The Physicochemical Characterization and In Vitro Digestibility of Maple Sugar Sand and Downgraded Maple Syrups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Maple By-Product Samples
2.2. Carbohydrate Analysis
2.3. Organic Acid Extraction and Analysis
2.4. Mineral Composition
2.5. Total Polyphenol Content
2.6. Physicochemical Properties
2.7. Evaluation of By-Product Digestibility
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties
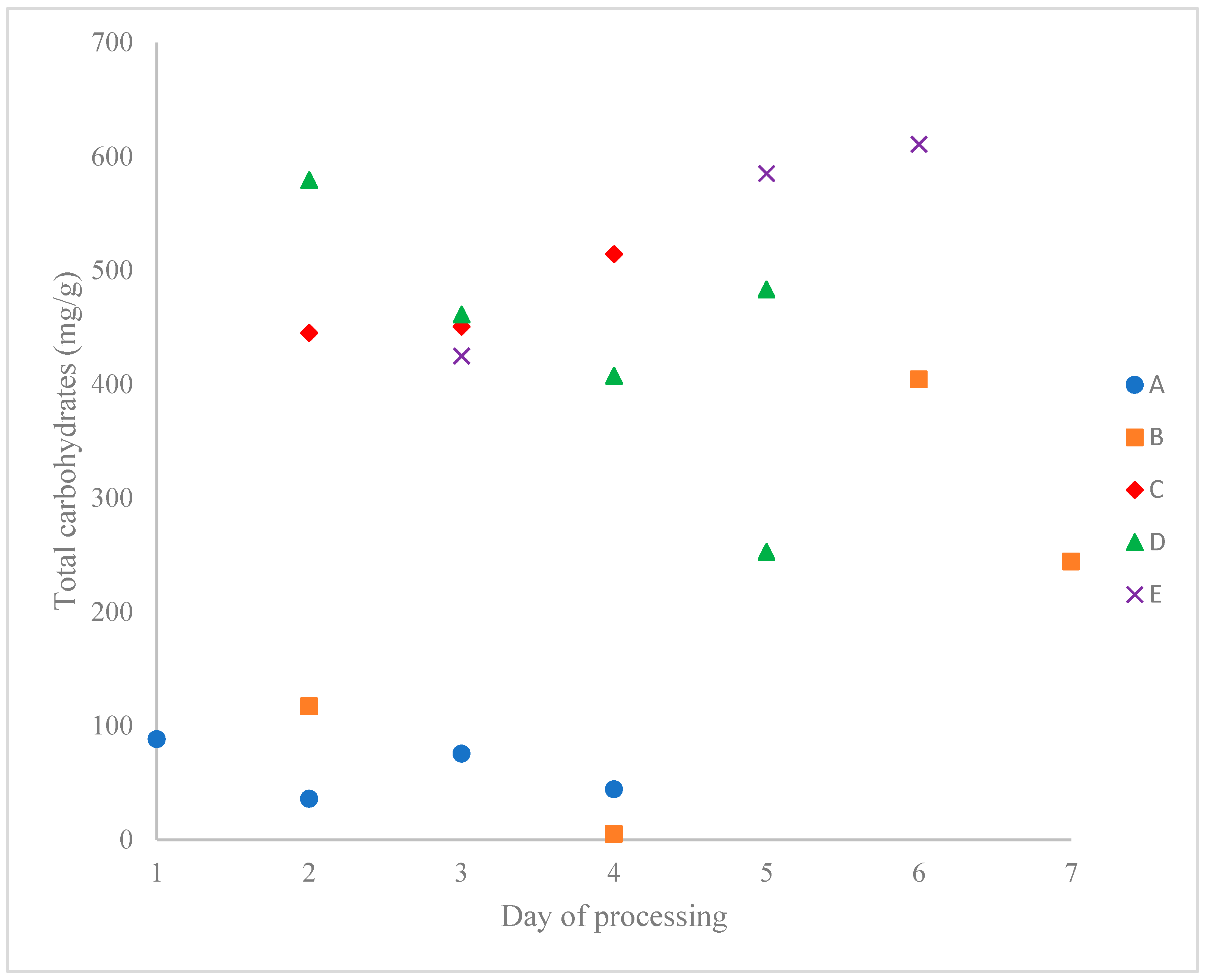
3.2. Carbohydrate Content
3.3. Organic Acids
3.4. Mineral Content
3.5. Total Polyphenol Content
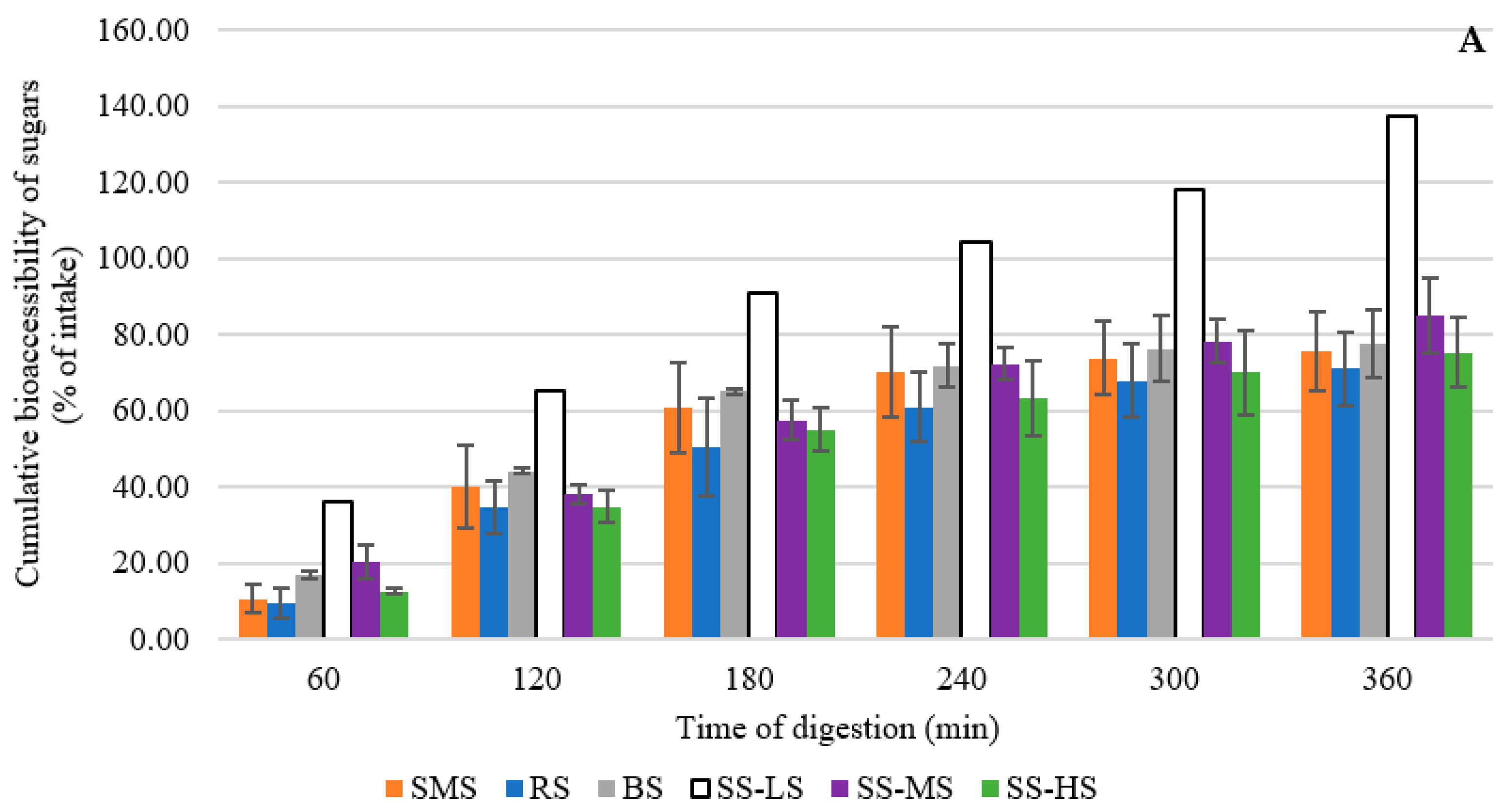
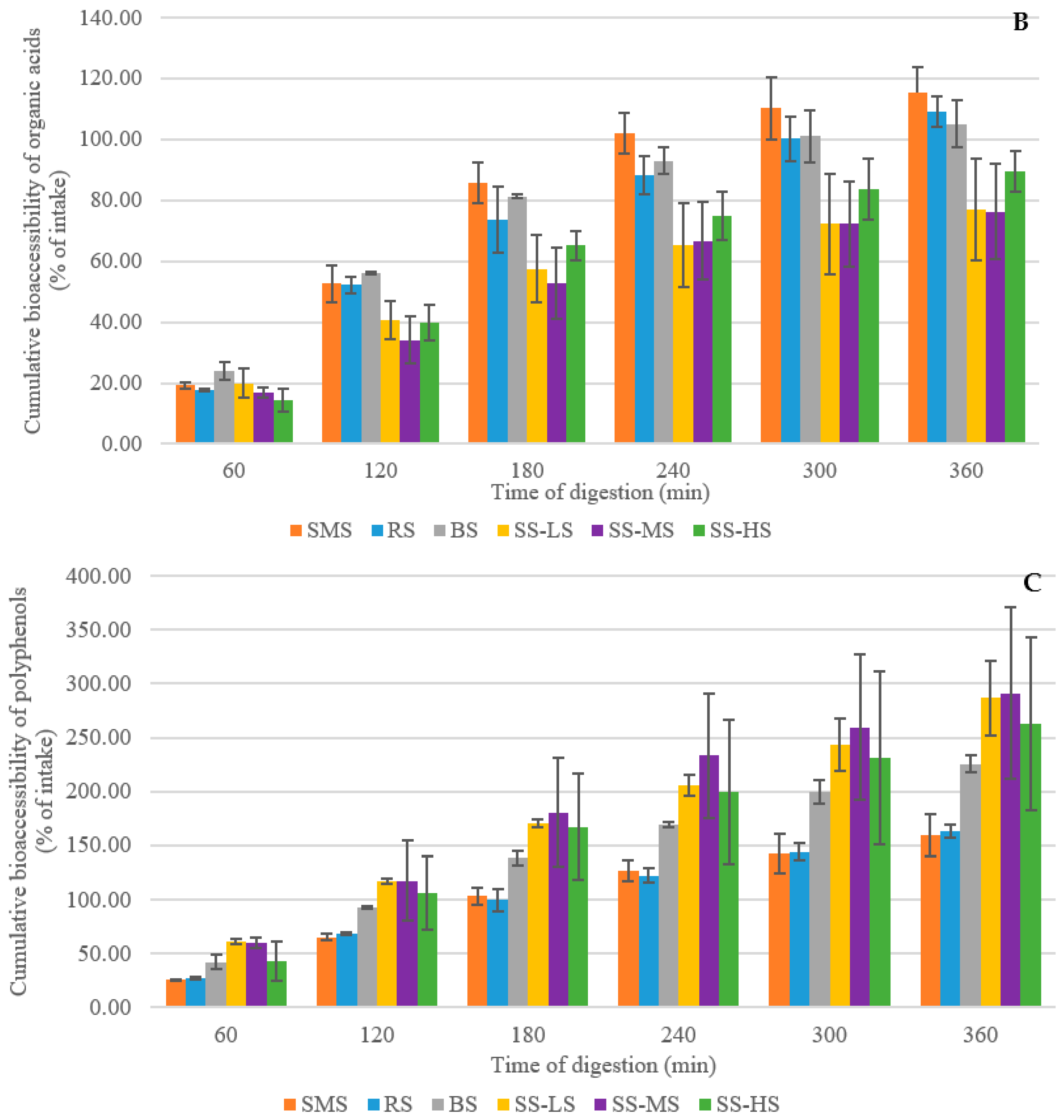
3.6. Evaluation of the Digestibility of Maple Syrup By-Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitney, G.G.; Upmeyer, M.M. Sweet trees, sour circumstances: The long search for sustainability in the North American maple products industry. For. Ecol. Manag. 2004, 200, 313–333. [Google Scholar] [CrossRef]
