; Jeon, S.L.; Seo, J. Preparation and characterization of pH-responsive poly (N, N-dimethyl acrylamide-co-methacryloyl sulfadimethoxine) hydrogels for application as food freshness indicators. React. Funct. Polym. 2017, 120, 57–65. [Google Scholar] [CrossRef]
- de Oliveira, J.P.; Bruni, G.P.; Lima, K.O.; El Halal, S.L.M.; da Rosa, G.S.; Dias, A.R.G.; da Rosa Zavareze, E. Cellulose fibers extracted from rice and oat husks and their application in hydrogel. Food Chem. 2017, 221, 153–160. [Google Scholar] [CrossRef]
- Kopjar, M.; Ivić, I.; Vukoja, J.; Šimunović, J.; Pichler, A. Retention of linalool and eugenol in hydrogels. Int. J. Food Sci. Technol. 2020, 55, 1416–1425. [Google Scholar] [CrossRef]
- Thivya, P.; Akalya, S.; Sinija, V. A comprehensive review on cellulose-based hydrogel and its potential application in the food industry. Appl. Food Res. 2022, 2, 100161. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Gregory, D.A.; Tripathi, L.; Fricker, A.T.; Asare, E.; Orlando, I.; Raghavendran, V.; Roy, I. Bacterial cellulose: A smart biomaterial with diverse applications. Mater. Sci. Eng. R Rep. 2021, 145, 100623. [Google Scholar] [CrossRef]
- Lin, S.-P.; Loira Calvar, I.; Catchmark, J.M.; Liu, J.-R.; Demirci, A.; Cheng, K.-C. Biosynthesis, production and applications of bacterial cellulose. Cellulose 2013, 20, 2191–2219. [Google Scholar] [CrossRef]
- Chen, C.; Xi, Y.; Weng, Y. Recent advances in cellulose-based hydrogels for tissue engineering applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I.; Pa’e, N.; Hashim, Z. Strategies in improving properties of cellulose-based hydrogels for smart applications. In Cellulose-Based Superabsorbent Hydrogels; Springer: Berlin/Heidelberg, Germany, 2019; pp. 887–908. [Google Scholar]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Hasan, S.; Kouzani, A.Z.; Adams, S.; Long, J.; Mahmud, M.A.P. Recent progress in hydrogel-based sensors and energy harvesters. Sens. Actuators A Phys. 2022, 335, 113382. [Google Scholar] [CrossRef]
- Cong, H.-P.; Wang, P.; Yu, S.-H. Stretchable and self-healing graphene oxide-polymer composite hydrogels: A dual-network design. Chem. Mater. 2013, 25, 3357–3362. [Google Scholar] [CrossRef]
- Yalpani, M. Polysaccharides: Syntheses, Modifications and Structure/property Relations; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Kloxin, A.M.; Kloxin, C.J.; Bowman, C.N.; Anseth, K.S. Mechanical properties of cellularly responsive hydrogels and their experimental determination. Adv. Mater. 2010, 22, 3484–3494. [Google Scholar] [CrossRef] [PubMed]
- Elbarbary, A.M.; Abd El-Rehim, H.A.; El-Sawy, N.M.; Hegazy, E.-S.A.; Soliman, E.-S.A. Radiation induced crosslinking of polyacrylamide incorporated low molecular weights natural polymers for possible use in the agricultural applications. Carbohydr. Polym. 2017, 176, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Sayed, S.M.; Liu, S.; Yao, F.; Oderinde, O.; Fu, G. Hydroxyethyl cellulose-based self-healing hydrogels with enhanced mechanical properties via metal-ligand bond interactions. Eur. Polym. J. 2018, 100, 219–227. [Google Scholar] [CrossRef]
- El Fawal, G.F.; Abu-Serie, M.M.; Hassan, M.A.; Elnouby, M.S. Hydroxyethyl cellulose hydrogel for wound dressing: Fabrication, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2018, 111, 649–659. [Google Scholar] [CrossRef]
- Sun, N.; Wang, T.; Yan, X. Self-assembled supermolecular hydrogel based on hydroxyethyl cellulose: Formation, in vitro release and bacteriostasis application. Carbohydr. Polym. 2017, 172, 49–59. [Google Scholar] [CrossRef]
- Li, J.; Chen, F.; Lin, X.; Ding, T. Hydrogen-bonding-assisted toughening of hierarchical carboxymethyl cellulose hydrogels for biomechanical sensing. Carbohydr. Polym. 2021, 269, 118252. [Google Scholar] [CrossRef]
- Lan, W.; He, L.; Liu, Y. Preparation and properties of sodium carboxymethyl cellulose/sodium alginate/chitosan composite film. Coatings 2018, 8, 291. [Google Scholar] [CrossRef]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef]
- Tran, T.H.; Okabe, H.; Hidaka, Y.; Hara, K. Removal of metal ions from aqueous solutions using carboxymethyl cellulose/sodium styrene sulfonate gels prepared by radiation grafting. Carbohydr. Polym. 2017, 157, 335–343. [Google Scholar] [CrossRef]
- Mandal, B.; Ray, S.K. Removal of safranine T and brilliant cresyl blue dyes from water by carboxy methyl cellulose incorporated acrylic hydrogels: Isotherms, kinetics and thermodynamic study. J. Taiwan Inst. Chem. Eng. 2016, 60, 313–327. [Google Scholar] [CrossRef]
- Rasoulzadeh, M.; Namazi, H. Carboxymethyl cellulose/graphene oxide bio-nanocomposite hydrogel beads as anticancer drug carrier agent. Carbohydr. Polym. 2017, 168, 320–326. [Google Scholar] [CrossRef]
- Sun, Y.; Le, X.; Zhou, S.; Chen, T. Recent Progress in Smart Polymeric Gel-Based Information Storage for Anti-Counterfeiting. Adv. Mater. 2022, 34, 2201262. [Google Scholar] [CrossRef]
- Tundisi, L.; Mostaço, G.; Carricondo, P.C.; Petri, D. Hydroxypropyl methylcellulose: Physicochemical properties and ocular drug delivery formulations. Eur. J. Pharm. Sci. 2021, 159, 105736. [Google Scholar] [CrossRef]
- kumar Kesavan, S.; Selvaraj, D.; Perumal, S.; Arunachalakasi, A.; Ganesan, N.; Chinnaiyan, S.K.; Balaraman, M. Fabrication of hybrid povidone-iodine impregnated collagen-hydroxypropyl methylcellulose composite scaffolds for wound-healing application. J. Drug Deliv. Sci. Technol. 2022, 70, 103247. [Google Scholar] [CrossRef]
