Potential of Modification of Techno-Functional Properties and Structural Characteristics of Citrus, Apple, Oat, and Pea Dietary Fiber by High-Intensity Ultrasound
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation Using High-Intensity Ultrasound
2.3. Characterization of Plant Dietary Fiber
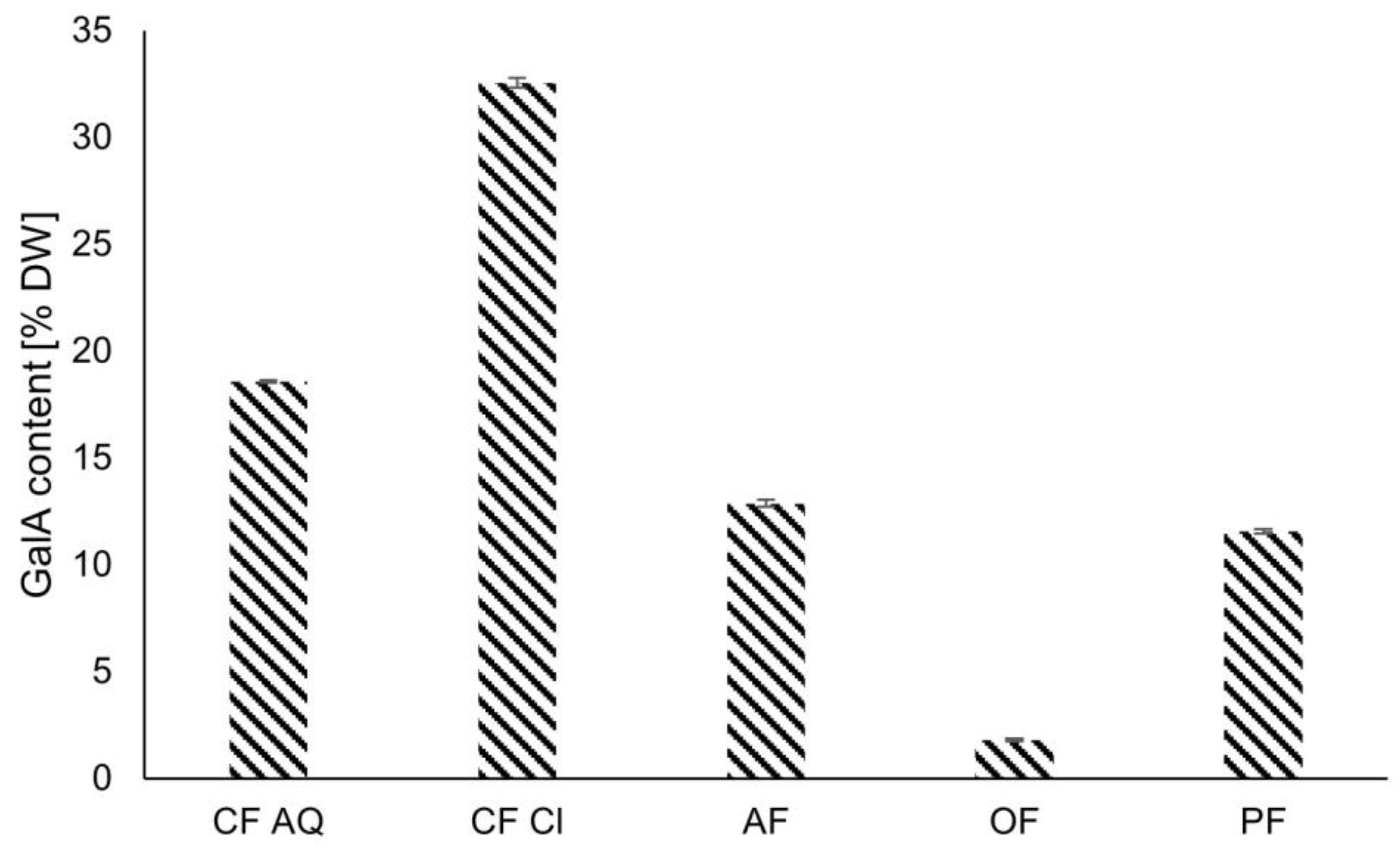
2.3.1. Composition of Plant Dietary Fibers
2.3.2. Determination of Pectin Content (GalA)
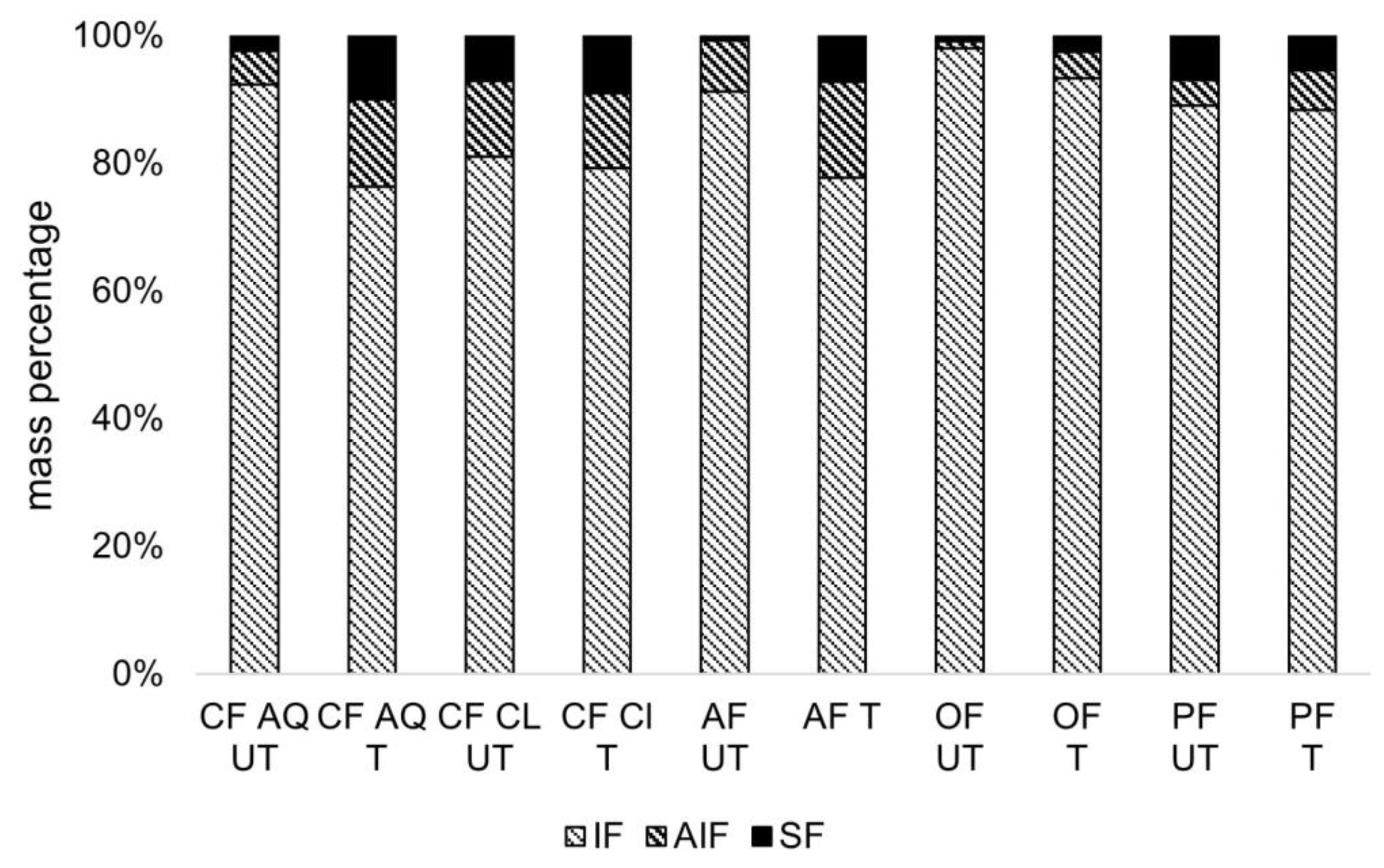
2.3.3. Determination of Insoluble, Alcohol-Insoluble, and Soluble Fractions in Dietary Fiber Suspensions before and after US-Treatment
2.3.4. Water Binding Capacity
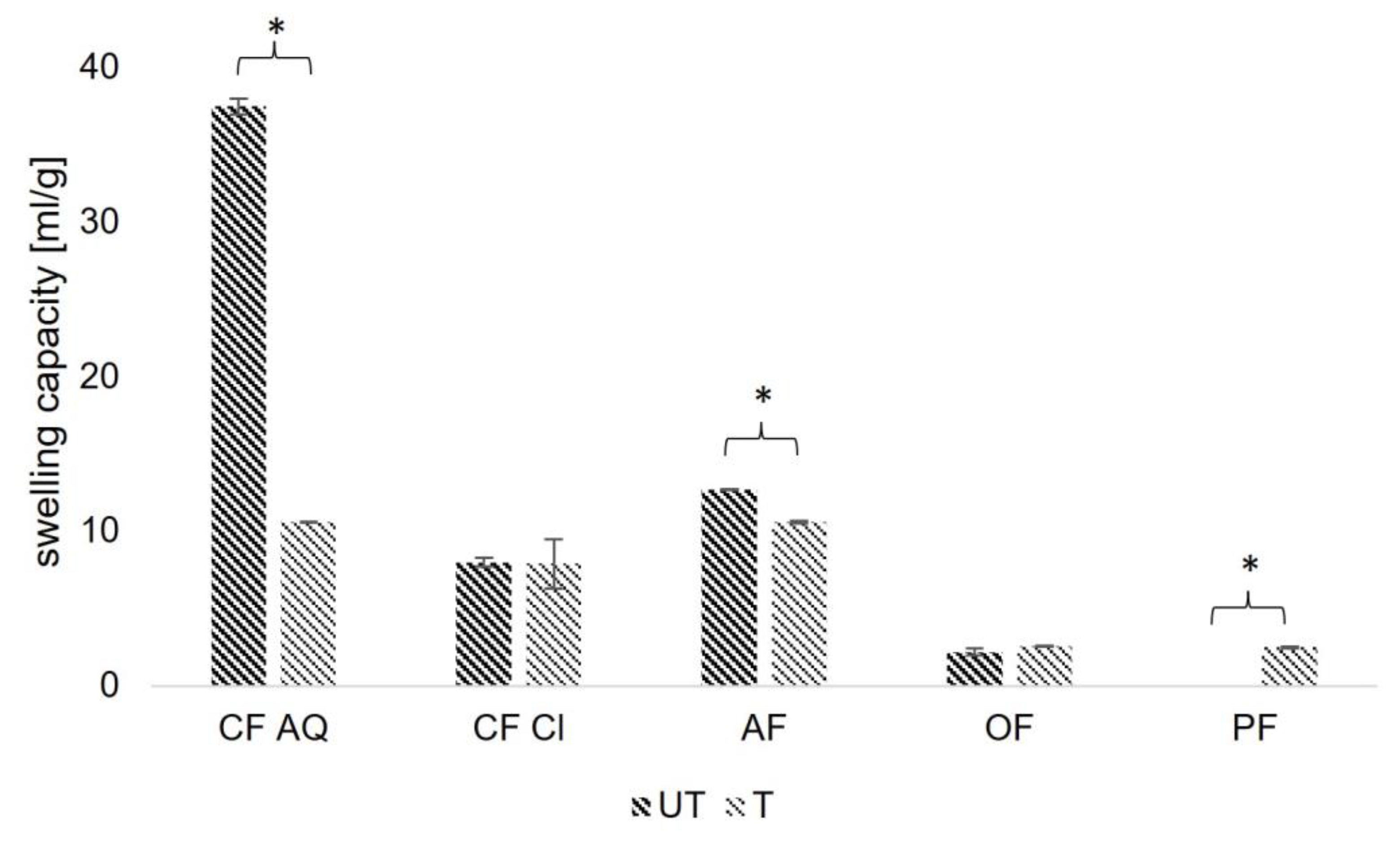
2.3.5. Swelling Capacity
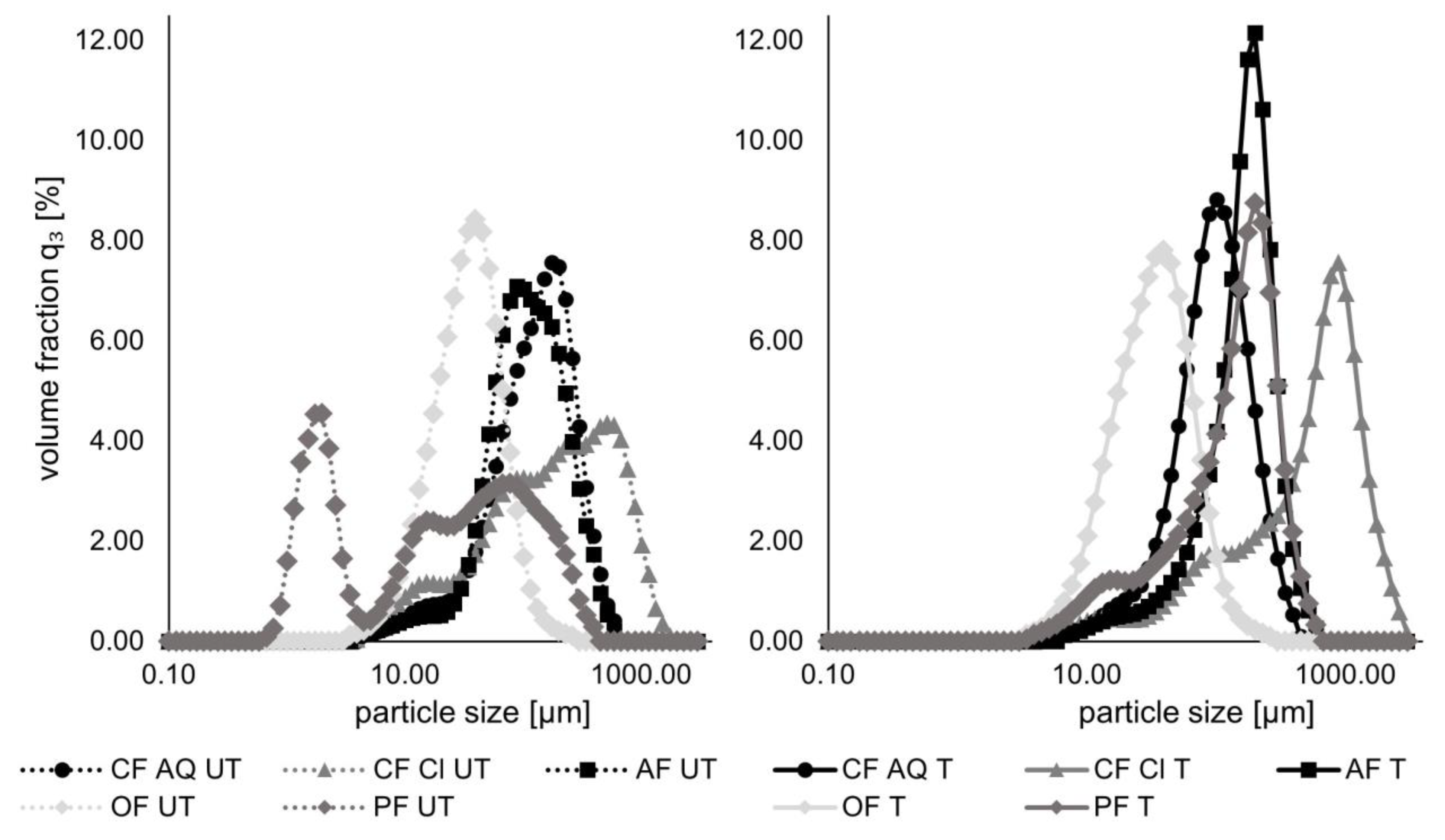
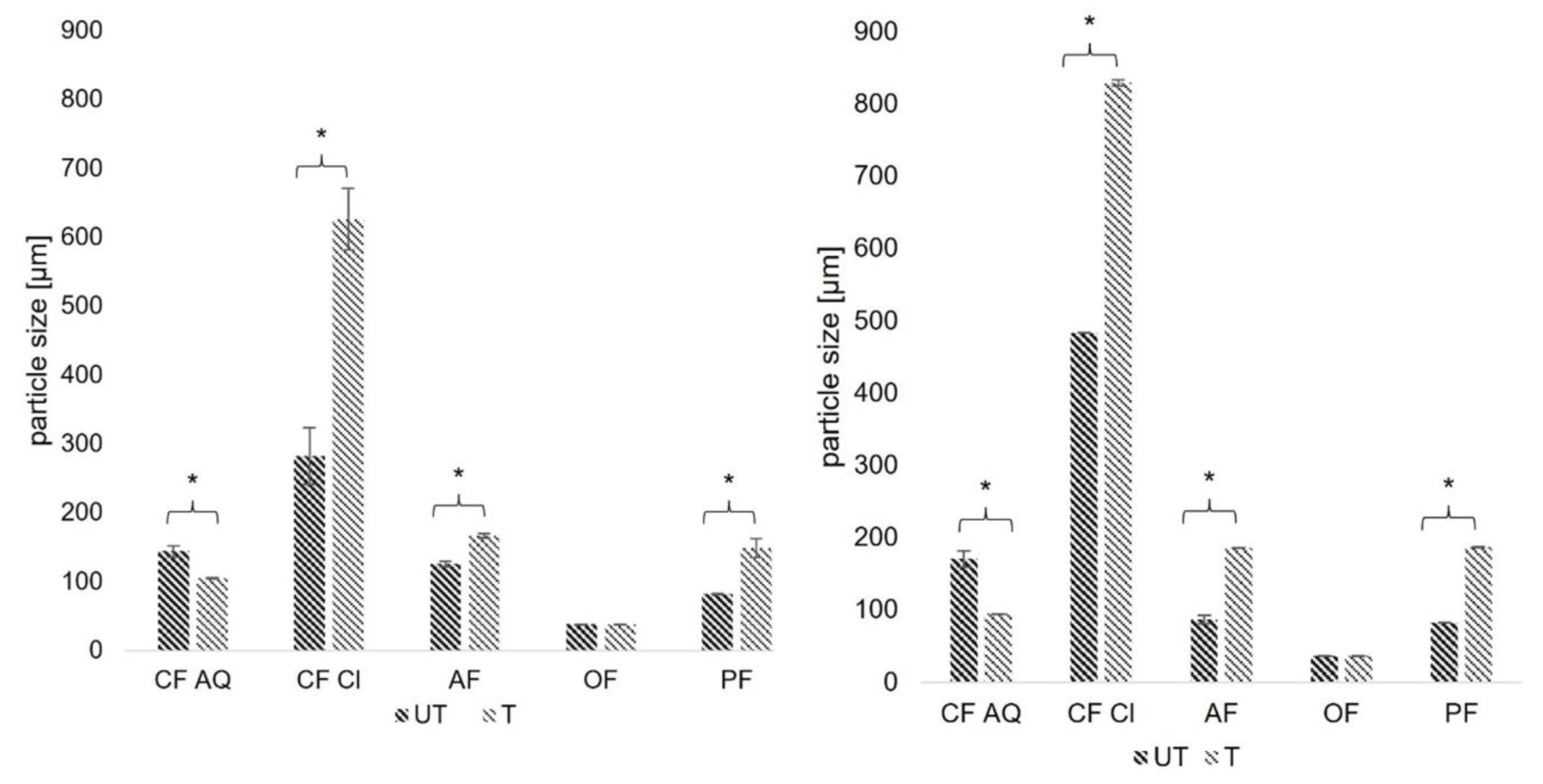
2.3.6. Determination of Particle Size Distribution
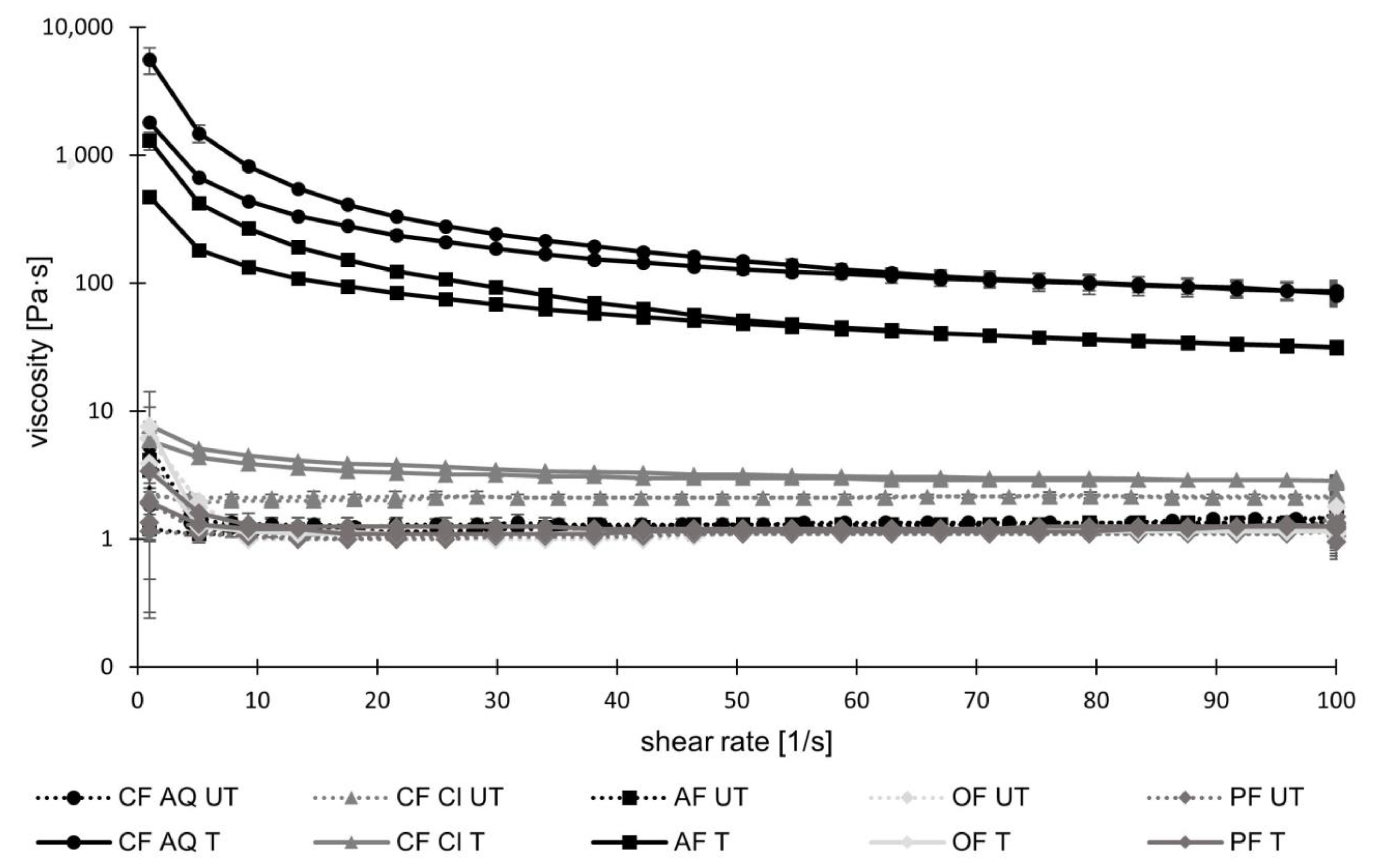
2.3.7. Rheological Characterization
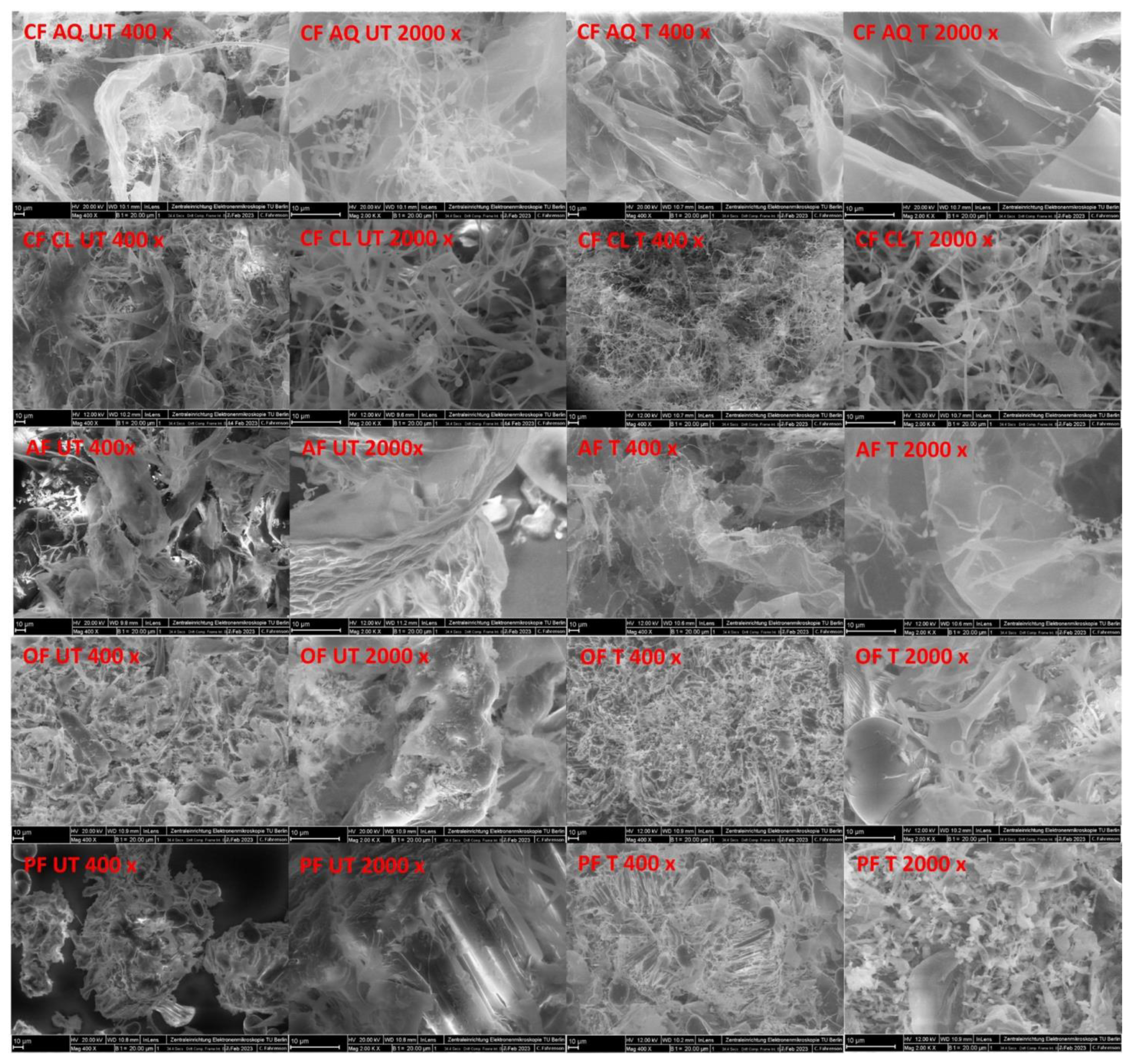
2.3.8. Field Emission Scanning Electron Microscopy (FE-SEM)
2.3.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2011, 49, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Kontogiorgos, V. Pectin: Technological and Physiological Properties, 1st ed.; Springer Nature: Cham, Switzerland, 2020; ISBN 9783030534219. [Google Scholar]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Dietary Fiber: Properties, Recovery, and Applications. In Dietary Fiber: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 27–58. ISBN 9780128164952. [Google Scholar]
- Dumanli, A.G. Nanocellulose and its Composites for Biomedical Applications. Curr. Med. Chem. 2017, 24, 512–528. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Edlund, U. Biopolymers, 1st ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2011; ISBN 9781119994312. [Google Scholar]
- Baltes, W.; Matissek, R. Lebensmittelchemie; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-16538-2. [Google Scholar]
- Jiang, Z.; Zhang, M.; Huang, Y.; Ma, C.; Mu, S.; Li, H.; Liu, X.; Ma, Y.; Liu, Y.; Hou, J. Comparison and Characterization of the Structure and Physicochemical Properties of Three Citrus Fibers: Effect of Ball Milling Treatment. Foods 2022, 11, 2665. [Google Scholar] [CrossRef] [PubMed]
