Crystallization Behavior and Physical Properties of Monoglycerides-Based Oleogels as Function of Oleogelator Concentration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oleogels
2.3. Determination of Onset Gelling Concentrations (OGC)
2.4. Characterization of Oleogels
2.4.1. Polarized Light Microscopy (PLM)
2.4.2. Hardness and Oil Binding Capacity (OBC)
2.4.3. Rheology Measurements
2.4.4. Differential Scanning Calorimetry (DSC)
2.4.5. X-ray Diffraction (XRD)
2.4.6. Crystal Morphology during Heating and Cooling Process
3. Results and Discussion
3.1. Oleogel Formation and Microstructure Observation
3.2. Oil Binding Capacity and Mechanical Strength
3.3. Rheological Analysis
3.4. Thermal Analysis
3.5. XRD Analysis
3.6. Effects of Temperature on Crystallization Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chaves, K.F.; Barrera-Arellano, D.; Ribeiro, A.P.B. Potential application of lipid organogels for food industry. Food Res. Int. 2018, 105, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Stender, S.; Dyerberg, J. Influence of trans fatty acids on health. Ann. Nutr. Metab. 2004, 48, 61–66. [Google Scholar] [CrossRef]
- Marangoni, A.G. Organogels: An Alternative Edible Oil-Structuring Method. JACS 2012, 89, 749–780. [Google Scholar] [CrossRef]
- Zhao, W.; Wei, Z.; Xue, C. Recent advances on food-grade oleogels: Fabrication, application and research trends. Crit. Rev. Food Sci. Nutr. 2022, 62, 7659–7676. [Google Scholar] [CrossRef]
- Luan, H.; Wu, Y.; Zheng, H.; Ke, X.; Mao, L. Preparation and physicochemical characterization of cinnamic acid based oleogels and oleogel emulsions. Food Sci. 2021, 42, 60–66. [Google Scholar] [CrossRef]
