Abstract
Zearalenone and its metabolites are mycotoxins generated by Fusarium species while crops are growing and can typically be found in various foods, posing a risk to human health. Governments have implemented stricter regulations concerning the permissible levels of zearalenone in food products to safeguard public health. Stricter regulations on zearalenone levels in food have been implemented. However, detecting zearalenone and its metabolites remains challenging due to sample complexity and interference. Surprisingly few reviews of sample preparation methods for zearalenone in food have appeared in the past decade. In this overview, we outline the most recent developments in the sample pre-treatment technology of zearalenone and its metabolites in food samples based on chromatography–mass spectrometry methods since 2012. This review covers some prominent technologies, such as liquid–liquid extraction-based methods, solid-phase extraction-based methods, and QuEChERS (quick, easy, cheap, effective, rugged, and safe) extraction, providing valuable insights into their advantages and limitations for potential applications. The assessment of the methods discussed, along with an overview of current challenges and prospects, will guide researchers in advancing the field and ensuring safer food quality for consumers worldwide.
1. Introduction
Zearalenone (ZEN) is a mycotoxin originating from specific Fusarium fungi, frequently detected in cereals and agricultural commodities like wheat, maize, barley, oats, and sorghum [1]. During the metabolism of ZEN in animals or food processing, it forms important metabolites, such as zearalanone (ZAN), α-zearalanol (α-ZAL), β-zearalanol (β-ZAL), α-zearalenol (α-ZEL), and β-zearalenol (β-ZEL), all of which have estrogenic activity [2]. These metabolites can specifically bind to estrogen receptors in domestic animals, thereby inducing a series of reproductive toxicities and teratogenic effects [2]. The impact of these phenomena encompasses the peroxidation of polyunsaturated fatty acids within the cellular membrane. Consequently, lipid peroxides emerge, causing subsequent disturbances in cellular metabolism and functionality, potentially culminating in cellular demise [1]. ZEN can also enter the human body through contaminated meat, milk, vegetable oil, and food products, causing obvious necrosis of peripheral blood mononuclear cells as well as adverse effects on the motility and function of sperm. These effects can impact the development of human reproductive organs and reproductive function [3]. As a result, the excessive intake of ZEN-contaminated food can negatively impact the well-being of both humans and animals [4].
The significant harm caused by zearalenone in corn has led to a surge in related research. A Web of Science search of scientific databases demonstrates the proliferation of zearalenone in academic literature since the year 2000, as depicted in Figure 1. The quantity of published papers incorporating zearalenone as a component of their research has increased over the years, with an annual average of more than 400 articles.
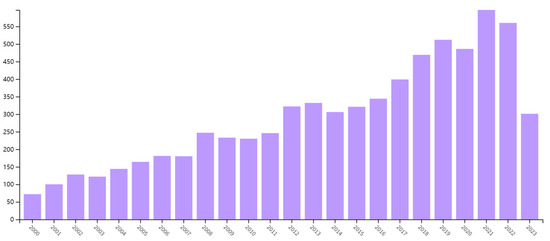
Figure 1.
Change in the number of publications from 2000 for the topic category “zearalenone”.
ZEN and its metabolites have been identified in 46 different foods, including corn, wheat, soybeans, beer, milk, cooking oil, and feedstuffs [5,6,7,8], prompting numerous countries worldwide to implement regulations on zearalenone levels [9]. For instance, China’s “Hygienical standard for feeds” (GB 13078-2017) sets a maximum allowable zearalenone content of 500 ng/g in corn feed, while the “Limits of Mycotoxins in Food” (GB2761-2017) prescribes that the content of zearalenone in wheat shall not exceed 60 ng/g [10,11]. Similarly, the European Commission specifies that all feedstuffs must have zearalenone limited to 5–20 ng/g. At the same time, the allowable limit of zearalenone in corn and grain is 100 ng/g, with a tolerable daily intake of 0.25 μg/kg body weight [12,13]. Given the potential health hazards associated with zearalenone and its metabolites and the existing regulations, strict monitoring and control of these compounds in food and feed are imperative to ensure food safety and animal wellbeing [14].
To effectively detect ZEN and its metabolites in food samples, various analytical techniques have been developed, including chromatography, mass spectrometry, immunoassay, Raman spectroscopy, electrochemical methods, and spectrophotometry [15,16,17,18,19,20,21,22]. However, these analytical techniques present certain limitations. For instance, distinguishing metabolites in immunoassays may be challenging due to potential false-positive effects caused by immunoaffinity reagents [23]. Raman spectroscopy may yield weak signals, making it difficult to distinguish certain samples [24]. Electrochemical analysis may suffer from poor selectivity [25], while spectrophotometry’s accuracy is relatively low [26]. In contrast, mass spectrometry and chromatography have become the most widely used detection methods, offering high efficiency, speed, resolution, micro-detection, and automation performance [27]. They can simultaneously detect multiple components with excellent selectivity and sensitivity. A variety of chromatographic methods, including capillary electrophoresis, liquid chromatography (LC), and gas chromatography (GC), combined with various detectors, have been employed to determine zearalenone and its metabolites [28,29,30]. Additionally, mass spectrometry, either alone or in combination with chromatographic separation, is suitable for the analysis of zearalenone and its metabolites [18].
Mass spectrometry and chromatography are crucial tools in mycotoxin detection due to their precision, sensitivity, and reliability [31]. While chromatographic and mass spectrometry techniques offer numerous advantages, directly applying them to analyze zearalenone and its metabolites in food samples proves challenging [32,33]. The complexity and diversity of chemical compounds in food matrices, coupled with the low analyte concentrations, pose practical difficulties in determining zearalenone and its metabolites without adequate sample preparation. Matrix interference further hampers successful detection and quantification of zearalenone at low abundance levels [34]. Moreover, incompatibilities may arise between complex food samples and instrumental analysis. To address these challenges, researchers have turned to effective sample preparation methods as a crucial step prior to instrumental detection. The use of effective sample preparation methods not only enhances the sensitivity of zearalenone and its metabolite detection but also helps in improving the overall accuracy and reliability of the analysis. By concentrating the analyte and eliminating interfering substances, these methods ensure more precise quantification and reliable results. As a result, these advancements in sample preparation techniques have led to significant progress in zearalenone and its metabolite analysis in food samples, ultimately contributing to the assurance of safer food quality for consumers worldwide.
At present, various methods for preparing samples have been developed, often in combination with mass spectrometry and chromatography techniques, to facilitate effective sample analysis. These methods include solid-phase extraction (SPE), QuEChERS, liquid–liquid extraction (LLE), and related methodologies derived from them [34,35,36,37,38]. Research continues to explore and optimize these methods, paving the way for further advancements in mycotoxin toxin testing and food safety analysis. Each sample preparation technology has its own applications, merits, and limitations. Proper sample preparation plays a crucial role in increasing the likelihood of successful sample analysis. This paper reviews the most recent advancements in sample preparation techniques for the analysis of ZEN and its metabolites in food samples, covering the period from 2012 to the present (see Figure 2). Table 1 lists the abbreviations and definitions used throughout this review.
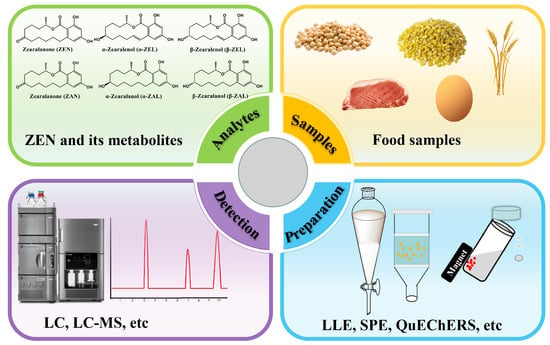
Figure 2.
A diagram outlining a standard analytical procedure for identifying ZEN and its metabolites in samples of food.

Table 1.
Abbreviations used in this review article.
2. Sample Preparation Methods
2.1. Liquid–Liquid Extraction-Based Methods
LLE is widely employed in the preparation of samples for food analysis. It relies on the principle that the analyte exhibits different partition coefficients between two immiscible solution bodies or phases [37]. LLE involves the use of an organic solvent that does not mix with water. After the extraction liquid is dried, the residue can be dissolved in a more suitable solvent for analysis and determination. It is currently the most widely used separation and purification technology, offering advantages such as a high recovery rate, large processing capacity, continuous operation, good separation efficiency, and easy automatic control. However, traditional LLE also has disadvantages, including time-consuming procedures, complex operations, and the consumption of large amounts of organic solvents. Additionally, it is less effective with highly polar analytes. To address these limitations, microextraction techniques like liquid–liquid microextraction (LLME) and dispersive liquid–liquid microextraction (DLLME) have been widely adopted. These techniques offer several advantages, including improved cost-effectiveness, high recovery, and efficient enrichment [35,36]. Analytical techniques such as GC, LC, and others, in combination with LLE, LLME, or DLLME techniques, have been used to determine ZEN and its metabolites in food commodities (Table 2).

