Streptococcus thermophilus JM905—Strain Carbon Source Utilization and Its Fermented Milk Metabolic Profile at Different Fermentation Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain, Culture Medium, and Growth Conditions
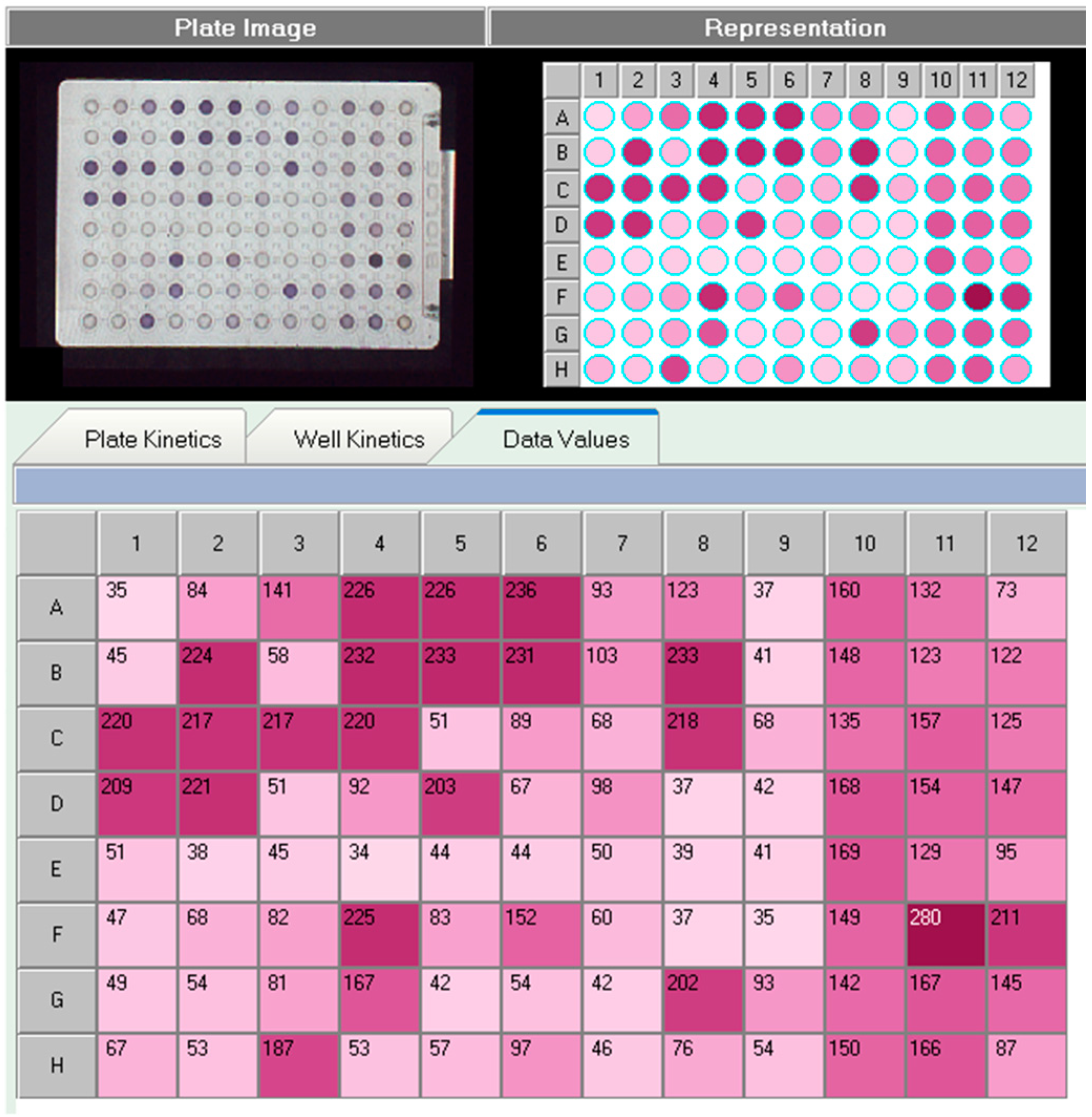
2.2. Utilization of Carbon Sources by the Strains
2.3. Fermented Milk Sample Preparation
2.4. Physico-Chemical Analysis
2.4.1. Fermented Milk pH, Acidity, and Post-Acidification
2.4.2. Fermented Milk Water-Holding Capacity, Viscosity, and Viable Bacteria Count
2.4.3. The Odor and Taste of Fermented Milk
2.5. Determination of Fermented Milk Protein and Free Amino Acids
2.6. Determination of Fermented Milk Fat and Fatty Acids
2.7. Sensory Evaluation
2.8. Metabolic Composition of Fermented Milk
2.9. Data Analysis
3. Results
3.1. Utilization of Carbon Sources by Strains
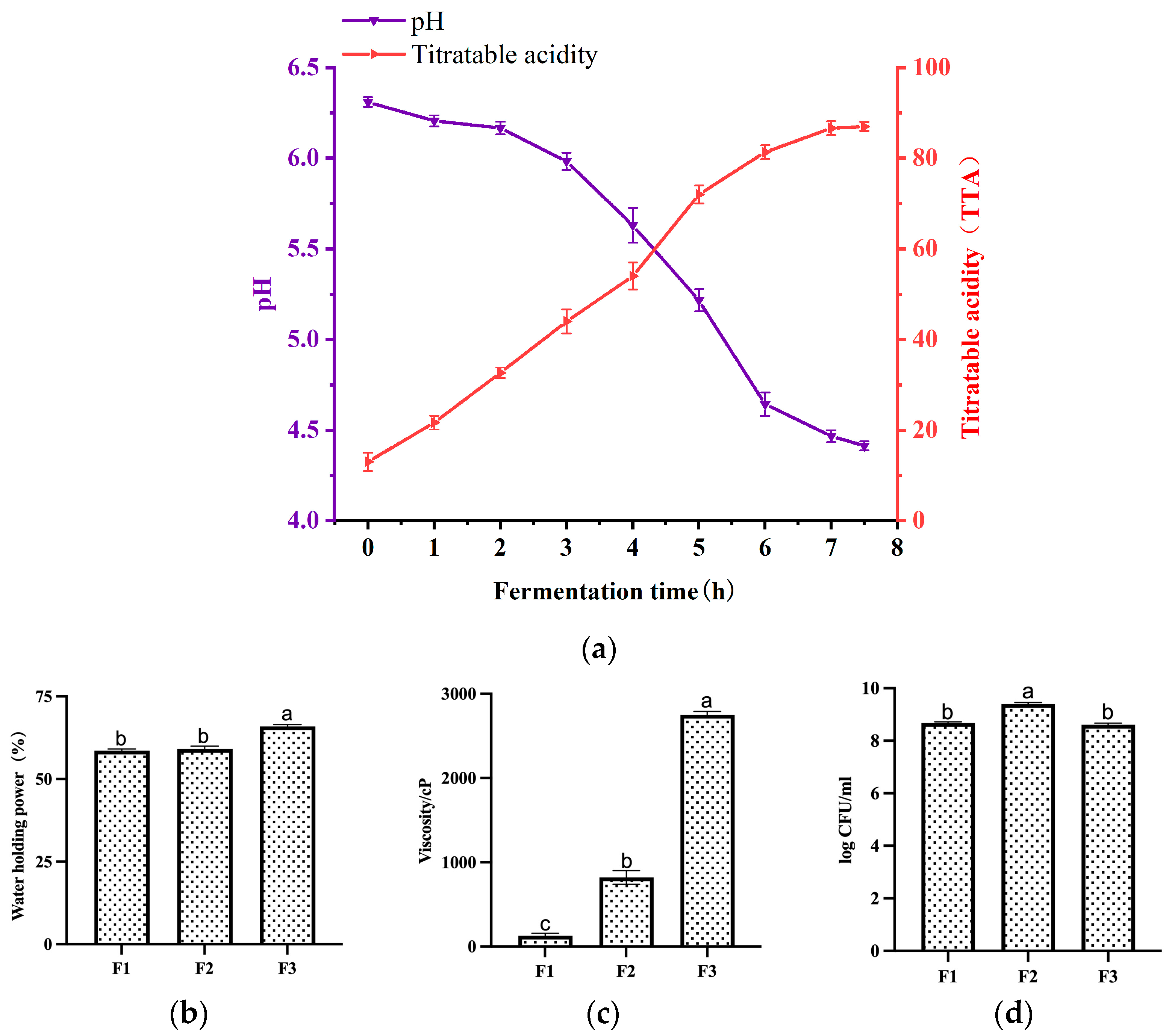
3.2. Changes in pH and Acidity during Fermentation
3.3. Changes in Water-Holding Capacity, Viscosity, and Viable Bacteria Count of Fermented Milk at Different Fermentation Stages
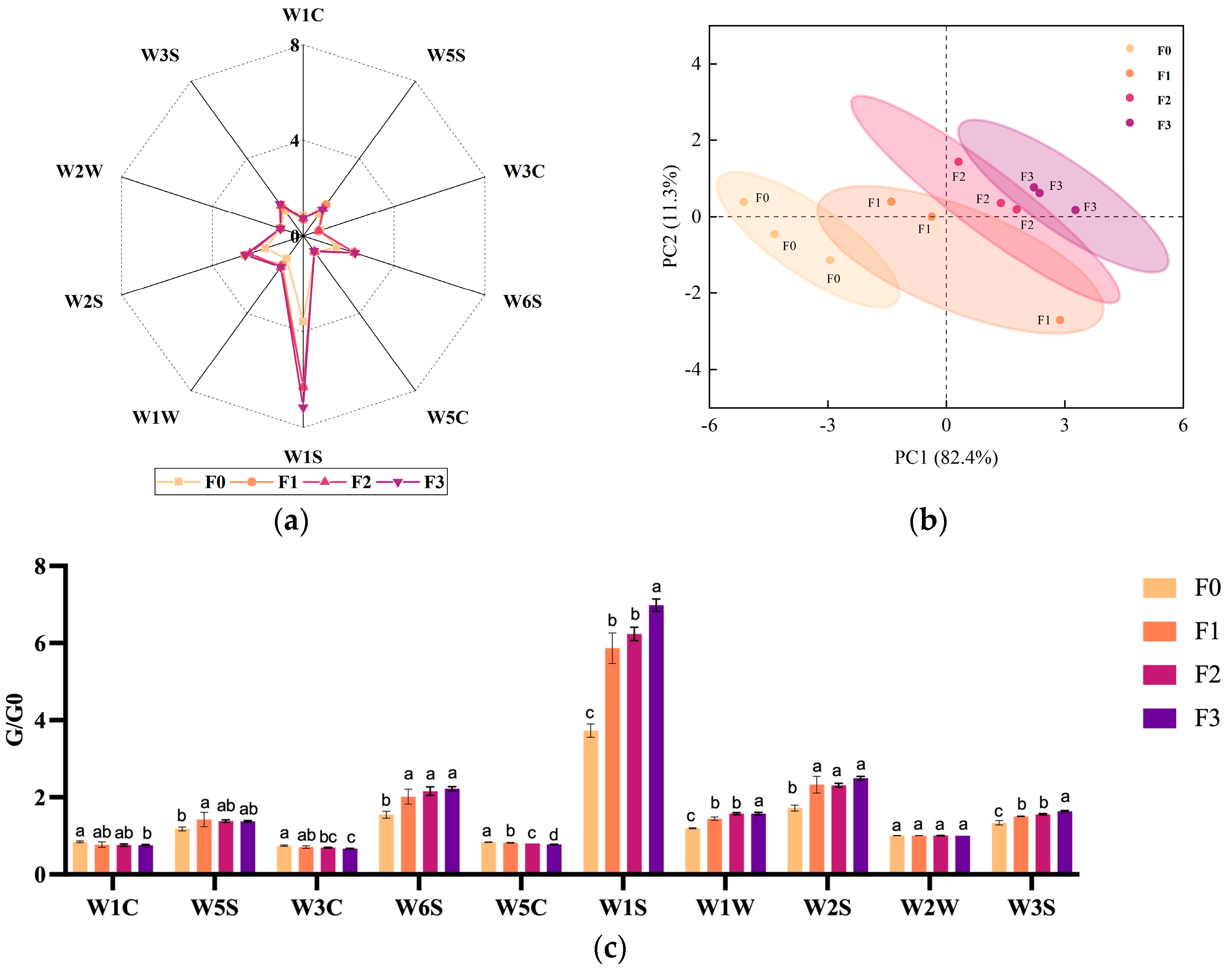
3.4. Changes in Odor and Taste of Fermented Milk
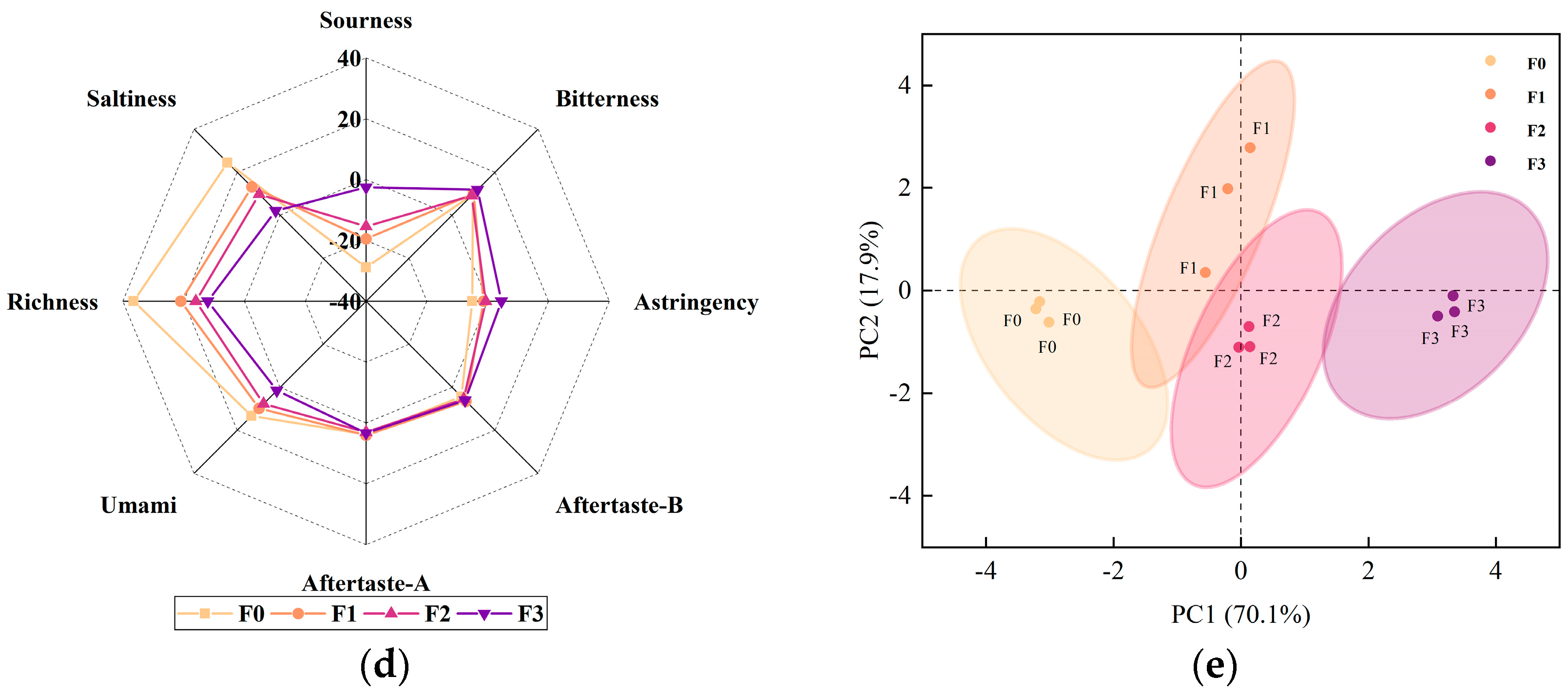
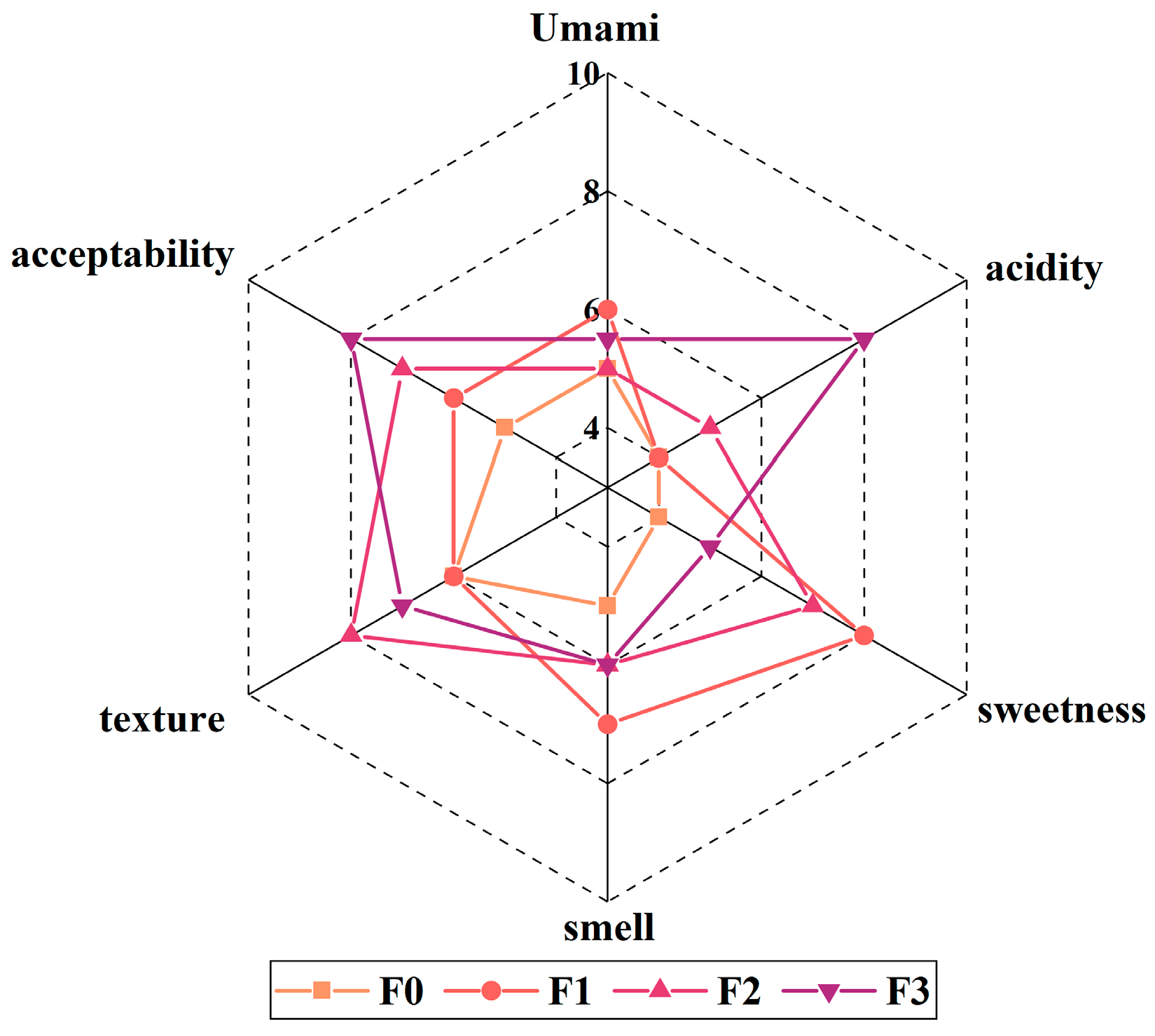
3.5. Sensory Evaluation
3.6. Fermented Milk Protein and Free Amino Acid Content
3.7. Fat and Fatty Acid Content of Fermented Milk
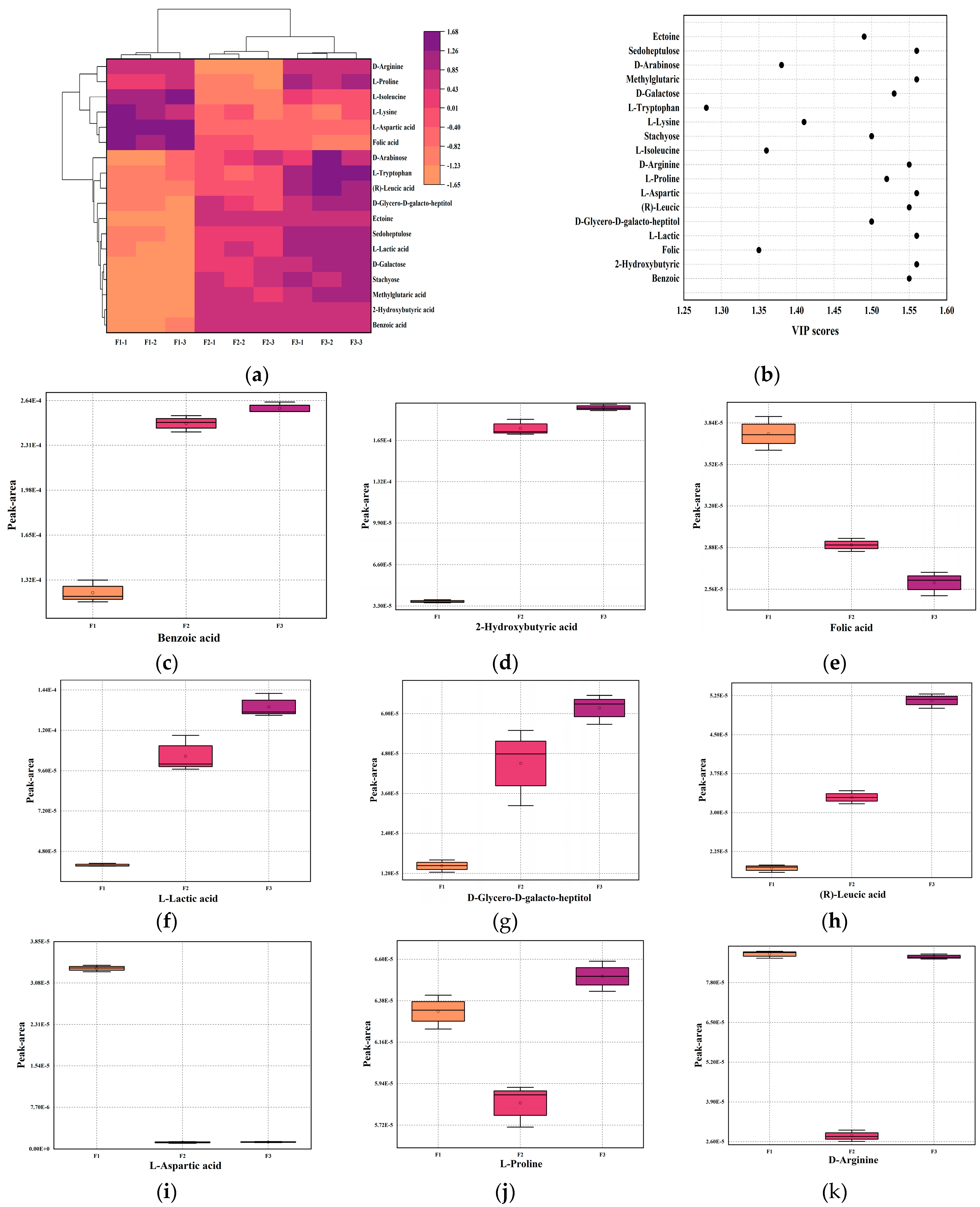
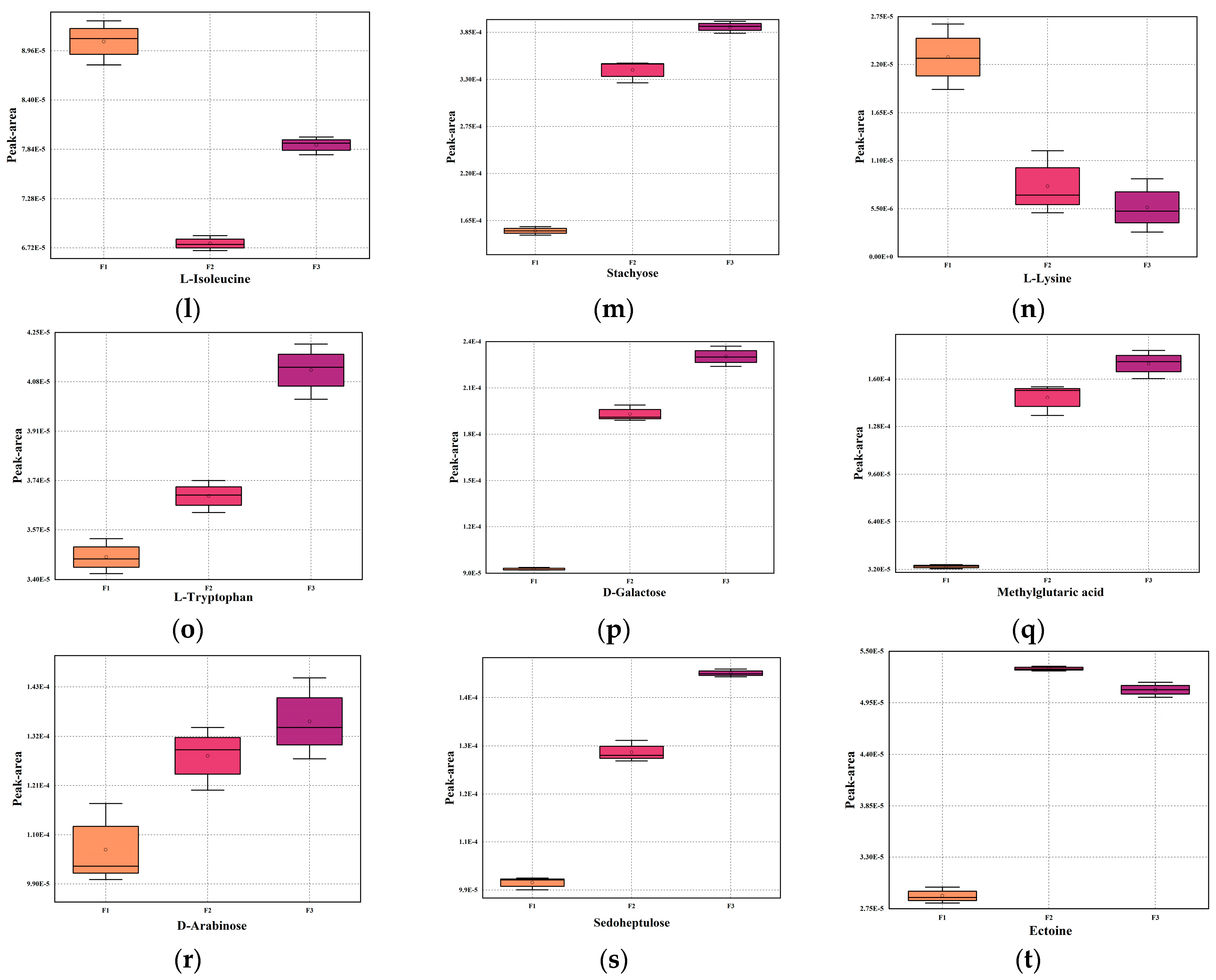
3.8. Changes in Key Metabolites of Fermented Milk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sakandar, H.A.; Zhang, H. Trends in Probiotic(s)-Fermented milks and their in vivo functionality: A review. Trends Food Sci. Technol. 2021, 110, 55–65. [Google Scholar] [CrossRef]