- Guenet, J.M. Physical Aspects of Organogelation: A Point of View. Gels 2021, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xu, J.; Lu, X.; Xu, Y.; Regenstein, J.M.; Zhang, Y.; Wang, F. Development and characterization of monoglyceride oleogels prepared with crude and refined walnut oil. LWT-Food 2022, 154, 112769. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, S.; Wang, Y.; Lee, W.J.; Fu, J.; Binks, B.P.; Wang, Y. Stabilisation of oleofoams by lauric acid and its glycerol esters. Food Chem. 2022, 386, 132776. [Google Scholar] [CrossRef]
- Li, S.; Wu, G.; Li, X.; Jin, Q.; Wang, X.; Zhang, H. Roles of gelator type and gelation technology on texture and sensory properties of cookies prepared with oleogels. Food Chem. 2021, 356, 129667. [Google Scholar] [CrossRef]
- Hossein Naeli, M.; Jafar, M.M.; Jamshid, F.; Azizollaah, Z. Developing and optimizing low-saturated oleogel shortening based on ethyl cellulose and hydroxypropyl methyl cellulose biopolymers. Food Chem. 2022, 369, 130963. [Google Scholar] [CrossRef]
- Sislioglu, K.; Gumus, C.E.; Koo, C.K.W.; Karabulut, I.; McClements, D.J. In vitro digestion of edible nanostructured lipid carriers: Impact of a Candelilla wax gelator on performance. Food Res. Int. 2021, 140, 110060. [Google Scholar] [CrossRef]
- Zhao, M.; Lan, Y.; Cui, L.; Monono, E.; Rao, J.; Chen, B. Formation, characterization, and potential food application of rice bran wax oleogels: Expeller-pressed corn germ oil versus refined corn oil. Food Chem. 2020, 309, 125704. [Google Scholar] [CrossRef]
- Guo, S.; Song, M.; Gao, X.; Dong, L.; Hou, T.; Lin, X.; Tan, W.; Cao, Y.; Rogers, M.; Lan, Y. Assembly pattern of multicomponent supramolecular oleogel composed of ceramide and lecithin in sunflower oil: Self-assembly or self-sorting? Food Funct. 2020, 11, 7651–7660. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, G.; Saleh, A.S.M.; Yang, H.; Wang, N.; Wang, P.; Yue, X.; Xiao, Z. Functional Characteristics of Oleogel Prepared from Sunflower Oil with β-Sitosterol and Stearic Acid. JACS 2017, 94, 1153–1164. [Google Scholar] [CrossRef]