- Tyree, M.T. Maple sap uptake, exudation, and pressure changes correlated with freezing exotherms and thawing endotherms. Plant Physiol. 1983, 73, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Ball, D.W. The Chemical Composition of Maple Syrup. J. Chem. Educ. 2007, 84, 1647. [Google Scholar] [CrossRef]
- Filteau, M.; Charron, G.; Landry, C.R. Identification of the fitness determinants of budding yeast on a natural substrate. ISME J. 2017, 11, 959–971. [Google Scholar] [CrossRef]
- Aaron, C.; Beaudry, G.; Parker, J.A.; Therrien, M. Maple Syrup Decreases TDP-43 Proteotoxicity in a Caenorhabditis elegans Model of Amyotrophic Lateral Sclerosis (ALS). J. Agric. Food Chem. 2016, 64, 3338–3344. [Google Scholar] [CrossRef]
- Hawco, C.L.; Wang, Y.; Taylor, M.; Weaver, D.F. A Maple Syrup Extract Prevents beta-Amyloid Aggregation. Can. J. Neurol. Sci. 2016, 43, 198–201. [Google Scholar] [CrossRef]
- Legault, J.; Girard-Lalancette, K.; Grenon, C.; Dussault, C.; Pichette, A. Antioxidant activity, inhibition of nitric oxide overproduction, and in vitro antiproliferative effect of maple sap and syrup from Acer saccharum. J. Med. Food 2010, 13, 460–468. [Google Scholar] [CrossRef]
- Li, L.; Seeram, N.P. Maple syrup phytochemicals include lignans, coumarins, a stilbene, and other previously unreported antioxidant phenolic compounds. J. Agric. Food Chem. 2010, 58, 11673–11679. [Google Scholar] [CrossRef]
- Liu, W.; Wei, Z.; Ma, H.; Cai, A.; Liu, Y.; Sun, J.; DaSilva, N.A.; Johnson, S.L.; Kirschenbaum, L.J.; Cho, B.P.; et al. Anti-glycation and anti-oxidative effects of a phenolic-enriched maple syrup extract and its protective effects on normal human colon cells. Food Funct. 2017, 8, 757–766. [Google Scholar] [CrossRef]
- Maisuria, V.B.; Hosseinidoust, Z.; Tufenkji, N. Polyphenolic extract from maple syrup potentiates antibiotic susceptibility and reduces biofilm formation of pathogenic bacteria. Appl. Environ. Microbiol. 2015, 81, 3782–3792. [Google Scholar] [CrossRef]
- Thériault, M.; Caillet, S.; Kermasha, S.; Lacroix, M. Antioxidant, antiradical and antimutagenic activities of phenolic compounds present in maple products. Food Chem. 2006, 98, 490–501. [Google Scholar] [CrossRef]
- Rose, K.N.; Barlock, B.J.; DaSilva, N.A.; Johnson, S.L.; Liu, C.; Ma, H.; Nelson, R.; Akhlaghi, F.; Seeram, N.P. Anti-neuroinflammatory effects of a food-grade phenolic-enriched maple syrup extract in a mouse model of Alzheimer’s disease. Nutr. Neurosci. 2019, 24, 710–719. [Google Scholar] [CrossRef] [PubMed]
- PPAQ. Statistiques Acéricoles; PPAQ: Quebec, QC, Canada, 2021.