Table 2.
Application of liquid–liquid extraction-based (LLE) methods for the determination of ZEN and its metabolites in foods.
DLLME comprises two steps: first, it involves extracting and dispersing the analyte; then, it requires centrifugation of the resulting mixture. The extraction takes place within a ternary blend consisting of an extraction solvent, a dispersion solvent, and an aqueous sample [49]. The nature of the dispersion solvent is crucial, as it disperses the fine drops of the extractant in the water medium. The choice of the appropriate extraction solvent is a key factor in the success of the extraction process. It affects both extraction recovery and selectivity. As an illustration, Pi et al. [40] utilized this technique by using 600 μL of 1-dodecanol as the extractant and 1.0 mL of acetonitrile (ACN) as the dispersant. They employed a method that combined ultrasonic-assisted aqueous two-phase extraction with solidifying organic drop-dispersive liquid–liquid microextraction. This approach enabled the simultaneous determination of nine mycotoxins, including ZEN, in medicinal and edible foods using LC with an in-series diode array detector (DAD) and fluorescence detector (FLD) [40].
In recent years, there has been a growing recognition of the importance of developing analytical methods that align with the principles of green chemistry in the field of food analysis. Green chemistry aims to minimize the environmental impact of chemical processes and promote sustainability. In the context of sample preparation methods for the chromatographic and mass spectrometric determination of zearalenone and its metabolites in food, it is crucial to consider the environmental implications of the techniques employed. This includes the choice of solvents, reagents, and extraction procedures. One notable approach that contributes to greener sample preparation is the use of deep eutectic solvents (DES). These solvents are formed by mixing two or more components, typically a hydrogen bond acceptor and a hydrogen bond donor, at a specific ratio that results in an eutectic mixture with a significantly lower melting point than the individual components. DES offer several advantages that make them attractive alternatives to traditional organic solvents. They possess low volatility, non-flammability, and biodegradability, thus reducing the release of harmful substances into the environment. Additionally, DES can be easily prepared from readily available and renewable components, further enhancing its sustainability.
By incorporating DES as an extraction solvent in the chromatographic and mass spectrometric determination of zearalenone and its metabolites in food, researchers can significantly reduce the environmental impact of the analytical process. DES not only provides improved extraction efficiency and selectivity but also minimizes the generation of hazardous waste. These characteristics make DES a promising option for achieving greener sample preparation and aligning with the principles of green chemistry. Pochivalov et al. [39] successfully used the analytical method of dispersive liquid–liquid microextraction gas chromatography flame ionization detector (DLLME-GC-FLD) to determine the content of zearalenone in grain samples. They used terpenoids and long-chain alcohols as extraction solvents and ACN as a dispersion solvent to separate dispersed solvents with smaller organic phase volumes (as shown in Figure 3). The DES extraction with DL-menthol and 1-hexanol as raw materials had a good effect, with a recovery rate of 93 ± 4% and an enrichment coefficient of 15.8 ± 0.7. The sensitivity of this method was also impressive, with a limit of detection (LOD) as low as 2 ng/g, highlighting the effectiveness of DES as a green and efficient extraction solvent for analytes in various samples. This makes it a promising choice for environmentally friendly and sensitive analytical applications.
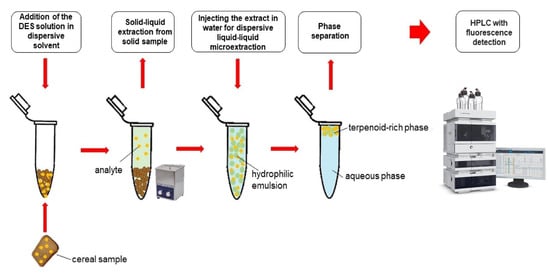
Figure 3.
Ultrasound-assisted solid–liquid extraction in combination with DLLME using deep eutectic solvents. Figure modified from [39].
To achieve higher extraction efficiency, it is essential to select suitable micro-extraction conditions based on the analyte. These factors include the volume and type of dispersion solvent, extraction solvent, and extraction time [50], as well as the viscosity, surface tension, and dielectric constant of the solvent. Generally, the dispersive solvent should dissolve in the extraction solvent but be insoluble in water, while the extraction solvent should be immiscible with water. It should have a low boiling point, a lower density than water, and be easily separable. Since choosing appropriate dispersion and extraction solvents is crucial for improving extraction efficiency, various solvents are often tested to optimize extraction conditions when developing extraction technologies. The specific extraction conditions may vary for different sample substrates. For instance, Emidio et al. [36] determined zearalenone in water samples using the DLLME-LC–MS/MS (tandem mass spectrometry) method. The optimal extraction conditions were as follows: 100 μL of bromocyclohexane was used as the extraction solvent (non-dispersible solvent), 10 mL of water sample (adjusted to pH 4), vortex extraction for 2 min, and centrifugation at 3500 rpm for 10 min without adjusting ionic strength. The LODs and limit of quantifications (LOQs) were in ranges of 4–20 ng/L and 8–40 ng/L, respectively. The results demonstrated that the method was suitable for determining ZEN in water samples [36].
Due to the fact that centrifugation can prolong sample preparation time, there is increasing interest in developing non-centrifugation methods to accelerate the extraction process. One such innovative approach gaining attention is solidifying organic drop-dispersive liquid–liquid microextraction (SOD-DLLME) without the need for centrifugal steps [51,52]. A novel method based on SOD-DLLME was reported for the simultaneous determination of nine mycotoxins in edible and medicinal foods using LC with DAD and FLD in series. The optimized conditions for the method involved using 600 μL of 1-dodecanol as the extractant, 1.0 mL of ACN as the dispersant, and a vortex-assisted time of 1.0 min. LODs for DAD and FLD were determined to be 0.1622 ng/mL and 0.04451 ng/mL, respectively.
DLLME is usually used to prepare liquid samples and is not usually used to prepare solid samples. However, the limitations of DLLME for solid samples can be overcome by combining it with other sample preparation methods such as SPE and QuEChERS [38,44]. For example, Zhou et al. [45] prepared samples by combining SPE with DLLME, used chloroform as the extraction solvent, and then conducted LC-FLD analysis to determine zearalenone in grains and beans. In addition, auxiliary energy fields such as ultrasonic and eddy currents can be added to the DLLME to replace artificial vibration with mechanical vibration to improve the efficiency of the dispersion step.
In recent years, ionic liquids have become increasingly popular as extraction solvents in DLLME because of their good properties, including high density, high thermal stability, low volatility, low water solubility, and toxicity. Bozkurt et al. [6] proposed a DLLME method utilizing ionic liquids and used this technology to extract zearalenone from beer and grain samples. Methanol was used as the dispersion solvent, while 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide and 1-methyl-3-octylimidazolium bis(trifluoromethanesulfonyl)imide were utilized as the extraction solvents [6]. By coupling with LC-FLD, the LOD of zearalenone was 0.25 ng/mL. In addition, Wang et al. [41] developed a method for zearalenone analysis in corn products by combining vortex-assisted ionic liquid dispersion liquid–liquid microextraction with LC-FLD. In the experiment, 10 g of a fully homogeneous corn sample and 50 mL of a methanol/water (80:20, v/v) mixture were extracted in the ultrasonic cleaning machine for 30 min, and the extraction solution was filtered using filter paper as a dispersion and [HMIM][PF6] as an extraction solvent. The LODs and LOQs were 0.3 and 1.0 ng/g, respectively. The average recoveries ranged from 83.5% to 94.9%, and the relative standard deviation (RSD) was less than 5.0%, showing that this method was applicable to the detection of zearalenone in corn products [41].
Recently, Ni et al. [42] measured zearalenone in corn oil by combining the immunomagnetic beads technique with the DLLME technique. Immunomagnetic beads have many advantages, such as easy surface modification, uniform particle size, and large specific surface area. Meanwhile, the purification method of immunomagnetic beads has the advantages of strong specificity, simple operation, and fast separation speed. By combining the advantages of immunomagnetic beads and DLLME technology and using LC-FLD for quantitative detection and analysis, zearalenone in concentrated corn oil was directly purified under the solubilization of the surfactant. With the characteristics of automation and high efficiency, the pre-treatment method of this technology is more environmentally friendly [42].
