Frying Performance of Gallic Acid and/or Methyl Gallate Accompanied by Phosphatidylcholine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Initial Quality Indicators of the Vegetable Oils
2.3. Oxidative Stability Index (OSI)
2.4. Frying Procedure
2.5. Kinetic Data Analysis
2.6. Statistical Analysis
3. Results
3.1. Characterization of the Vegetable Oils
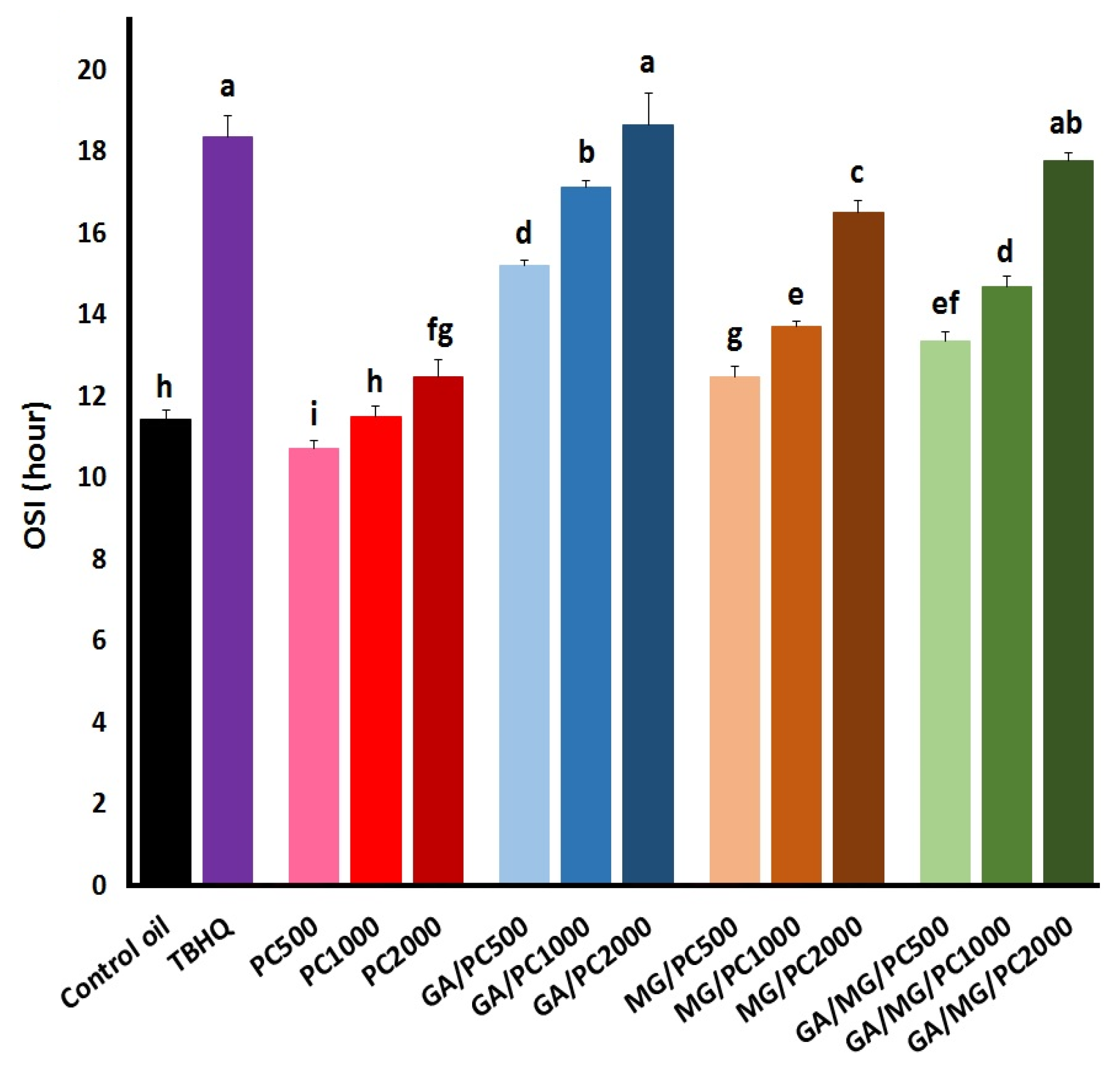
3.2. OSI Values
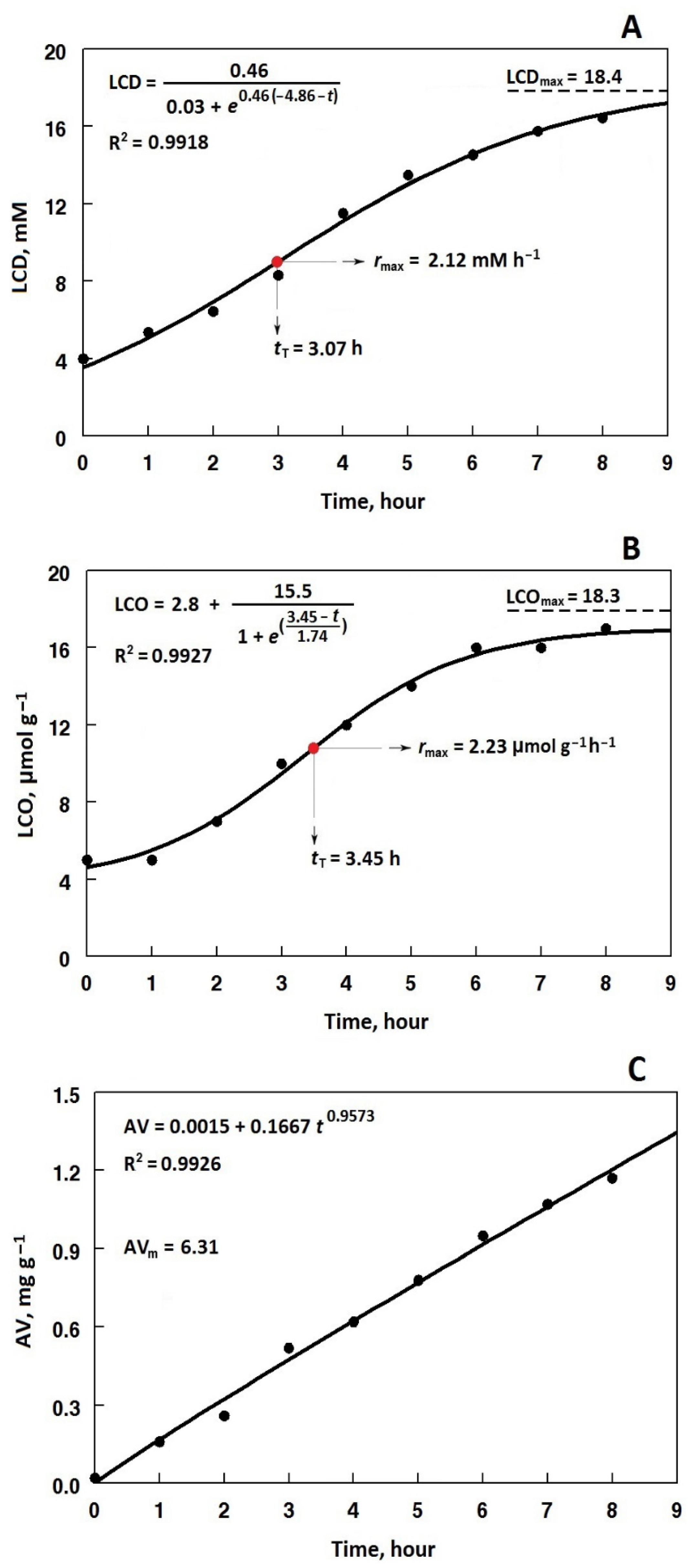
3.3. Kinetics of Change in the Total LCD Content
3.4. Kinetics of Change in the Total LCO Content
3.5. Kinetics of Change in AV
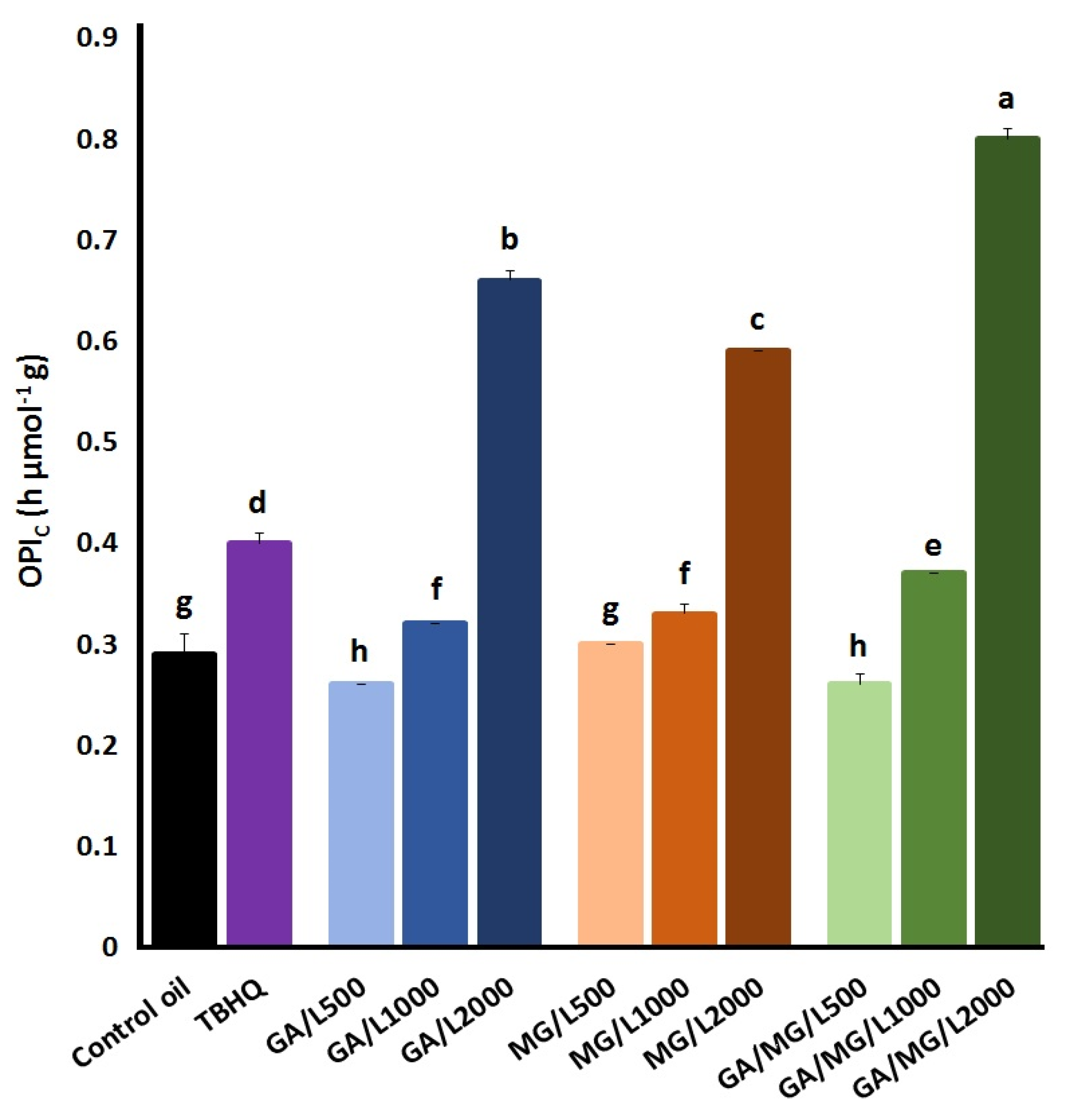
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Choe, E.; Min, D. Chemistry of deep-fat frying oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef] [PubMed]
- Bordin, K.; Kunitake, M.T.; Aracava, K.K.; Trindade, C.S.F. Changes in food caused by deep fat frying—A review. Arch. Latinoam. Nutr. 2013, 63, 5–13. [Google Scholar] [PubMed]
- Wang, W.; Wu, N.; Zu, Y.; Fu, Y. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.C.M.; Szeto, Y.T.; Benzie, I.F. Antioxidant protection of edible oils. Plant Foods Hum. Nutr. 2007, 62, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Jeon, M.; Kim, Y.S. Methyl gallate, a potent antioxidant inhibits mouse and human adipocyte differentiation and oxidative stress in adipocytes through impairment of mitotic clonal expansion. Biofactors 2016, 42, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.Y.; Choi, D.S. Protective effect of gallic acid on the thermal oxidation of corn and soybean oils during high temperature heating. Food Sci. Biotechnol. 2016, 25, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Farhoosh, R.; Nyström, L. Antioxidant potency of gallic acid, methyl gallate and their combinations in sunflower oil triacylglycerols at high temperature. Food Chem. 2018, 244, 29–35. [Google Scholar] [CrossRef]
- Hosseinkhani, M.; Farhoosh, R. Kinetics of chemical deteriorations over the frying protected by gallic acid and methyl gallate. Sci. Rep. 2023, 13, 11059. [Google Scholar] [CrossRef]