- Narvhus, J.A.; Abrahamsen, R.K. Traditional and modern Nordic fermented milk products: A review. Int. Dairy J. 2023, 142, 105641. [Google Scholar] [CrossRef]
- Mallappa, R.H.; Balasubramaniam, C.; Nataraj, B.H.; Ramesh, C.; Kadyan, S.; Pradhan, D.; Muniyappa, S.K.; Grover, S. Microbial diversity and functionality of traditional fermented milk products of India: Current scenario and future perspectives. Int. Dairy J. 2021, 114, 104941. [Google Scholar] [CrossRef]
- Martin, N.H.; Evanowski, R.L.; Wiedmann, M. Invited review: Redefining raw milk quality—Evaluation of raw milk microbiological parameters to ensure high-quality processed dairy products. J. Dairy Sci. 2023, 106, 1502–1517. [Google Scholar] [CrossRef]
- Zebib, H.; Abate, D.; Woldegiorgis, A.Z. Nutritional quality and adulterants of cow raw milk, pasteurized and cottage cheese collected along value chain from three regions of Ethiopia. Heliyon 2023, 9, e15922. [Google Scholar] [CrossRef]
- Polanowska, K.; Grygier, A.; Kuligowski, M.; Rudzińska, M.; Nowak, J. Effect of tempe fermentation by three different strains of Rhizopus oligosporus on nutritional characteristics of faba beans. LWT 2020, 122, 109024. [Google Scholar] [CrossRef]
- Shen, X.; Li, W.; Cai, H.; Guo, S.; Li, M.; Liu, Y.; Sun, Z. Metabolomics analysis reveals differences in milk metabolism and fermentation rate between individual Lactococcus lactis subsp. lactis strains. Food Res. Int. 2022, 162, 111920. [Google Scholar] [CrossRef]
- Hossain, S.; Khetra, Y.; Dularia, C.; Meena, G.S.; Arora, S. Symbiotic fermentation study of Acetobacter orientalis and lactic acid bacteria for lactobionic acid enriched yoghurt production. Food Biosci. 2023, 53, 102612. [Google Scholar] [CrossRef]
