Partial Replacement of NaCl by KCl, MgCl2 and CaCl2 Chloride Salts in the Production of Sucuk: Effects on Volatile Compounds, Lipid Oxidation, Microbiological and Sensory Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Sucuk Production
2.3. Microbiological Analysis
2.4. aw, pH and TBARS
2.5. Sensory Evaluation
2.6. Volatile Compounds
2.7. Statistical Analysis
3. Results and Discussion
3.1. Microbiological Properties
3.2. aw, pH and TBARS
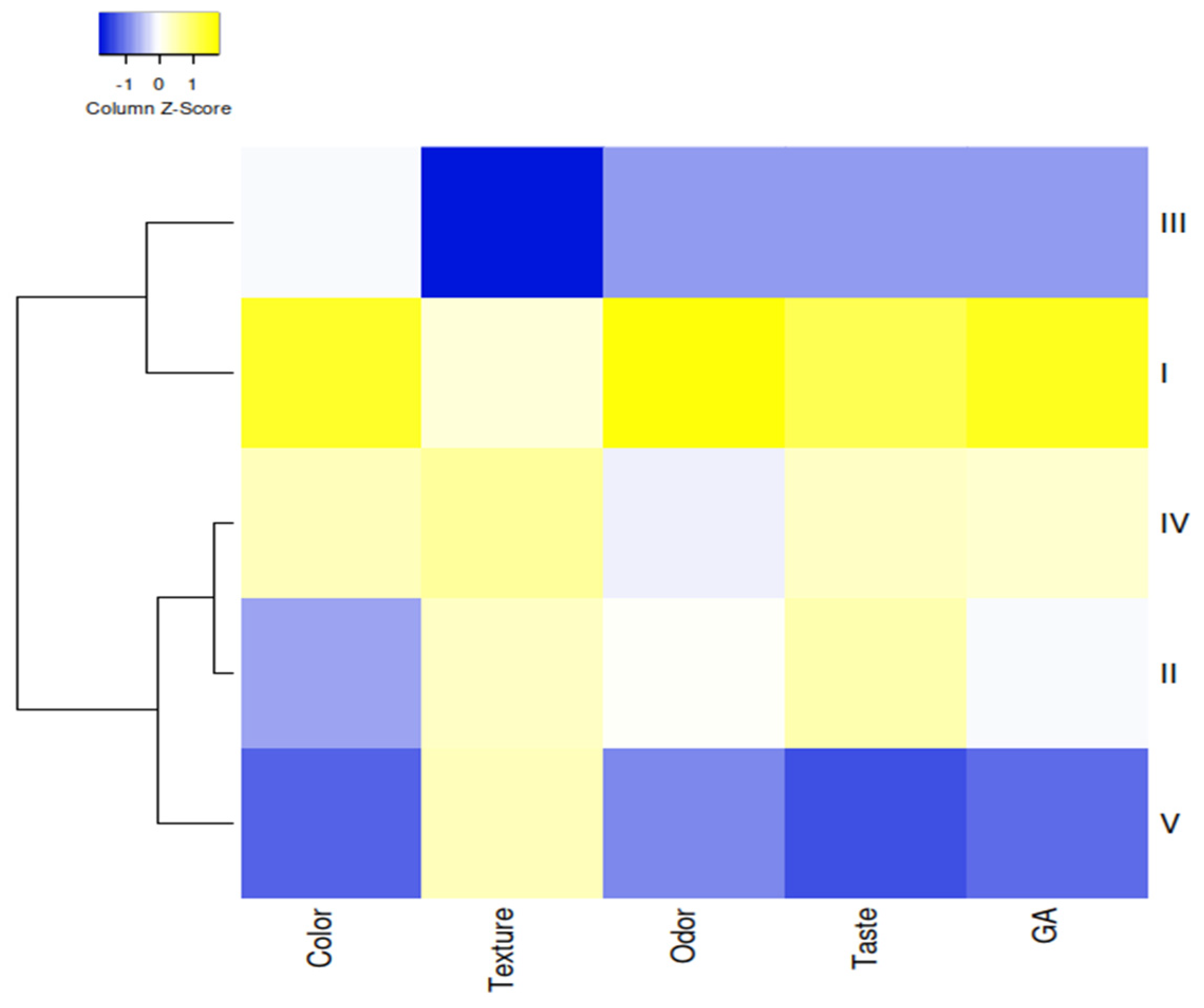
3.3. Sensory Evaluation
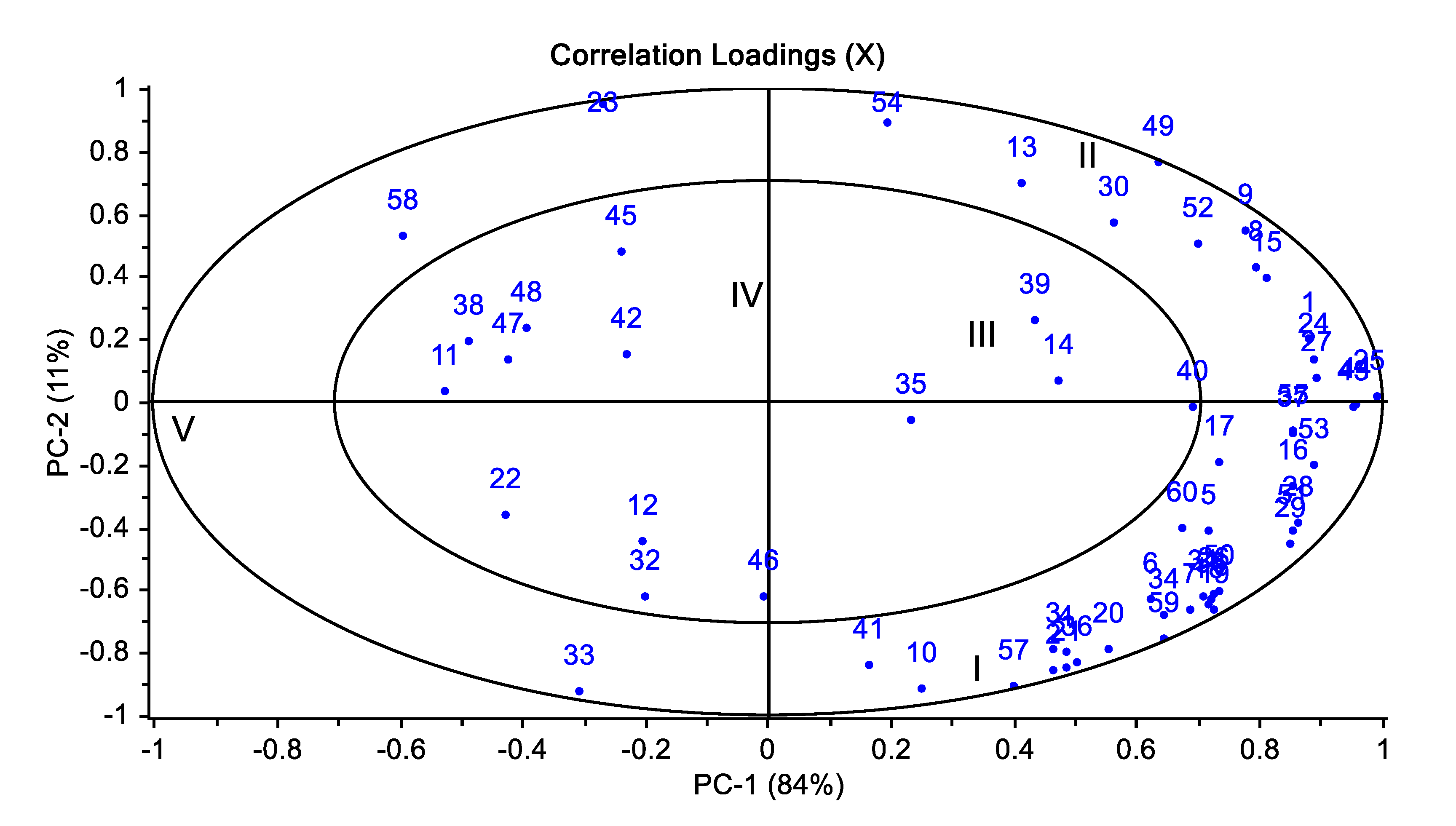
3.4. Volatile Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaban, G.; Kaya, M. Effects of Lactobacillus plantarum and Staphylococcus xylosus on the quality characteristics of dry fermented sausage “sucuk”. J. Food Sci. 2009, 74, S58–S63. [Google Scholar] [CrossRef] [PubMed]
- Kaban, G.; Yılmaz Oral, Z.F.; Kaya, M. Sucuk. In Production of Traditional Mediterranean Meat Products; Lorenzo, J.M., Dominguez, R., Pateiro, M., Munekata, P.E.S., Eds.; Humana Press, Springer Nature: New York, NY, USA, 2022; Chapter 16; pp. 143–152. [Google Scholar]
