Docosahexaenoic Acid Stability in Ready-to-Use Therapeutic Food
Abstract
1. Introduction
2. Materials and Methods
2.1. DHA Materials
2.2. RUTF Essential Fatty Acid Reformulation
2.3. DHA Stability in Stored RUTF
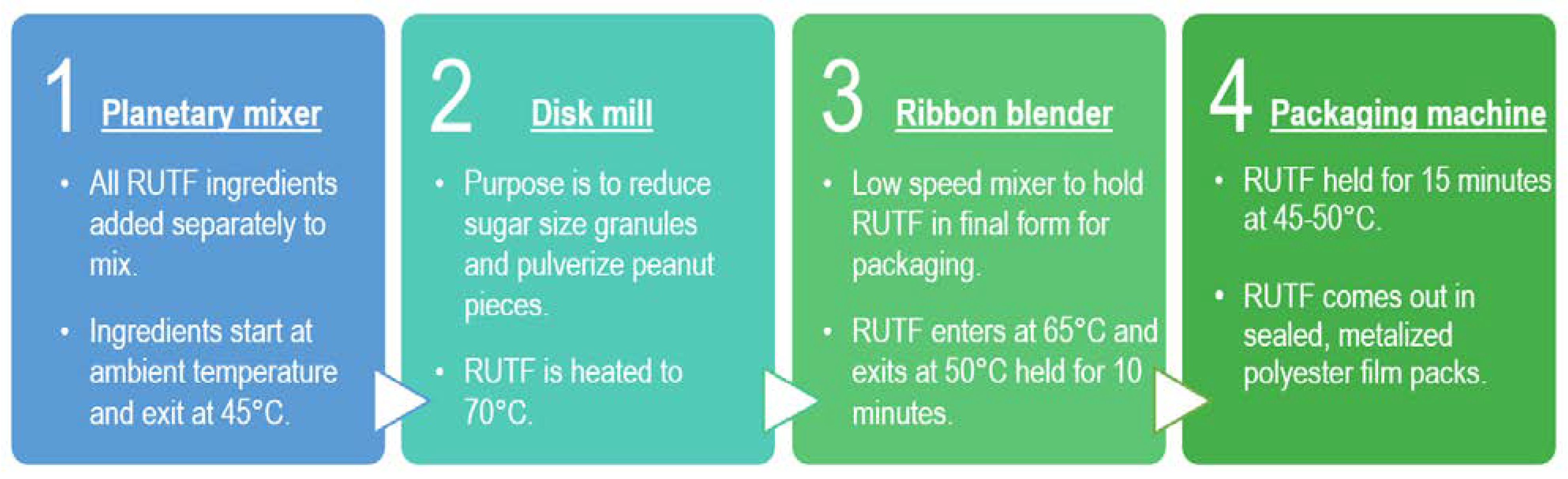
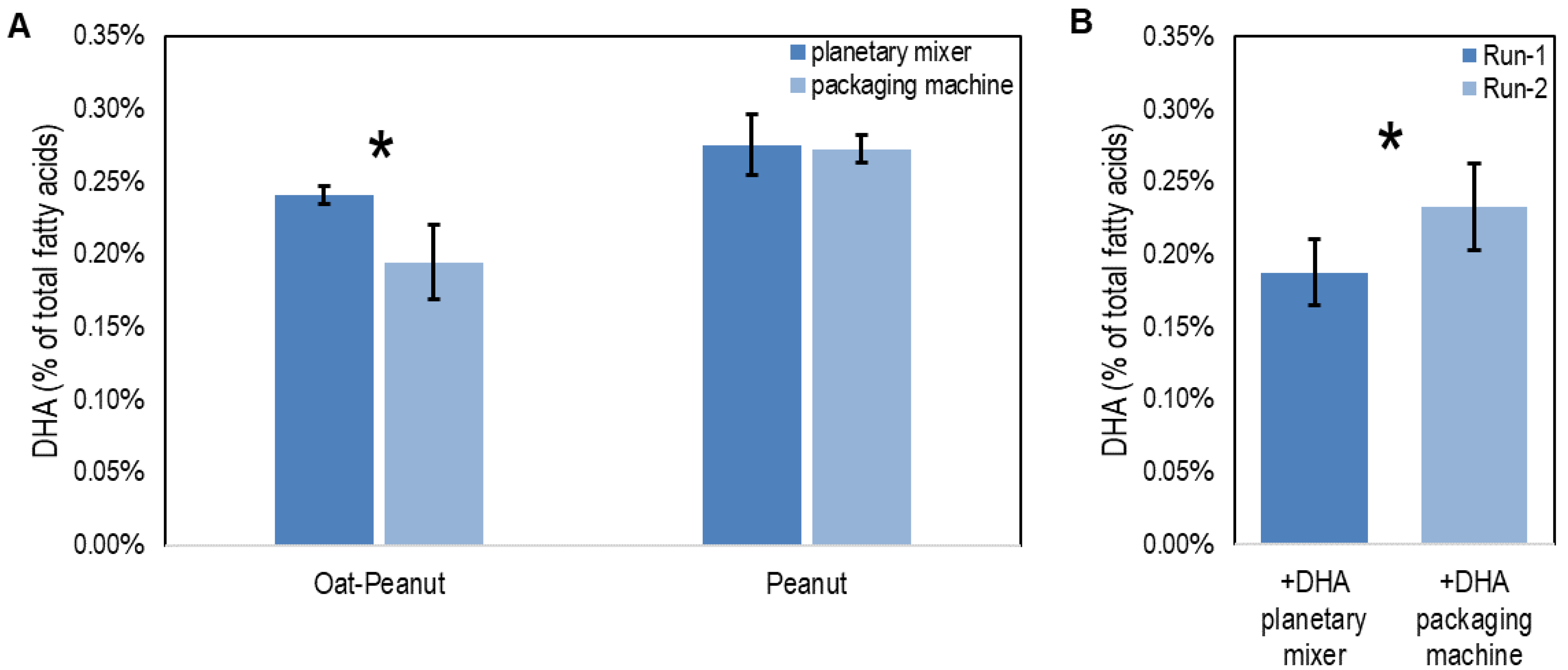
2.4. DHA Stability in the RUTF Manufacturing Process
2.5. Fatty Acid Analysis
2.6. Statistical Analysis
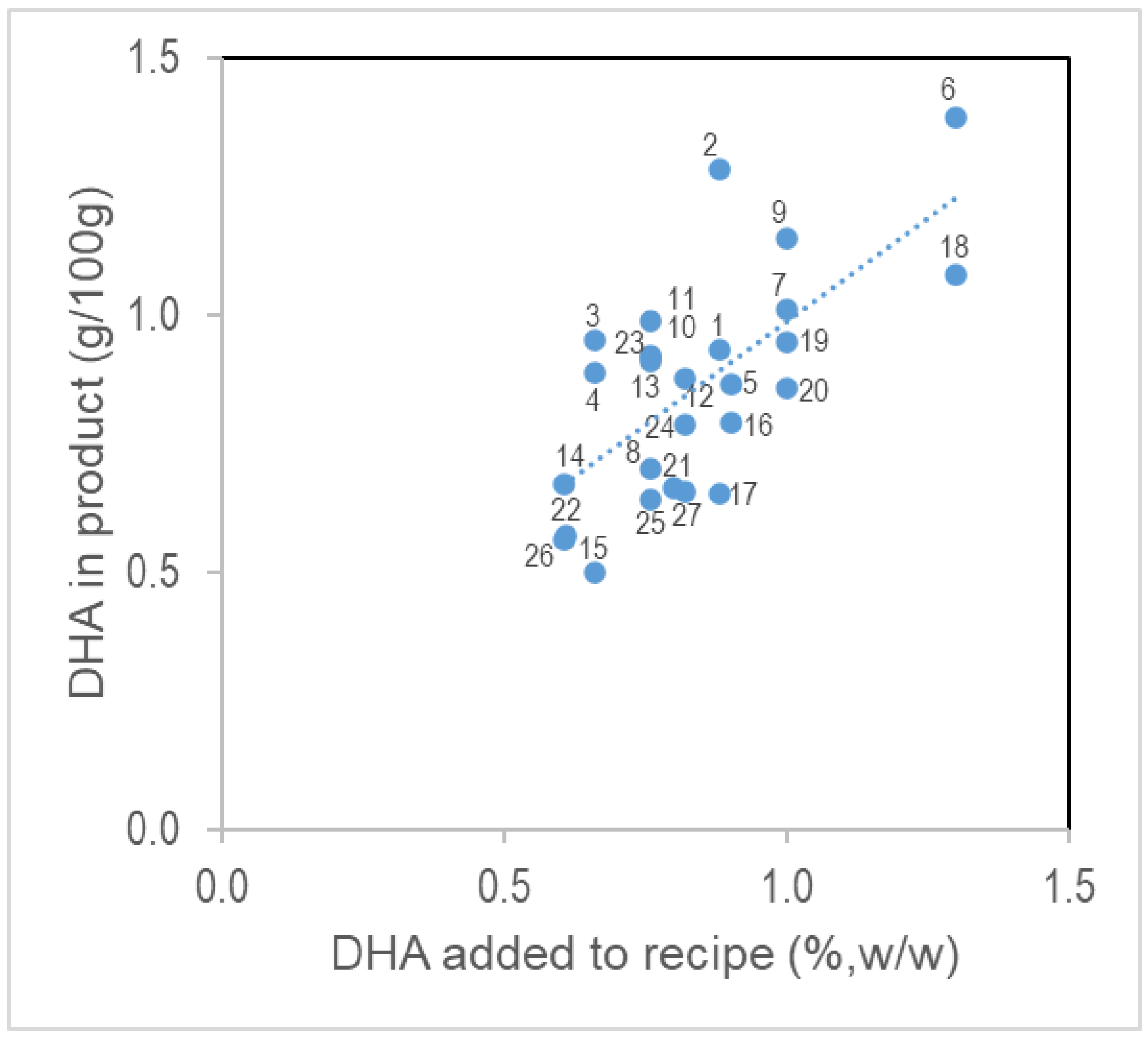
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanyero, B.; Namusoke, H.; Nabukeera-Barungi, N.; Grenov, B.; Mupere, E.; Michaelsen, K.F.; Mølgaard, C.; Christensen, V.B.; Friis, H.; Briend, A. Transition from F-75 to ready-to-use therapeutic food in children with severe acute malnutrition, an observational study in Uganda. Nutr. J. 2017, 16, 52. [Google Scholar] [CrossRef] [PubMed]