- Zadeike, D.; Vaitkeviciene, R.; Degutyte, R.; Bendoraitiene, J.; Rukuiziene, Z.; Cernauskas, D.; Svazas, M.; Juodeikiene, G. A comparative study on the structural and functional properties of water-soluble and alkali-soluble dietary fibres from rice bran after hot-water, ultrasound, hydrolysis by cellulase, and combined pre-treatments. Int. J. Food Sci. Technol. 2022, 57, 1137–1149. [Google Scholar] [CrossRef]
- Benam, N.S.; Goli, M.; Ardebili, S.M.S.; Vaezshoushtari, N. The quality characteristics of dough and toast bread prepared with wheat flour containing different levels of Portulaca oleracea leaf powder. Food Sci. Technol. 2022, 42, e60820. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, S.; Narale, B.A.; Shanmugam, A.; Mettu, S.; Ashokkumar, M. Ultrasonic Processing of Food Waste to Generate Value-Added Products. Foods 2022, 11, 2035. [Google Scholar] [CrossRef]
- São José, J.F.B.; de Andrade, N.J.; de Ramos, A.M.; Vanetti, M.C.D.; Stringheta, P.C.; Chaves, J.B.P. Decontamination by ultrasound application in fresh fruits and vegetables. Food Control 2014, 45, 36–50. [Google Scholar] [CrossRef]
- Canselier, J.P.; Delmas, H.; Wilhelm, A.M.; Abismaïl, B. Ultrasound Emulsification—An Overview. J. Dispers. Sci. Technol. 2002, 23, 333–349. [Google Scholar] [CrossRef]
- Chen, T.-T.; Zhang, Z.-H.; Wang, Z.-W.; Chen, Z.-L.; Ma, H.; Yan, J.-K. Effects of ultrasound modification at different frequency modes on physicochemical, structural, functional, and biological properties of citrus pectin. Food Hydrocoll. 2021, 113, 106484. [Google Scholar] [CrossRef]
- Wei, C.; Ge, Y.; Liu, D.; Zhao, S.; Wei, M.; Jiliu, J.; Hu, X.; Quan, Z.; Wu, Y.; Su, Y.; et al. Effects of High-Temperature, High-Pressure, and Ultrasonic Treatment on the Physicochemical Properties and Structure of Soluble Dietary Fibers of Millet Bran. Front. Nutr. 2021, 8, 820715. [Google Scholar] [CrossRef] [PubMed]
- Khawas, P.; Deka, S.C. Isolation and characterization of cellulose nanofibers from culinary banana peel using high-intensity ultrasonication combined with chemical treatment. Carbohydr. Polym. 2016, 137, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Sumari, S.; Roesyadi, A.; Sumarno, S. Effects of Ultrasound on the Morphology, Particle Size, Crystallinity, and Crystallite Size of CelluloSE. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2013, 14, 229–239. [Google Scholar]