- Yoshida, K.; Terao, J.; Suzuki, T.; Takama, K. Inhibitory effect of phosphatidylserine on iron-dependent lipid peroxidation. Biochem. Biophys. Res. Comm. 1991, 179, 1077–1081. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Leon, M.M.; Zamora, R. Antioxidative activity of amino phospholipids and phospholipid/amino acid mixtures in edible oils as determined by the Rancimat method. J. Agric. Food Chem. 2006, 4, 5461–5467. [Google Scholar] [CrossRef]
- Cui, L.; McClements, D.J.; Decker, E.A. Impact of phosphatidylethanolamine on the antioxidant activity of α-tocopherol and trolox in bulk oil. J. Agric. Food Chem. 2015, 63, 3288–3294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, M.; Zhang, W.; Zhao, W.; Yang, R. Impact of phosphatidylcholine and phosphatidylethanolamine on the oxidative stability of stripped peanut oil and bulk peanut oil. Food Chem. 2020, 311, 125962. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, H.; Farhoosh, R.; Rezaie, M. Interfacial performance of gallic acid and methyl gallate accompanied by lecithin in inhibiting bulk phase oil peroxidation. Food Chem. 2020, 328, 127128. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; McClements, D.J.; Decker, E.A. Minor components in food oils: A critical review of their roles on lipid oxidation chemistry in bulk oils and emulsions. Crit. Rev. Food Sci. Nutr. 2011, 51, 901–916. [Google Scholar] [CrossRef] [PubMed]
- Aladedunye, F.A.; Przybylski, R. Frying performance of canola oil triacylglycerides as affected by vegetable oils minor components. J. Am. Oil Chem. Soc. 2012, 89, 41–53. [Google Scholar] [CrossRef]
- Aladedunye, F.; Przybylski, R. Phosphatidylcholine and dihydrocaffeic acid amide mixture enhanced the thermo-oxidative stability of canola oil. Food Chem. 2014, 150, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Hammond, E.G. Analysis of oleate, linoleate and linolenate hydroperoxides in oxidized ester mixtures. Lipids 1980, 15, 379–385. [Google Scholar] [CrossRef]
- Shantha, N.C.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS Press: Champaign, IL, USA, 1993. [Google Scholar]
- Wong, M.L.; Timms, R.E.; Goh, E.M. Colorimetric determination of total tocopherols in palm oil, olein and stearin. J. Am. Oil Chem. Soc. 1988, 65, 258–261. [Google Scholar] [CrossRef]