- Sun, P.; Xia, B.; Ni, Z.-J.; Wang, Y.; Elam, E.; Thakur, K.; Ma, Y.-L.; Wei, Z.-J. Characterization of functional chocolate formulated using oleogels derived from beta-sitosterol with gamma-oryzanol/lecithin/stearic acid. Food Chem. 2021, 360, 130017. [Google Scholar] [CrossRef]
- Cui, M.; Mao, L.; Lu, Y.; Yuan, F.; Gao, Y. Effect of monoglyceride content on the solubility and chemical stability of carotene in organogels. LWT-Food 2019, 106, 83–91. [Google Scholar] [CrossRef]
- Giacomozzi, A.S.; Palla, C.A.; Carrin, M.E.; Martini, S. Physical Properties of Monoglycerides Oleogels Modified by Concentration, Cooling Rate, and High-Intensity Ultrasound. J. Food Sci. 2019, 84, 2549–2561. [Google Scholar] [CrossRef]
- Lopez-Martinez, A.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charo-Alonso, M.A.; Marangoni, A.G.; Toro-Vazquez, J.F. Comparing the crystallization and rheological behavior of organogels developed by pure and commercial monoglycerides in vegetable oil. Food Res. Int. 2014, 64, 946–957. [Google Scholar] [CrossRef]
- Chen, C.H.; Terentjev, E.M. Aging and metastability of monoglycerides in hydrophobic solutions. Langmuir 2009, 25, 6717–6724. [Google Scholar] [CrossRef]
- Chen, C.H.; Van Damme, I.; Terentjev, E.M. Phase behavior of C18 monoglyceride in hydrophobic solutions. Soft Matter 2009, 5, 432–439. [Google Scholar] [CrossRef]
- Lopez-Martínez, A.; Charó-Alonso, M.A.; Marangoni, A.G.; Toro-Vazquez, J.F. Monoglyceride organogels developed in vegetable oil with and without ethylcellulose. Food Res. Int. 2015, 72, 37–46. [Google Scholar] [CrossRef]
- Pan, J.; Tang, L.; Dong, Q.; Li, Y.; Zhang, H. Effect of oleogelation on physical properties and oxidative stability of camellia oil-based oleogels and oleogel emulsions. Food Res. Int. 2021, 140, 110057. [Google Scholar] [CrossRef] [PubMed]
- Valoppi, F.; Calligaris, S.; Barba, L.; Šegatin, N.; Poklar Ulrih, N.; Nicoli, M.C. Influence of oil type on formation, structure, thermal, and physical properties of monoglyceride-based organogel. Eur. J. Lipid Sci. Technol. 2016, 119, 1500549. [Google Scholar] [CrossRef]