- Kalla-Bertholdt, A.-M.; Nguyen, P.-V.; Baier, A.K.; Rauh, C. Influence of dietary fiber on in-vitro lipid digestion of emulsions prepared with high-intensity ultrasound. Innov. Food Sci. Emerg. Technol. 2021, 73, 102799. [Google Scholar] [CrossRef]
- Kalla-Bertholdt, A.-M.; Baier, A.K.; Rauh, C. Influence of High-Intensity Ultrasound on Characteristics and Bioaccessibility of Pea Protein in Fiber-Enriched Suspensions. Foods 2023, 12, 3160. [Google Scholar] [CrossRef] [PubMed]
- Blumenkrantz, N.; Asboe-Hansen, G. New Method for Quantitative Determination of Uronic Acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Morales-Medina, R.; Dong, D.; Schalow, S.; Drusch, S. Impact of microfluidization on the microstructure and functional properties of pea hull fibre. Food Hydrocoll. 2020, 103, 105660. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, J.; Qi, J. Functional and structural properties of dietary fiber from citrus peel affected by the alkali combined with high-speed homogenization treatment. LWT 2020, 128, 109397. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, W.; Cheng, J.; Lu, Z. Antioxidant and physicochemical properties of soluble dietary fiber from garlic straw as treated by energy-gathered ultrasound. Int. J. Food Prop. 2019, 22, 678–688. [Google Scholar] [CrossRef]
- Morales-Medina, R.; Drusch, S.; Acevedo, F.; Castro-Alvarez, A.; Benie, A.; Poncelet, D.; Dragosavac, M.M.; Defain Tesoriero, M.V.; Löwenstein, P.; Yonaha, V.; et al. Structure, controlled release mechanisms and health benefits of pectins as an encapsulation material for bioactive food components. Food Funct. 2022, 13, 10870–10881. [Google Scholar] [CrossRef] [PubMed]
- Sucharipa, R. Protopectin and some other constituents of lemon peel. J. Am. Chem. Soc. 1924, 46, 145–156. [Google Scholar] [CrossRef]
- Pathirannehelage, N.P.V.; Joye, I.J. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. [Google Scholar] [CrossRef]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef] [PubMed]