- Kareem, S.A.; Dere, I.; Gungula, D.T.; Andrew, F.P.; Saddiq, A.M.; Adebayo, E.F.; Tame, V.T.; Kefas, H.M.; Joseph, J.; Patrick, D.O. Synthesis and Characterization of Slow-Release Fertilizer Hydrogel Based on Hydroxy Propyl Methyl Cellulose, Polyvinyl Alcohol, Glycerol and Blended Paper. Gels 2021, 7, 262. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Q.; Cheng, Q.; Ai, J.; Feng, M.; Wang, C.; Lv, X.; He, M.; Chen, Y. Construction of conductive hydroxyethyl cellulose/soy protein isolate/polypyrrole composite sponges and their performances. Cellulose 2021, 28, 8527–8539. [Google Scholar] [CrossRef]
- Han, Q.-Y.; Wen, X.; Gao, J.-Y.; Zhong, C.-S.; Ni, Y.-Y. Application of plasma-activated water in the food industry: A review of recent research developments. Food Chem. 2022, 405, 134797. [Google Scholar] [CrossRef]
- Yuan, M.; Bi, B.; Huang, J.; Zhuo, R.; Jiang, X. Thermosensitive and photocrosslinkable hydroxypropyl chitin-based hydrogels for biomedical applications. Carbohydr. Polym. 2018, 192, 10–18. [Google Scholar] [CrossRef]
- Butylina, S.; Geng, S.; Oksman, K. Properties of as-prepared and freeze-dried hydrogels made from poly (vinyl alcohol) and cellulose nanocrystals using freeze-thaw technique. Eur. Polym. J. 2016, 81, 386–396. [Google Scholar] [CrossRef]
- Cao, X.; Li, F.; Li, Y.; Chen, S.; Li, X.; Lu, Y. Preparation and application of cellulose-based hydrogels derived from bamboo. ChemRxiv® 2022, 1–29. [Google Scholar] [CrossRef]
- Chee, B.S.; de Lima, G.G.; Devine, D.M.; Nugent, M.J. Investigation of the effects of orientation on freeze/thawed Polyvinyl alcohol hydrogel properties. Mater. Today Commun. 2018, 17, 82–93. [Google Scholar] [CrossRef]
- Zhang, X.; Huo, W.; Yan, S.; Chen, Y.; Gan, K.; Liu, J.; Yang, J. Innovative application of PVA hydrogel for the forming of porous Si3N4 ceramics via freeze-thaw technique. Ceram. Int. 2018, 44, 13409–13413. [Google Scholar] [CrossRef]
- Butnaru, E.; Cheaburu, C.N.; Yilmaz, O.; Pricope, G.M.; Vasile, C. Poly (vinyl alcohol)/chitosan/montmorillonite nanocomposites for food packaging applications: Influence of montmorillonite content. High Perform. Polym. 2016, 28, 1124–1138. [Google Scholar] [CrossRef]
- de Sousa Iwamoto, L.A.; Duailibi, M.T.; Iwamoto, G.Y.; de Oliveira, D.C.; Duailibi, S.E. Evaluation of ethylene oxide, gamma radiation, dry heat and autoclave sterilization processes on extracellular matrix of biomaterial dental scaffolds. Sci. Rep. 2022, 12, 4299. [Google Scholar] [CrossRef]
- Khoushabi, A.; Schmocker, A.; Pioletti, D.; Moser, C.; Schizas, C.; Månson, J.-A.; Bourban, P.-E. Photo-polymerization, swelling and mechanical properties of cellulose fibre reinforced poly (ethylene glycol) hydrogels. Compos. Sci. Technol. 2015, 119, 93–99. [Google Scholar] [CrossRef]
- Naskar, A.; Khan, H.; Sarkar, R.; Kumar, S.; Halder, D.; Jana, S. Anti-biofilm activity and food packaging application of room temperature solution process based polyethylene glycol capped Ag-ZnO-graphene nanocomposite. Mater. Sci. Eng. C 2018, 91, 743–753. [Google Scholar] [CrossRef]
- Pin, L.; Airul, A.; Muntaz, A.B.; Wan, Y.; Mohd, A. Preparation and characterization of starch/acrylamide-based hydrogel from Stemona curtissi Tuber. Malays. J. Anal. Sci. 2016, 20, 157–170. [Google Scholar]
- Zhao, Y.; Ma, L.; Zeng, R.; Tu, M.; Zhao, J. Preparation, characterization and protein sorption of photo-crosslinked cell membrane-mimicking chitosan-based hydrogels. Carbohydr. Polym. 2016, 151, 237–244. [Google Scholar] [CrossRef]
- Müller, C.M.; Laurindo, J.B.; Yamashita, F. Effect of cellulose fibers addition on the mechanical properties and water vapor barrier of starch-based films. Food Hydrocoll. 2009, 23, 1328–1333. [Google Scholar] [CrossRef]
- Baniasadi, H.; Madani, Z.; Ajdary, R.; Rojas, O.J.; Seppälä, J. Ascorbic acid-loaded polyvinyl alcohol/cellulose nanofibril hydrogels as precursors for 3D printed materials. Mater. Sci. Eng. C 2021, 130, 112424. [Google Scholar] [CrossRef]
- George, J.; Hsu, C.-C.; Nguyen, L.T.B.; Ye, H.; Cui, Z. Neural tissue engineering with structured hydrogels in CNS models and therapies. Biotechnol. Adv. 2020, 42, 107370. [Google Scholar] [CrossRef]
- Farshbaf, M.; Davaran, S.; Zarebkohan, A.; Annabi, N.; Akbarzadeh, A.; Salehi, R. Significant role of cationic polymers in drug delivery systems. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1872–1891. [Google Scholar] [CrossRef]
- Percec, V.; Bera, T.K.; Butera, R.J. A new strategy for the preparation of supramolecular neutral hydrogels. Biomacromolecules 2002, 3, 272–279. [Google Scholar] [CrossRef]
- Sağlam, D.; Venema, P.; de Vries, R.; van der Linden, E. The influence of pH and ionic strength on the swelling of dense protein particles. Soft Matter 2013, 9, 4598–4606. [Google Scholar] [CrossRef]
- Duan, J.; Jiang, J.; Han, C.; Yang, J.; Liu, L.; Li, J. The study of intermolecular inclusion in cellulose physical gels. BioResources 2014, 9, 4006–4013. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, H.; Feng, A.; Huo, M.; Wang, Z.; Hu, J.; Gao, W.; Yuan, J. Electrochemical redox responsive supramolecular self-healing hydrogels based on host-guest interaction. Polym. Chem. 2015, 6, 3652–3659. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Supramolecular hydrogels from in situ host-guest inclusion between chemically modified cellulose nanocrystals and cyclodextrin. Biomacromolecules 2013, 14, 871–880. [Google Scholar] [CrossRef]
