Evaluation of In Vitro Antimicrobial, Antioxidant, and Anti-Quorum Sensing Activity of Edible Mushroom (Agrocybe aegerita)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extract Preparation
2.3. Characterization
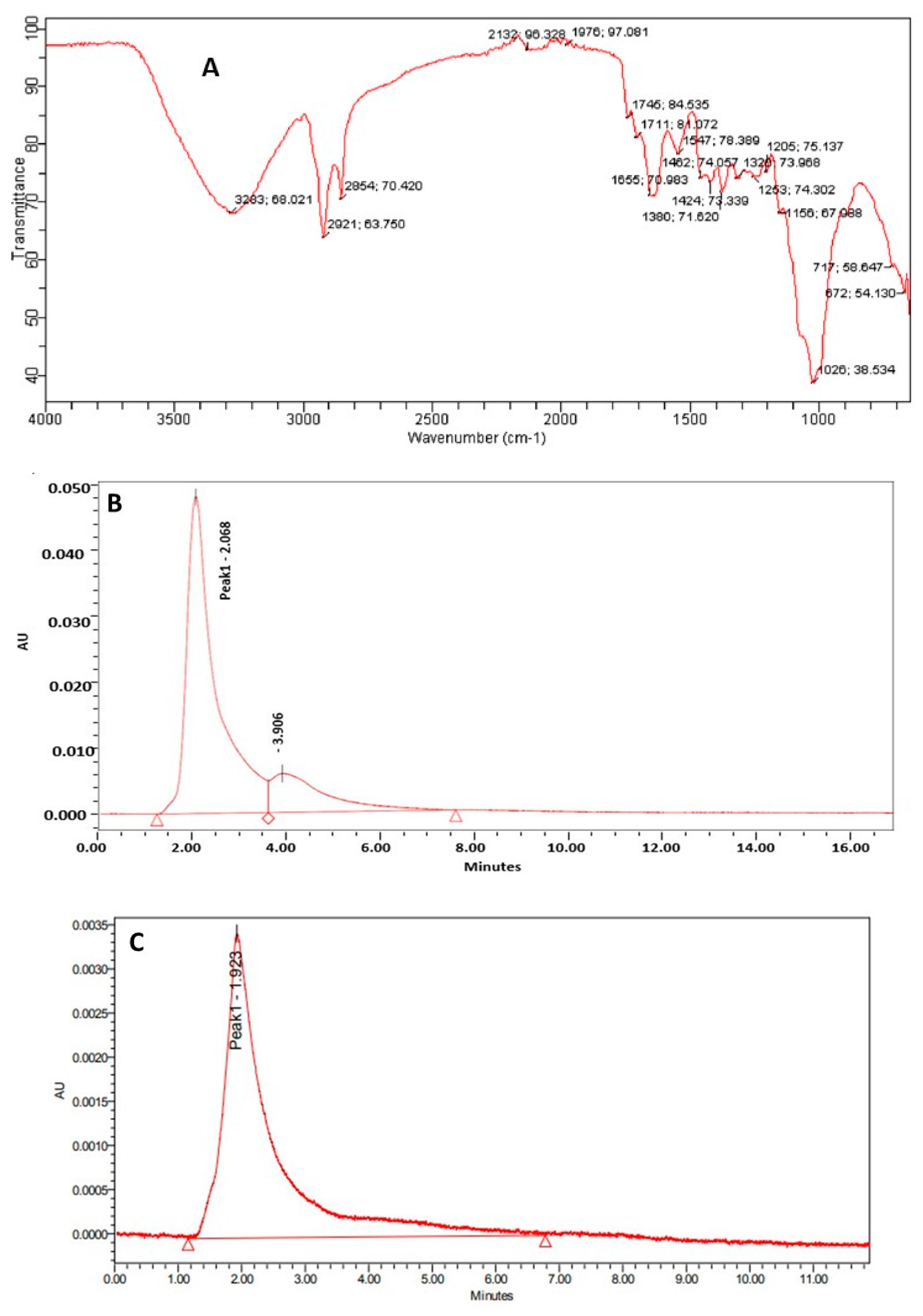
2.3.1. Determination of Functional Group and Quantification of Phenolic Compounds
2.3.2. Identification of Bioactive Compounds Using GC-MS Technique
2.4. Antioxidant Assay
2.4.1. DPPH Free Radical Scavenging Assay
2.4.2. Nitric Oxide Assay
2.5. Determination of Virulence Factor in P. aeruginosa Responsible for Quorum Sensing
2.5.1. Estimation of Pyocyanin Production
2.5.2. Estimation of Pyoverdine Production
2.5.3. Swarming Motility
2.6. Antibiofilm Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization and Estimation of Bioactive Compounds of Agrocybe aegerita
3.2. Identification of Mushroom Bioactive Compounds Using GC-MS Analysis
3.3. Antioxidant Activity
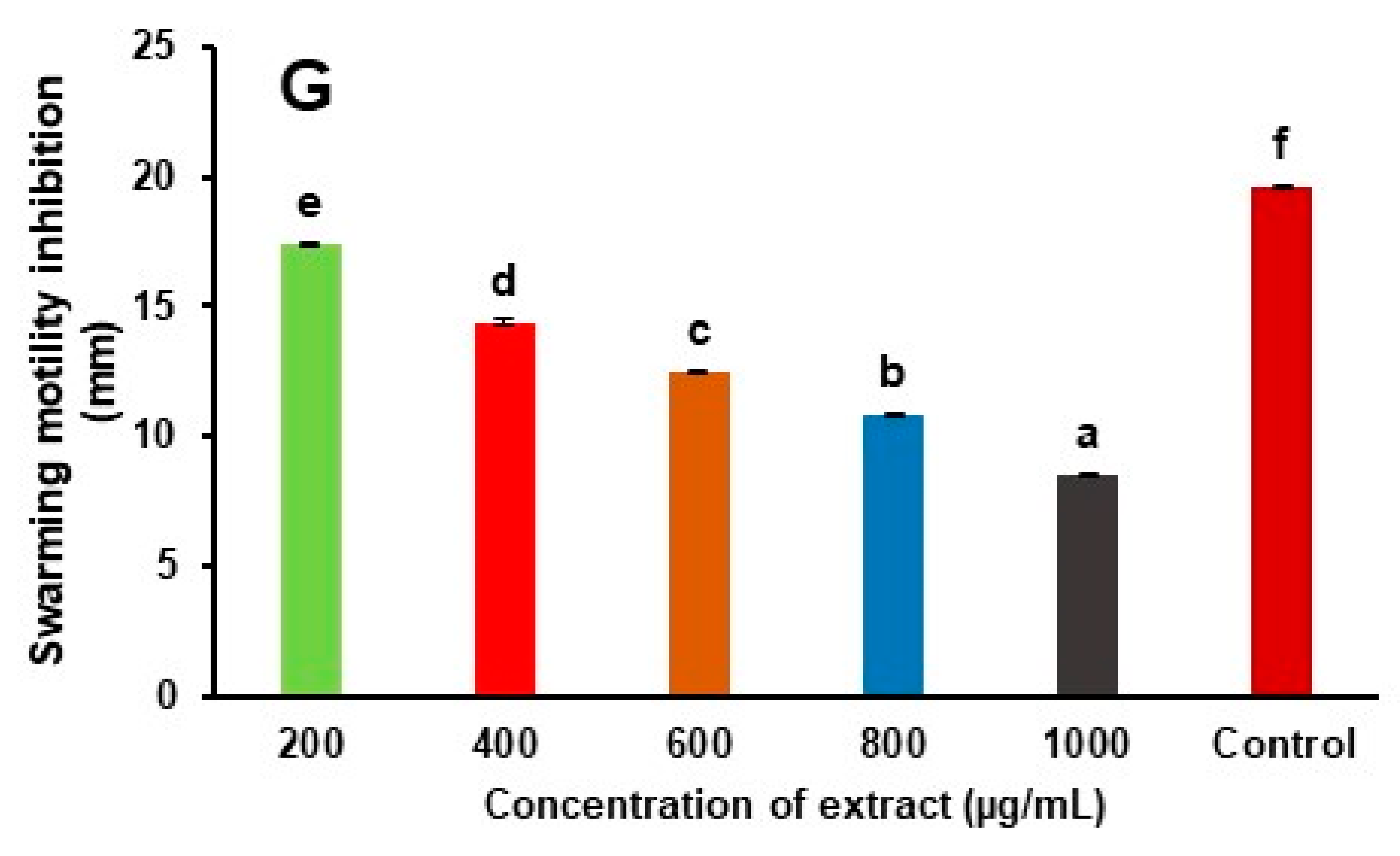
3.4. Reduction of Quorum Sensing Linked Virulence Factor
3.5. Antibiofilm Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takahashi, J.A.; Barbosa, B.V.R.; Martins, B.d.A.; P. Guirlanda, C.; A. F. Moura, M. Use of the versatility of fungal metabolism to meet modern demands for healthy aging, functional foods, and sustainability. J. Fungi 2020, 6, 223. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; MMS, C.P.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Yadav, K.K. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef] [PubMed]
- Paloi, S.; Kumla, J.; Paloi, B.P.; Srinuanpan, S.; Hoijang, S.; Karunarathna, S.C.; Lumyong, S. Termite Mushrooms (Termitomyces), a Potential Source of Nutrients and Bioactive Compounds Exhibiting Human Health Benefits: A Review. J. Fungi 2023, 9, 112. [Google Scholar] [CrossRef]