- Vijaya Vahini, R.; Lamiya, F.; Sowmya, C. A study on preparation and quality assessment of fermented banana blossom (Musa Acuminate Colla). Food Humanit. 2023, 1, 1188–1193. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Guo, S.; Sun, Y.; Kwok, L.-Y.; Zhang, H.; Peng, C. Comparison of the effects of single probiotic strains Lactobacillus casei Zhang and Bifidobacterium animalis ssp. lactis Probio-M8 and their combination on volatile and nonvolatile metabolomic profiles of yogurt. J. Dairy Sci. 2021, 104, 7509–7521. [Google Scholar] [CrossRef]
- Evivie, S.; Ogwu, M.; Evivie, E.; Md, M.; Huo, G. PBT-001—Unraveling the genomic, antibiotic susceptibility, and antioxidant properties of the dairy starter Streptococcus thermophilus SMQ-301. Int. J. Antimicrob. Agents 2021, 58, 21003216. [Google Scholar] [CrossRef]
- Zhuo, J.; Xuan, J.; Chen, Y.; Tu, J.; Mu, H.; Wang, J.; Liu, G. Increase of γ-aminobutyric acid content and improvement of physicochemical characteristics of mulberry leaf powder by fermentation with a selected lactic acid bacteria strain. LWT 2023, 187, 115250. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, R.; Zhao, J.; Li, C.; Guo, T.; Yang, S.; Han, T.; Kong, J. Fast-acidification promotes GABA synthesis in response to acid stress in Streptococcus thermophilus. LWT 2022, 164, 113671. [Google Scholar] [CrossRef]
- Xia, W.; Han, J.; Zhu, S.; Wang, Y.; Zhang, W.; Wu, Z. Structural elucidation of the exopolysaccharide from Streptococcus thermophilus XJ53 and the effect of its molecular weight on immune activity. Int. J. Biol. Macromol. 2023, 230, 123177. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, W.; Guo, S.; Sun, Y.; Yao, K.; Liu, Z.; Sun, Z.; Kwok, L.-Y.; Peng, C. Different growth behaviors and metabolomic profiles in yogurts induced by multistrain probiotics of Lactobacillus casei Zhang and Bifidobacterium lactis V9 under different fermentation temperatures. J. Dairy Sci. 2021, 104, 10528–10539. [Google Scholar] [CrossRef]
- Shi, Z.; Fan, X.; Tu, M.; Wu, Z.; Pan, D. Comparison of changes in fermented milk quality due to differences in the proteolytic system between Lactobacillus helveticus R0052 and Lactococcus lactis subsp. lactis JCM5805. Food Biosci. 2023, 51, 102271. [Google Scholar] [CrossRef]
- Al-Dhabaan, F.A.M.; Bakhali, A.H. Analysis of the bacterial strains using Biolog plates in the contaminated soil from Riyadh community. Saudi J. Biol. Sci. 2017, 24, 901–906. [Google Scholar] [CrossRef]
- Sun, M.; Yu, J.; Song, Y.; Li, X.; Mu, G.; Tuo, Y. Metabolomic analysis of fermented milk with Lactobacillus delbrueckii subsp. bulgaricus, Lacticaseibacillus paracasei cocultured with Kluyveromyces marxianus during storage. Food Biosci. 2023, 54, 102901. [Google Scholar] [CrossRef]
- Khakhariya, R.; Sakure, A.A.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Padhi, S.; Rai, A.K.; Liu, Z.; Patil, G.B.; Mankad, M.; et al. A comparative study of fermented buffalo and camel milk with anti-inflammatory, ACE-inhibitory and anti-diabetic properties and release of bio active peptides with molecular interactions: In vitro, in silico and molecular study. Food Biosci. 2023, 52, 102373. [Google Scholar] [CrossRef]