- Capannesi, C.; Palchetti, I.; Mascini, M.; Parenti, A. Electrochemical sensor and biosensor for polyphenols detection in olive oils. Food Chem. 2000, 71, 553–562. [Google Scholar] [CrossRef]
- Saguy, I.S.; Shani, A.; Weinberg, P.; Garti, N. Utilization of jojoba oil for deep-fat frying of foods. LWT—Food Sci. Technol. 1996, 29, 573–577. [Google Scholar] [CrossRef]
- Endo, Y.; Li, C.M.; Tagiri-Endo, M.; Fugimoto, K. A modified method for the estimation of total carbonyl compounds in heated and frying oils using 2-propanol as a solvent. J. Am. Oil Chem. Soc. 2001, 10, 1021–1024. [Google Scholar] [CrossRef]
- Farhoosh, R. Reliable determination of the induction period and critical reverse micelle concentration of lipid hydroperoxides exploiting a model composed of pseudo-first and -second order reaction kinetics. LWT—Food Sci. Technol. 2018, 98, 406–410. [Google Scholar] [CrossRef]
- Farhoosh, R. A reconsidered approach providing kinetic parameters and rate constants to analyze the oxidative stability of bulk lipid systems. Food Chem. 2020, 327, 127088. [Google Scholar] [CrossRef] [PubMed]
- Farhoosh, R. Quantitative criteria characterizing the time change pattern of total lipid-peroxidation carbonyls. Sci. Rep. 2022, 12, 22345. [Google Scholar] [CrossRef] [PubMed]
- Grompone, M.A. Sunflower oil. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; Wiley: New York, NY, USA, 2005; pp. 654–730. [Google Scholar]
- Basiron, Y. Palm oil. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; Wiley: New York, NY, USA, 2005; pp. 333–429. [Google Scholar]
- Hsieh, R.J.; Kinsella, J.E. Oxidation of polyunsaturated fatty acids: Mechanisms, products, and inhibition with emphasis on fish. Adv. Food Nutr. Res. 1989, 33, 233–341. [Google Scholar] [PubMed]
- De Marco, E.; Savarese, M.; Parisini, C.; Battimo, I.; Falco, S.; Sacchi, R. Frying performance of a sunflower/palm oil blend in comparison with pure palm oil. Eur. J. Lipid Sci. Technol. 2007, 109, 237–246. [Google Scholar] [CrossRef]
- Gunstone, F.D. Vegetable sources of lipids. In Modifying Lipids for Use in Food; Gunstone, F.D., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2006; pp. 11–27. [Google Scholar]