- Fernandes, T.; Garrine, C.; Ferrão, J.; Bell, V.; Varzakas, T. Mushroom nutrition as preventative healthcare in Sub-Saharan Africa. Appl. Sci. 2021, 11, 4221. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.H.; El Omari, N.; Bouyahya, A. Health benefits and pharmacological properties of stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Mannan, A. A review on nigella sativa: A marvel herb. J. Drug Deliv. Ther. 2020, 10, 213–219. [Google Scholar] [CrossRef]
- Shrikhandia, S.P.; Devi, S.; Sumbali, G. Lignocellulosic waste management through cultivation of certain commercially useful and medicinal mushrooms: Recent scenario. In Biology, Cultivation and Applications of Mushrooms, 1st ed.; Arya, A., Rusevska, K., Eds.; Springer: Singapore, 2022; pp. 497–534. [Google Scholar]
- Díaz-Godínez, G.; Téllez-Téllez, M. Fungi in Sustainable Food Production, 1st ed.; Springer: New York, NY, USA, 2021; pp. 143–164. [Google Scholar]
- Yong, T.; Chen, S.; Xie, Y.; Shuai, O.; Li, X.; Chen, D.; Liang, Y. Hypouricemic effects of extracts from Agrocybe aegerita on hyperuricemia mice and virtual prediction of bioactives by molecular docking. Front. Pharmacol. 2018, 9, 498. [Google Scholar] [CrossRef]
- Anusiya, G.; Gowthama Prabu, U.; Yamini, N.V.; Sivarajasekar, N.; Rambabu, K.; Bharath, G.; Banat, F. A review of the therapeutic and biological effects of edible and wild mushrooms. Bioengineered 2021, 12, 11239–11268. [Google Scholar] [CrossRef]
- Wu, L.; Luo, Y. Bacterial quorum-sensing systems and their role in intestinal bacteria-host crosstalk. Front. Microbiol. 2021, 12, 611413. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal–response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- AlYammahi, J.; Rambabu, K.; Thanigaivelan, A.; Bharath, G.; Hasan, S.W.; Show, P.L.; Banat, F. Advances of non-conventional green technologies for phyto-saccharides extraction: Current status and future perspectives. Phytochem. Rev. 2023, 22, 1067–1088. [Google Scholar] [CrossRef]