- Ferro, A.C.; Okuro, P.K.; Badan, A.P.; Cunha, R.L. Role of the oil on glyceryl monostearate based oleogels. Food Res. Int. 2019, 120, 610–619. [Google Scholar] [CrossRef]
- Zheng, H.; Deng, L.; Que, F.; Feng, F.; Zhang, H. Physical characterization and antimicrobial evaluation of glycerol monolaurate organogels. Colloid Surface 2016, 502, 19–25. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Wan, L.; Mao, L.; Li, B.; Zhang, X. Addition of glyceryl monostearate affects the crystallization behavior and polymorphism of palm stearin. Bioprocess Biosyst. Eng. 2021, 44, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Vereecken, J.; Meeussen, W.; Foubert, I.; Lesaffer, A.; Wouters, J.; Dewettinck, K. Comparing the crystallization and polymorphic behaviour of saturated and unsaturated monoglycerides. Food Res. Int. 2009, 42, 1415–1425. [Google Scholar] [CrossRef]
- Li, J.; Guo, R.; Bi, Y.; Zhang, H.; Xu, X. Comprehensive evaluation of saturated monoglycerides for the forming of oleogels. LWT 2021, 151, 112061. [Google Scholar] [CrossRef]
- Himawan, C.; Starov, V.M.; Stapley, A.G. Thermodynamic and kinetic aspects of fat crystallization. Adv. Colloid Interface Sci. 2006, 122, 3–33. [Google Scholar] [CrossRef]
- Omonov, T.S.; Bouzidi, L.; Narine, S.S. Quantification of oil binding capacity of structuring fats: A novel method and its application. Chem. Phys. Lipids. 2010, 163, 728–740. [Google Scholar] [CrossRef]
- Doan, C.D.; Van de Walle, D.; Dewettinck, K.; Patel, A.R. Evaluating the Oil-Gelling Properties of Natural Waxes in Rice Bran Oil: Rheological, Thermal, and Microstructural Study. JACS 2015, 92, 801–811. [Google Scholar] [CrossRef]
- Palla, C.; de Vicente, J.; Carrín, M.E.; Gálvez Ruiz, M.J. Effects of cooling temperature profiles on the monoglycerides oleogel properties: A rheo-microscopy study. Food Res. Int. 2019, 125, 108613. [Google Scholar] [CrossRef] [PubMed]