- Himmelein, S.; Lewe, V.; Stuart, M.C.; Ravoo, B.J. A carbohydrate-based hydrogel containing vesicles as responsive non-covalent cross-linkers. Chem. Sci. 2014, 5, 1054–1058. [Google Scholar] [CrossRef]
- Appel, E.A.; Biedermann, F.; Rauwald, U.; Jones, S.T.; Zayed, J.M.; Scherman, O.A. Supramolecular cross-linked networks via host-guest complexation with cucurbit [8] uril. J. Am. Chem. Soc. 2010, 132, 14251–14260. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, F.; Elmalem, E.; Ghosh, I.; Nau, W.M.; Scherman, O.A. Strongly fluorescent, switchable perylene bis (diimide) host-guest complexes with cucurbit [8] uril in water. Angew. Chem. 2012, 124, 7859–7863. [Google Scholar] [CrossRef]
- McKee, J.R.; Appel, E.A.; Seitsonen, J.; Kontturi, E.; Scherman, O.A.; Ikkala, O. Healable, stable and stiff hydrogels: Combining conflicting properties using dynamic and selective three-component recognition with reinforcing cellulose nanorods. Adv. Funct. Mater. 2014, 24, 2706–2713. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, J.; Coulston, R.J.; Parker, R.M.; Biedermann, F.; Liu, X.; Scherman, O.A.; Abell, C. Supramolecular hydrogel microcapsules via cucurbit [8] uril host-guest interactions with triggered and UV-controlled molecular permeability. Chem. Sci. 2015, 6, 4929–4933. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Tsang, K.; Mithieux, S.M.; Nikkhah, M.; Ameri, A.; Khademhosseini, A.; Weiss, A.S. Highly elastic micropatterned hydrogel for engineering functional cardiac tissue. Adv. Funct. Mater. 2013, 23, 4950–4959. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, F.; Li, M.; Wang, E. pH switching on-off semi-IPN hydrogel based on cross-linked poly (acrylamide-co-acrylic acid) and linear polyallyamine. Polymer 2005, 46, 7695–7700. [Google Scholar] [CrossRef]
- Baroli, B. Photopolymerization of biomaterials: Issues and potentialities in drug delivery, tissue engineering, and cell encapsulation applications. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2006, 81, 491–499. [Google Scholar] [CrossRef]
- Garcia, Y.; Collighan, R.; Griffin, M.; Pandit, A. Assessment of cell viability in a three-dimensional enzymatically cross-linked collagen scaffold. J. Mater. Sci. Mater. Med. 2007, 18, 1991–2001. [Google Scholar] [CrossRef]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly (vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. In Biopolymers PVA Hydrogels Anionic Polymerisation Nanocomposites; Chang, J.Y., Chang, D.Y., Godovsky, M.J., Han, C.M., Hassan, J., Kim, B., Lee, Y., Lee, N.A., Peppas, R.P., Quirk, T.Y., Eds.; Springer: Heidelberg, Germany, 2000; Volume 153, pp. 37–65. [Google Scholar]
- Raza, F.; Zafar, H.; Zhu, Y.; Ren, Y.; -Ullah, A.; Khan, A.U.; He, X.; Han, H.; Aquib, M.; Boakye-Yiadom, K.O. A review on recent advances in stabilizing peptides/proteins upon fabrication in hydrogels from biodegradable polymers. Pharmaceutics 2018, 10, 16. [Google Scholar] [CrossRef]
- Takigami, M.; Amada, H.; Nagasawa, N.; Yagi, T.; Kasahara, T.; Takigami, S.; Tamada, M. Preparation and properties of CMC gel. Trans. Mater. Res. Soc. Jpn. 2007, 32, 713–716. [Google Scholar] [CrossRef]
- Sibaja-Hernández, R.; Román-Guerrero, A.; Sepúlveda-Jiménez, G.; Rodríguez-Monroy, M. Physicochemical, shear flow behaviour and emulsifying properties of Acacia cochliacantha and Acacia farnesiana gums. Ind. Crops Prod. 2015, 67, 161–168. [Google Scholar] [CrossRef]
- Mahendran, T.; Williams, P.; Phillips, G.; Al-Assaf, S.; Baldwin, T. New insights into the structural characteristics of the arabinogalactan− protein (AGP) fraction of gum arabic. J. Agric. Food Chem. 2008, 56, 9269–9276. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels classification according to the physical or chemical interactions and as stimuli-sensitive materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Ranganathan, N.; Bensingh, R.J.; Kader, M.A.; Nayak, S.K. Synthesis and properties of hydrogels prepared by various polymerization reaction systems. In Cellulose-Based Superabsorbent Hydrogels; Springer: Berlin/Heidelberg, Germany, 2019; pp. 487–511. [Google Scholar]
- Gyawali, D.; Nair, P.; Zhang, Y.; Tran, R.T.; Zhang, C.; Samchukov, M.; Makarov, M.; Kim, H.K.; Yang, J. Citric acid-derived in situ crosslinkable biodegradable polymers for cell delivery. Biomaterials 2010, 31, 9092–9105. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Stone, S.A.; Gosavi, P.; Athauda, T.J.; Ozer, R.R. In situ citric acid crosslinking of alginate/polyvinyl alcohol electrospun nanofibers. Mater. Lett. 2013, 112, 32–35. [Google Scholar] [CrossRef]
- Menzel, C.; Olsson, E.; Plivelic, T.S.; Andersson, R.; Johansson, C.; Kuktaite, R.; Järnström, L.; Koch, K. Molecular structure of citric acid cross-linked starch films. Carbohydr. Polym. 2013, 96, 270–276. [Google Scholar] [CrossRef]
- Wang, S.; Ren, J.; Li, W.; Sun, R.; Liu, S. Properties of polyvinyl alcohol/xylan composite films with citric acid. Carbohydr. Polym. 2014, 103, 94–99. [Google Scholar] [CrossRef]