- Shashikant, M.; Bains, A.; Chawla, P.; Sharma, M.; Kaushik, R.; Kandi, S.; Kuhad, R.C. In-vitro antimicrobial and anti-inflammatory activity of modified solvent evaporated ethanolic extract of Calocybe indica: GCMS and HPLC characterization. Int. J. Food Microbiol. 2022, 376, 109741. [Google Scholar] [CrossRef] [PubMed]
- Bekiaris, G.; Tagkouli, D.; Koutrotsios, G.; Kalogeropoulos, N.; Zervakis, G.I. Pleurotus mushrooms content in glucans and ergosterol assessed by ATR-FTIR spectroscopy and multivariate analysis. Foods 2020, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Bristy, A.T.; Islam, T.; Ahmed, R.; Hossain, J.; Reza, H.M.; Jain, P. Evaluation of total phenolic content, HPLC analysis, and antioxidant potential of three local varieties of mushroom: A comparative study. Int. J. Food Sci. 2022, 2022, 3834936. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Adebayo, S.A.; Ondua, M.; Shai, L.J.; Lebelo, S.L. Inhibition of nitric oxide production and free radical scavenging activities of four South African medicinal plants. J. Inflamm. Res. 2019, 12, 195–203. [Google Scholar] [CrossRef]
- Rodriguez-Urretavizcaya, B.; Pascual, N.; Pastells, C.; Martin-Gomez, M.T.; Vilaplana, L.; Marco, M.P. Diagnosis and stratification of Pseudomonas aeruginosa infected patients by immunochemical quantitative determination of Pyocyanin from clinical bacterial isolates. Front. Cell. Infect. Microbiol. 2021, 11, 786929. [Google Scholar] [CrossRef]
- Shukla, A.; Parmar, P.; Patel, B.; Goswami, D.; Saraf, M. Breaking bad: Better call gingerol for improving antibiotic susceptibility of Pseudomonas aeruginosa by inhibiting multiple quorum sensing pathways. Microbiol. Res. 2021, 252, 126863. [Google Scholar] [CrossRef]
- Hou, L.; Debru, A.; Chen, Q.; Bao, Q.; Li, K. AmrZ regulates swarming motility through cyclic di-GMP-dependent motility inhibition and controlling Pel polysaccharide production in Pseudomonas aeruginosa PA14. Front. Microbiol. 2019, 10, 1847. [Google Scholar] [CrossRef]