- Lücke, F.K. Fermented sausages. In Microbiology of Fermented Foods; Wood, B.J.B., Ed.; Blackie Academic and Professional: London, UK, 1998; Volume 2, pp. 441–483. [Google Scholar]
- Öksüztepe, G.; Güran, H.Ş.; İncili, G.K.; Gül, S.B. Elazığ’da tüketime sunulan fermente sucukların mikrobiyolojik ve kimyasal kalitesi. Fırat Üniv. Sağlık Bilim. Vet. Derg. 2011, 25, 107–114. [Google Scholar]
- Erdoğrul, Ö.; Ergün, Ö. Kahramanmaraş piyasasında tüketilen sucukların bazı fiziksel, kimyasal, duyusal ve mikrobiyolojik özellikleri. İstanbul Üniv. Vet. Fak. Derg. 2015, 31, 55–65. [Google Scholar]
- Campagnol, P.C.B.; dos Santos, B.A.; Wagner, R.; Terra, N.N.; Pollonio, M.A.R. The effect of yeast extract addition on quality of fermented sausages at low NaCl content. Meat Sci. 2011, 87, 290–298. [Google Scholar] [CrossRef] [PubMed]
- who.int. WHO Global Sodium Benchmarks for Different Food Categories. 2021, pp. 1–20. Available online: https://iris.who.int/handle/10665/341081 (accessed on 9 July 2023).