- Lao, L.; Yang, G.; Zhang, A.; Liu, L.; Guo, Y.; Lian, L.; Pan, D.; Wu, Z. Anti-inflammation and gut microbiota regulation properties of fatty acids derived from fermented milk in mice with dextran sulfate sodium-induced colitis. J. Dairy Sci. 2022, 105, 7865–7877. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, L.; Liu, X.; Cui, S.; Tang, X.; Chen, W.; Zhao, J. Screening of lactic acid bacteria for soymilk fermentation based on sucrose utilization ability. Food Ferment. Ind. 2020, 46, 43–49. [Google Scholar]
- Macharia, J.M.; Kaposztas, Z.; Varjas, T.; Budán, F.; Zand, A.; Bodnar, I.; Bence, R.L. Targeted lactate dehydrogenase genes silencing in probiotic lactic acid bacteria: A possible paradigm shift in colorectal cancer treatment? Biomed. Pharmacother. 2023, 160, 114371. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Jakobsen, L.M.A.; Geiker, N.R.W.; Bertram, H.C. Chemically acidified, live and heat-inactivated fermented dairy yoghurt show distinct bioactive peptides, free amino acids and small compounds profiles. Food Chem. 2022, 376, 131919. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Dalziel, J.E.; Nguyen, H.T.H.; Rounce, J.; Day, L.; Maes, E. Investigation of free amino acids in lactic acid bacteria fermented milk and their ability to activate the calcium sensing receptor. Int. Dairy J. 2023, 141, 105568. [Google Scholar] [CrossRef]
- Li, D.; Peng, J.; Kwok, L.-y.; Zhang, W.; Sun, T. Metabolomic analysis of Streptococcus thermophilus S10-fermented milk. LWT 2022, 161, 113368. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, J.; Cao, P.; Jin, Y.; Pan, D.; Zeng, X.; Guo, Y. Characterization of probiotic bacteria involved in fermented milk processing enriched with folic acid. J. Dairy Sci. 2017, 100, 4223–4229. [Google Scholar] [CrossRef]