- Shahidi, F.; Zhong, Y. Edible oil and fat products, chemistry, properties and health effects, lipid oxidation: Measurement methods. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; Wiley: New York, NY, USA, 2005; pp. 370–371. [Google Scholar]
- Houhoula, D.P.; Oreopoulou, V.; Tzai, C. A kinetic study of oil deterioration during frying and a comparison with heating. J. Am. Oil Chem. Soc. 2002, 79, 133–137. [Google Scholar] [CrossRef]
- Pantzaris, T.P. Comparison of monounsaturated and polyunsaturated oils in continuous frying. Gras. Acei. 1998, 49, 319–352. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Tanii, K.; Tanaka, T. Peroxidation of polyunsaturated fatty acids, initiated by the direct interaction between a cobalt porphyrin complex and polyunsaturated fatty acids. J. Chem. Soc., Perkin Trans. 2 1989, 12, 2035–2039. [Google Scholar] [CrossRef]
- White, P.J. Methods for measuring changes in deep-fat frying oils. Food Technol. 1991, 45, 75–80. [Google Scholar]
- Moosavi, S.M.R.; Farhoosh, R. A new insight into the evaluation of used frying oils based on the kinetics of chemical changes during the process. Foods 2023, 12, 316. [Google Scholar] [CrossRef] [PubMed]
- Damanik, M.; Murkovic, M. Formation of potentially toxic carbonyls during oxidation of triolein in the presence of alimentary antioxidants. Monatsh. Chem. 2017, 148, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. Hydroperoxide decomposition. In Lipid Oxidation; Frankel, E.N., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 67–98. [Google Scholar]
- Crosby, G. Do cooking oils present a health risk? Food Technol. 2018, 2, 50–57. [Google Scholar]
- Frega, N.; Mozzon, M.; Lercker, G. Effect of free fatty acids on the oxidative stability of vegetable oils. J. Am. Oil Chem. Soc. 1999, 76, 325–329. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit. Rev. Food Sci. Nutr. 2005, 45, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, F.J.; Nogales, F.; Zamora, R. Determination of pyrrolized phospholipids in oxidized phospholipid vesicles and lipoproteins. Anal. Biochem. 2004, 334, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, F.J.; Nogales, F.; Zamora, R. Changes produced in the antioxidative activity of phospholipids as a consequence of their oxidation. J. Agric. Food Chem. 2005, 53, 659–662. [Google Scholar] [CrossRef]