- Raybaudi-Massilia, R.; Mosqueda-Melgar, J.; Rosales-Oballos, Y.; de Petricone, R.C.; Frágenas, N.N.; Zambrano-Durán, A.; Sayago, K.; Lara, M.; Urbina, G. New alternative to reduce sodium chloride in meat products: Sensory and microbiological evaluation. LWT—Food Sci. Technol. 2019, 108, 253–260. [Google Scholar] [CrossRef]
- De Almeida, M.A.; Saldaña, E.; da Silva Pinto, J.S.; Palacios, J.; Contreras-Castillo, C.J.; Sentandreu, M.A.; Fadda, S.G. A peptidomic approach of meat protein degradation in a low-sodium fermented sausage model using autochthonous starter cultures. Food Res. Int. 2018, 109, 368–379. [Google Scholar] [CrossRef]
- Cittadini, A.; Domínguez, R.; Gómez, B.; Pateiro, M.; Pérez-Santaescolástica, C.; López-Fernández, O.; Sarries, M.V.; Lorenzo, J.M. Effect of NaCl replacement by other chloride salts on physicochemical parameters, proteolysis and lipolysis of dry-cured foal “cecina”. J. Food Sci. Technol. 2020, 57, 1628–1635. [Google Scholar] [CrossRef]
- Campagnol, P.C.B.; Santos, B.A.D.; Terra, N.N.; Pollonio, M.A.R. Lysine, disodium guanylate and disodium inosinate as flavor enhancers in low-sodium fermented sausages. Meat Sci. 2012, 91, 334–338. [Google Scholar] [CrossRef]
- Zheng, J.; Han, Y.; Ge, G.; Zhao, M.; Sun, W. Partial substitution of NaCl with chloride salt mixtures: Impact on oxidative characteristics of meat myofibrillar protein and their rheological properties. Food Hydrocol. 2019, 96, 36–42. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Wen, R.; Liu, Q.; Chen, Q.; Kong, B. Effect of NaCl substitutes on the physical, microbial and sensory characteristics of Harbin dry sausage. Meat Sci. 2019, 156, 205–213. [Google Scholar] [CrossRef]
- Armenteros, M.; Aristoy, M.C.; Barat, C.M.; Toldra, N.F. Biochemical and sensory properties of dry-cured loins as affected by partial replacement of sodium by potassium, calcium, and magnesium. J. Agric. Food Chem. 2009, 57, 9699–9705. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, B.A.D.; Campagnol, P.C.B.; Fagundes, R.W.; Pollonio, M.A.R. Generation of volatile compounds in Brazilian low-sodium dry fermented sausages containing blends of NaCl, KCl, CaCl2 during processing and storage. Food Res. Int. 2015, 74, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Gelabert, J.; Gou, P.; Guerrero, L.; Arnau, J. Effect of sodium chloride replacement on some characteristics of fermented sausages. Meat Sci. 2003, 65, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Guardia, M.D.; Guerrero, L.; Gelabert, J.; Gou, P.; Arnau, J. Sensory characterisation and consumer acceptability of small calibre fermented sausages with 50% substitution of NaCl by mixtures of KCl and potassium lactate. Meat Sci. 2008, 80, 1225–1230. [Google Scholar] [CrossRef]