- UNICEF; WHO; World Bank Group. Levels and Trends in Child Malnutrition: Key Findings of the 2019 Edition; UNICEF, WHO, and the World Bank Group Joint Child Malnutrition Estimates. 2019. Available online: https://www.who.int/publications/i/item/WHO-NMH-NHD-19.20 (accessed on 10 November 2022).
- Moustiés, C.; Bourlieu-Lacanal, C.; Hemery, Y.M.; Baréa, B.; Villeneuve, P.; Servent, A.; Alter, P.; Lebrun, M.; Laillou, A.; Wieringa, F.T.; et al. Nutritional quality of ready-to-use therapeutic foods: Focus on lipid composition and vitamin content. OCL 2022, 29, 13. [Google Scholar] [CrossRef]
- Report of the 42nd Session of the Codex Committee on Nutrition and Foods for Special Dietary Uses. 2021. Available online: https://www.usda.gov/sites/default/files/documents/ccnfsdu-delegates-report.pdf (accessed on 10 November 2022).
- Brenna, J.T.; Salem, N.; Sinclair, A.J.; Cunnane, S.C. α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.-C.; Liu, L.; Zeilani, M.; Ickes, S.; Trehan, I.; Maleta, K.; Craig, C.; Thakwalakwa, C.; Singh, L.; Brenna, J.T.; et al. High-oleic ready-to-use therapeutic food maintains docosahexaenoic acid status in severe malnutrition. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Akomo, P.; Bahwere, P.; Berkley, J.A.; Calder, P.C.; Jones, K.D.; Liu, L.; Manary, M.; Trehan, I.; Briend, A. Balancing omega-6 and omega-3 fatty acids in ready-to-use therapeutic foods (RUTF). BMC Med. 2015, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, K.; Callaghan-Gillespie, M.; Maleta, K.; Nkhoma, M.; George, M.; Park, H.G.; Lee, R.; Humphries-Cuff, I.; Lacombe, R.J.S.; Wegner, D.R.; et al. Low linoleic acid foods with added DHA given to Malawian children with severe acute malnutrition improve cognition: A randomized, triple-blinded, controlled clinical trial. Am. J. Clin. Nutr. 2022, 115, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture (USDA). FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 10 November 2022).