- Tosh, S.M.; Yada, S. Dietary fibres in pulse seeds and fractions: Characterization, functional attributes, and applications. Food Res. Int. 2010, 43, 450–460. [Google Scholar] [CrossRef]
- Hall, C.; Hillen, C.; Garden Robinson, J. Composition, Nutritional Value, and Health Benefits of Pulses. Cereal Chem. 2017, 94, 11–31. [Google Scholar] [CrossRef]
- Ohkuma, K.; Matsuda, I.; Katta, Y. New Method for Determining Total Dietary Fiber by Liquid Chromatography. J. AOAC Int. 2000, 83, 1013–1019. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, M.; Adhikari, B.; Liu, Y. Effect of homogenization and ultrasonication on the physical properties of insoluble wheat bran fibres. Int. Agrophysics 2015, 29, 423–432. [Google Scholar] [CrossRef]
- Hassan, Z.; Imran, M.; Ahmad, M.H.; Khan, M.K. Ultrasound-Assisted Modification of Insoluble Dietary Fiber from Chia (Salvia hispanica L.) Seeds. J. Food Qual. 2021, 2021, 5035299. [Google Scholar] [CrossRef]
- Ma, Q.; Yu, Y.; Zhou, Z.; Wang, L.; Cao, R. Effects of different treatments on composition, physicochemical and biological properties of soluble dietary fiber in buckwheat bran. Food Biosci. 2023, 53, 102517. [Google Scholar] [CrossRef]
- Spotti, M.J.; Campanella, O.H. Functional modifications by physical treatments of dietary fibers used in food formulations. Curr. Opin. Food Sci. 2017, 15, 70–78. [Google Scholar] [CrossRef]
- Téllez-Morales, J.A.; Hernández-Santo, B.; Rodríguez-Miranda, J. Effect of ultrasound on the techno-functional properties of food components/ingredients: A review. Ultrason. Sonochemistry 2020, 61, 104787. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, N.; Zhu, J.; Zheng, M.; Liu, H.; Liu, J. Influence of modification methods on physicochemical and structural properties of soluble dietary fiber from corn bran. Food Chem. X 2022, 14, 100298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ye, X.; Xue, S.J.; Zhang, X.; Liu, D.; Meng, R.; Chen, S. Effect of high-intensity ultrasound on the physicochemical properties and nanostructure of citrus pectin. J. Sci. Food Agric. 2013, 93, 2028–2036. [Google Scholar] [CrossRef]
- Ismail, N.A.; Zhao, J. Effects of Ultrasound and Steam Explosion Treatments on the Physicochemical Properties of Rice Bran Fibre. Pertanika J. Trop. Agric. Sci. 2022, 45, 893–918. [Google Scholar] [CrossRef]