- Kaban, G.; Bayraktar, F.; Jaberi, R.; Fettahoğlu, K.; Kaya, M. Effects of NaCl substitution with KCl on quality properties of heat-treated sucuk during the production stages. J. Food Nutrit. Res. 2022, 61, 43–52. [Google Scholar]
- Wen, R.; Hu, Y.; Zhang, L.; Wang, Y.; Chen, Q.; Kong, B. Effect of NaCl substitutes on lipid and protein oxidation and flavor development of Harbin dry sausage. Meat Sci. 2019, 156, 33–43. [Google Scholar] [CrossRef]
- Gimeno, O.; Astiasaran, I.; Bello, J. Influence of partial replacement of NaCl with KCl and CaCl2 on microbiological evolution of dry fermented sausages. Food Micro 2001, 18, 329–334. [Google Scholar] [CrossRef]
- Fieira, C.; Marchi, J.F.; Alfaro, A.D.T. Partial replacement of sodium chloride in Italian salami and the influence on the sensory properties and texture. Acta Sci. Technol. 2015, 37, 293–299. [Google Scholar] [CrossRef]
- Kaban, G.; Kaya, M. Identification of lactic acid bacteria and gram-positive catalase-positive cocci ısolated from naturally fermented sausage (sucuk). J. Food Sci. 2008, 73, M385–M388. [Google Scholar] [CrossRef]
- Baumgart, J.; Eigener, V.; Firnhaber, J.; Hildebrant, G.; Reenen Hoekstra, E.S.; Samson, R.A.; Spicher, G.; Timm, F.; Yarrow, D.; Zschaler, R. Mikrobilogische Unterschung von Lebensmitteln, (3., Aktualisierte und erw. Aufl.); Behr’s Verlag: Hamburg, Germany, 1993. [Google Scholar]
- Erge, A.; Eren, Ö. Effect of gelatin/chitosan coating on chicken patty quality during frozen storage: A response surface methodology application. Kafkas Univ. Vet. Fak. Derg. 2021, 27, 165–171. [Google Scholar] [CrossRef]
- Lemon, D.W. An Improved TBA Test for Rancidity New Series Circular, No: 51; Halifax-Laboratory: Halifax, Nova Scatia, 1975. [Google Scholar]
- Kaban, G.; Kaya, M. Effect of starter culture on growth of Staphylococcus aureus in sucuk. Food Cont. 2006, 17, 797–801. [Google Scholar] [CrossRef]