While LLE-based methods are recognized for their ease of use and efficiency, they can lead to environmental pollution due to the organic solvents used. Additionally, the potential for cross-contamination arises when dealing with samples of complex composition. To address these concerns, advancements in this method should prioritize environmentally friendly and sustainable practices, such as exploring greener alternatives like DES, which can enhance analyte selectivity while maintaining environmental integrity. By adopting such strategies, researchers can contribute to the development of more eco-friendly and sustainable sample preparation methods for the chromatographic and mass spectrometric determination of zearalenone and its metabolites in food.
2.2. Solid-Phase Extraction-Based Methods
SPE employs a solid adsorbent to capture the desired compound within the liquid sample. This process effectively isolates the target compound from any interfering substances present in the sample or its matrix. Subsequently, the compound is eluted using an eluent or through thermal desorption. This elution process serves the purpose of separating and concentrating the target compound (see Figure 4). SPE has undergone significant development in many related branches, such as dispersive solid-phase extraction (d-SPE), solid-phase microextraction (SPME), magnetic solid-phase extraction (MSPE), and stir-bar sorptive extraction (SBSE); all are receiving increasing attention [53,54]. SPE is a superior alternative to LLE due to its enhanced selectivity, reduced solvent usage, automation-friendly nature, and minimal cross-contamination risk. It enables efficient pre-concentration, sample cleanup, and compatibility with various detection techniques, making it a preferred choice in modern analytical chemistry.
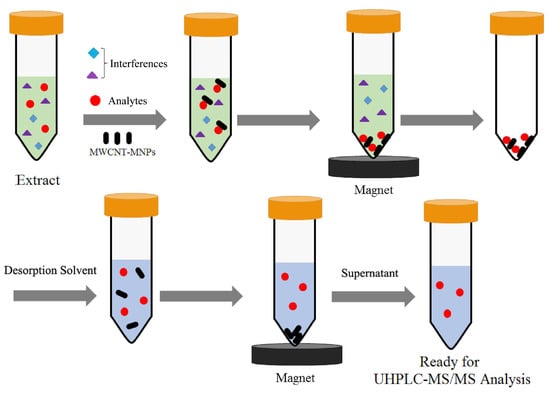
Figure 4.
Schematic diagram of the magnetic solid-phase extraction (MSPE) procedure based on multi-walled carbon nanotubes-magnetic nanoparticles (MWCNT-MNPs). Figure modified from [55].
Although these pre-treatment technologies have greatly improved the sample yield in terms of solvent consumption, extraction time, enrichment factor, and reproducibility, conventional adsorbents (such as octadecyl silicon and polymer reverse phase materials) in common use lack good selectivity [56]. This is an analytical deficiency that requires extensive optimization to minimize matrix adsorption/interference and co-elution of non-target compounds. Obviously, it is very important to develop a better selective adsorbent for the detection of zearalenone and its metabolites. In the chemical structure of zearalenone, the seventh carbon contains a separate ketone group, which is easy to react with a hydrazine group to form zearalenone hydrazone on the solid phase. Drzymala et al. [57] combined dynamic covalent hydrazine chemistry with LC-FLD analysis technology to quantitatively detect the content of zearalenone in edible oil. The resin skeleton, particle size, pore size, specific surface area, resin skeleton, and load performance of seven different hydrazine materials were evaluated. Therefore, we chose a hydrazine-based functional silica gel. Decoupling was achieved by introducing 20% acetone, which reacted with the hydrazine group, thereby replacing and releasing zearalenone for detection. The final experimental results indicated that the LOD is 10 ng/g and the LOQ is 30 ng/g. It is worth noting that by applying a mixture of acetic acid (AcOH) and methanol, the recovery and activation of the hydrazine cylinder can be achieved in one step. Therefore, acetone was removed, and the hydrazine portion was converted into the corresponding acetate. A hydrazine cartridge can be repeatedly applied 15 times without affecting its performance [57].
In recent years, nanomaterials have played an increasingly important role in the fields of chromatography and mass spectrometry technology. To some extent, magnetic nanoparticles are very helpful for extraction and preconcentration techniques, mainly because they can be easily separated from the matrix through external magnets without retaining the remaining magnetization. Due to their exceptional physical and chemical properties (among other good properties) of iron oxides (magnetite, Fe3O4, and maghemite, γ-Fe2O3), they have become the most widely used magnetic nanoparticle in d-SPE as well as in other applications. Gonzalez-Salamo et al. [58] synthesized Fe3O4@pDA m-NPs in their laboratory. Prior to liquid chromatography–mass spectrometry (LC–MS) analysis, it was used as a d-SPE adsorbent to extract zearalenone and its metabolites from complex substrates such as milk (full-fat skimmed milk and semi-skimmed goat milk) and yogurt (an unsweetened natural yogurt). Because this kind of food sample matrix is complex, it is not possible to directly apply previously developed methods. To reduce the matrix effect of the sample and the problem of contamination, clogging or damaging the LC column, etc., the team added the initial deproteinization step to remove the milk protein. The experimental results showed that when the sample was 1.5 mL of milk, the best removal effect of milk protein was obtained by using 3 mL of ACN and 75 μL of acetic acid, and a slightly higher reproducible relative recovery can be obtained by using 80 mg of Fe3O4@pDA NPs as adsorbent and 8 mL of methanol as eluent. For the yogurt sample, using the same volume of AcOH, change the sample/ACN ratio from 1/2 to 1/3 w/v. Under these conditions, taking 1.5 g of yogurt, 4.5 mL of ACN, and 75 μL of AcOH, a good deproteinization effect can be obtained. The amount of adsorbent and eluent extracted is the same as that of milk samples. Finally, they performed matrix matching calibration and recovery studies on the selected matrix. The results showed that the linearity was good, the relative recovery was in the range of 70–120%, the RSD was lower than 16%, the LOD of the milk sample was in the range of 0.21–4.77 ng/mL, and the LOD of the yogurt sample was in the range of 0.29–4.54 ng/g [58]. In another study, Zhao et al. [59] prepared PEGylated multi-walled carbon nanotube magnetic nanoparticles (PEG-MWCNTs-MNP) as adsorbents and used the MSPE method for sample pre-treatment. It was shown that when the sample was 4 g of liquid milk and 10 mg of adsorbent was used, the extraction efficiency was the highest. However, when the desorption agent was ethyl acetate containing 1% formic acid, the desorption efficiency was the best. The extracted zearalenone and its metabolites were quantified with LC-Q-Exactive HRMS. The results showed that the LOD and LOQ of liquid milk were in the range of 0.005–0.050 ng/g and 0.015–0.150 ng/g, respectively [59].
In addition to magnetic nanomaterials as adsorbents, many examples of different adsorbents used in these methods have been reported in the literature, including reduced graphene oxide and gold nanoparticle composites [60], graphitized carbon black [61], chitosan nanofibers [62], immunosorbents [63], and molecularly imprinted polymers (MIPs) [64,65]. Utilizing immunoaffinity columns (IACs) for mycotoxin purification and concentration has garnered significant research attention. The notable benefit of IAC lies in the exceptional specificity exhibited by imprinted antibodies towards their intended analytes. Regrettably, the majority of commercially accessible IAC contain antibodies tailored for just a single mycotoxin or a narrow cluster of closely associated mycotoxins, rendering them expensive and non-reusable. More in-depth studies are needed on the application of IAC to sample pre-treatment of zearalenone and its metabolites [66]. MIPs, being a synthetic polymer material, have demonstrated their excellence as adsorbents in the realm of molecularly imprinted solid-phase extraction (MISPE). This method offers considerable benefits, including specific molecular recognition, exceptional predetermined selectivity, remarkable stability, reusability, and the capacity to effectively mitigate background interference during detection. Moya-Cavas et al. [67] polymerized N-(2-aminoethyl) methylacrylamide as a functional monomer, methylacrylamide as a comonomer, ethylene glycol dimethacrylate as a crosslinking agent, and silicon beads as a sacrificial scaffold. The polymerized compound cyclododecyl 2,4-dihydroxybenzoate (CDHB) was similar to zearalenone in size, function, and shape and was used as a template substitute for MIP synthesis. The eluent was eluted with 2.5 mL of trifluoroacetic acid/methanol (3/97, v/v), and zearalenone was analyzed by LC-FLD. The results show that the LOD of the oil sample is 5 ng/g [67]. Zhang et al. [68] prepared hydroxyapatite-supported surface imprinted polymers (HAP@MIPs) using coumarin-3-carboxylic acid and naringenin as pseudotemplate molecules for zearalenone. They characterized the sorbent using various methods and found that it achieved adsorption equilibrium within 6 min, with an adsorption capacity of 6.77 µg/mg. HAP@MIPs were employed as the sorbent, and 3 mL of methanol was used as the eluent in SPE. This was coupled with LC to identify zearalenone in different cereal samples. The sample recoveries obtained ranged from 70.09% to 101.88%, with standard deviations of 2.06% to 8.47% [68]. Table 3 presents a variety of materials used as SPE sorbents for determining ZEN and its metabolites in food samples in recent years.