- Seligra, P.G.; Jaramillo, C.M.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch-glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J. Citric acid crosslinked β-cyclodextrin/carboxymethylcellulose hydrogel films for controlled delivery of poorly soluble drugs. Carbohydr. Polym. 2017, 164, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J. Citric acid crosslinked cyclodextrin/hydroxypropylmethylcellulose hydrogel films for hydrophobic drug delivery. Int. J. Biol. Macromol. 2016, 93, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Prakash, P.; Rout, P.K.; Bhaladhare, S. Synthesis and Characterization of Superabsorbent Cellulose-Based Hydrogel for Agriculture Application. Starch-Stärke 2021, 73, 1900284. [Google Scholar] [CrossRef]
- Koschella, A.; Hartlieb, M.; Heinze, T. A “click-chemistry” approach to cellulose-based hydrogels. Carbohydr. Polym. 2011, 86, 154–161. [Google Scholar] [CrossRef]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J.; Mali, K.K.; Pargaonkar, S.S.; Shinde, P.V.; Dhane, N.S. Citric acid crosslinked carboxymethylcellulose-poly (ethylene glycol) hydrogel films for delivery of poorly soluble drugs. Int. J. Biol. Macromol. 2018, 118, 783–791. [Google Scholar] [CrossRef]
- Sampatrao Ghorpade, V.; Vyankatrao Yadav, A.; Jacky Dias, R.; Krishnat Mali, K. Fabrication of citric acid crosslinked β-cyclodextrin/hydroxyethylcellulose hydrogel films for controlled delivery of poorly soluble drugs. J. Appl. Polym. Sci. 2018, 135, 46452. [Google Scholar] [CrossRef]
- Laus, R.; De Favere, V.T. Competitive adsorption of Cu (II) and Cd (II) ions by chitosan crosslinked with epichlorohydrin-triphosphate. Bioresour. Technol. 2011, 102, 8769–8776. [Google Scholar] [CrossRef]
- Jawad, A.H.; Nawi, M. Oxidation of crosslinked chitosan-epichlorohydrine film and its application with TiO2 for phenol removal. Carbohydr. Polym. 2012, 90, 87–94. [Google Scholar] [CrossRef]
- Kittipongpatana, O.S.; Kittipongpatana, N. Physicochemical, in vitro digestibility and functional properties of carboxymethyl rice starch cross-linked with epichlorohydrin. Food Chem. 2013, 141, 1438–1444. [Google Scholar] [CrossRef]
- Meybodi, Z.E.; Imani, M.; Atai, M. Kinetics of dextran crosslinking by epichlorohydrin: A rheometry and equilibrium swelling study. Carbohydr. Polym. 2013, 92, 1792–1798. [Google Scholar] [CrossRef]
- Wang, W.; Jin, X.; Zhu, Y.; Zhu, C.; Yang, J.; Wang, H.; Lin, T. Effect of vapor-phase glutaraldehyde crosslinking on electrospun starch fibers. Carbohydr. Polym. 2016, 140, 356–361. [Google Scholar] [CrossRef]
- Nagireddi, S.; Katiyar, V.; Uppaluri, R. Pd (II) adsorption characteristics of glutaraldehyde cross-linked chitosan copolymer resin. Int. J. Biol. Macromol. 2017, 94, 72–84. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, W.; Li, G. The microstructure and stability of collagen hydrogel cross-linked by glutaraldehyde. Polym. Degrad. Stab. 2016, 130, 264–270. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid Interface Sci. 2011, 166, 36–59. [Google Scholar] [CrossRef]
- Khairuddin, N.M.A.; Afifi, A.M.; Hashim, N.A.; Mohamad, S.E.; Kalantari, K. Immobilization of bovine serum albumin on the chitosan/PVA film. Sains Malays. 2018, 47, 1311–1318. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, X.; Tan, G.; Tian, L.; Liu, D.; Liu, Y.; Yang, X.; Pan, W. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr. Polym. 2017, 155, 208–217. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Chen, F.; Yu, S.; Liu, B.; Ni, Y.; Yu, C.; Su, Y.; Zhu, X.; Yu, X.; Zhou, Y.; Yan, D. An injectable enzymatically crosslinked carboxymethylated pullulan/chondroitin sulfate hydrogel for cartilage tissue engineering. Sci. Rep. 2016, 6, 20014. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, X.; Yang, Y.; Zhao, X.; Chen, X.; Jing, T.; Zhou, Y.; Xu, J.; Zhang, Y.; Cheng, Y. Construction of supramolecular hydrogels using imidazolidinyl urea as hydrogen bonding reinforced factor. J. Mater. Chem. B 2020, 8, 3058–3063. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating polymer networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Kang, H.; Liu, R.; Wang, D.; Jin, X.; Li, Q.; Huang, Y. Dual-stimuli sensitive nanogels fabricated by self-association of thiolated hydroxypropyl cellulose. Polym. Chem. 2011, 2, 672–678. [Google Scholar] [CrossRef]
- Cudjoe, E.; Herbert, K.M.; Rowan, S.J. Strong, rebondable, dynamic cross-linked cellulose nanocrystal polymer nanocomposite adhesives. ACS Appl. Mater. Interfaces 2018, 10, 30723–30731. [Google Scholar] [CrossRef]
- Hou, X.; Pan, Y.; Xiao, H.; Liu, J. Controlled release of agrochemicals using pH and redox dual-responsive cellulose nanogels. J. Agric. Food Chem. 2019, 67, 6700–6707. [Google Scholar] [CrossRef]
- Shao, C.; Yang, J. Dynamics in Cellulose-Based Hydrogels with Reversible Cross-Links. Self-Heal. Self-Recover. Hydrogels 2020, 285, 319–354. [Google Scholar]
- Matsumoto, S.; Christie, R.J.; Nishiyama, N.; Miyata, K.; Ishii, A.; Oba, M.; Koyama, H.; Yamasaki, Y.; Kataoka, K. Environment-responsive block copolymer micelles with a disulfide cross-linked core for enhanced siRNA delivery. Biomacromolecules 2009, 10, 119–127. [Google Scholar] [CrossRef]
- Cerritelli, S.; Velluto, D.; Hubbell, J.A. PEG-SS-PPS: Reduction-sensitive disulfide block copolymer vesicles for intracellular drug delivery. Biomacromolecules 2007, 8, 1966–1972. [Google Scholar] [CrossRef]
- Liu, H.; Rong, L.; Wang, B.; Xie, R.; Sui, X.; Xu, H.; Zhang, L.; Zhong, Y.; Mao, Z. Facile fabrication of redox/pH dual stimuli responsive cellulose hydrogel. Carbohydr. Polym. 2017, 176, 299–306. [Google Scholar] [CrossRef]