- Teixeira, A.; Domínguez, R.; Ferreira, I.; Pereira, E.; Estevinho, L.; Rodrigues, S.; Lorenzo, J.M. Effect of NaCl replacement by other salts on the quality of Bísaro pork sausages (PGI Chouriça de Vinhais). Foods 2021, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, B.A.; Campagnol, P.C.; da Cruz, A.G.; Morgano, M.A.; Wagner, R.; Pollonio, M.A. Is there a potential consumer market for low-sodium fermented sausages? J. Food Sci. 2015, 80, S1093–S1099. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, O.; Astiasaran, I.; Bello, J. A mixture of potassium, magnesium, and calcium chlorides as a partial replacement of sodium chloride in dry fermented sausages. Food Chem. 1998, 46, 4372–4375. [Google Scholar] [CrossRef]
- Dos Santos, B.A.; Campagnol, P.C.B.; Fagundes, M.B.; Wagner, R.; Pollonio, M.A.R. Adding blends of NaCl, KCl, and CaCl2 to low-sodium dry fermented sausages: Effects on lipid oxidation on curing process and shelf life. J. Food Qual. 2017, 2017, 70855798. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Tang, J.; Huang, M.; Zhao, J.; Zhang, J. Influence of partial replacement of NaCl with KCl on formation of volatile compounds in Jinhua ham during processing. Food Sci. Biotechnol. 2016, 25, 379–391. [Google Scholar] [CrossRef]
- Zanardi, E.; Ghidini, S.; Conter, M.; Ianieri, A. Mineral composition of Italian salami and effect of NaCl partial replacement on compositional, physico-chemical and sensory parameters. Meat Sci. 2010, 86, 742–747. [Google Scholar] [CrossRef]
- Qin, L.G.; Li, X.A.; Huang, Y.X.; Li, Y.J.; Chen, Q. Flavour profile of traditional dry sausage prepared with partial substitution of NaCl with KCl. Foods 2023, 12, 388. [Google Scholar] [CrossRef]
- Spaziani, M.; Del Torre, M.; Stecchini, M.L. Changes of physicochemical, microbiological, and textural properties during ripening of Italian low-asid sausages. proteolysis, sensory and volatile profiles. Meat Sci. 2009, 81, 77–85. [Google Scholar] [CrossRef]
- Kaban, G.; Kaya, M. Effects of Staphylococcus carnosus on quality characteristics of sucuk (Turkish dry-fermented sausage) during ripening. Food Sci. Biotechnol. 2009, 18, 150–156. [Google Scholar]
- Marco, A.; Navarro, J.L.; Flores, M. The sensory quality of dry fermented sausages as affected by fermentation stage and curing agents. Europ. Food Res. Technol. 2008, 226, 449–458. [Google Scholar] [CrossRef]
- Kaban, G. Volatile compounds of traditional Turkish dry fermented sausage (sucuk). Int. J. Food Proper. 2010, 13, 525–534. [Google Scholar] [CrossRef]
- Meynier, A.; Novelli, E.; Chizzolini, R.; Zanardi, E.; Gandemer, G. Volatile compounds of commercial milano salami. Meat Sci. 1999, 51, 175–183. [Google Scholar] [CrossRef] [PubMed]
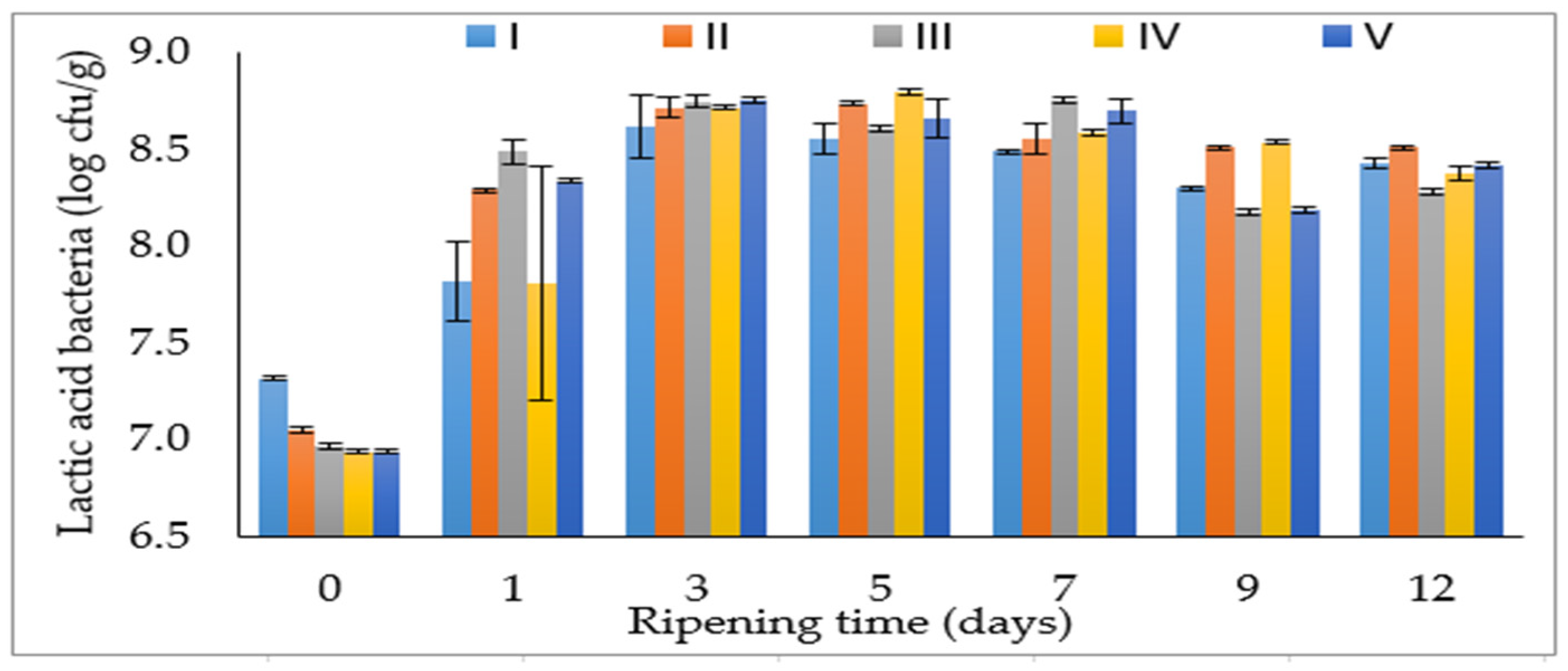
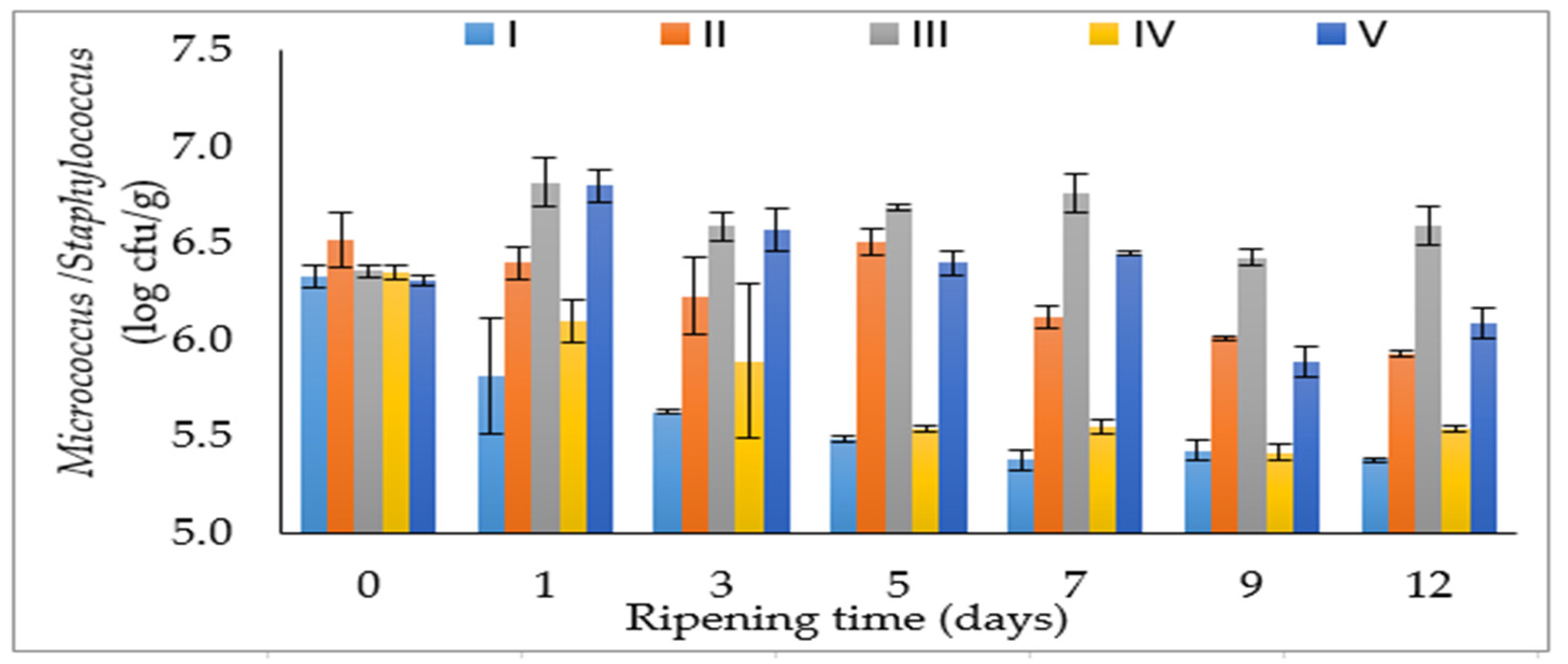
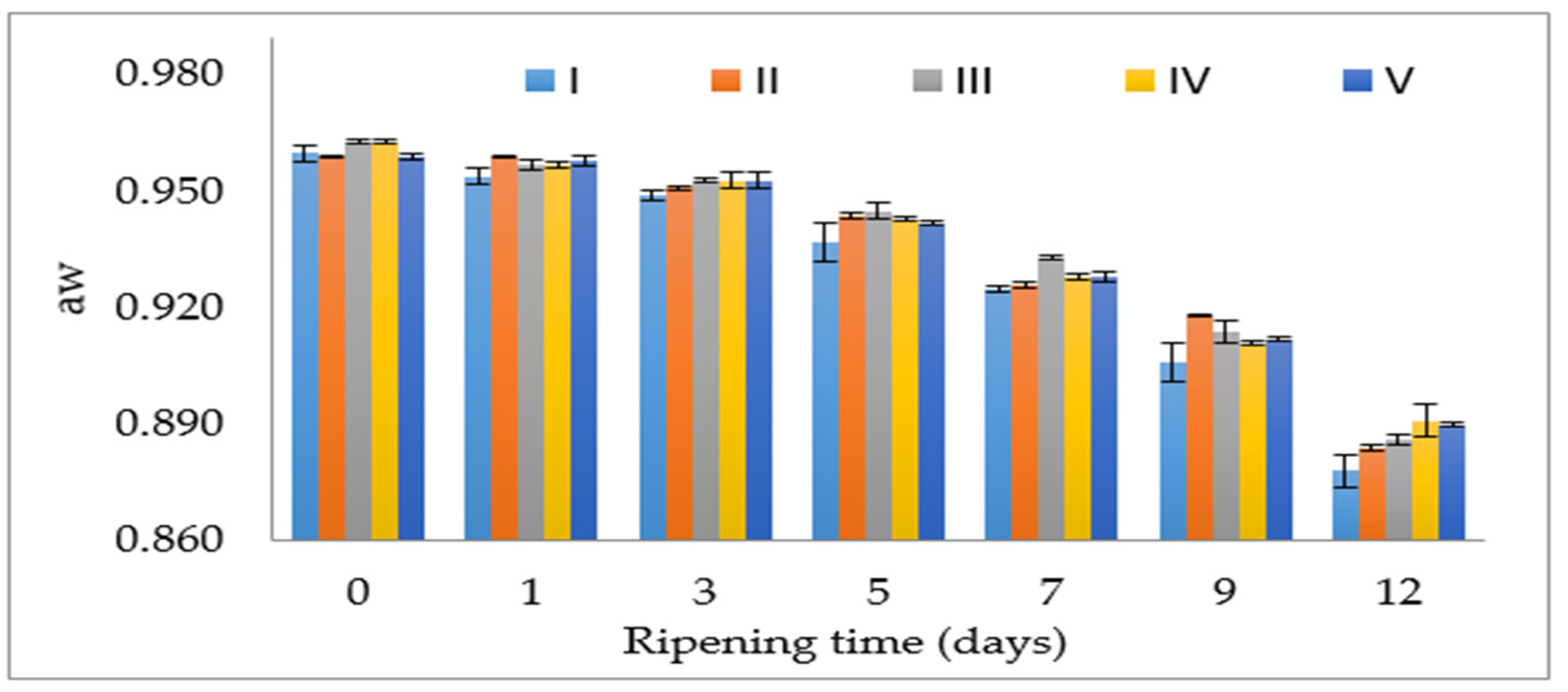