- Yang, W.; Kong, X.; Zheng, Y.; Sun, W.; Chen, S.; Liu, D.; Zhang, H.; Fang, H.; Tian, J.; Ye, X. Controlled ultrasound treatments modify the morphology and physical properties of rice starch rather than the fine structure. Ultrason. Sonochem. 2019, 59, 104709. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Maghsoudlou, Y.; Rostamabadi, H.; Rostamabadi, M.M.; Hamedi, H.; Hosseini, S.M.H. Preparation of physically modified oat starch with different sonication treatments. Food Hydrocoll. 2019, 89, 311–320. [Google Scholar] [CrossRef]
- Fan, X.; Li, S.; Zhang, A.; Chang, H.; Zhao, X.; Lin, Y.; Feng, Z. Mechanism of change of the physicochemical characteristics, gelation process, water state, and microstructure of okara tofu analogues induced by high-intensity ultrasound treatment. Food Hydrocoll. 2021, 111, 106241. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef]
- Chen, J.Y.; Piva, M.; Labuza, T.P. Evaluation of Water Binding Capacity (WBC) of Food Fiber Sources. J. Food Sci. 1984, 49, 59–63. [Google Scholar] [CrossRef]
- Mudgil, D. Dietary Fiber for the Prevention of Cardiovascular Disease. In Dietary Fiber for the Prevention of Cardiovascular Disease; Elsevier: Amsterdam, The Netherlands, 2017; pp. 35–59. ISBN 9780128051306. [Google Scholar]
- He, Y.; Li, W.; Zhang, X.; Li, T.; Ren, D.; Lu, J. Physicochemical, functional, and microstructural properties of modified insoluble dietary fiber extracted from rose pomace. J. Food Sci. Technol. 2020, 57, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, Y.; Jiang, X.; Gan, D.; Fan, J.; Sun, Y.; Liu, W.; Li, X. Dietary fiber extraction from citrus peel pomace: Yield optimization and evaluation of its functionality, rheological behavior, and microstructure properties. J. Food Sci. 2023, 88, 3507–3523. [Google Scholar] [CrossRef]
- Grgić, T.; Pavišić, Z.; Maltar-Strmečki, N.; Voučko, B.; Čukelj Mustač, N.; Ćurić, D.; Le-Bail, A.; Novotni, D. Ultrasound-assisted Modification of Enzymatic and Antioxidant Activities, Functional and Rheological Properties of Oat and Barley Bran. Food Bioprocess. Technol. 2023, 16, 2416–2429. [Google Scholar] [CrossRef]