- Bouaouina, S.; Aouf, A.; Touati, A.; Ali, H.; Elkhadragy, M.; Yehia, H.; Farouk, A. Effect of Nanoencapsulation on the Antimicrobial and Antibiofilm Activities of Algerian Origanum glandulosum Desf. against Multidrug-Resistant Clinical Isolates. Nanomaterials 2022, 12, 2630. [Google Scholar] [CrossRef]
- Das, A.; Bai, C.-H.; Chang, J.-S.; Huang, Y.-L.; Wang, F.-F.; Chen, Y.-C.; Chao, J.C.-J. Associations of dietary patterns and vitamin D levels with iron status in pregnant women: A cross-sectional study in Taiwan. Nutrients 2023, 15, 1805. [Google Scholar] [CrossRef] [PubMed]
- Osei Akoto, C.; Acheampong, A.; Boakye, Y.D.; Asante, B.; Ohene, S.; Amankwah, F. Anthelminthic, anti-inflammatory, antioxidant, and antimicrobial activities and FTIR analyses of Vernonia camporum stem-bark. J. Chem. 2021, 2021, 3328073. [Google Scholar] [CrossRef]
- Lu, X.; Tong, S.; Liu, P. Enhanced α-glucosidase inhibition activity of exopolysaccharides fractions from mycelium of Inonotus obliquus under addition of birch sawdust lignocellulose component. Int. J. Biol. Macromol. 2023, 242, 124699. [Google Scholar] [CrossRef]
- Boukir, A.; Fellak, S.; Doumenq, P. Structural characterization of Argania spinosa Moroccan wooden artifacts during natural degradation progress using infrared spectroscopy (ATR-FTIR) and X-ray diffraction (XRD). Heliyon 2019, 5, e02477. [Google Scholar] [CrossRef]
- Li, J.; Yang, W.; Ren, J.; Cao, B.; Zhu, X.; Lin, L.; Zhao, R. A New Species Agrocybe striatipes, also a Newly Commercially Cultivated Mushroom with Highly Nutritional and Healthy Values. J. Fungi 2023, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.; Gopal, J.; Muthu, M. Antioxidant activity of mushroom extracts/polysaccharides—Their antiviral properties and plausible antiCOVID-19 properties. Antioxidants 2021, 10, 1899. [Google Scholar] [CrossRef]