| LAB (log cfu/g) | M/S (log cfu/g) | aw | pH | TBARS (µmol MDA/kg) | |
|---|---|---|---|---|---|
| Salt Mixture (SM) | |||||
| I | 8.22 | 5.64 e | 0.930 b | 5.08 b | 6.62 |
| II | 8.34 | 6.25 c | 0.934 a | 5.14 a | 6.66 |
| III | 8.29 | 6.61 a | 0.936 a | 5.04 bc | 6.52 |
| IV | 8.25 | 5.77 d | 0.935 a | 4.97 d | 6.97 |
| V | 8.29 | 6.36 b | 0.934 a | 5.02 c | 6.34 |
| Significance | ns | ** | * | ** | ns |
| Ripening Time (RT) | |||||
| 0 | 7.04 e | 6.37 a | 0.960 a | 5.79 a | 6.60 cb |
| 1 | 8.15 d | 6.39 a | 0.957 b | 5.47 b | 6.13 c |
| 3 | 8.71 a | 6.18 b | 0.952 c | 4.76 e | 6.03 c |
| 5 | 8.47 b | 6.12 bc | 0.942 d | 4.80 cd | 6.88 b |
| 7 | 8.62 a | 6.05 c | 0.928 e | 4.80 cd | 6.94 ab |
| 9 | 8.34 c | 5.83 d | 0.912 f | 4.83 d | 6.31 c |
| 12 | 8.40 bc | 5.91 d | 0.886 g | 4.88 c | 7.47 a |
| Significance | ** | ** | ** | ** | ** |
| SM × RT | ** | ** | ** | ns | ns |
| SEM | 0.01 | 0.01 | 0.001 | 0.01 | 0.07 |
| Compounds | No | KI | Salt Mixtures | SEM | Sig. | ||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | |||||
| Aliphatic hydrocarbons | |||||||||
| Undecane | 1 | 1100 | 0.97 | 1.24 | 1.06 | 0.59 | 0.35 | 0.12 | ns |
| Dodecane | 2 | 1200 | 3.70 a | 0.57 b | 0.63 b | 0.34 b | 0.33 b | 0.27 | * |
| Tridecane | 3 | 1300 | 4.59 a | 1.64 b | 0.83 b | 0.81 b | 0.93 b | 0.24 | * |
| Tetradecane | 4 | 1400 | 3.27 a | 1.30 b | 1.11 b | 0.64 b | 0.90 b | 0.15 | * |
| Pentadecane | 5 | 1500 | 0.61 | 0.47 | 0.49 | 0.21 | 0.26 | 0.08 | ns |
| Aromatic hydrocarbons | |||||||||
| Toluene | 6 | 796 | 2.28 | 1.72 | 1.70 | 1.22 | 1.37 | 0.14 | ns |
| p-xylene | 7 | 898 | 1.36 a | 0.24 c | 0.91 ab | 0.40 cb | 0.00 c | 0.09 | * |
| Styrene | 8 | 935 | 0.47 | 0.60 | 0.71 | 0.63 | 0.21 | 0.10 | ns |
| 1-methyl-2-(1-methylethyl)-benzene | 9 | 1062 | 65.34 | 81.45 | 67.54 | 68.58 | 53.0 | 12.22 | ns |
| 1-methoxy-4-(1-propenyl)-benzene | 10 | 1230 | 1.69 | 0.54 | 0.59 | 0.88 | 0.75 | 0.25 | ns |
| 1-methoxy-4-(2-propenyl)-benzene | 11 | 1238 | 0.50 | 0.41 | 0.92 | 0.66 | 0.84 | 0.25 | ns |
| 1,2-dimethoxy-4-(2-propenyl)-benzene | 12 | 1482 | 1.81 | 1.58 | 1.76 | 1.91 | 1.77 | 0.15 | ns |
| Ketones | |||||||||
| 2,3-Butanedione | 13 | 657 | 1.15 b | 4.38 a | 1.33 b | 2.05 b | 0.94 b | 0.22 | * |
| 3-hydroxy-2-butanone | 14 | 779 | 6.44 ab | 5.22 b | 8.63 a | 8.13 a | 4.57 b | 0.29 | * |
| 6-methyl-5-hepten-2-one | 15 | 1050 | 0.37 | 0.44 | 0.52 | 0.44 | 0.21 | 0.09 | ns |
| Aldehydes | |||||||||
| Acetaldehyde | 16 | 623 | 12.49 a | 9.00 b | 12.73 a | 9.97 a | 5.45 c | 0.21 | ** |