- Ulbrich, M.; Flöter, E. Impact of high pressure homogenization modification of a cellulose based fiber product on water binding properties. Food Hydrocoll. 2014, 41, 281–289. [Google Scholar] [CrossRef]
- Dikeman, C.L.; Fahey, G.C. Viscosity as related to dietary fiber: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 649–663. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4987-2669-6. [Google Scholar]
- Mezger, T. Das Rheologie Handbuch, 5th ed.; Vincentz: Hannover, Germany, 2016; ISBN 978-3-86630-633-2. [Google Scholar]
- Lepaus, B.M.; Valiati, B.S.; Machado, B.G.; Domingos, M.M.; Silva, M.N.; Faria-Silva, L.; Bernardes, P.C.; Da Oliveira, D.S.; de São José, J.F.B. Impact of ultrasound processing on the nutritional components of fruit and vegetable juices. Trends Food Sci. Technol. 2023, 138, 752–765. [Google Scholar] [CrossRef]
- Wang, W.; Feng, Y.; Chen, W.; Adie, K.; Liu, D.; Yin, Y. Citrus pectin modified by microfluidization and ultrasonication: Improved emulsifying and encapsulation properties. Ultrason. Sonochem. 2021, 70, 105322. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, K.; Zhang, Z.; Liu, F.; Hu, H.; Pan, S. Changes on the rheological properties of pectin-enriched mango nectar by high intensity ultrasound. LWT 2018, 91, 414–422. [Google Scholar] [CrossRef]
- Chen, L.; Chen, L.; Zhu, K.; Bi, X.; Xing, Y.; Che, Z. The effect of high-power ultrasound on the rheological properties of strawberry pulp. Ultrason. Sonochem. 2020, 67, 105144. [Google Scholar] [CrossRef]
- Seshadri, R.; Weiss, J.; Hulbert, G.; Mount, J. Ultrasonic processing influences rheological and optical properties of high-methoxyl pectin dispersions. Food Hydrocoll. 2002, 17, 191–197. [Google Scholar] [CrossRef]
- Bi, X.; Hemar, Y.; Balaban, M.O.; Liao, X. The effect of ultrasound on particle size, color, viscosity and polyphenol oxidase activity of diluted avocado puree. Ultrason. Sonochem. 2015, 27, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Juodeikiene, G.; Trakselyte-Rupsiene, K.; Navickaite, B.; Zadeike, D.; Bendoraitiene, J.; Bartkiene, E.; Lele, V.; Rueller, L.; Robert, J.; Arnoldi, A.; et al. Functionalization of soya press cake (okara) by ultrasonication for enhancement of submerged fermentation with Lactobacillus paracasei LUHS244 for wheat bread production. LWT 2021, 152, 112337. [Google Scholar] [CrossRef]