- Government-of-Canada. Canadian Grade Compendium: Volume 7—Maple Syrup; Government-of-Canada: Ottawa, ON, Canada, 2021. [Google Scholar]
- Gouvernement-du-Québec. Règlement des Producteurs Acéricoles sur les Normes de Qualité et le Classement; Gouvernement-du-Québec: Québec, QC, Canada, 2022. [Google Scholar]
- Camara, M.; Cournoyer, M.; Sadiki, M.; Martin, N. Characterization and Removal of Buddy Off-Flavor in Maple Syrup. J. Food Sci. 2019, 84, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- N’Guyen, G.Q.; Martin, N.; Jain, M.; Lagace, L.; Landry, C.R.; Filteau, M. A systems biology approach to explore the impact of maple tree dormancy release on sap variation and maple syrup quality. Sci. Rep. 2018, 8, 14658. [Google Scholar] [CrossRef] [PubMed]
- N’Guyen, G.Q.; Roblet, C.; Lagacé, L.; Filteau, M. A metataxonomic analysis of maple sap microbial communities reveals new insights into maple syrup complexity. Front. Syst. Biol. 2022, 2, 893007. [Google Scholar] [CrossRef]
- Hwang, H.-I.; Hartman, T.G.; Ho, C.-T. Relative Reactivities of Amino Acids in Pyrazine Formation. J. Agric. Food Chem. 1995, 43, 179–184. [Google Scholar] [CrossRef]
- Amrani-Hemaimi, M.; Cerny, C.; Fay, L.B. Mechanisms of Formation of Alkylpyrazines in the Maillard Reaction. J. Agric. Food Chem. 1995, 43, 2818–2822. [Google Scholar] [CrossRef]
- Perkins, T.D.; van den Berg, A.K. Chapter 4 Maple Syrup—Production, Composition, Chemistry, and Sensory Characteristics. Adv. Food Nutr. Res. 2009, 56, 101–143. [Google Scholar]
- Lagace, L.; Camara, M.; Leclerc, S.; Charron, C.; Sadiki, M. Chemical and microbial characterization of ropy maple sap and syrup. In Maple Syrup Digest; North American Maple Syrup Council: Sturbridge, MA, USA, 2018; pp. 9–19. [Google Scholar]
- Sun, J.; Ma, H.; Seeram, N.P.; Rowley, D.C. Detection of Inulin, a Prebiotic Polysaccharide, in Maple Syrup. J. Agric. Food Chem. 2016, 64, 7142–7147. [Google Scholar] [CrossRef]
- Britten, M.; Morin, A. Functional characterization of the exopolysaccharide from Enterobacter agglomerans grown on low-grade maple sap. LWT—Food Sci. Technol. 1995, 28, 264–271. [Google Scholar] [CrossRef]
- Fabian, F.W.; Buskirk, H.H. Aërobacter aërogenes as a Cause of Ropiness in Maple Sirup. Ind. Eng. Chem. 1935, 27, 349–350. [Google Scholar] [CrossRef]
- Arzate, A.; Robaire, S.; Batungwanayo, A. L’entartrage des Échangeurs de Chaleur dans L’industrie Alimentaire: Problématique Acéricole; Centre ACER: Camberwell, VIC, Australia, 2013. [Google Scholar]
- Heiligmann, R.B.; Koelling, M.R.; Perkins, T.D.; Ohio State, U. North American Maple Syrup Producers Manual; Ohio State University: Ohio, OH, USA, 2006. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Renaud, V.; Faucher, M.; Perreault, V.; Serre, E.; Dube, P.; Boutin, Y.; Bazinet, L. Evolution of cranberry juice compounds during in vitro digestion and identification of the organic acid responsible for the disruption of in vitro intestinal cell barrier integrity. J. Food Sci. Technol. 2020, 57, 2329–2342. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Bhatta, S.; Ratti, C.; Stevanovic, T. Impact of drying processes on properties of polyphenol-enriched maple sugar powders. J. Food Process Eng. 2019, 42, e13239. [Google Scholar] [CrossRef]