| Pentanal | 17 | 742 | 1.32 | 1.15 | 0.81 | 1.22 | 0.62 | 0.34 | ns |
| Hexanal | 18 | 849 | 4.54 a | 2.25 b | 1.92 b | 2.03 b | 0.85 b | 0.24 | * |
| Heptanal | 19 | 955 | 1.22 | 0.59 | 0.63 | 0.65 | 0.29 | 0.09 | ns |
| Octanal | 20 | 1051 | 1.94 | 0.75 | 0.86 | 0.42 | 0.49 | 0.17 | ns |
| Nonanal | 21 | 1143 | 5.14 | 1.05 | 1.16 | 0.83 | 0.64 | 0.55 | ns |
| 2-methyl-3-phenylpropanal | 22 | 1333 | 4.62 | 3.45 | 5.03 | 5.14 | 5.00 | 0.82 | ns |
| Acids | |||||||||
| Acetic acid | 23 | 710 | 35.08 b | 46.83 a | 44.46 a | 46.65 a | 44.30 a | 0.70 | * |
| Propionic acid | 24 | 889 | 7.61 a | 8.86 a | 8.60 a | 4.58 b | 2.93 c | 0.17 | ** |
| Sulphur compounds | |||||||||
| 2-propene-1-thiol | 25 | 570 | 93.09 a | 89.70 a | 92.59 a | 76.47 b | 37.12 c | 1.52 | ** |
| Allyl methyl sulfide | 26 | 730 | 12.02 a | 8.17 b | 7.09 b | 7.64 b | 5.35 b | 0.46 | * |
| 3-methylthiophene | 27 | 874 | 1.38 | 1.40 | 1.17 | 1.37 | 0.80 | 0.07 | ns |
| 3,3-thiobis -1-propene | 28 | 888 | 11.20 a | 8.27 b | 8.72 ab | 4.78 c | 3.33 c | 0.34 | ** |
| Disulfide methyl 2-propenyl | 29 | 958 | 2.94 | 2.23 | 2.33 | 1.66 | 1.32 | 0.13 | ns |
| Diallyl disulfide | 30 | 1138 | 8.08 | 9.02 | 8.64 | 7.89 | 7.90 | 0.66 | ns |
| Esters | |||||||||
| Ethyl acetate | 31 | 648 | 3.18 | 0.92 | 2.08 | 0.29 | 0.00 | 0.34 | ns |
| Butyl propionate | 32 | 952 | 1.33 a | 0.67 b | 1.30 a | 0.38 b | 1.33 a | 0.07 | * |
| 3-hexenoic acid. methyl ester | 33 | 954 | 0.76 | 0.59 | 0.68 | 0.66 | 0.73 | 0.07 | ns |
| Propyl hexanoate | 34 | 1151 | 4.62 | 1.96 | 3.44 | 1.58 | 1.37 | 0.29 | ns |
| 2.4-hexadienoic acid ethyl ester | 35 | 1142 | 2.05 | 1.71 | 2.66 | 2.18 | 1.89 | 0.35 | ns |
| Hexyl butanoate | 36 | 1221 | 4.62 | 1.12 | 1.04 | 0.73 | 0.58 | 0.41 | ns |
| Hexyl hexanoate | 37 | 1133 | 1.05 | 1.08 | 0.76 | 0.43 | 0.29 | 0.15 | ns |
| Alcohols | |||||||||
| Ethyl alcohol | 38 | 539 | 0.35 | 0.47 | 0.64 | 0.29 | 0.66 | 0.07 | ns |
| 2-ethyl-1-hexanol | 39 | 1084 | 0.21 | 0.19 | 0.51 | 0.39 | 0.11 | 0.08 | ns |
| 1-decanol | 40 | 1218 | 0.68 ab | 0.91 a | 0.24 bc | 0.00 c | 0.00 c | 0.07 | * |
| 4-(1-methylethyl)-benzenemethanol | 41 | 1383 | 3.64 | 2.56 | 3.29 | 3.18 | 3.03 | 0.41 | ns |
| Terpenes | |||||||||
| α-thujene | 42 | 944 | 0.90 | 0.81 | 1.31 | 1.23 | 1.08 | 0.16 | ns |
| α-pinene | 43 | 950 | 3.33 | 3.13 | 3.62 | 3.21 | 2.16 | 0.22 | ns |
| Camphene | 44 | 970 | 0.58 | 0.59 | 0.43 | 0.42 | 0.15 | 0.04 | ns |
| β-thujene | 45 | 994 | 0.77 | 0.90 | 1.01 | 1.35 | 0.98 | 0.19 | ns |