- Lyu, S.; Yang, Q.; Duan, X.; Liu, J.; Yu, Y.; Pan, F.; Zhang, T. Impact of different cooling times during post-maturation on physicochemical and texture properties of fermented egg-milk beverage. Food Biosci. 2023, 54, 102906. [Google Scholar] [CrossRef]
- Lin, X.-t.; Xiao, B.-x.; Liu, J.-p.; Cao, M.-y.; Yang, Z.-p.; Zhao, L.-y.; Chen, G.-t. An acidic heteropolysaccharide rich in galactose and arabinose derived from ginger: Structure and dynamics. Food Biosci. 2023, 56, 103127. [Google Scholar] [CrossRef]
- Li, S.; Shah, N.P. Effects of Pleurotus eryngii polysaccharides on bacterial growth, texture properties, proteolytic capacity, and angiotensin-I-converting enzyme–inhibitory activities of fermented milk. J. Dairy Sci. 2015, 98, 2949–2961. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, Y.; Ou, W.; Yu, G.; Ai, Q.; Zhang, W.; Mai, K. Stachyose protects intestinal mucosal barrier via promotion of tight junction and Lactobacillus casei-drived inhibition of apoptosis in juvenile turbot, Scophthalmus maximus L. Aquaculture 2022, 556, 738280. [Google Scholar] [CrossRef]
- Mantel, M.; da Silva, T.F.; Gloria, R.; Vassaux, D.; Vital, K.D.; Cardoso, V.N.; Fernandes, S.O.A.; Guédon, É.; Le Loir, Y.; Faria, A.M.C.; et al. Fat matters: Fermented whole milk potentiates the anti-colitis effect of Propionibacterium freudenreichii. J. Funct. Foods 2023, 106, 105614. [Google Scholar] [CrossRef]
- Oliveira, F.L.d.; Arruda, T.Y.P.; Morzelle, M.C.; Pereira, A.P.A.; Casarotti, S.N. Fruit by-products as potential prebiotics and promising functional ingredients to produce fermented milk. Food Res. Int. 2022, 161, 111841. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Park, J.; Li, R.; Ji, G.E.; Johnston, T.V.; Choe, D.; Park, S.