- Minekus, M.; Marteau, P.; Havenaar, R.; Veld, J.H.J.H.i.t. A Multicompartmental Dynamic Computer-controlled Model Simulating the Stomach and Small Intestine. Altern. Lab. Anim. 1995, 23, 197–209. [Google Scholar] [CrossRef]
- Meunier, J.P.; Manzanilla, E.G.; Anguita, M.; Denis, S.; Perez, J.F.; Gasa, J.; Cardot, J.M.; Garcia, F.; Moll, X.; Alric, M. Evaluation of a dynamic in vitro model to simulate the porcine ileal digestion of diets differing in carbohydrate composition. J. Anim. Sci. 2008, 86, 1156–1163. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Blackburn, J.; Lada, R.R. A comparative physicochemical analysis of maple (Acer saccharum Marsh.) syrup produced in North America with special emphasis on seasonal changes in Nova Scotia maple syrup composition. J. Food Compos. Anal. 2020, 92, 103573. [Google Scholar] [CrossRef]
- Robinson, R.A.; Maclean, K.S.; Macconnell, H.M. Heavy metal, pH, and total solid content of maple sap and syrup produced in eastern Canada. J. Assoc. Off. Anal. Chem. 1989, 72, 674–676. [Google Scholar] [CrossRef]
- Filteau, M.; Lagace, L.; Lapointe, G.; Roy, D. Maple sap predominant microbial contaminants are correlated with the physicochemical and sensorial properties of maple syrup. Int. J. Food Microbiol. 2012, 154, 30–36. [Google Scholar] [CrossRef]
- Stuckel, J.G.; Low, N.H. The chemical composition of 80 pure maple syrup samples produced in North America. Food Res. Int. 1996, 29, 373–379. [Google Scholar] [CrossRef]
- Morselli, M.F. Chemical composition of maple syrup. J. Natl. Maple Syrup Dig. 1975, 14, 12. [Google Scholar]
- PPAQ. Industry Sheet Maple Syrup of Québec; PPAQ: Quebec, QC, Canada, 2018.
- Singh, A.S.; Jones, A.M.; Saxena, P.K. Variation and correlation of properties in different grades of maple syrup. Plant Foods Hum. Nutr. 2014, 69, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Morselli, M.F.; Whalen, M.L.; Baggett, K.L. Characteristics of Maple Syrup Processed from Bleach-Treated Sap (1). J. Food Prot. 1985, 48, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Gallander, J.F.; Hacskaylo, J.; Gould, W.A.; Willits, C.O. Environmental and Chemical Factors Associated with Maple Sugar Sand Formation; Ohio Agricultural Research and Development Center: Wooster, OH, USA, 1967. [Google Scholar]
- Willits, C.O.; Hills, C.H. Maple Sirup Producers Manual; Agricultural Research Service, US Department of Agriculture: Washington, DC, USA, 1976.
- Medina, M.B. Determination of the total phenolics in juices and superfruits by a novel chemical method. J. Funct. Foods 2011, 3, 79–87. [Google Scholar] [CrossRef]
- Lester, G.E.; Lewers, K.S.; Medina, M.B.; Saftner, R.A. Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin–Ciocalteu: Assay interference by ascorbic acid. J. Food Compos. Anal. 2012, 27, 102–107. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- MAPAQ. Unité de Conversion “Sirop D’érable”; MAPAQ: Québec, QC, Canada, 2020.