Table 3.
Application of solid-phase extraction-based (SPE) methods for the determination of ZEN and its metabolites in food.
Ever since its inception as a technique for preparing samples, especially liquid samples, SPE-based approaches have found widespread application in separating, enriching, and purifying various compounds. This encompasses eliminating interfering substances [78]. The rapid progress of SPE-based methods can be attributed to the synthesis of numerous new sorbents in recent years. SPME requires fewer consumables, involves simpler handling, and encounters less interference from the sample matrix. Consequently, SPME can be a suitable alternative to SPE in many scenarios [18]. Future research in this field should prioritize the development of sorbents with enhanced selectivity and higher adsorption capacity while also aiming to reduce the amount of sorbent required for extraction. This reduction would contribute to minimizing or eliminating the reliance on organic solvents. Additionally, ensuring the repeatability and reproducibility of the obtained sorbents remains an important consideration.
2.3. QuEChERS
QuEChERS (quick, easy, cheap, effective, rugged, and safe), a swift sample preparation technique, emerged in recent years as an innovative advancement renowned for its speed, accuracy, and efficiency [79]. Since the introduction of the QuEChERS method, it has gained substantial popularity. It is widely employed for the qualitative and quantitative detection of pesticide residues in diverse agricultural products. Through extensive research, the QuEChERS method has found widespread application in various food testing scenarios [80].
The typical procedure for the QuEChERS method is as follows: (1) sample grinding; (2) using ACN extraction separation; (3) adding MgSO4 and other salts for water removal; (4) adding adsorbents such as ethylenediamine-n-propyl silane to remove impurities; (5) detection of the supernatant by appropriate detection technique. The general procedure for this method is depicted in Figure 5. Compared to traditional sample preparation methods, QuEChERS offers several advantages, including fast analysis speed, minimal contamination, low cost, and high recovery. Originally designed for identifying pesticide residues in fruits and vegetables, the QuEChERS method has more recently found application in the analysis of zearalenone and its metabolites in food. This is frequently conducted in combination with LC and LC–MS/MS. He et al. [81] used an enzyme-linked immunosorbent assay (ELISA) and LC to detect ZEN after QuEChERS extraction. The optimal composition for QuEChERS extraction is 6 g of MgSO4, 1.5 g of sodium chloride, 1.5 g of sodium citrate dihydrate, and 1 g of citric acid sesquihydrate. The optimal adsorbent is 300 mg of primary secondary amine [81].
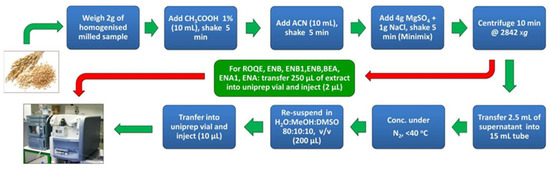
Figure 5.
General procedure for analyzing zearalenone in cereals based on QuEChERS [82].
Ferreira et al. [82] developed a rapid sample preparation method that relied on the pre-extraction of fatty compounds using n-hexane, ACN extraction of target analytes, simple d-SPE using MgSO4 and C18 adsorbents to remove matrix co-extracts, followed by glycosylation and further gas chromatography–mass spectrometry (GC–MS) analysis of the analytes [83]. Prior to residue analysis, the authors verified the analytical method for LOD, LOQ, linearity, and recovery, all of which fell within acceptable ranges [82]. Table 4 demonstrates the application of QuEChERS to the extraction of ZEN and its metabolites in various food samples. These examples illustrate the suitability of QuEChERS for sample preparation in various analytical methods.

Table 4.
Utilizing QuEChERS-derived techniques to analyze ZEN and its metabolites in diverse food samples.
QuEChERS technology has gained widespread adoption across various fields since its inception. Its advantages of being fast, simple, inexpensive, efficient, reliable, and safe are widely recognized. However, there are still some challenges that need to be addressed. One such challenge is the limited sampling volume, which can impact the detection limit to some extent. Additionally, the use of ACN or acidified ACN for extraction in this technology can potentially cause instrument damage when combined with chromatographic techniques. Furthermore, the inherent limitations of the technology in eliminating matrix effects in complex matrices require additional correction processes, which may introduce errors in the analysis. Therefore, further advancements and improvements should be pursued in future studies. These may include exploring new purification agents, optimizing extraction reagents, and developing correction methods to mitigate the influence of matrix effects.
3. Selecting a Sample Preparation Strategy
As previously discussed, a variety of sample preparation methods have played a crucial role in preparing samples for the precise quantification and identification of zearalenone and its metabolites in diverse food samples, particularly those susceptible to mycotoxin contamination. Each of these methods comes with its own distinct advantages and disadvantages, necessitating careful consideration based on the specific circumstances and characteristics of the sample matrix. For instance, SPE is well-regarded for its exceptional selectivity and cleanliness, making it suitable for highly precise analyses, though it can be time-consuming and involve higher costs. On the other hand, the QuEChERS method offers a rapid and cost-effective solution, particularly ideal for screening purposes in extensive sample surveys, even though it may exhibit reduced selectivity and robustness. LLE, commonly chosen for its simplicity and efficiency, may have environmental concerns due to solvent use, but the employment of environmentally friendly solvents such as DES could address this issue. It is important to note that there is no one-size-fits-all method; suitability is paramount and depends on factors such as sample type, analysis cost, the specific goals of the research, and the technological resources available.
To determine the most suitable sample preparation method for a specific scenario, one must carefully weigh these advantages and disadvantages against the specific analysis requirements. Factors like sample complexity, analyte concentration, available resources, and the desired level of sensitivity and selectivity all play a pivotal role in method selection. Considering the potential presence of other mycotoxins or contaminants within the sample matrix is also crucial. By conducting a comprehensive assessment of these variables and tailoring the choice of sample preparation method accordingly, researchers can ensure the most accurate and effective determination of zearalenone and its metabolites in food, contributing to safer and more dependable food quality control measures.
4. Chromatographic and Mass Spectrometric Analysis
The complexity of food matrices will potentially interfere with the selectivity of zearalenone detection. Thus, apart from implementing effective sample preprocessing techniques for purification and enrichment prior to instrumental analysis, the choice of an appropriate detection instrument is equally essential. Presently, LC-FLD and LC–MS are extensively utilized for detecting ZEN in food samples [89,90]. However, LC-FLD detection relies on chromatographic separation of the analyte and coexisting matrix, which results in poor selectivity. In contrast, LC–MS offers high sensitivity and selectivity for analysis. LC–MS showcases exceptional sensitivity and unparalleled selectivity for analysis [91]. This inherent capability of LC–MS to precisely differentiate target compounds from background interferences increases its efficacy in ZEA and metabolite determination, thereby significantly increasing the reliability of results obtained. LC-FLD has lower analysis costs and can be used for preliminary screening. However, for more accurate results, it is usually necessary to confirm the potentially positive samples identified in the preliminary screening using LC–MS.
For both LC-FLD and LC–MS, optimal chromatographic separation conditions that distinguish the target analyte from the matrix are pivotal for enhancing analysis selectivity. When opting for an LC column to detect ZEN and its metabolites, the C18 column is the usual choice. Moreover, the utilization of an ultra-high-performance liquid chromatography (UPLC) column, featuring a smaller stationary phase particle size, has gained traction. This selection heightens column efficiency and yields satisfactory analytical outcomes, with higher resolution and narrower chromatographic peaks achievable in a shorter timeframe. The choice of the mobile phase significantly influences peak shape and separation effects. Typically, ACN and methanol in conjunction with water are widely employed for the reverse-phase chromatographic separation of ZEN and its metabolites. During LC–MS analysis, ZEN and its metabolites are commonly detected using negative ion mode, employing either ESI or APCI ionization methods. However, the complex nature of food matrices can greatly suppress the analyte ionization response, impacting the accuracy, precision, and uncertainty of detection in LC–MS analysis. Overcoming matrix effects is crucial for achieving accurate and reliable quantitative analysis in LC–MS [92].