- Boora, F.; Chirisa, E.; Mukanganyama, S. Evaluation of nitrite radical scavenging properties of selected Zimbabwean plant extracts and their phytoconstituents. J. Food Process. 2014, 2014, 918018. [Google Scholar] [CrossRef]
- Sifat, N.; Lovely, F.; Zihad, S.N.K.; Hossain, M.G.; Shilpi, J.A.; Grice, I.D.; Uddin, S.J. Investigation of the nutritional value and antioxidant activities of common Bangladeshi edible mushrooms. Clin. Phytoscience 2020, 6, 88. [Google Scholar] [CrossRef]
- Stojanova, M.; Pantić, M.; Karadelev, M.; Čuleva, B.; Nikšić, M. Antioxidant potential of extracts of three mushroom species collected from the Republic of North Macedonia. J. Food Process. Preserv. 2021, 45, e15155. [Google Scholar] [CrossRef]
- De Sousa, T.; Hébraud, M.; Dapkevicius, M.L.E.; Maltez, L.; Pereira, J.E.; Capita, R.; Poeta, P. Genomic and Metabolic Characteristics of the Pathogenicity in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2021, 22, 12892. [Google Scholar] [CrossRef]
- Llamas, M.A.; Sánchez-Jiménez, A. Iron homeostasis in Pseudomonas aeruginosa: Targeting iron acquisition and storage as an antimicrobial strategy. In Pseudomonas aeruginosa: Biology, Pathogenesis and Control Strategies, 1st ed.; Filloux, A., Ramos, J.L., Eds.; Springer International Publishing: Singapore; Cham, Switzerland, 2022; pp. 29–68. [Google Scholar]
- Tchatchedre, M.; Amoussa, A.M.O.; Atindehou, M.; Nacoulma, A.P.; Sanni, A.; Lagnika, L. Anti-quorum sensing, antibacterial, antioxidant activities, and phytoconstituents analysis of medicinal plants used in Bénin: Acacia macrostachya (Rchb. ex DC.). J. Appl. Biol. Biotechnol. 2020, 8, 84–93. [Google Scholar]
- Jagani, S.; Chelikani, R.; Kim, D.S. Effects of phenol and natural phenolic compounds on biofilm formation by Pseudomonas aeruginosa. Biofouling 2009, 25, 321–324. [Google Scholar] [CrossRef]