- Aliasl Khiabani, A.; Tabibiazar, M.; Roufegarinejad, L.; Hamishehkar, H.; Alizadeh, A. Preparation and characterization of carnauba wax/adipic acid oleogel: A new reinforced oleogel for application in cake and beef burger. Food Chem. 2020, 333, 127446. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Huang, Q.; Wu, J. Rheological behaviors of an exopolysaccharide from fermentation medium of a Cordyceps sinensis fungus (Cs-HK1). Carbohydr. Polym. 2014, 114, 506–513. [Google Scholar] [CrossRef]
- Moreira, H.R.; Munarin, F.; Gentilini, R.; Visai, L.; Granja, P.L.; Tanzi, M.C.; Petrini, P. Injectable pectin hydrogels produced by internal gelation: pH dependence of gelling and rheological properties. Carbohydr. Polym. 2014, 103, 339–347. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Shrestha, R.G.; Sharma, S.C.; Aramaki, K. Stabilization of nonaqueous foam with lamellar liquid crystal particles in diglycerol monolaurate/olive oil system. J. Colloid Interf. Sci. 2008, 328, 172–179. [Google Scholar] [CrossRef]
- Anandha Rao, M. Rheology of Fluid, Semisolid, and Solid Foods. Principles and Applications, 3rd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2014; p. xiii-461. [Google Scholar]
- Meng, Z.; Qi, K.; Guo, Y.; Wang, Y.; Liu, Y. Effects of thickening agents on the formation and properties of edible oleogels based on hydroxypropyl methyl cellulose. Food Chem. 2018, 246, 137–149. [Google Scholar] [CrossRef]
- Uslu, E.K.; Yılmaz, E. Preparation and characterization of oleogels and emulgels with glycerol monooleate–cholesterol mixtures. Chem. Pap. 2021, 75, 2075–2085. [Google Scholar] [CrossRef]
- Da Pieve, S.; Calligaris, S.; Panozzo, A.; Arrighetti, G.; Nicoli, M.C. Effect of monoglyceride organogel structure on cod liver oil stability. Food Res. Int. 2011, 44, 2978–2983. [Google Scholar] [CrossRef]