For LC–MS analysis of food samples, using isotopically labeled internal standards for matrix effect correction is an ideal approach. Isotopically labeled analytes closely resemble the analytes of interest in structure and properties. Stable isotope dilution methods involve using molecules labeled with stable isotopes such as 13C, 15N, or 2H. These labeled molecules possess the same structure as the target analyte, serving as internal standards or diluents. This effectively minimizes matrix effects on the signal, offering high sensitivity in the nanogram or lower range. This method proves highly advantageous for the accurate quantitative analysis of zearalenone in food. The stable isotope dilution method has been successfully applied to detect zearalenone in various food products, including beer, maize, wheat flour, dairy products, peanut butter, food-grade gums, baby food, and animal feed, yielding positive results. For instance, Lijalem et al. [9] developed a stable isotope dilution-LC–MS to determine zearalenone and its derivatives in maize. They employed 13C or 2H -labeled molecules with the same chemical structure as the target mycotoxin as internal standards, coupled with LC–MS for detection [9]. This approach had good selectivity, sensitivity (LODs: 0.14 to 0.33 ng/g), accuracy (relative recoveries: 96.7–103.6%), and precision (RSDs < 4%) within the concentration range of 20–400 ng/g. Thus, meeting the requirements of most applications.
However, applying stable isotope dilution methods for zearalenone determination in food is hindered by the high costs associated with synthesizing stable isotope internal standards specific to zearalenone and its metabolites. Additionally, most of its metabolites lack commercially available isotope-labeled analyte standards. Therefore, recent advancements in stable isotope labeling strategies have aimed to address these limitations [93]. Zhang et al. [94] developed an isotope-coded derivatization method to quantify six mycotoxins, including ZEN, in various complex matrices using LC–MS/MS. This involved derivatizing both actual samples and standards with d0-dansyl chloride and its heavy counterpart, d4-dansyl chloride. A mixture of derivatives labeled with light and heavy isotopes was then analyzed using LC–MS/MS, allowing for isotopic internal calibration. This method achieved high sensitivity, with detection limits ranging from 31.03 to 38.15 ng/L. Accuracy percentages ranged from 97.13% to 104.1%, with precision below 3.42%. Successful application of this method was demonstrated by analyzing mycotoxins in three cereal samples (corn, wheat, and sorghum), yielding recoveries ranging from 71.3% to 92.6%. Future advancements in stable isotope labeling technology should focus on improving existing labeling techniques and reagents. Progress and refinement in isotope labeling technology are expected to greatly contribute to mycotoxin detection in food and enhance global food safety.
In addition to LC-FLD and LC–MS, GC–MS has also been employed for the detection of ZEN and its metabolites. However, in contrast, GC–MS necessitates the process of derivatization when analyzing polar ZEN and its metabolites [95]. This is due to the fact that ZEN compounds are nonvolatile and cannot be directly detected upon entering a gas chromatograph–mass spectrometer. Volatile derivatives are formed to enable their analysis, which is achieved by reducing the polarity of the target compounds through silylation derivatization. Principal silylation reagents, such as N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA), N-(trimethylsilyl)imidazole (TSIM), and N-methyl-N-trimethylsilyl trifluoroacetamide (MSTFA), are commonly employed for the chemical derivatization of ZEN compounds. As a result, this can result in GC–MS being relatively time-consuming. It might be precisely this reason that there are fewer studies utilizing GC–MS for the detection of ZEN and its metabolites compared to articles employing LC-FLD and LC–MS analytical techniques.
5. Conclusions and Future Perspectives
The precise determination of ZEN and its metabolites within food matrices has evolved into a critical imperative for upholding food safety standards. However, this task is far from straightforward due to the inherent challenges posed by factors such as the minute concentrations of ZEN and its metabolites in complex food matrices, exacerbated by the presence of an assay of interfering compounds. As a result, the necessity to enhance sample purity and enrichment for quantification emerges as paramount, ensuring the acquisition of dependable quantitative and qualitative insights. To tackle these complexities, a diverse array of sample preparation methodologies has found application in the analysis of zearalenone and its metabolites. Among these techniques, LLE and LLME, SPE and SPME, QuEChERS approaches, and derivatization strategies have gained widespread use. The recent integration of novel materials within microextraction methodologies has introduced streamlined, cost-efficient, and high-efficiency avenues for the analysis of zearalenone in food matrices. However, the horizon of zearalenone determination methods extends beyond current achievements, as researchers are propelling the field toward the amalgamation of distinct sample preparation techniques with automated, reproducible, and precision-oriented assays. An important facet of this trajectory lies in the challenge of miniaturizing the sample processing workflow. This must be achieved while preserving accuracy and robustness and reducing time expenditure, solvent usage, and costs. This formidable goal is underscored not only by economic considerations but also by a conscientious commitment to environmentally sustainable practices. In this evolving landscape, future advancements are poised to revolutionize zearalenone and its metabolite analysis, fostering enhanced food safety regulation and safeguarding public health against mycotoxin contamination.
Author Contributions
Writing—original draft preparation, Y.L., Q.X. and J.C.; writing—review and editing, S.Y., Z.Z. and D.C.; funding acquisition, D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “China Postdoctoral Science Foundation, grant number 2021M702937”, and the “Team of Professors from Zhengzhou University Assisting the Innovation-driven Development of Enterprises Project, grant number JSZLQY2022096”.
Data Availability Statement
The data used to support the findings of this study can be made available by the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ropejko, K.; Twarużek, M. Zearalenone and Its Metabolites—General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Applegate, T. Zearalenone (ZEN) in Livestock and Poultry: Dose, Toxicokinetics, Toxicity and Estrogenicity. Toxins 2020, 12, 377. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef]
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K.; et al. Occurrence, Impact on Agriculture, Human Health, and Management Strategies of Zearalenone in Food and Feed: A Review. Toxins 2021, 13, 92. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, S.S.; Isik, G. Ionic Liquid Based Dispersive Liquid-Liquid Microextraction for Preconcentration of Zearalenone and Its Determination in Beer and Cereal Samples by High-Performance Liquid Chromatography with Fluorescence Detection. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1601–1607. [Google Scholar] [CrossRef]
- Jorquera-Pereira, D.; Pavón-Pérez, J.; Ríos-Gajardo, G. Identification of type B trichothecenes and zearalenone in Chilean cereals by planar chromatography coupled to mass spectroscopy. Food Addit. Contam. Part A 2021, 38, 1778–1787. [Google Scholar] [CrossRef]
- Junsai, T.; Poapolathep, S.; Sutjarit, S.; Giorgi, M.; Zhang, Z.; Logrieco, A.F.; Li, P.; Poapolathep, A. Determination of Multiple Mycotoxins and Their Natural Occurrence in Edible Vegetable Oils Using Liquid Chromatography–Tandem Mass Spectrometry. Foods 2021, 10, 2795. [Google Scholar] [CrossRef]