- Vercet, A.; Sánchez, C.; Burgos, J.; Montanes, L.; Lopez Buesa, P. The effects of manothermosonication on tomato pectic enzymes and tomato paste rheological properties. J. Food Eng. 2002, 53, 273–278. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Vanga, S.K.; Raghavan, V. High-intensity ultrasound processing of kiwifruit juice: Effects on the microstructure, pectin, carbohydrates and rheological properties. Food Chem. 2020, 313, 126121. [Google Scholar] [CrossRef] [PubMed]
- Muneer, F.; Johansson, E.; Hedenqvist, M.S.; Plivelic, T.S.; Markedal, K.E.; Petersen, I.L.; Sørensen, J.C.; Kuktaite, R. The impact of newly produced protein and dietary fiber rich fractions of yellow pea (Pisum sativum L.) on the structure and mechanical properties of pasta-like sheets. Food Res. Int. 2018, 106, 607–618. [Google Scholar] [CrossRef]
- Han, L.; Cao, S.; Yu, Y.; Xu, X.; Cao, X.; Chen, W. Modification in physicochemical, structural and digestive properties of pea starch during heat-moisture process assisted by pre- and post-treatment of ultrasound. Food Chem. 2021, 360, 129929. [Google Scholar] [CrossRef]
- Kaur, H.; Gill, B.S. Effect of high-intensity ultrasound treatment on nutritional, rheological and structural properties of starches obtained from different cereals. Int. J. Biol. Macromol. 2019, 126, 367–375. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Xue, F.; Adhikari, B. Application of ultrasound treatment to improve the technofunctional properties of hemp protein isolate. Future Foods 2022, 6, 100176. [Google Scholar] [CrossRef]
- Bussemaker, M.J.; Zhang, D. Effect of Ultrasound on Lignocellulosic Biomass as a Pretreatment for Biorefinery and Biofuel Applications. Ind. Eng. Chem. Res. 2013, 52, 3563–3580. [Google Scholar] [CrossRef]
- Liu, Y.; Yi, S.; Ye, T.; Leng, Y.; Alomgir Hossen, M.; Sameen, D.E.; Dai, J.; Li, S.; Qin, W. Effects of ultrasonic treatment and homogenization on physicochemical properties of okara dietary fibers for 3D printing cookies. Ultrason. Sonochem. 2021, 77, 105693. [Google Scholar] [CrossRef] [PubMed]