- Sittiprapaporn, P.; Bumrungpert, A.; Suyajai, P.; Stough, C. Effectiveness of fish Oil-DHA supplementation for cognitive function in Thai children: A randomized, doubled-blind, two-dose, placebo-controlled clinical trial. Foods 2022, 11, 2595. [Google Scholar] [CrossRef]
- Wallace, J.M.; McCabe, A.J.; Robson, P.J.; Keogh, M.K.; Murray, C.A.; Kelly, P.M.; Márquez-Ruiz, G.; McGlynn, H.; Gilmore, W.S.; Strain, J.J. Bioavailability of n-3 polyunsaturated fatty acids (PUFA) in foods enriched with microencapsulated fish oil. Ann. Nutr. Metab. 2000, 44, 157–162. [Google Scholar] [CrossRef]
- Sanguansri, L.; Augustin, M.A.; Lockett, T.J.; Abeywardena, M.Y.; Royle, P.J.; Mano, M.T.; Patten, G.S. Bioequivalence of n-3 fatty acids from microencapsulated fish oil formulations in human subjects. Br. J. Nutr. 2015, 113, 822–831. [Google Scholar] [CrossRef]
- Zuzarte, A.; Mui, M.; Ordiz, M.I.; Weber, J.; Ryan, K.; Manary, M.J. Reducing oil separation in ready-to-use therapeutic food. Foods 2020, 9, 706. [Google Scholar] [CrossRef]
- Garces, R.; Mancha, M. One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal. Biochem. 1993, 211, 139–143. [Google Scholar] [CrossRef]
- Zhou, Y.; Nijland, M.; Miller, M.; Ford, S.; Nathanielsz, P.W.; Brenna, J.T. The influence of maternal early to mid-gestation nutrient restriction on long chain polyunsaturated fatty acids in fetal sheep. Lipids 2008, 43, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T. Animal studies of the functional consequences of suboptimal polyunsaturated fatty acid status during pregnancy, lactation and early post-natal life. Matern. Child Nutr. 2011, 7, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Varamini, B.; Jensen, R.G.; Diersen-Schade, D.A.; Boettcher, J.A.; Arterburn, L.M. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Babirekere-Iriso, E.; Mortensen, C.G.; Mupere, E.; Rytter, M.J.H.; Namusoke, H.; Michaelsen, K.F.; Briend, A.; Stark, K.D.; Friis, H.; Lauritzen, L. Changes in whole-blood PUFA and their predictors during recovery from severe acute malnutrition. Br. J. Nutr. 2016, 115, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.D.; Ali, R.; Khasira, M.A.; Odera, D.; West, A.L.; Koster, G.; Akomo, P.; Talbert, A.W.; Goss, V.M.; Ngari, M.; et al. Ready-to-use therapeutic food with elevated n-3 polyunsaturated fatty acid content, with or without fish oil, to treat severe acute malnutrition: A randomized controlled trial. BMC Med. 2015, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.T. The slow discovery of the importance of omega 3 essential fatty acids in human health. J. Nutr. 1998, 128, 427s–433s. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.T. Biological activities of and requirements for polyunsaturated acids. Prog. Chem. Fats Other Lipids 1971, 9, 607–682. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Kothapalli, K.S.; Brenna, J.T. Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 103–110. [Google Scholar] [CrossRef]
- Lattka, E.; Illig, T.; Koletzko, B.; Heinrich, J. Genetic variants of the FADS1 FADS2 gene cluster as related to essential fatty acid metabolism. Curr. Opin. Lipidol. 2010, 21, 64–69. [Google Scholar] [CrossRef]
- Hibbeln, J.R.; Nieminen, L.R.; Blasbalg, T.L.; Riggs, J.A.; Lands, W.E. Healthy intakes of n-3 and n-6 fatty acids: Estimations considering worldwide diversity. Am. J. Clin. Nutr. 2006, 83, 1483s–1493s. [Google Scholar] [CrossRef] [PubMed]
- Riveros, C.G.; Mestrallet, M.G.; Gayol, M.F.; Quiroga, P.R.; Nepote, V.; Grosso, N.R. Effect of storage on chemical and sensory profiles of peanut pastes prepared with high-oleic and normal peanuts. J. Sci. Food Agric. 2010, 90, 2694–2699. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.B.; Ciappolino, V.; Agostoni, C. DHA effects in brain development and function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 1992, 120, S129–S138. [Google Scholar] [CrossRef]