- Wettlaufer, T.; Flöter, E. Effect of Cooling Rate on the Oleogel Properties of Wax–Wax-Hydrolyzate Mixtures. Food Biophys. 2022, 17, 344–359. [Google Scholar] [CrossRef]
- Hartel, R.W. Advances in food crystallization. Annu. Rev. Food Sci. Technol. 2013, 4, 277–292. [Google Scholar] [CrossRef] [PubMed]
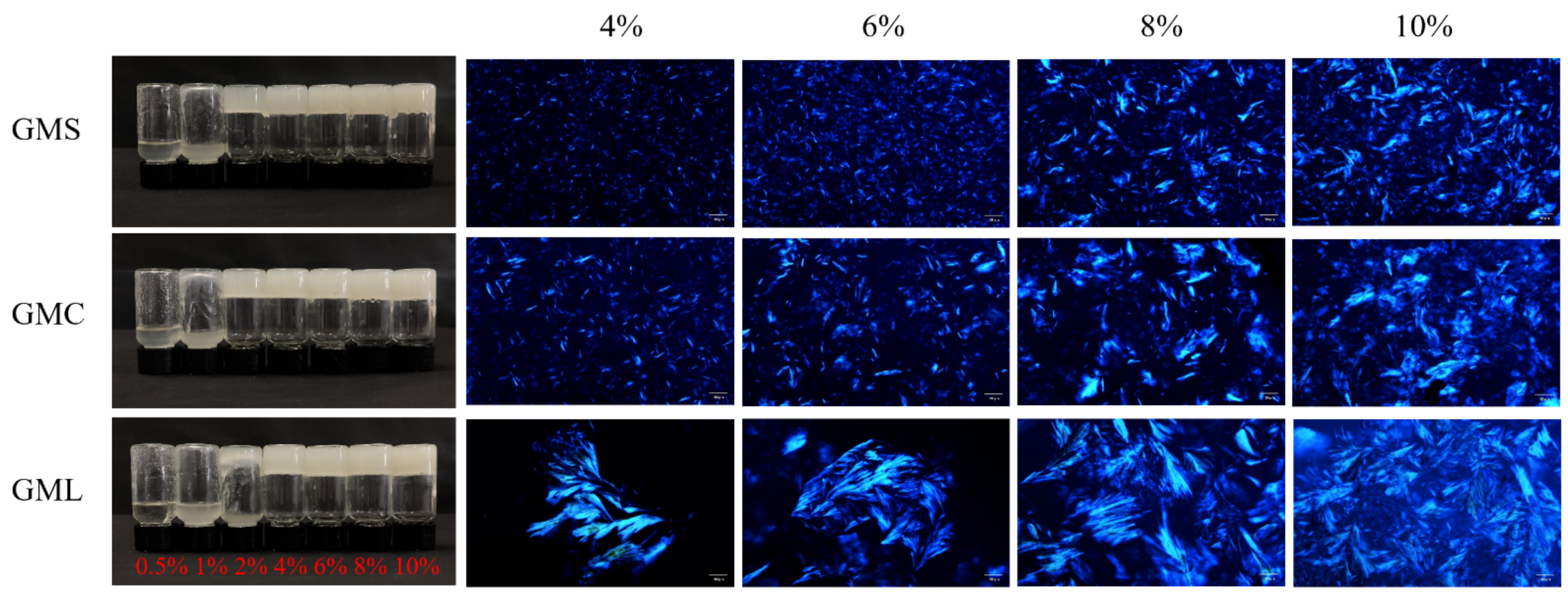
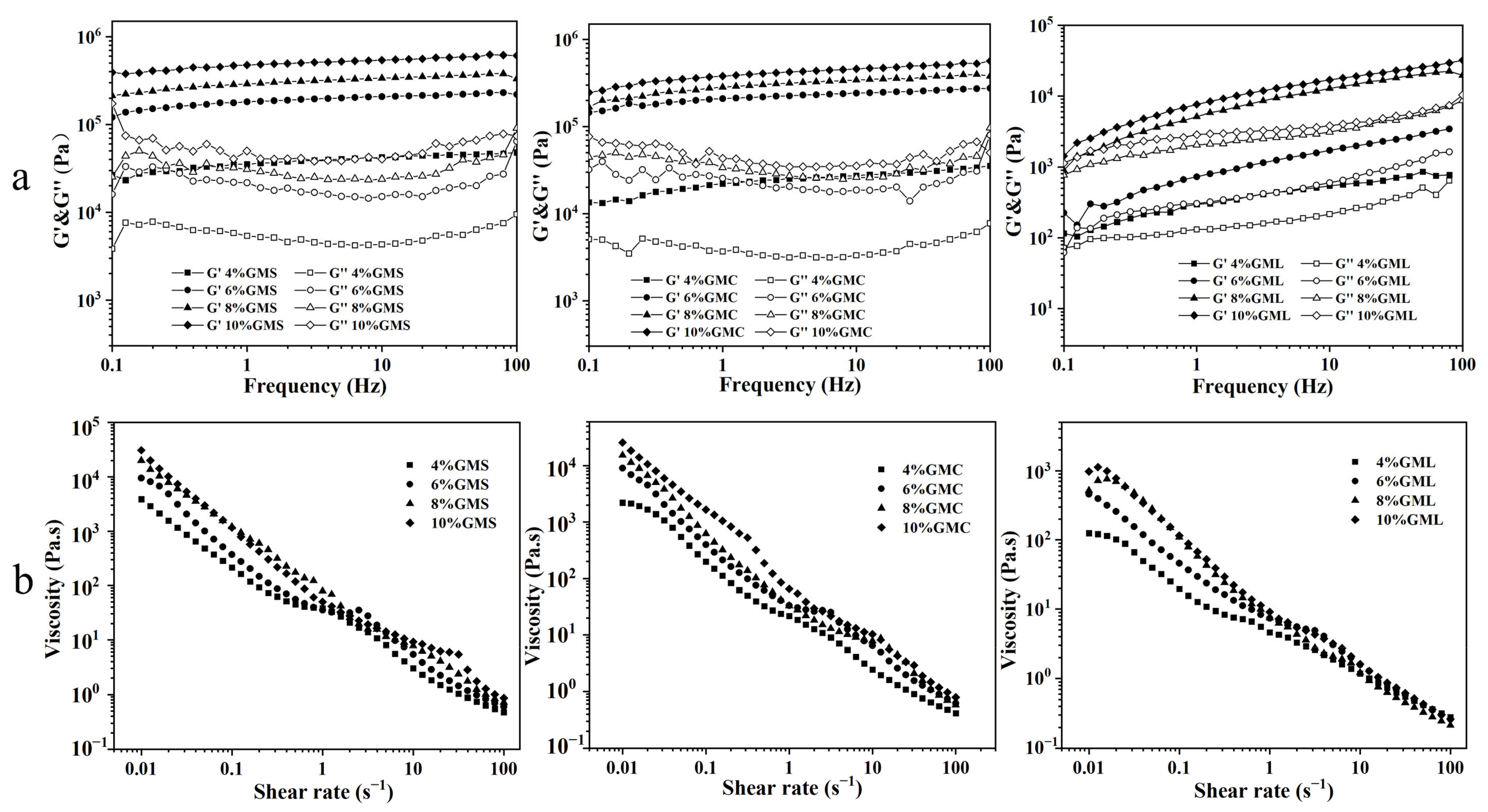
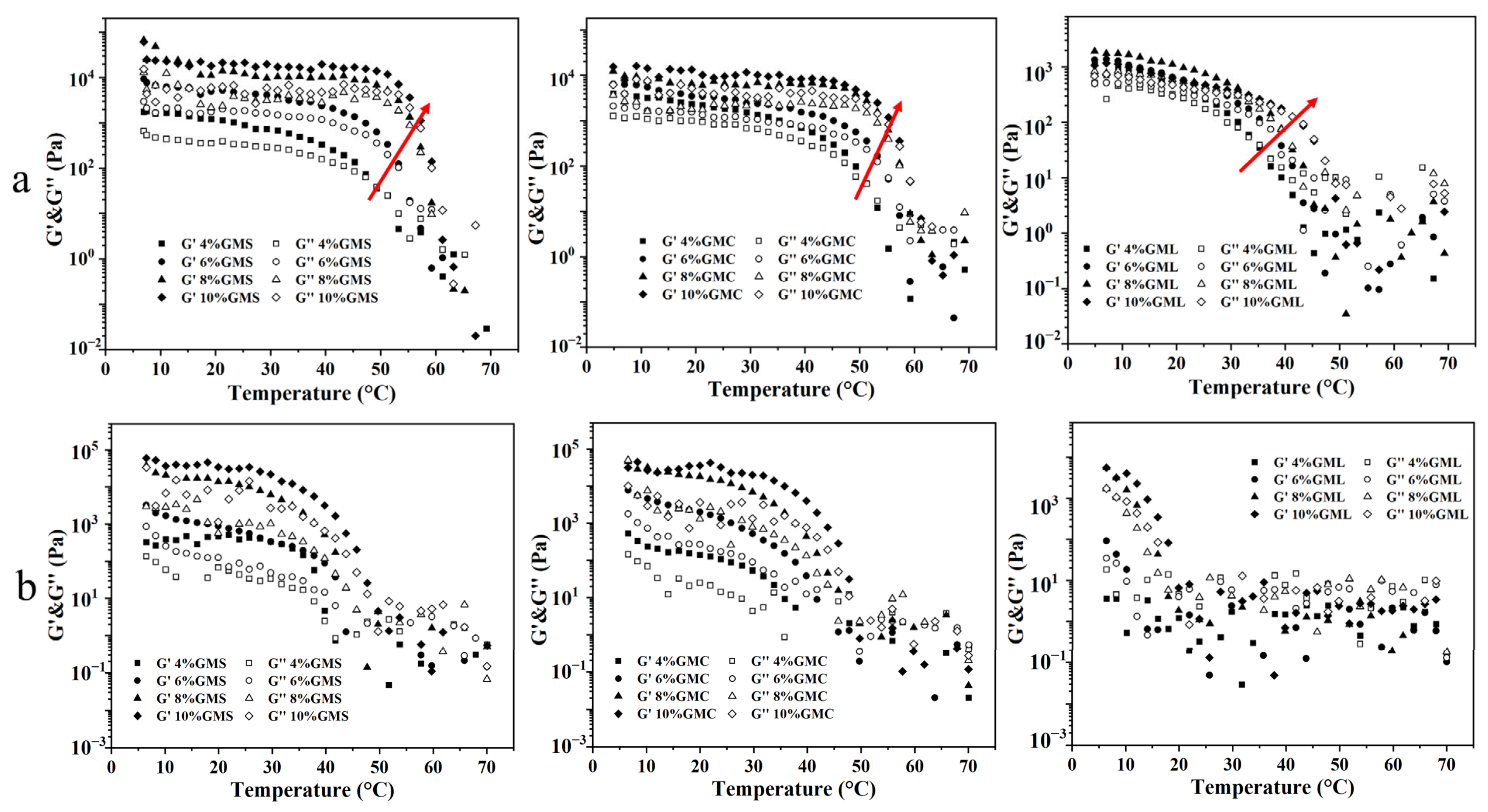


| Sample | OBC (%) | Hardness (g) | ||||
|---|---|---|---|---|---|---|
| GMS | GMC | GML | GMS | GMC | GML | |
| 4% | 79.88 ± 1.18 a | 77.06 ± 0.74 a | 46.51 ± 2.13 a | 20.29 ± 1.86 a | 16.15 ± 0.51 a | 7.73 ± 0.45 a |
| 6% | 93.61 ± 0.55 b | 85.11 ± 0.25 b | 58.39 ± 1.88 b | 47.47 ± 4.83 b | 48.62 ± 0.69 b | 18.98 ± 0.44 b |
| 8% | 98.22 ± 0.50 c | 92.10 ± 1.95 c | 65.07 ± 0.86 c | 92.16 ± 4.19 c | 77.59 ± 3.18 c | 32.24 ± 2.19 c |
| 10% | 98.06 ± 0.21 c | 93.75 ± 2.13 c | 74.85 ± 2.35 d | 106.25 ± 2.45 d | 98.75 ± 1.21 d | 40.23 ± 0.56 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xu, J.; Tang, C.; Li, Y. Crystallization Behavior and Physical Properties of Monoglycerides-Based Oleogels as Function of Oleogelator Concentration. Foods 2023, 12, 345. https://doi.org/10.3390/foods12020345
Zhang Y, Xu J, Tang C, Li Y. Crystallization Behavior and Physical Properties of Monoglycerides-Based Oleogels as Function of Oleogelator Concentration. Foods. 2023; 12(2):345. https://doi.org/10.3390/foods12020345
Chicago/Turabian StyleZhang, Yingzhu, Jinqi Xu, Cuie Tang, and Yan Li. 2023. "Crystallization Behavior and Physical Properties of Monoglycerides-Based Oleogels as Function of Oleogelator Concentration" Foods 12, no. 2: 345. https://doi.org/10.3390/foods12020345
APA StyleZhang, Y., Xu, J., Tang, C., & Li, Y. (2023). Crystallization Behavior and Physical Properties of Monoglycerides-Based Oleogels as Function of Oleogelator Concentration. Foods, 12(2), 345. https://doi.org/10.3390/foods12020345