- Blumenthal, M.M. A new look at the chemistry and physics of deep-fat frying. Food Technol. 1991, 2, 68–71. [Google Scholar]
- Kourimska, L.; Pokorny, J.; Reblova, Z. Phospholipids as inhibitors of oxidation during food storage and frying. Prehrambeno Technol. Biotechnol. Rev. 1994, 32, 91–94. [Google Scholar]
- Akoh, C.C.; Min, D.B. Food lipids: Chemistry, Nutritin, and Biotechnology; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Gordon, M.H.; Kourimska, L. The effects of antioxidants on changes in oils during heating and deep frying. J. Sci. Food Agric. 1995, 68, 347–353. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Leon, M.M.; Zamora, R. Effect of tocopherols in the antioxidative activity of oxidized lipid-amine reaction products. J. Agric. Food Chem. 2007, 55, 4436–4442. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Woo, Y.; Choi, H.; Kim, M.-J.; Lee, J. Dioleylphosphatidylcholine increases the antioxidant properties of ascorbyl palmitate in bulk oils compared to other hydrophilic and lipophilic antioxidants. Food Chem. 2021, 349, 129082. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, J.R.; Graf, E. Role of alpha-tocopherol, ascorbic acid, citric acid and EDTA as oxidants in model systems. J. Food Sci. 1986, 51, 1293–1296. [Google Scholar] [CrossRef]
- Cui, L.; Decker, E.A. Phospholipids in foods: Prooxidants or antioxidants? J. Sci. Food Agric. 2016, 96, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Sievers, R.E.; Bailar, J.C. Some metal chelates of ethylenediaminetetraacetic acid, diethylenetriaminepentaacetic acid, and triethylenetetraminehexaacetic acid. Inorg. Chem. 1962, 1, 174–182. [Google Scholar] [CrossRef]
- Pan, X.; Irwin, J.A.; Leonard, M.; Welsby, D. Choline and ethanolamine decompose lipid hydroperoxides into hydroxyl lipids. J. Am. Oil Chem. Soc. 2010, 87, 1235–1245. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Yuki, E.; Kato, H.; Fujimaki, M. The mechanism of synergism between tocopherols and trimethylamine oxide in the inhibition of the autoxidation of methyl linoleate. Agric. Biol. Chem. 1978, 42, 711–716. [Google Scholar] [CrossRef][Green Version]