- Ashfaq, A.; Clochard, M.-C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M. Polymerization reactions and modifications of polymers by ionizing radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.; Mujtaba, M.; Alghamdi, N.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Jose, G.; Shalumon, K.; Chen, J.-P. Natural polymers based hydrogels for cell culture applications. Curr. Med. Chem. 2020, 27, 2734–2776. [Google Scholar] [CrossRef]
- Kumar, A.; Sood, A.; Han, S.S. Potential of magnetic nano cellulose in biomedical applications: Recent Advances. Biomater. Polym. Horiz. 2022, 1, 32–47. [Google Scholar] [CrossRef]
- Gårdebjer, S.; Larsson, M.; Gebäck, T.; Skepö, M.; Larsson, A. An overview of the transport of liquid molecules through structured polymer films, barriers and composites–Experiments correlated to structure-based simulations. Adv. Colloid Interface Sci. 2018, 256, 48–64. [Google Scholar] [CrossRef]
- Hu, M.; Yang, J.; Xu, J. Structural and biological investigation of chitosan/hyaluronic acid with silanized-hydroxypropyl methylcellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering. Drug Deliv. 2021, 28, 607–619. [Google Scholar] [CrossRef]
- Yin, J.; Fang, Y.; Xu, L.; Ahmed, A. High-throughput fabrication of silk fibroin/hydroxypropyl methylcellulose (SF/HPMC) nanofibrous scaffolds for skin tissue engineering. Int. J. Biol. Macromol. 2021, 183, 1210–1221. [Google Scholar] [CrossRef]
- Sharma, D.; Dev, D.; Prasad, D.; Hans, M. Sustained release drug delivery system with the role of natural polymers: A review. J. Drug Deliv. Ther. 2019, 9, 913–923. [Google Scholar]
- Powell, S.K.; Cruz, R.L.; Ross, M.T.; Woodruff, M.A. Past, present, and future of soft-tissue prosthetics: Advanced polymers and advanced manufacturing. Adv. Mater. 2020, 32, 2001122. [Google Scholar] [CrossRef]
- Kayra, N.; Aytekin, A.Ö. Synthesis of carboxymethyl cellulose: Production, modification, and novel preparation methods. In Carboxymethyl Cellulose: Synthesis and Charracterization, Mondal, M.I.H., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2019; Volume 1, pp. 1–18. [Google Scholar]
- Jang, G.-W.B.; Hsieh, C.-H.; Lai, A.; Chan, S. Manufacturing Methods of PLA Composites. In Polylactic Acid-Based Nanocellulose and Cellulose Composites; CRC Press: Boca Raton, FL, USA, 2022; pp. 51–82. [Google Scholar]
- He, X.; Lu, Q. Design and fabrication strategies of cellulose nanocrystal-based hydrogel and its highlighted application using 3D printing: A review. Carbohydr. Polym. 2022, 301, 120351. [Google Scholar] [CrossRef]
- Davis, C.A. Advanced Overview of Bacterial Nanocellulose Manufacturing for Novel Applications. In Analysis of Biosynthesis, Chemical Treatments, Processing, and Properties; Universitat Politècnica de Catalunya: Barcelona, Spain, 2022. [Google Scholar]
- Teo, S.H.; Chee, C.Y.; Fahmi, M.Z.; Wibawa Sakti, S.C.; Lee, H.V. Review of Functional Aspects of Nanocellulose-Based Pickering Emulsifier for Non-Toxic Application and Its Colloid Stabilization Mechanism. Molecules 2022, 27, 7170. [Google Scholar] [CrossRef] [PubMed]
- Fucina, G.; Cesca, K.; Berti, F.V.; Biavatti, M.W.; Porto, L.M. Melanoma growth in non-chemically modified translucid bacterial nanocellulose hollow and compartimentalized spheres. Biochim. Biophys. Acta (BBA) Gen. Subj. 2022, 1866, 130183. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.B.; Kamal, T. Bacterial Cellulose: Synthesis, Production, and Applications; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Oprea, M.; Voicu, S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef] [PubMed]
- Nikolsky, S.N.; Zlenko, D.V.; Melnikov, V.P.; Stovbun, S.V. The fibrils untwisting limits the rate of cellulose nitration process. Carbohydr. Polym. 2019, 204, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Thiangtham, S.; Runt, J.; Manuspiya, H. Sulfonation of dialdehyde cellulose extracted from sugarcane bagasse for synergistically enhanced water solubility. Carbohydr. Polym. 2019, 208, 314–322. [Google Scholar] [CrossRef]
- Willems, C.; Trutschel, M.L.; Mazaikina, V.; Strätz, J.; Mäder, K.; Fischer, S.; Groth, T. Hydrogels Based on Oxidized Cellulose Sulfates and Carboxymethyl Chitosan: Studies on Intrinsic Gel Properties, Stability, and Biocompatibility. Macromol. Biosci. 2021, 21, 2100098. [Google Scholar] [CrossRef]
- Strätz, J.; Fischer, S. Tailored covalently cross-linked hydrogels based on oxidized cellulose sulfate and carboxymethyl chitosan by targeted adjustment of the storage modulus. Cellulose 2020, 27, 7535–7542. [Google Scholar] [CrossRef]
- Su, T.; Wu, Q.-X.; Chen, Y.; Zhao, J.; Cheng, X.-D.; Chen, J. Fabrication of the polyphosphates patched cellulose sulfate-chitosan hydrochloride microcapsules and as vehicles for sustained drug release. Int. J. Pharm. 2019, 555, 291–302. [Google Scholar] [CrossRef]
- Senna, A.M.; Novack, K.M.; Botaro, V.R. Synthesis and characterization of hydrogels from cellulose acetate by esterification crosslinking with EDTA dianhydride. Carbohydr. Polym. 2014, 114, 260–268. [Google Scholar] [CrossRef]
- Senna, A.M.; Botaro, V.R. Biodegradable hydrogel derived from cellulose acetate and EDTA as a reduction substrate of leaching NPK compound fertilizer and water retention in soil. J. Control. Release 2017, 260, 194–201. [Google Scholar] [CrossRef]