-H.; Park, M.S.; Ku, S. A randomized, double-blind, controlled human study: The efficacy of exopolysaccharides in milk fermented by Weissella confusa VP30 (VP30-EPS) to ameliorate functional constipation. J. Funct. Foods 2023, 104, 105491. [Google Scholar] [CrossRef]
- Hayashida, S.; Hagi, T.; Kobayashi, M.; Kusumoto, K.-I.; Ohmori, H.; Tomita, S.; Suzuki, S.; Yamashita, H.; Sato, K.; Miura, T.; et al. Comparison of taste characteristics between koji mold–ripened cheese and Camembert cheese using an electronic tongue system. J. Dairy Sci. 2023, 106, 6701–6709. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Copelin, J.E.; Park, T.; Mitchell, K.E.; Firkins, J.L.; Socha, M.T.; Luchini, D. Effects of diet fermentability and supplementation of 2-hydroxy-4-(methylthio)-butanoic acid and isoacids on milk fat depression: 2. Ruminal fermentation, fatty acid, and bacterial community structure. J. Dairy Sci. 2021, 104, 1604–1619. [Google Scholar] [CrossRef]
- Pahumunto, N.; Piwat, S.; Chanvitan, S.; Ongwande, W.; Uraipan, S.; Teanpaisan, R. Fermented milk containing a potential probiotic Lactobacillus rhamnosus SD11 with maltitol reduces Streptococcus mutans: A double-blind, randomized, controlled study. J. Dent. Sci. 2020, 15, 403–410. [Google Scholar] [CrossRef]
| Fermentation Time (h) | Lactic Acid (g/100 g) |
|---|---|
| 0 | 0.18 |
| 1 | 0.14 |
| 2 | |
| 3 | |
| 4 | |
| 5 | 0.18 |
| 6 | 0.14 |
| 7 | 0.14 |
| 7.5 | 0.09 |
| Free Amino Acid | Type (by Flavor) | Content (g/100 mL) |
|---|---|---|
| Aspartic acid | Flavourful amino acid | 0.0036 |
| Glutamic acid | 0.0011 | |
| Threonine | Sweetening amino acid | 0.0042 |
| Serine | 0.0040 | |