- Chen, B.; Han, A.; McClements, D.J.; Decker, E.A. Physical structures in soybean oil and their impact on lipid oxidation. J. Agric. Food Chem. 2010, 58, 11993–11999. [Google Scholar] [CrossRef]
- Cui, L.; Kittipongpittaya, K.; McClements, D.J.; Decker, E.A. Impact of phosphoethanolamine reverse micelles on lipid oxidation in bulk oils. J. Am. Oil Chem. Soc. 2014, 91, 1931–1937. [Google Scholar] [CrossRef]
- Laguerre, M.; Chen, B.; Lecomte, J.; Villeneuve, P.; McClements, D.J.; Decker, E.A. Antioxidant properties of chlorogenic acid and its alkyl esters in stripped corn oil in combination with phospholipids and/or water. J. Agric. Food Chem. 2011, 59, 10361–10366. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Han, A.; Laguerre, M.; McClements, D.J.; Decker, E.A. Role of reverse micelles on lipid oxidation in bulk oils—impact of phospholipids on antioxidant activity of a-tocopherol and Trolox. Food Funct. 2011, 2, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Doert, M.; Jaworska, K.; Moersel, J.; Kroh, L.W. Synergistic effect of lecithins for tocopherols: Lecithin based regeneration of alpha-tocopherol. Eur. Food Res. Technol. 2012, 235, 915–928. [Google Scholar] [CrossRef]
- Takenaka, A.; Hosokawa, M.; Miyashita, K. Unsaturated phosphatidylethanolamine as effective synergist in combination with α-tocopherol. J. Oleo Sci. 2007, 56, 511–516. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kittipongpittaya, K.; Panya, A.; Cui, L.; McClements, D.J.; Decker, E.A. Association colloids formed by multiple surface active minor components and their effect on lipid oxidation in bulk oil. J. Am. Oil Chem. Soc. 2014, 91, 1955–1965. [Google Scholar] [CrossRef]
- Kittipongpittaya, K.; Panya, A.; McClements, D.J.; Decker, E.A. Impact of free fatty acids and phospholipids on reverse micelles formation and lipid oxidation in bulk oil. J. Am. Oil Chem. Soc. 2014, 91, 453–462. [Google Scholar] [CrossRef]
- Koga, T.; Terao, J. Antioxidant activity of a novel phosphatidyl derivative of vitamin E in lard and its model system. J. Agric. Food Chem. 1994, 42, 1291–1294. [Google Scholar] [CrossRef]
- Koga, T.; Terao, J. Phospholipids increase radical-scavenging activity of vitamin E in a bulk oil model system. J. Agric. Food Chem. 1995, 43, 1450–1454. [Google Scholar] [CrossRef]