- Maharjan, B.; Park, J.; Kaliannagounder, V.K.; Awasthi, G.P.; Joshi, M.K.; Park, C.H.; Kim, C.S. Regenerated cellulose nanofiber reinforced chitosan hydrogel scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 251, 117023. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Otoni, C.G.; Espitia, P.J.; Avena-Bustillos, R.J.; McHugh, T.H. Trends in antimicrobial food packaging systems: Emitting sachets and absorbent pads. Food Res. Int. 2016, 83, 60–73. [Google Scholar] [CrossRef]
- Roy, N.; Saha, N.; Kitano, T.; Saha, P. Biodegradation of PVP-CMC hydrogel film: A useful food packaging material. Carbohydr. Polym. 2012, 89, 346–353. [Google Scholar] [CrossRef]
- Fernández, A.; Soriano, E.; López-Carballo, G.; Picouet, P.; Lloret, E.; Gavara, R.; Hernández-Muñoz, P. Preservation of aseptic conditions in absorbent pads by using silver nanotechnology. Food Res. Int. 2009, 42, 1105–1112. [Google Scholar] [CrossRef]
- Bodbodak, S.; Rafiee, Z. Recent trends in active packaging in fruits and vegetables. In Eco-friendly Technology for Postharvest Produce Quality; Elsevier: Amsterdam, The Netherlands, 2016; pp. 77–125. [Google Scholar]
- Oun, A.A.; Rhim, J.-W. Carrageenan-based hydrogels and films: Effect of ZnO and CuO nanoparticles on the physical, mechanical, and antimicrobial properties. Food Hydrocoll. 2017, 67, 45–53. [Google Scholar] [CrossRef]
- Martín, M.C.; López, O.V.; Ciolino, A.E.; Morata, V.I.; Villar, M.A.; Ninago, M.D. Immobilization of enological pectinase in calcium alginate hydrogels: A potential biocatalyst for winemaking. Biocatal. Agric. Biotechnol. 2019, 18, 101091. [Google Scholar] [CrossRef]
- Wolf, M.; Belfiore, L.A.; Tambourgi, E.B.; Paulino, A.T. Production of low-dosage lactose milk using lactase immobilised in hydrogel. Int. Dairy J. 2019, 92, 77–83. [Google Scholar] [CrossRef]
- Facin, B.R.; Moret, B.; Baretta, D.; Belfiore, L.A.; Paulino, A.T. Immobilization and controlled release of β-galactosidase from chitosan-grafted hydrogels. Food Chem. 2015, 179, 44–51. [Google Scholar] [CrossRef]
- Zhang, Z.; Decker, E.A.; McClements, D.J. Encapsulation, protection, and release of polyunsaturated lipids using biopolymer-based hydrogel particles. Food Res. Int. 2014, 64, 520–526. [Google Scholar] [CrossRef]
- Heidebach, T.; Först, P.; Kulozik, U. Microencapsulation of probiotic cells for food applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 291–311. [Google Scholar] [CrossRef]
- Shewan, H.M.; Stokes, J.R. Review of techniques to manufacture micro-hydrogel particles for the food industry and their applications. J. Food Eng. 2013, 119, 781–792. [Google Scholar] [CrossRef]
- Rostami, H.; Nikoo, A.M.; Rajabzadeh, G.; Niknia, N.; Salehi, S. Development of cumin essential oil nanoemulsions and its emulsion filled hydrogels. Food Biosci. 2018, 26, 126–132. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, W.; Kong, F.; Lin, M.; Mustapha, A. Cellulose nanofibril/silver nanoparticle composite as an active food packaging system and its toxicity to human colon cells. Int. J. Biol. Macromol. 2019, 129, 887–894. [Google Scholar] [CrossRef]
- Mao, L.; Lu, Y.; Cui, M.; Miao, S.; Gao, Y. Design of gel structures in water and oil phases for improved delivery of bioactive food ingredients. Crit. Rev. Food Sci. Nutr. 2020, 60, 1651–1666. [Google Scholar] [CrossRef] [PubMed]
- Shit, S.C.; Shah, P.M. Edible polymers: Challenges and opportunities. J. Polym. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Chen, L.; Remondetto, G.E.; Subirade, M. Food protein-based materials as nutraceutical delivery systems. Trends Food Sci. Technol. 2006, 17, 272–283. [Google Scholar] [CrossRef]
- Farris, S.; Schaich, K.M.; Liu, L.; Piergiovanni, L.; Yam, K.L. Development of polyion-complex hydrogels as an alternative approach for the production of bio-based polymers for food packaging applications: A review. Trends Food Sci. Technol. 2009, 20, 316–332. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Effect of fish gelatin coating enriched with oregano essential oil on the quality of refrigerated rainbow trout fillet. J. Aquat. Food Prod. Technol. 2016, 25, 835–842. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Mohan, C.; Ravishankar, C.; Lalitha, K.; Gopal, T.S. Effect of chitosan edible coating on the quality of double filleted Indian oil sardine (Sardinella longiceps) during chilled storage. Food Hydrocoll. 2012, 26, 167–174. [Google Scholar] [CrossRef]
- Bazargani-Gilani, B. Activating sodium alginate-based edible coating using a dietary supplement for increasing the shelf life of rainbow trout fillet during refrigerated storage (4±1 C). J. Food Saf. 2018, 38, e12395. [Google Scholar] [CrossRef]
- Yıldız, P.O.; Yangılar, F. Effects of different whey protein concentrate coating on selected properties of rainbow trout (Oncorhynchus mykiss) during cold storage (4 C). Int. J. Food Prop. 2016, 19, 2007–2015. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Marangoni, A.G. Organogels: An alternative edible oil-structuring method. J. Am. Oil Chem. Soc. 2012, 89, 749–780. [Google Scholar] [CrossRef]
- Moschakis, T.; Panagiotopoulou, E.; Katsanidis, E. Sunflower oil organogels and organogel-in-water emulsions (part I): Microstructure and mechanical properties. LWT 2016, 73, 153–161. [Google Scholar] [CrossRef]