- Lijalem, Y.G.; Gab-Allah, M.A.; Choi, K.; Kim, B. Development of isotope dilution-liquid chromatography/tandem mass spectrometry for the accurate determination of zearalenone and its metabolites in corn. Food Chem. 2022, 384, 132483. [Google Scholar] [CrossRef]
- Zhang, Y.; Kuang, F.; Liu, C.; Ma, K.; Liu, T.; Zhao, M.; Lv, G.; Huang, H. Contamination and Health Risk Assessment of Multiple Mycotoxins in Edible and Medicinal Plants. Toxins 2023, 15, 209. [Google Scholar] [CrossRef]
- Li, R.; Wen, Y.; Yang, L.; Liu, A.; Wang, F.; He, P. Dual quantum dot nanobeads-based fluorescence-linked immunosorbent assay for simultaneous detection of aflatoxin B1 and zearalenone in feedstuffs. Food Chem. 2022, 366, 130527. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Asi, M.R.; Jinap, S.; Rashid, U. Detection of aflatoxins and zearalenone contamination in wheat derived products. Food Control 2014, 35, 223–226. [Google Scholar] [CrossRef]
- Danicke, S.; Winkler, J. Invited review: Diagnosis of zearalenone (ZEN) exposure of farm animals and transfer of its residues into edible tissues (carry over). Food Chem. Toxicol. 2015, 84, 225–249. [Google Scholar] [CrossRef]
- Zheng, W.; Feng, N.; Wang, Y.; Noll, L.; Xu, S.; Liu, X.; Lu, N.; Zou, H.; Gu, J.; Yuan, Y.; et al. Effects of zearalenone and its derivatives on the synthesis and secretion of mammalian sex steroid hormones: A review. Food Chem. Toxicol. 2019, 126, 262–276. [Google Scholar] [CrossRef]
- Appell, M.; Compton, D.L.; Bosma, W.B. Raman spectral analysis for rapid determination of zearalenone and alpha-zearalanol. Spectrochim. Acta A 2022, 270, 120842. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Chertovich, J.O.; Zherdev, A.V.; Sveshnikov, P.G.; Dzantiev, B.B. Ultrasensitive magnetic ELISA of zearalenone with pre-concentration and chemiluminescent detection. Food Control 2018, 84, 330–338. [Google Scholar] [CrossRef]
- Sadrabadi, N.R.; Ensafi, A.A.; Heydari-Bafrooei, E.; Fazilati, M. Screening of Food Samples for Zearalenone Toxin Using an Electrochemical Bioassay Based on DNA-Zearalenone Interaction. Food Anal. Methods 2016, 9, 2463–2470. [Google Scholar] [CrossRef]
- Wang, W.; Liu, T.; Wang, Y.; Mu, G.; Zhang, F.; Yang, Q.; Hou, X. Hydrophilic Covalent Organic Frameworks Coated Steel Sheet as a Mass Spectrometric Ionization Source for the Direct Determination of Zearalenone and Its Derivatives. J. Agric. Food Chem. 2022, 70, 12211–12219. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, W.T.; Liu, Z.J.; Fu, X.L.; Du, D.L. High-performance liquid chromatography for the sensitive zearalenone determination by the automated immunomagnetic beads purifier for one-step sample pre-treatment. Eur. Food Res. Technol. 2022, 248, 109–117. [Google Scholar] [CrossRef]
- Urusov, A.E.; Petrakova, A.V.; Kuzmin, P.G.; Zherdev, A.V.; Sveshnikov, P.G.; Shafeev, G.A.; Dzantiev, B.B. Application of gold nanoparticles produced by laser ablation for immunochromatographic assay labeling. Anal. Biochem. 2015, 491, 65–71. [Google Scholar] [CrossRef]
- Liu, G.; Han, Z.; Nie, D.; Yang, J.; Zhao, Z.; Zhang, J.; Li, H.; Liao, Y.; Song, S.; De Saeger, S.; et al. Rapid and sensitive quantitation of zearalenone in food and feed by lateral flow immunoassay. Food Control 2012, 27, 200–205. [Google Scholar] [CrossRef]
- Kong, W.-J.; Shen, H.-H.; Zhang, X.-F.; Yang, X.-L.; Qiu, F.; Ou-yang, Z.; Yang, M.-H. Analysis of zearalenone and α-zearalenol in 100 foods and medicinal plants determined by HPLC-FLD and positive confirmation by LC-MS-MS. J. Sci. Food Agric. 2013, 93, 1584–1590. [Google Scholar] [CrossRef]
- Guzman, N.A.; Guzman, D.E. Immunoaffinity Capillary Electrophoresis in the Era of Proteoforms, Liquid Biopsy and Preventive Medicine: A Potential Impact in the Diagnosis and Monitoring of Disease Progression. Biomolecules 2021, 11, 1443. [Google Scholar] [CrossRef]
- Zhai, W.; You, T.; Ouyang, X.; Wang, M. Recent progress in mycotoxins detection based on surface-enhanced Raman spectroscopy. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1887–1909. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.; Lu, H.; Shi, L.; Wang, P.; Ali, Z.; Li, J. Electrochemical detection of bisphenols in food: A review. Food Chem. 2021, 346, 128895. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Dong, Y.; Peng, W.; Fu, Y.; Li, Q.; Fan, Q.; Wang, Y.; Wang, Z. Current analytical methods for the determination of persulfate in aqueous solutions: A historical review. Chem. Eng. J. 2021, 416, 129143. [Google Scholar] [CrossRef]
- Rigano, F.; Tranchida, P.Q.; Dugo, P.; Mondello, L. High-performance liquid chromatography combined with electron ionization mass spectrometry: A review. TrAC Trends Anal. Chem. 2019, 118, 112–122. [Google Scholar] [CrossRef]
- Li, M.; Tong, Z.; Gao, X.; Zhang, L.; Li, S. Simultaneous detection of zearalenone, citrinin, and ochratoxin A in pepper by capillary zone electrophoresis. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2020, 37, 1388–1398. [Google Scholar] [CrossRef]
- Qian, M.; Zhang, H.; Wu, L.; Jin, N.; Wang, J.; Jiang, K. Simultaneous determination of zearalenone and its derivatives in edible vege oil by gel permeation chromatography and gas chromatography-triple quadrupole mass spectrometry. Food Chem. 2015, 166, 23–28. [Google Scholar] [CrossRef]
- Medina, B.G.; Sartori, A.V.; de Moraes, M.H.P.; Cardoso, M.H.W.M.; Jacob, S.D. Validation and application of an analytical method for the determination of mycotoxins in crackers by UPLC-MS/MS. Food Sci. Technol. 2019, 39, 583–591. [Google Scholar] [CrossRef]
- Vargas Medina, D.A.; Bassolli Borsatto, J.V.; Maciel, E.V.S.; Lanças, F.M. Current role of modern chromatography and mass spectrometry in the analysis of mycotoxins in food. TrAC Trends Anal. Chem. 2021, 135, 116156. [Google Scholar] [CrossRef]
- Rausch, A.K.; Brockmeyer, R.; Schwerdtle, T. Development and validation of a liquid chromatography tandem mass spectrometry multi-method for the determination of 41 free and modified mycotoxins in beer. Food Chem. 2021, 338, 127801. [Google Scholar] [CrossRef] [PubMed]
- Bessaire, T.; Ernest, M.; Christinat, N.; Carreres, B.; Panchaud, A.; Badoud, F. High resolution mass spectrometry workflow for the analysis of food contaminants: Application to plant toxins, mycotoxins and phytoestrogens in plant-based ingredients. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2021, 38, 978–996. [Google Scholar] [CrossRef] [PubMed]
- Al-Taher, F.; Banaszewski, K.; Jackson, L.; Zweigenbaum, J.; Ryu, D.; Cappozzo, J. Rapid Method for the Determination of Multiple Mycotoxins in Wines and Beers by LC-MS/MS Using a Stable Isotope Dilution Assay. J. Agric. Food Chem. 2013, 61, 2378–2384. [Google Scholar] [CrossRef]
- D’Orazio, G.; Asensio-Ramos, M.; Hernandez-Borges, J.; Fanali, S.; Rodriguez-Delgado, M.A. Estrogenic compounds determination in water samples by dispersive liquid-liquid microextraction and micellar electrokinetic chromatography coupled to mass spectrometry. J. Chromatogr. A 2014, 1344, 109–121. [Google Scholar] [CrossRef]
- Emidio, E.S.; da Silva, C.P.; de Marchi, M.R. Determination of estrogenic mycotoxins in environmental water samples by low-toxicity dispersive liquid-liquid microextraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2015, 1391, 1–8. [Google Scholar] [CrossRef]
- Khan, M.R.; Alothman, Z.A.; Ghfar, A.A.; Wabaidur, S.M. Analysis of aflatoxins in nonalcoholic beer using liquid-liquid extraction and ultraperformance LC-MS/MS. J. Sep. Sci. 2013, 36, 572–577. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Gámiz-Gracia, L.; García-Campaña, A.M. Alternative sample treatments for the determination of sulfonamides in milk by HPLC with fluorescence detection. Food Chem. 2014, 143, 459–464. [Google Scholar] [CrossRef]