| β-pinene | 46 | 996 | 8.41 | 7.43 | 7.95 | 8.50 | 7.95 | 0.57 | ns |
| β-myrcene | 47 | 1005 | 8.50 | 7.79 | 11.51 | 11.45 | 10.65 | 1.57 | ns |
| α-phellandrene | 48 | 1019 | 4.16 | 3.98 | 7.04 | 6.78 | 6.10 | 1.15 | ns |
| 3-carene | 49 | 1026 | 10.92 | 17.19 | 14.52 | 14.55 | 9.22 | 2.27 | ns |
| α-terpinene | 50 | 1030 | 3.67 | 2.33 | 1.91 | 1.80 | 1.21 | 0.45 | ns |
| D-limonene | 51 | 1054 | 47.37 | 37.95 | 32.62 | 34.53 | 22.75 | 6.88 | ns |
| β-phellandrene | 52 | 1065 | 3.17 | 3.96 | 3.30 | 3.02 | 2.74 | 0.61 | ns |
| ɣ-terpinene | 53 | 1105 | 34.69 | 31.84 | 25.70 | 28.40 | 18.79 | 6.41 | ns |
| α-terpinolene | 54 | 1131 | 0.01 | 0.52 | 0.18 | 0.25 | 0.14 | 0.10 | ns |
| p-cymenene | 55 | 1157 | 3.23 | 3.27 | 2.98 | 2.56 | 2.44 | 0.57 | ns |
| Linalool | 56 | 1161 | 11.73 | 8.55 | 8.38 | 8.88 | 6.98 | 1.95 | ns |
| Terpinen-4-ol | 57 | 1233 | 3.23 | 0.91 | 1.18 | 1.07 | 1.03 | 0.54 | ns |
| α-terpineol | 58 | 1258 | 0.83 | 0.99 | 1.07 | 1.27 | 1.17 | 0.25 | ns |
| Copaene | 59 | 1433 | 0.80 | 0.45 | 0.56 | 0.48 | 0.38 | 0.17 | ns |
| Caryophyllene | 60 | 1490 | 5.40 | 4.44 | 5.06 | 5.26 | 4.09 | 0.61 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şimşek, D.; Yılmaz Oral, Z.F.; Jaberi, R.; Kaya, M.; Kaban, G. Partial Replacement of NaCl by KCl, MgCl2 and CaCl2 Chloride Salts in the Production of Sucuk: Effects on Volatile Compounds, Lipid Oxidation, Microbiological and Sensory Properties. Foods 2023, 12, 3525. https://doi.org/10.3390/foods12193525
Şimşek D, Yılmaz Oral ZF, Jaberi R, Kaya M, Kaban G. Partial Replacement of NaCl by KCl, MgCl2 and CaCl2 Chloride Salts in the Production of Sucuk: Effects on Volatile Compounds, Lipid Oxidation, Microbiological and Sensory Properties. Foods. 2023; 12(19):3525. https://doi.org/10.3390/foods12193525
Chicago/Turabian StyleŞimşek, Derya, Zeynep Feyza Yılmaz Oral, Rahimeh Jaberi, Mükerrem Kaya, and Güzin Kaban. 2023. "Partial Replacement of NaCl by KCl, MgCl2 and CaCl2 Chloride Salts in the Production of Sucuk: Effects on Volatile Compounds, Lipid Oxidation, Microbiological and Sensory Properties" Foods 12, no. 19: 3525. https://doi.org/10.3390/foods12193525
APA StyleŞimşek, D., Yılmaz Oral, Z. F., Jaberi, R., Kaya, M., & Kaban, G. (2023). Partial Replacement of NaCl by KCl, MgCl2 and CaCl2 Chloride Salts in the Production of Sucuk: Effects on Volatile Compounds, Lipid Oxidation, Microbiological and Sensory Properties. Foods, 12(19), 3525. https://doi.org/10.3390/foods12193525