- Bayram, I.; Decker, E.A. Underlying mechanisms of synergistic antioxidant interactions during lipid oxidation. Trends Food Sci. Technol. 2023, 133, 219–230. [Google Scholar] [CrossRef]
- Lu, Z.; Nie, G.; Belton, P.S.; Tang, H.; Zhao, B. Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem. Int. 2006, 48, 263–274. [Google Scholar] [CrossRef]
- Niki, E.; Saito, T.; Kawakami, A.; Kamiya, Y. Inhibition of oxidation of methyl linoleate in solution by vitamin E and vitamin C. J. Biol. Chem. 1984, 259, 4177–4182. [Google Scholar] [CrossRef]
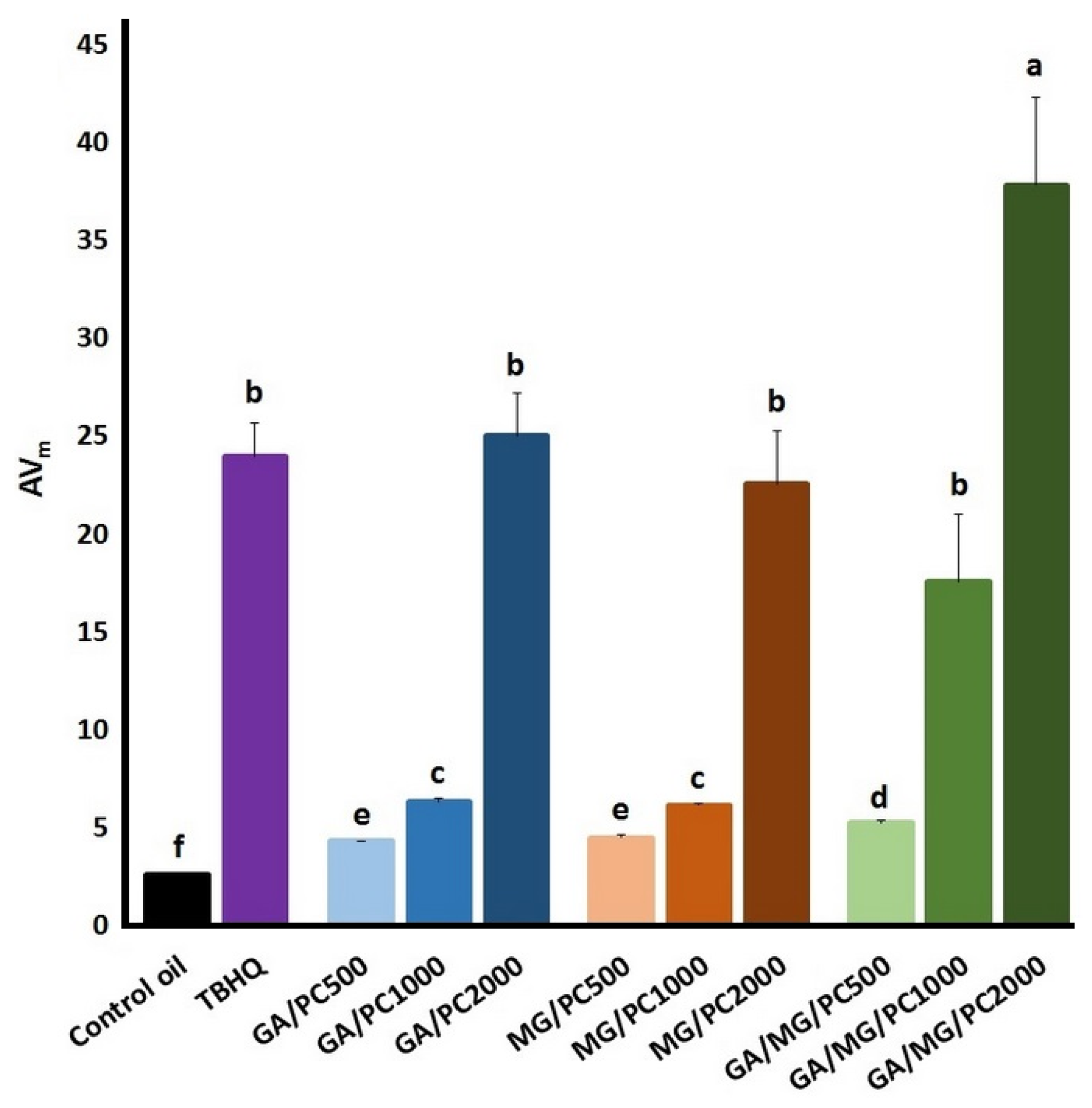
| Oil Sample | ||
|---|---|---|
| Sunflower | Palm Olein | |
| Major fatty acids (%) | ||
| C14:0 | – | 1.0 ± 0.0 |
| C16:0 | 7.0 ± 0.3 | 38.9 ± 0.2 |
| C18:0 | 3.8 ± 0.1 | 4.4 ± 0.1 |
| C18:1Δ9 | 25.5 ± 0.3 | 42.0 ± 0.6 |
| C18:2Δ9,12 | 61.8 ± 0.4 | 12.2 ± 0.1 |
| C18:3Δ9,12,15 | 0.2 ± 0.0 | 0.3 ± 0.0 |
| Calculated oxidizability (COX) value | 6.7 ± 0.1 | 1.7 ± 0.0 |
| Peroxide value (PV, meq/kg) | 1.53 ± 0.01 | 0.40 ± 0.00 |
| Acid value (AV, mg/g) | 0.15 ± 0.01 | 0.26 ± 0.03 |
| Total tocopherols content (mg/kg) | 490 ± 1 | 185 ± 1 |
| Total phenolics content (mg/kg) | 36.0 ± 1.0 | 53.1 ± 0.2 |
| Treatment | LCDmax (mM) | tT (h) | kc (/h) | kd (/mM h) | rn (/h) |
|---|---|---|---|---|---|
| Control oil | 27.3 ± 0.5 c | 2.22 ± 0.06 g | 0.6639 ± 0.0081 ab | 0.0243 ± 0.0008 c | 0.1660 ± 0.0020 a |
| TBHQ (1.2 mM) | 26.1 ± 0.2 c | 4.48 ± 0.05 d | 0.3905 ± 0.0038 f | 0.0149 ± 0.0003 e | 0.0976 ± 0.0009 d |
| GA (1.2 mM) + PC (mg/kg) | |||||
| 500 | 17.4 ± 0.2 fg | 2.07 ± 0.02 h | 0.6790 ± 0.0101 a | 0.0391 ± 0.0009 a | 0.1697 ± 0.0025 a |
| 1000 | 18.4 ± 0.1 e | 3.07 ± 0.01 f | 0.4620 ± 0.0017 d | 0.0252 ± 0.0002 c | 0.1115 ± 0.0004 c |
| 2000 | 30.1 ± 0.5 b | 8.23 ± 0.12 b | 0.2415 ± 0.0005 h | 0.0080 ± 0.0002 g | 0.0604 ± 0.0001 f |
| MG (1.2 mM) + PC (mg/kg) | |||||
| 500 | 16.6 ± 0.1 h | 2.21 ± 0.01 g | 0.6521 ± 0.0018 b | 0.0393 ± 0.0002 a | 0.1630 ± 0.0017 a |
| 1000 | 17.2 ± 0.0 g | 3.02 ± 0.03 f | 0.4405 ± 0.0020 e | 0.0256 ± 0.0001 c | 0.1101 ± 0.0005 c |
| 2000 | 24.6 ± 0.0 d | 6.71 ± 0.01 c | 0.2421 ± 0.0001 h | 0.0099 ± 0.0000 f | 0.0605 ± 0.0000 f |
| GA/MG 50:50 (1.2 mM) + PC (mg/kg) | |||||