- Pochivalov, A.; Pavlova, K.; Garmonov, S.; Bulatov, A. Behaviour of deep eutectic solvent based on terpenoid and long-chain alcohol during dispersive liquid-liquid microextraction: Determination of zearalenone in cereal samples. J. Mol. Liq. 2022, 366, 120231. [Google Scholar] [CrossRef]
- Pi, J.; Jin, P.; Zhou, S.; Wang, L.; Wang, H.; Huang, J.; Gan, L.; Yuan, T.; Fan, H. Combination of Ultrasonic-assisted Aqueous Two-phase Extraction with Solidifying Organic Drop-dispersive Liquid–liquid Microextraction for Simultaneous Determination of Nine Mycotoxins in Medicinal and Edible Foods by HPLC with In-series DAD and FLD. Food Anal. Methods 2021, 15, 428–439. [Google Scholar] [CrossRef]
- Wang, L.; Luan, C.; Chen, F.; Wang, R.; Shao, L. Determination of zearalenone in maize products by vortex-assisted ionic-liquid-based dispersive liquid-liquid microextraction with high-performance liquid chromatography. J. Sep. Sci. 2015, 38, 2126–2131. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Ye, J.; Xuan, Z.; Li, L.; Wen, X.; Li, Z.; Liu, H.; Wang, S. Automatic Pretreatment of Dispersive Liquid Liquid Microextraction Based on Immunomagnetic Beads Coupled with UPLC-FLD for the Determination of Zearalenone in Corn Oils. Toxins 2023, 15, 337. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.A.; Sukor, R.; Ismail, M.N.; Selamat, J. Dispersive Liquid-Liquid Microextraction (DLLME) and LC-MS/MS Analysis for Multi-Mycotoxin in Rice Bran: Method Development, Optimization and Validation. Toxins 2021, 13, 280. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Taherimaslak, Z.; Parvizi, S.; Torkejokar, M. Spectrofluorimetric determination of zearalenone using dispersive liquid-liquid microextraction coupled to micro-solid phase extraction onto magnetic nanoparticles. RSC Adv. 2014, 4, 45065–45073. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, J.J.; Huang, B.F.; Cai, Z.X.; Ren, Y.P. High-performance liquid chromatographic determination of multi-mycotoxin in cereals and bean foodstuffs using interference-removal solid-phase extraction combined with optimized dispersive liquid-liquid microextraction. J. Sep. Sci. 2017, 40, 2141–2150. [Google Scholar] [CrossRef]
- Antep, H.M.; Merdivan, M. Development of new dispersive liquid–liquid microextraction technique for the identification of zearalenone in beer. Anal. Methods 2012, 4, 4129–4134. [Google Scholar] [CrossRef]
- Bochetto, A.; Merino, N.; Kaplan, M.; Guiñez, M.; Cerutti, S. Design of a combined microextraction and back-extraction technique for the analysis of mycotoxins in amaranth seeds. J. Food Compost. Anal. 2021, 98, 103818. [Google Scholar] [CrossRef]
- D’Orazio, G.; Asensio-Ramos, M.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á.; Fanali, S. Evaluation of the combination of a dispersive liquid-liquid microextraction method with micellar electrokinetic chromatography coupled to mass spectrometry for the determination of estrogenic compounds in milk and yogurt. Electrophoresis 2015, 36, 615–625. [Google Scholar] [CrossRef]
- Callejon, R.M.; Ubeda, C.; Rios-Reina, R.; Morales, M.L.; Troncoso, A.M. Recent developments in the analysis of musty odour compounds in water and wine: A review. J. Chromatogr. A 2016, 1428, 72–85. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Zhang, Q. Advances in the development of detection techniques for mycotoxins in vegetable oil. Se Pu 2019, 37, 569–580. [Google Scholar] [CrossRef]
- Song, S.; Ediage, E.N.; Wu, A.; De Saeger, S. Development and application of salting-out assisted liquid/liquid extraction for multi-mycotoxin biomarkers analysis in pig urine with high performance liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2013, 1292, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.M.; Arroyo-Manzanares, N.; Garcia-Campana, A.M.; Gamiz-Gracia, L. Determination of Fusarium toxins in functional vegetable milks applying salting-out-assisted liquid-liquid extraction combined with ultra-high-performance liquid chromatography tandem mass spectrometry. Food Addit. Contam. Part A 2017, 34, 2033–2041. [Google Scholar] [CrossRef] [PubMed]
- Khalifehzadeh, E.; Ahmadi, S.; Beigmohammadi, F. Magnetic dispersive solid phase extraction of ZEAralenone using Fe3O4@ hydroxy propyl methyl cellulose nanocomposite from wheat flour samples prior to fluorescence determination: Multivariate optimization by Taguchi design. Microchem. J. 2021, 170, 106682. [Google Scholar] [CrossRef]
- Calahorra-Rio, L.; Guadaño-Sánchez, M.; Moya-Cavas, T.; Urraca, J.L. Magnetic Core-Shell Nanoparticles Using Molecularly Imprinted Polymers for Zearalenone Determination. Molecules 2022, 27, 8166. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Jiang, K.; Fan, Z.; Diana Di Mavungu, J.; Dong, M.; Guo, W.; Fan, K.; Campbell, K.; Zhao, Z.; Wu, Y. Multi-walled carbon nanotubes-based magnetic solid-phase extraction for the determination of zearalenone and its derivatives in maize by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Control 2017, 79, 177–184. [Google Scholar] [CrossRef]
- Speltini, A.; Scalabrini, A.; Maraschi, F.; Sturini, M.; Profumo, A. Newest applications of molecularly imprinted polymers for extraction of contaminants from environmental and food matrices: A review. Anal. Chim. Acta 2017, 974, 1–26. [Google Scholar] [CrossRef]
- Drzymala, S.S.; Weiz, S.; Heinze, J.; Marten, S.; Prinz, C.; Zimathies, A.; Garbe, L.A.; Koch, M. Automated solid-phase extraction coupled online with HPLC-FLD for the quantification of zearalenone in edible oil. Anal. Bioanal. Chem. 2015, 407, 3489–3497. [Google Scholar] [CrossRef]
- Gonzalez-Salamo, J.; Socas-Rodriguez, B.; Hernandez-Borges, J.; Rodriguez-Delgado, M.A. Core-shell poly(dopamine) magnetic nanoparticles for the extraction of estrogenic mycotoxins from milk and yogurt prior to LC-MS analysis. Food Chem. 2017, 215, 362–368. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Y.C.; Bai, X.L.; Liu, Y.M.; Wu, G.F.; Yang, F.S.; Liao, X. Multi-mycotoxins analysis in liquid milk by UHPLC-Q-Exactive HRMS after magnetic solid-phase extraction based on PEGylated multi-walled carbon nanotubes. Food Chem. 2020, 305, 125429. [Google Scholar] [CrossRef]
- Jiang, K.; Huang, Q.; Fan, K.; Wu, L.; Nie, D.; Guo, W.; Wu, Y.; Han, Z. Reduced graphene oxide and gold nanoparticle composite-based solid-phase extraction coupled with ultra-high-performance liquid chromatography-tandem mass spectrometry for the determination of 9 mycotoxins in milk. Food Chem. 2018, 264, 218–225. [Google Scholar] [CrossRef]
- Barbera, G.; Capriotti, A.L.; Cavaliere, C.; Foglia, P.; Montone, C.M.; Chiozzi, R.Z.; Lagana, A. A Rapid Magnetic Solid Phase Extraction Method Followed by Liquid Chromatography-Tandem Mass Spectrometry Analysis for the Determination of Mycotoxins in Cereals. Toxins 2017, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ma, Y.; Zhang, X.; Yang, X.; Hu, X. A dispersive solid phase extraction adsorbent based on aptamer modified chitosan nanofibers for zearalenone separation in corn, wheat, and beer samples. Anal. Methods 2020, 12, 5852–5860. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jin, Y.; Guo, Q.; Wang, X.; Luo, S.; Yang, W.; Li, J.; Chen, Y. Immunoaffinity Cleanup and Isotope Dilution-Based Liquid Chromatography Tandem Mass Spectrometry for the Determination of Six Major Mycotoxins in Feed and Feedstuff. Toxins 2022, 14, 631. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Ge, W.; Liu, X.; Zhu, Y. Preconcentration and Determination of Zearalenone in Corn Oil by a One-Step Prepared Polydopamine-Based Magnetic Molecularly Imprinted Polymer (MIP) with High-Performance Liquid Chromatography with Fluorescence (HPLC-FLD) Detection. Anal. Lett. 2022, 55, 343–354. [Google Scholar] [CrossRef]
- Kholová, A.; Lhotská, I.; Erben, J.; Chvojka, J.; Švec, F.; Solich, P.; Šatínský, D. Comparison of nanofibers, microfibers, nano/microfiber graphene doped composites, molecularly imprinted polymers, and restricted access materials for on-line extraction and chromatographic determination of citrinin, zearalenone, and ochratoxin A in plant-based milk beverages. Microchem. J. 2023, 191, 108937. [Google Scholar]