- Krishnan, T.; Yin, W.F.; Chan, K.G. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa PAO1 by ayurveda spice clove (Syzygium Aromaticum) bud extract. Sensors 2012, 12, 4016–4030. [Google Scholar] [CrossRef]
- Qais, F.A.; Ahmad, I. Anti-quorum sensing and biofilm inhibitory effect of some medicinal plants against gram-negative bacterial pathogens: In vitro and in silico investigations. Heliyon 2022, 8, e11113. [Google Scholar]
- Nassima, B.; Nassima, B.; Riadh, K. Antimicrobial and antibiofilm activities of phenolic compounds extracted from Populus nigra and Populus alba buds (Algeria). Braz. J. Pharm. Sci. 2019, 55, e18114. [Google Scholar] [CrossRef]
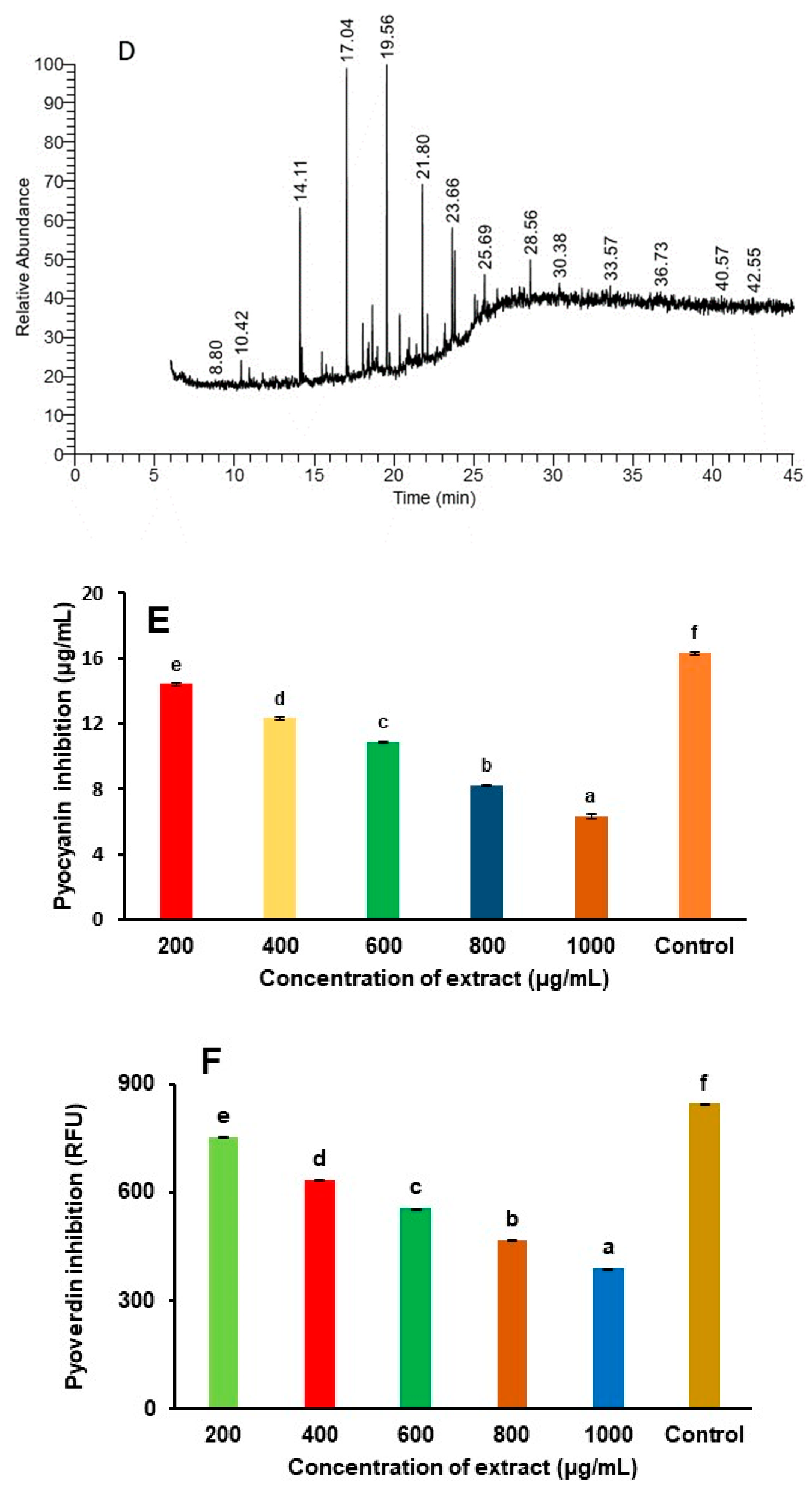
| Retention Time (min) | Compound Name | Molecular Formula |
|---|---|---|
| 10.42 | 5-Methyl-1-heptanol | C8H18O |
| 14.11 | 4-Nonene, 3-methyl-, (Z)- | C10H20 |
| 17.04 | 1-Hexadecanol | C16H34O |
| 19.56 | 2-Heptadecenal | C17H32O |
| 21.80 | 10-Heneicosene (c,t) | C21H42 |
| 23.66 | Phthalic acid, butyl hept-4-yl ester | C19H28O4 |
| 25.69 | 2-Dodecanol | C12H26O |
| 28.56 | 9H-Carbazole-1-carboxylic acid, 4-(1H-indol-3-yl)-, methyl ester | C22H16N2O2 |
| 30.38 | Ethanol, 2-(methylamino)- | C3H9NO |
| 33.57 | Benzoic acid, TMS derivative | C10H14O2Si |
| 36.73 | Acetamide, 2,2,2-trifluoro- | C2H2F3NO |
| 40.57 | 7-Hydroxy-7,8,9,10-tetramethyl-7, 8-dihydrocyclohepta[d,e]naphthale | C18H20O |
| 42.55 | Pyrazolo[1,5-a]pyridine, 3-methyl-2-phenyl | C14H12N2 |
| Concentration of Mushroom Extract (µg/mL) | DPPH Scavenging Assay (%) | Control Ascorbic Acid (%) | Nitric Oxide Scavenging Assay (%) | Control Ascorbic Acid (%) |
|---|---|---|---|---|
| 50 | 38.53 ± 0.11 a | 42.33 ± 0.06 a | 38.82 ± 0.04 a | 43.47 ± 0.09 a |
| 100 | 54.31 ± 0.12 b | 57.43 ± 0.05 b | 51.63 ± 0.11 b | 53.67 ± 0.12 b |
| 150 | 72.83 ± 0.11 c | 78.60 ± 0.11 c | 69.91 ± 0.69 c | 68.37 ± 0.17 c |
| 200 | 85.63 ± 0.12 d | 95.90 ± 0.21 d | 82.02 ± 0.12 d | 83.34 ± 0.35 d |