| 500 | 15.6 ± 0.4 i | 1.96 ± 0.05 h | 0.5075 ± 0.0008 c | 0.0326 ± 0.0008 b | 0.1269 ± 0.0002 b |
| 1000 | 17.8 ± 0.0 f | 3.63 ± 0.01 e | 0.3376 ± 0.0004 g | 0.0190 ± 0.0001 d | 0.0844 ± 0.0001 e |
| 2000 | 35.5 ± 0.6 a | 10.56 ± 0.11 a | 0.2067 ± 0.0003 i | 0.0058 ± 0.0001 h | 0.0517 ± 0.0001 g |
| Treatment | LCOmax (μmol/g) | tT (h) | rmax (μmol/g h) | rn (/h) |
|---|---|---|---|---|
| Control oil | 27.0 ± 0.8 b | 4.51 ± 0.08 c | 4.51 ± 0.18 a | 0.1668 ± 0.0019 a |
| TBHQ (1.2 mM) | 16.4 ± 0.0 g | 4.26 ± 0.04 d | 2.46 ± 0.03 c | 0.1500 ± 0.0016 b |
| GA (1.2 mM) + PC (mg/kg) | ||||
| 500 | 19.2 ± 0.1 d | 3.20 ± 0.05 h | 3.08 ± 0.05 b | 0.1607 ± 0.0037 a |
| 1000 | 18.3 ± 0.0 f | 3.45 ± 0.05 g | 2.23 ± 0.02 ef | 0.1215 ± 0.0012 c |
| 2000 | 13.2 ± 0.2 h | 4.80 ± 0.03 b | 1.39 ± 0.11 hi | 0.1049 ± 0.0014 d |
| MG (1.2 mM) + PC (mg/kg) | ||||
| 500 | 19.9 ± 0.1 c | 3.87 ± 0.05 e | 3.24 ± 0.05 b | 0.1631 ± 0.0016 a |
| 1000 | 18.1 ± 0.2 f | 3.59 ± 0.01 f | 2.21 ± 0.01 f | 0.1222 ± 0.0018 c |
| 2000 | 14.2 ± 0.6 h | 5.26 ± 0.37 b | 1.52 ± 0.09 h | 0.1073 ± 0.0020 d |
| GA/MG 50:50 (1.2 mM) + PC (mg/kg) | ||||
| 500 | 18.9 ± 0.1 e | 3.27 ± 0.01 h | 2.28 ± 0.02 de | 0.1204 ± 0.0008 c |
| 1000 | 19.9 ± 0.4 cd | 4.37 ± 0.15 cd | 1.96 ± 0.07 g | 0.0958 ± 0.0032 e |
| 2000 | 29.2 ± 0.3 a | 12.74 ± 0.16 a | 1.20 ± 0.05 i | 0.0411 ± 0.0013 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadeghi Vahid, G.; Farhoosh, R. Frying Performance of Gallic Acid and/or Methyl Gallate Accompanied by Phosphatidylcholine. Foods 2023, 12, 3560. https://doi.org/10.3390/foods12193560
Sadeghi Vahid G, Farhoosh R. Frying Performance of Gallic Acid and/or Methyl Gallate Accompanied by Phosphatidylcholine. Foods. 2023; 12(19):3560. https://doi.org/10.3390/foods12193560
Chicago/Turabian StyleSadeghi Vahid, Ghazaleh, and Reza Farhoosh. 2023. "Frying Performance of Gallic Acid and/or Methyl Gallate Accompanied by Phosphatidylcholine" Foods 12, no. 19: 3560. https://doi.org/10.3390/foods12193560
APA StyleSadeghi Vahid, G., & Farhoosh, R. (2023). Frying Performance of Gallic Acid and/or Methyl Gallate Accompanied by Phosphatidylcholine. Foods, 12(19), 3560. https://doi.org/10.3390/foods12193560