- Vaclavikova, M.; MacMahon, S.; Zhang, K.; Begley, T.H. Application of single immunoaffinity clean-up for simultaneous determination of regulated mycotoxins in cereals and nuts. Talanta 2013, 117, 345–351. [Google Scholar] [CrossRef]
- Moya-Cavas, T.; Navarro-Villoslada, F.; Lucas Urraca, J.; Antonio Serrano, L.; Orellana, G.; Cruz Moreno-Bondi, M. Simultaneous determination of zearalenone and alternariol mycotoxins in oil samples using mixed molecularly imprinted polymer beads. Food Chem. 2023, 412, 135538. [Google Scholar] [CrossRef]
- Zhang, Y.X.; He, J.; Song, L.X.; Wang, H.G.; Huang, Z.P.; Sun, Q.Y.; Ba, X.; Li, Y.Y.; You, L.Q.; Zhang, S.S. Application of surface-imprinted polymers supported by hydroxyapatite in the extraction of zearalenone in various cereals. Anal. Bioanal. Chem. 2020, 412, 4045–4055. [Google Scholar] [CrossRef]
- Zhao, Y.; Wan, L.H.; Bai, X.L.; Liu, Y.M.; Zhang, F.P.; Liu, Y.M.; Liao, X. Quantification of mycotoxins in vegetable oil by UPLC-MS/MS after magnetic solid-phase extraction. Food Addit. Contam. Part A 2017, 34, 1201–1210. [Google Scholar] [CrossRef]
- Moreno, V.; Zougagh, M.; Rios, A. Hybrid nanoparticles based on magnetic multiwalled carbon nanotube-nanoC(18)SiO(2) composites for solid phase extraction of mycotoxins prior to their determination by LC-MS. Microchim. Acta 2016, 183, 871–880. [Google Scholar] [CrossRef]
- Thongprapai, P.; Cheewasedtham, W.; Chong, K.F.; Rujiralai, T. Selective magnetic nanographene oxide solid-phase extraction with high-performance liquid chromatography and fluorescence detection for the determination of zearalenone in corn samples. J. Sep. Sci. 2018, 41, 4348–4354. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Mu, G.D.; Wang, X.J.; Zhang, F.; Li, Y.L.; Lu, D.J.; Chen, F.M.; Yang, M.L.; He, M.Y.; Liu, T. Fast construction of core-shell structured magnetic covalent organic framework as sorbent for solid-phase extraction of zearalenone and its derivatives prior to their determination by UHPLC-MS/MS. Microchim. Acta 2021, 188, 246. [Google Scholar] [CrossRef] [PubMed]
- Lucci, P.; David, S.; Conchione, C.; Milani, A.; Moret, S.; Pacetti, D.; Conte, L. Molecularly Imprinted Polymer as Selective Sorbent for the Extraction of Zearalenone in Edible Vegetable Oils. Foods 2020, 9, 1439. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Feng, Y.; Suo, D.; Xiao, Z.; Wang, S.; Liang, Y.; Fan, X. Simultaneous Determination of 11 Mycotoxins in Maize via Multiple-Impurity Adsorption Combined with Liquid Chromatography–Tandem Mass Spectrometry. Foods 2022, 11, 3624. [Google Scholar] [CrossRef]
- Xu, X.-L.; Xu, S.-J.; Wang, B.; Liu, Y.-W.; Li, W.-X.; Fan, M.; Xu, X.; Ma, L.; Chen, D. In-syringe cotton fiber solid-phase extraction coupled with stable-isotope-dilution liquid chromatography-tandem mass spectrometry for the determination of zearalenone. Microchem. J. 2023, 193, 109264. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, X.; Yang, Y.; Shi, R.; Ruan, G.; Huang, Y. Amphiphilic polymers facilitated solid-phase extraction coupled with ultra-performance liquid chromatography-tandem mass spectrometry for direct extraction and analysis of zearalenone and zearalanone in corn juice samples. J. Sep. Sci. 2023, 46, 2300112. [Google Scholar] [CrossRef]
- Wang, M.; He, J.; Zhang, Y.; Tian, Y.; Xu, P.; Zhang, X.; Li, Y.; Chen, J.; He, L. Application of magnetic hydroxyapatite surface-imprinted polymers in pretreatment for detection of zearalenone in cereal samples. J. Chromatogr. B 2022, 1201–1202, 123297. [Google Scholar] [CrossRef]
- Gadzala-Kopciuch, R.; Kuzniewska, A.; Buszewski, B. Analytical approaches and preparation of biological, food and environmental samples for analyses of zearalenone and its metabolites. Rev. Anal. Chem. 2020, 39, 157–167. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Camara, J.S. QuEChERS—Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Deng, Y.C.; Zheng, J.F.; Zhang, Y.; Yang, L.H.; Liao, C.J.; Su, L.; Zhou, Y.Y.; Gong, D.X.; Chen, L.; et al. The application of the QuEChERS methodology in the determination of antibiotics in food: A review. TRAC-Trends Anal. Chem. 2019, 118, 517–537. [Google Scholar] [CrossRef]
- He, Q.R.; Peng, H.W.; Yang, J.Y.; Xu, Z.L.; Fan, C.C.; Sun, Y.M. QuEChERS extraction followed by enzyme-linked immunosorbent assay for determination of deoxynivalenol and zearalenone in cereals. Food Agric. Immunol. 2017, 28, 1477–1495. [Google Scholar] [CrossRef]
- De Colli, L.; Elliott, C.; Finnan, J.; Grant, J.; Arendt, E.K.; McCormick, S.P.; Danaher, M. Determination of 42 mycotoxins in oats using a mechanically assisted QuEChERS sample preparation and UHPLC-MS/MS detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1150, 122187. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Fernandes, J.O.; Cunha, S.C. Optimization and validation of a method based in a QuEChERS procedure and gas chromatography-mass spectrometry for the determination of multi-mycotoxins in popcorn. Food Control 2012, 27, 188–193. [Google Scholar] [CrossRef]
- Zhao, H.X.; Chen, X.Y.; Shen, C.; Qu, B.C. Determination of 16 mycotoxins in vegetable oils using a QuEChERS method combined with high-performance liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part A 2017, 34, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Hou, C.; Lu, Y.; Jiang, J.; Zhang, X. Simultaneous Screening for 14 Mycotoxin Contaminants in Foods by QuEChERS-LC-MS-MS. Food Sci. 2014, 35, 190–196. [Google Scholar]
- Yan, Z.; Wang, L.; Wang, J.; Tan, Y.L.; Yu, D.Z.; Chang, X.J.; Fan, Y.Y.; Zhao, D.Y.; Wang, C.; De Boevre, M.; et al. A QuEChERS-Based Liquid Chromatography-Tandem Mass Spectrometry Method for the Simultaneous Determination of Nine Zearalenone-Like Mycotoxins in Pigs. Toxins 2018, 10, 129. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, N.; Yang, L.; Deng, Y.; Wang, J.; Song, S.; Lin, S.; Wu, A.; Zhou, Z.; Hou, J. Multi-mycotoxin analysis of animal feed and animal-derived food using LC-MS/MS system with timed and highly selective reaction monitoring. Anal. Bioanal. Chem. 2015, 407, 7359–7368. [Google Scholar] [CrossRef]
- Jettanajit, A.; Nhujak, T. Determination of Mycotoxins in Brown Rice Using QuEChERS Sample Preparation and UHPLC-MS-MS. J. Chromatogr. Sci. 2016, 54, 720–729. [Google Scholar] [CrossRef]
- Han, X.; Huangfu, B.; Xu, T.; Xu, W.-T.; Asakiya, C.; Huang, K.; Xiaoyun, H. Research Progress of Safety of Zearalenone: A Review. Toxins 2022, 14, 386. [Google Scholar] [CrossRef]
- Mazaheri, M.; Maymand, M.M.; Gilasgar, A.; Akbarzadeh, A.; Manafi, M.H. Quantification of the zearalenone in maize oil with no clean-up. Food Control 2021, 127, 108166. [Google Scholar] [CrossRef]
- Lv, S.; Wu, X.; Guan, J.; Yan, Y.; Ge, M.; Zhu, G. Quantification and Confirmation of Zearalenone Using a LC-MS/MS QTRAP System in Multiple Reaction Monitoring and Enhanced Product Ion Scan Modes. Food Anal. Methods 2021, 14, 1843–1851. [Google Scholar] [CrossRef]
- Trufelli, H.; Palma, P.; Famiglini, G.; Cappiello, A. An overview of matrix effects in liquid chromatography–mass spectrometry. Mass Spectrom. Rev. 2011, 30, 491–509. [Google Scholar] [CrossRef]
- Tian, X.; Permentier, H.P.; Bischoff, R. Chemical isotope labeling for quantitative proteomics. Mass Spectrom. Rev. 2023, 42, 546–576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, J.; Shi, X.; Sun, Z.; You, J. Development of a method for the quantification of six mycotoxins in cereal samples using isotope-coded derivatization combined with ultra-high-performance liquid chromatography–tandem mass spectrometry. J. Food Compost. Anal. 2023, 120, 105347. [Google Scholar] [CrossRef]
- Luo, S.; Liu, Y.; Guo, Q.; Wang, X.; Tian, Y.; Yang, W.; Li, J.; Chen, Y. Determination of Zearalenone and Its Derivatives in Feed by Gas Chromatography-Mass Spectrometry with Immunoaffinity Column Cleanup and Isotope Dilution. Toxins 2022, 14, 764. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).