- Zampouni, K.; Soniadis, A.; Moschakis, T.; Biliaderis, C.; Lazaridou, A.; Katsanidis, E. Crystalline microstructure and physicochemical properties of olive oil oleogels formulated with monoglycerides and phytosterols. LWT 2022, 154, 112815. [Google Scholar] [CrossRef]
- Demirkesen, I.; Mert, B. Recent developments of oleogel utilizations in bakery products. Crit. Rev. Food Sci. Nutr. 2020, 60, 2460–2479. [Google Scholar] [CrossRef]
- Kouzounis, D.; Lazaridou, A.; Katsanidis, E. Partial replacement of animal fat by oleogels structured with monoglycerides and phytosterols in frankfurter sausages. Meat Sci. 2017, 130, 38–46. [Google Scholar] [CrossRef]
- Martins, A.J.; Lorenzo, J.M.; Franco, D.; Pateiro, M.; Domínguez, R.; Munekata, P.E.; Pastrana, L.M.; Vicente, A.A.; Cunha, R.L.; Cerqueira, M.A. Characterization of enriched meat-based pâté manufactured with oleogels as fat substitutes. Gels 2020, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Panagiotopoulou, E.; Moschakis, T.; Katsanidis, E. Sunflower oil organogels and organogel-in-water emulsions (part II): Implementation in frankfurter sausages. LWT 2016, 73, 351–356. [Google Scholar] [CrossRef]
- Pinto, T.C.; Martins, A.J.; Pastrana, L.; Pereira, M.C.; Cerqueira, M.A. Oleogel-based systems for the delivery of bioactive compounds in foods. Gels 2021, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, A.; Lupi, F.R.; Gabriele, D.; Baldino, N.; De Cindio, B. Bigels: A unique class of materials for drug delivery applications. Soft Mater. 2018, 16, 77–93. [Google Scholar] [CrossRef]
- Kodela, S.P.; Pandey, P.M.; Nayak, S.K.; Uvanesh, K.; Anis, A.; Pal, K. Novel agar-stearyl alcohol oleogel-based bigels as structured delivery vehicles. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 669–678. [Google Scholar] [CrossRef]
- Shakeel, A.; Farooq, U.; Iqbal, T.; Yasin, S.; Lupi, F.R.; Gabriele, D. Key characteristics and modelling of bigels systems: A review. Mater. Sci. Eng. C 2019, 97, 932–953. [Google Scholar] [CrossRef]
- Behera, B.; Sagiri, S.S.; Pal, K.; Pramanik, K.; Rana, U.A.; Shakir, I.; Anis, A. Sunflower oil and protein-based novel bigels as matrices for drug delivery applications—Characterization and in vitro antimicrobial efficiency. Polym. Technol. Eng. 2015, 54, 837–850. [Google Scholar] [CrossRef]
- Rehman, K.; Amin, M.C.I.M.; Zulfakar, M.H. Development and physical characterization of polymer-fish oil bigel (hydrogel/oleogel) system as a transdermal drug delivery vehicle. J. Oleo Sci. 2014, 63, 961–970. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Singh, V.K.; Kulanthaivel, S.; Banerjee, I.; Basak, P.; Battachrya, M.; Pal, K. Stearate organogel-gelatin hydrogel based bigels: Physicochemical, thermal, mechanical characterizations and in vitro drug delivery applications. J. Mech. Behav. Biomed. Mater. 2015, 43, 1–17. [Google Scholar] [CrossRef]
- Ghiasi, F.; Golmakani, M.-T. Fabrication and characterization of a novel biphasic system based on starch and ethylcellulose as an alternative fat replacer in a model food system. Innov. Food Sci. Emerg. Technol. 2022, 78, 103028. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Iturra, N.; Contardo, I.; Millao, S.; Morales, E.; Rubilar, M. Food-Grade Bigels with Potential to Replace Saturated and Trans Fats in Cookies. Gels 2022, 8, 445. [Google Scholar] [CrossRef]
- Singla, D.; Singh, A.; Gupta, R. Texture analysis of fruits for its deteriorated classification. In Proceedings of the International Conference on Wireless Intelligent and Distributed Environment for Communication, Sonepat, India, 16–18 February 2018; pp. 131–142. [Google Scholar]
- Hu, B.; Chen, L.; Lan, S.; Ren, P.; Wu, S.; Liu, X.; Shi, X.; Li, H.; Du, Y.; Ding, F. Layer-by-layer assembly of polysaccharide films with self-healing and antifogging properties for food packaging applications. ACS Appl. Nano Mater. 2018, 1, 3733–3740. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. The use of packaging techniques to maintain freshness in fresh-cut fruits and vegetables: A review. Int. J. Food Sci. Technol. 2009, 44, 875–889. [Google Scholar] [CrossRef]
- Jin, K.; Tang, Y.; Liu, J.; Wang, J.; Ye, C. Nanofibrillated cellulose as coating agent for food packaging paper. Int. J. Biol. Macromol. 2021, 168, 331–338. [Google Scholar] [CrossRef]
- Singh, M.; Sahareen, T. Investigation of cellulosic packets impregnated with silver nanoparticles for enhancing shelf-life of vegetables. LWT 2017, 86, 116–122. [Google Scholar] [CrossRef]
- Moradian, S.; Almasi, H.; Moini, S. Development of bacterial cellulose-based active membranes containing herbal extracts for shelf life extension of button mushrooms (Agaricus bisporus). J. Food Process. Preserv. 2018, 42, e13537. [Google Scholar] [CrossRef]
- Patanè, C.; Malvuccio, A.; Saita, A.; Rizzarelli, P.; Siracusa, L.; Rizzo, V.; Muratore, G. Nutritional changes during storage in fresh-cut long storage tomato as affected by biocompostable polylactide and cellulose based packaging. LWT 2019, 101, 618–624. [Google Scholar] [CrossRef]
- Dehnad, D.; Mirzaei, H.; Emam-Djomeh, Z.; Jafari, S.-M.; Dadashi, S. Thermal and antimicrobial properties of chitosan–nanocellulose films for extending shelf life of ground meat. Carbohydr. Polym. 2014, 109, 148–154. [Google Scholar] [CrossRef]