- Zoghi, A.; Vedadi, S.; Esfahani, Z.H.; Gavlighi, H.A.; Khosravi-Darani, K. A review on pectin extraction methods using lignocellulosic wastes. Biomass Conv. Bioref. 2021, 13, 5577–5589. [Google Scholar] [CrossRef]

| Fat Content [%] | Carbohydrate Content [%] | Protein Content [%] | Salt Content [%] | Total Dietary Fiber Content [%] | |
|---|---|---|---|---|---|
| CF AQ | ≤1 | ≤1 | 5 | 1.30 | 90 |
| CF Cl | <1 | 6 | 6 | 0.40 | 64–82 |
| AF | ≤1 | ≤1 | 9 | 1.00 | 85 |
| OF | ≤1 | ≤1 | ≤1 | 0.10 | ≥90 |
| PF | 1 | 7 | 7 | 0.03 | 70–90 |
| Insoluble Dietary Fiber Content [% DM] | Soluble Dietary Fiber Content [% DM] | |
|---|---|---|
| CF AQ | 74.56 ± 0.34 | 14.57 ± 0.83 |
| CF Cl | 39.80 ± 0.16 | 23.30 ± 0.22 |
| AF | 71.91 ± 1.05 | 11.14 ± 0.66 |
| OF | 94.27 ± 0.40 | 0.21 ± 0.50 |
| PF | 72.91 ± 0.01 | 4.39 ± 0.35 |
| Sample | Mass of Fiber in Untreated Suspensions [g/100 g] | Mass of Fiber in Treated Suspensions [g/100 g] |
|---|---|---|
| CF AQ | 1.00 ± 0.01 | 0.25 ± 0.01 |
| CF Cl | 0.50 ± 0.01 | 0.50 ± 0.01 |
| AF | 1.00 ± 0.01 | 0.50 ± 0.01 |
| OF | 1.00 ± 0.01 | 1.00 ± 0.01 |
| PF | 1.00 ± 0.01 | 1.00 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalla-Bertholdt, A.-M.; Baier, A.K.; Rauh, C. Potential of Modification of Techno-Functional Properties and Structural Characteristics of Citrus, Apple, Oat, and Pea Dietary Fiber by High-Intensity Ultrasound. Foods 2023, 12, 3663. https://doi.org/10.3390/foods12193663
Kalla-Bertholdt A-M, Baier AK, Rauh C. Potential of Modification of Techno-Functional Properties and Structural Characteristics of Citrus, Apple, Oat, and Pea Dietary Fiber by High-Intensity Ultrasound. Foods. 2023; 12(19):3663. https://doi.org/10.3390/foods12193663
Chicago/Turabian StyleKalla-Bertholdt, Ann-Marie, Anne Kathrin Baier, and Cornelia Rauh. 2023. "Potential of Modification of Techno-Functional Properties and Structural Characteristics of Citrus, Apple, Oat, and Pea Dietary Fiber by High-Intensity Ultrasound" Foods 12, no. 19: 3663. https://doi.org/10.3390/foods12193663
APA StyleKalla-Bertholdt, A.-M., Baier, A. K., & Rauh, C. (2023). Potential of Modification of Techno-Functional Properties and Structural Characteristics of Citrus, Apple, Oat, and Pea Dietary Fiber by High-Intensity Ultrasound. Foods, 12(19), 3663. https://doi.org/10.3390/foods12193663