| Glycine | 0.0001 | |
| Alanine | 0.0003 | |
| Proline | 0.0007 | |
| Isoleucine | Bitter amino acid | 0.0023 |
| Leucine | 0.0020 | |
| Phenylalanine | 0.0003 | |
| Histidine | 0.0002 | |
| Tyrosine | 0.0001 | |
| Valine | 0.0017 | |
| Methionine | - | 0.0002 |
| Lysine | 0.0011 | |
| Arginine | 0.0015 |
| Type | Content (g/100 g) |
|---|---|
| Fat | 0.161 0.0030 |
| Monounsaturated fatty acid | 0.0129 0.0022 |
| Polyunsaturated fatty acid | 0.0026 0.00015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, Y.; Li, B.; Hou, B.; Hung, W.; He, J.; Jiang, Y.; Zhang, Y.; Man, C. Streptococcus thermophilus JM905—Strain Carbon Source Utilization and Its Fermented Milk Metabolic Profile at Different Fermentation Stages. Foods 2023, 12, 3690. https://doi.org/10.3390/foods12193690
Li Y, Wang Y, Li B, Hou B, Hung W, He J, Jiang Y, Zhang Y, Man C. Streptococcus thermophilus JM905—Strain Carbon Source Utilization and Its Fermented Milk Metabolic Profile at Different Fermentation Stages. Foods. 2023; 12(19):3690. https://doi.org/10.3390/foods12193690
Chicago/Turabian StyleLi, Yu, Ye Wang, Baolei Li, Baochao Hou, Weilian Hung, Jian He, Yujun Jiang, Yu Zhang, and Chaoxin Man. 2023. "Streptococcus thermophilus JM905—Strain Carbon Source Utilization and Its Fermented Milk Metabolic Profile at Different Fermentation Stages" Foods 12, no. 19: 3690. https://doi.org/10.3390/foods12193690
APA StyleLi, Y., Wang, Y., Li, B., Hou, B., Hung, W., He, J., Jiang, Y., Zhang, Y., & Man, C. (2023). Streptococcus thermophilus JM905—Strain Carbon Source Utilization and Its Fermented Milk Metabolic Profile at Different Fermentation Stages. Foods, 12(19), 3690. https://doi.org/10.3390/foods12193690