- Khezrian, A.; Shahbazi, Y. Application of nanocompostie chitosan and carboxymethyl cellulose films containing natural preservative compounds in minced camel’s meat. Int. J. Biol. Macromol. 2018, 106, 1146–1158. [Google Scholar] [CrossRef]
- Al-Moghazy, M.; Mahmoud, M.; Nada, A.A. Fabrication of cellulose-based adhesive composite as an active packaging material to extend the shelf life of cheese. Int. J. Biol. Macromol. 2020, 160, 264–275. [Google Scholar] [CrossRef]
- Pacaphol, K.; Seraypheap, K.; Aht-Ong, D. Development and application of nanofibrillated cellulose coating for shelf life extension of fresh-cut vegetable during postharvest storage. Carbohydr. Polym. 2019, 224, 115167. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Dharini, V.; Selvam, S.P.; Sadiku, E.R.; Kumar, M.M.; Jayaramudu, J.; Gupta, U.N. Physical, antifungal, and biodegradable properties of cellulose nanocrystals and chitosan nanoparticles for food packaging application. Mater. Today: Proc. 2021, 38, 860–869. [Google Scholar] [CrossRef]
- Pirsa, S.; Chavoshizadeh, S. Design of an optical sensor for ethylene based on nanofiber bacterial cellulose film and its application for determination of banana storage time. Polym. Adv. Technol. 2018, 29, 1385–1393. [Google Scholar] [CrossRef]
| Materials | Methods | Applications | References |
|---|---|---|---|
| Polyacryl amide Hydroxyethyl cellulose | Radiation-induced Chemical crosslinking | Agricultural product processing Wound dressing | [48] [49] |
| Free-radical polymerization | Self-healing | [50] | |
| Grafting | Bacteriostasis | [51] | |
| Carboxymethyl cellulose | Freeze–thaw Layer-by-layer assembly (Fabrication method) | Enzyme immobilization Improved preservation of beef | [52] [53] |
| Gamma radiation | Hemostat hydrogel | [54] | |
| Grafting | Metal ions removal | [55] | |
| Co-polymerization | Pigment removal | [56] | |
| Chemical crosslinking | Drug carrier agent | [57] | |
| Chemical crosslinking | Anti-counterfeiting and labelling | [58] | |
| Hydroxypropyl methylcellulose | Chemical crosslinking | Drug delivery | [59] |
| Radiation | Scaffolds | [60] | |
| Chemical crosslinking | Controlled release | [61] | |
| Hydroxypropyl cellulose | Pre-polymerization | Anti-fouling | [62] |
| Freeze–thaw | Biomedical | [63] | |
| Chemical crosslinking | Thermoresponsive hydrogel | [64] | |
| Photo-crosslinking | Biomedical | [65] | |
| Polyvinyl alcohol | Freeze–thaw | Biomedical | [66] |
| Freeze–thaw | Regenerative medicines | [67] | |
| Freeze–thaw | Drug release | [68] | |
| Freeze–thaw Freeze–thaw | Radome materials Food packaging | [69] [70] | |
| Polyethylene glycol | Gamma-radiation | Scaffolds | [71] |
| Photo-polymerization | Implants | [72] | |
| Chemical crosslinking | Anti-biofilm activity and food packaging | [73] | |
| Starch Cellulose nanofibril | Radical polymerization | Wound dressing | [74] |
| Freeze–thaw Air-drying castingCombined with Polyvinyl alcohol and ascorbic acid | Biomedical Food packaging Food 3D-printing materials | [75] [76] [77] |
| Polymers | Role of Cellulose | Film’s Activity | References |
|---|---|---|---|
| Cellulose/silver nanoparticles | Add silver particles for antibacterial protection and to increase shelf life. | The film demonstrated significant antibacterial action against Aeromonas hydrophila | [208] |
| Bacterial cellulose | Cellulose was used to transport plant extracts and ensure their delayed release. | Prolonged shelf life and decreased post-harvest microbial storage | [209] |
| Cellulose/polylactide | Provide coatings with enhanced antioxidant characteristics. | Add enhances the flavor and freshness of tomatoes. | [210] |
| Cellulose and chitosan | Enhanced thermal and anti-bacterial attributes. | Prolonged shelf life for ground meat. | [211] |
| Carboxymethyl cellulose and chitosan | Increase chitosan solubility. | Enhance antimicrobial effect by adjusting chitosan concentrations. | [212] |
| Cellulose | Adhesive derived from cellulose for active packaging. | Increased cheese freshness and shelf life. | [213] |
| Nanocellulose | Nanocellulose has gas barrier qualities that limit leaf respiration. | A longer storage life and larger capacity for storing the product were achieved. | [214] |
| Cellulose nanocrystals/chitosan | Enhancement of mechanical and barrier characteristics. | Increased shelf life to 20 days. | [215] |
| Cellulose based on ethylene | Enhanced water vapor transmission and moisture absorption. | The trigger was the manufacture of ethylene. | [216] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nath, P.C.; Debnath, S.; Sharma, M.; Sridhar, K.; Nayak, P.K.; Inbaraj, B.S. Recent Advances in Cellulose-Based Hydrogels: Food Applications. Foods 2023, 12, 350. https://doi.org/10.3390/foods12020350
Nath PC, Debnath S, Sharma M, Sridhar K, Nayak PK, Inbaraj BS. Recent Advances in Cellulose-Based Hydrogels: Food Applications. Foods. 2023; 12(2):350. https://doi.org/10.3390/foods12020350
Chicago/Turabian StyleNath, Pinku Chandra, Shubhankar Debnath, Minaxi Sharma, Kandi Sridhar, Prakash Kumar Nayak, and Baskaran Stephen Inbaraj. 2023. "Recent Advances in Cellulose-Based Hydrogels: Food Applications" Foods 12, no. 2: 350. https://doi.org/10.3390/foods12020350
APA StyleNath, P. C., Debnath, S., Sharma, M., Sridhar, K., Nayak, P. K., & Inbaraj, B. S. (2023). Recent Advances in Cellulose-Based Hydrogels: Food Applications. Foods, 12(2), 350. https://doi.org/10.3390/foods12020350