- Brenna, J.T. Long-chain polyunsaturated fatty acids and the preterm infant: A case study in developmentally sensitive nutrient needs in the United States1–4. Am. J. Clin. Nutr. 2016, 103, 606S–615S. [Google Scholar] [CrossRef]
- Marín, M.C.; Rey, G.E.; Pedersolí, L.C.; Rodrigo, M.A.; de Alaniz, M.J. Dietary long-chain fatty acids and visual response in malnourished nursing infants. Prostaglandins Leukot Essent Fat. Acids 2000, 63, 385–390. [Google Scholar] [CrossRef]
- Lelijveld, N.; Jalloh, A.A.; Kampondeni, S.D.; Seal, A.; Wells, J.C.; Goyheneix, M.; Chimwezi, E.; Mallewa, M.; Nyirenda, M.J.; Heyderman, R.S.; et al. Brain MRI and cognitive function seven years after surviving an episode of severe acute malnutrition in a cohort of Malawian children. Public Health Nutr. 2019, 22, 1406–1414. [Google Scholar] [CrossRef]
| Nutrient | % Total Energy | kcal/100 g | g/100 g | mg/100 kcal |
|---|---|---|---|---|
| Energy | 520–550 | |||
| Lipids | 45–60 | 234–330 | 26–36.7 | 5–7 |
| n-6 fatty acids | 2.9–6.7 | 15.3–36.9 | 1.7–4.1 | 330–780 |
| n-3 fatty acids | 1–2.5 | 5.4–13.5 | 0.6–1.5 | 110–280 |
| Code | Encapsulation Source Material | Source of Formulation Ingredients | Allergens Beyond Fish | Storage and Stability | Product |
|---|---|---|---|---|---|
| A | Sucrose | modified food starch, mixed natural tocopherols, sucrose | mixed tocopherols | Sensitive to air, heat, light, and humidity. May be stored for 24 months from the date of manufacture in an unopened original container (which is sealed under inert gas) and at temperatures below 25 °C (77 °F). Once open use contents quickly. | MEG-3® ‘15’ n-3 High DHA Powder S/SD |
| B | Fish gelatin | sunflower oil, mixed natural tocopherals | mixed tocopherols, soybean oil | Unopened packages in refrigerated conditions 36–46 °F for up to 18 months. Open packages in refrigerated conditions up to 1 week. | MEG-3™ DHA rf Powder |
| C | Dairy | sodium caseinate, dextrose monohydrate, dried glucose syrup | contains milk, soy, and fish | May be stored for 24 months from the data of manufacture in the unopened original package in dry, cool conditions (10–25 °C). After opening bag, the gas headspace should be flushed with inert gas prior to being resealed. Contents of resealed bag should be used within 3 months after resealing. | Nu-Mega Driphorm HiDHA 50 |
| D | Fish oil Concentrate | concentrated wild Alaskan pollock oil omega-3 triglyceride, natural mixed tocopherols | mixed tocopherols | May be stored for three years from the date of manufacture in an unopened original container (which is sealed under inert gas) and at temperatures below 25 °C (77 °F). Once open use contents quickly. | AlaskOmega TG 230460 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
James, G.; Stephenson, K.; Callaghan-Gillespie, M.; Kamara, M.T.; Park, H.G.; Brenna, J.T.; Manary, M.J. Docosahexaenoic Acid Stability in Ready-to-Use Therapeutic Food. Foods 2023, 12, 308. https://doi.org/10.3390/foods12020308
James G, Stephenson K, Callaghan-Gillespie M, Kamara MT, Park HG, Brenna JT, Manary MJ. Docosahexaenoic Acid Stability in Ready-to-Use Therapeutic Food. Foods. 2023; 12(2):308. https://doi.org/10.3390/foods12020308
Chicago/Turabian StyleJames, Genevieve, Kevin Stephenson, Meghan Callaghan-Gillespie, Mohamed Tabita Kamara, Hui Gyu Park, J. Thomas Brenna, and Mark J. Manary. 2023. "Docosahexaenoic Acid Stability in Ready-to-Use Therapeutic Food" Foods 12, no. 2: 308. https://doi.org/10.3390/foods12020308
APA StyleJames, G., Stephenson, K., Callaghan-Gillespie, M., Kamara, M. T., Park, H. G., Brenna, J. T., & Manary, M. J. (2023). Docosahexaenoic Acid Stability in Ready-to-Use Therapeutic Food. Foods, 12(2), 308. https://doi.org/10.3390/foods12020308