| Mushroom Extract (µg/mL) | Pyocyanin (µg/mL) | Pyoverdine (RFU) | Swarming Motility (mm) |
|---|---|---|---|
| 200 | 14.43 ± 0.09 (11.68%) | 754.92 ± 0.17 (10.78%) | 17.37 ± 0.09 (11.3%) |
| 400 | 12.34 ± 0.12 (24.47%) | 634.51 ± 0.24 (25.01%) | 14.36 ±0.12 (26.7%) |
| 600 | 10.87 ± 0.05 (33.47%) | 556.82 ± 0.15 (34.19%) | 12.54 ± 0.05 (36.02%) |
| 800 | 8.24 ± 0.07 (49.57%) | 468.31 ± 0.12 (44.65%) | 10.82 ± 0.07 (44.7%) |
| 1000 | 6.32 ± 0.12 (61.32%) | 389.02 ± 0.015 (54.02%) | 8.56 ± 0.06 (56.32%) |
| Control | 16.34 ± 0.10 | 846.21 ± 0.18 | 19.62 ± 0.05 |
| Concentration of Mushroom Extract (µg/mL) | Biofilm Inhibition Extract (%) | Biofilm Inhibition Streptomycin (Control) (%) |
|---|---|---|
| 200 | 22.34 ± 0.21 a | 36.45 ± 0.56 b |
| 400 | 32.67 ± 0.17 a | 47.34 ± 0.32 b |
| 600 | 49.48 ± 0.22 a | 58.67 ± 0.26 b |
| 800 | 61.24 ± 0.28 a | 69.56 ± 0.19 b |
| 1000 | 72.35 ± 0.14 a | 84.23 ± 0.18 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bains, A.; Chawla, P.; Inbaraj, B.S. Evaluation of In Vitro Antimicrobial, Antioxidant, and Anti-Quorum Sensing Activity of Edible Mushroom (Agrocybe aegerita). Foods 2023, 12, 3562. https://doi.org/10.3390/foods12193562
Bains A, Chawla P, Inbaraj BS. Evaluation of In Vitro Antimicrobial, Antioxidant, and Anti-Quorum Sensing Activity of Edible Mushroom (Agrocybe aegerita). Foods. 2023; 12(19):3562. https://doi.org/10.3390/foods12193562
Chicago/Turabian StyleBains, Aarti, Prince Chawla, and Baskaran Stephen Inbaraj. 2023. "Evaluation of In Vitro Antimicrobial, Antioxidant, and Anti-Quorum Sensing Activity of Edible Mushroom (Agrocybe aegerita)" Foods 12, no. 19: 3562. https://doi.org/10.3390/foods12193562
APA StyleBains, A., Chawla, P., & Inbaraj, B. S. (2023). Evaluation of In Vitro Antimicrobial, Antioxidant, and Anti-Quorum Sensing Activity of Edible Mushroom (Agrocybe aegerita). Foods, 12(19), 3562. https://doi.org/10.3390/foods12193562