- Kardum, N.; Glibetic, M. Polyphenols and Their Interactions with Other Dietary Compounds: Implications for Human Health. Adv. Food Nutr. Res. 2018, 84, 103–144. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Lobo, M.; Gonzalez, M. Factors affecting sample extraction in the liquid chromatographic determination of organic acids in papaya and pineapple. Food Chem. 2009, 114, 734–741. [Google Scholar] [CrossRef]
- Xu, W.-J.; Zhai, J.-W.; Cui, Q.; Liu, J.-Z.; Luo, M.; Fu, Y.-J.; Zu, Y.-G. Ultra-turrax based ultrasound-assisted extraction of five organic acids from honeysuckle (Lonicera japonica Thunb.) and optimization of extraction process. Sep. Purif. Technol. 2016, 166, 73–82. [Google Scholar] [CrossRef]
- Sun, C.; Liu, F.; Sun, J.; Li, J.; Wang, X. Optimisation and establishment of separation conditions of organic acids from Usnea longissima Ach. by pH-zone-refining counter-current chromatography: Discussion of the eluotropic sequence. J. Chromatogr. A 2016, 1427, 96–101. [Google Scholar] [CrossRef]
- Faraloni, C.; Albanese, L.; Chini Zittelli, G.; Meneguzzo, F.; Tagliavento, L.; Zabini, F. New Route to the Production of Almond Beverages Using Hydrodynamic Cavitation. Foods 2023, 12, 935. [Google Scholar] [CrossRef] [PubMed]
- Pesic, M.B.; Milincic, D.D.; Kostic, A.Z.; Stanisavljevic, N.S.; Vukotic, G.N.; Kojic, M.O.; Gasic, U.M.; Barac, M.B.; Stanojevic, S.P.; Popovic, D.A.; et al. In vitro digestion of meat- and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; McDougall, G.J.; Alegria, A.; Alminger, M.; Arrigoni, E.; Aura, A.M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the gap-deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites—A position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Deng, J.; Wen, L.; You, L.; Zhao, Z.; Zhou, L. Release of phenolic compounds and antioxidant capacity of Chinese hawthorn “Crataegus pinnatifida” during in vitro digestion. J. Funct. Foods 2018, 40, 76–85. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Gumienna, M.; Lasik, M.; Czarnecki, Z. Bioconversion of grape and chokeberry wine polyphenols during simulated gastrointestinal in vitro digestion. Int. J. Food Sci. Nutr. 2011, 62, 226–233. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Liu, Y.; Zhang, Q.; Li, Y.; Wu, Z. Release of phenolics compounds from Rubus idaeus L. dried fruits and seeds during simulated in vitro digestion and their bio-activities. J. Funct. Foods 2018, 46, 57–65. [Google Scholar] [CrossRef]
- Mišan, A.; Mimica-Dukić, N.; Mandić, A.; Sakač, M.; Milovanović, I.; Sedej, I. Development of a rapid resolution HPLC method for the separation and determination of 17 phenolic compounds in crude plant extracts. Open Chem. 2011, 9, 133–142. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Adebooye, O.C.; Alashi, A.M.; Aluko, R.E. A brief review on emerging trends in global polyphenol research. J. Food Biochem. 2018, 42, e12519. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- de Medeiros Dantas, J.M.; Álvares Monge Neto, A.; Ghislain, T.; Lavoie, J.-M. Bioethanol Production as an Alternative End for Maple Syrups with Flavor Defects. Fermentation 2022, 8, 58. [Google Scholar] [CrossRef]
- Chua, K.J.; Chou, S.K. Low-cost drying methods for developing countries. Trends Food Sci. Technol. 2003, 14, 519–528. [Google Scholar] [CrossRef]
- Cherrington, C.A.; Hinton, M.; Mead, G.C.; Chopra, I. Organic Acids: Chemistry, Antibacterial Activity and Practical Applications. Adv. Microb. Physiol. 1991, 32, 87–108. [Google Scholar]
- Jongbloed, A.; Mroz, Z.; Van der Weij-Jongbloed, R.; Kemme, P. The effects of microbial phytase, organic acids and their interaction in diets for growing pigs. Livest. Prod. Sci. 2000, 67, 113–122. [Google Scholar] [CrossRef]
- Papatsiros, V.G. The use of organic acids in monogastric animals (swine and rabbits). J. Cell Anim. Biol. 2012, 6, 154–159. [Google Scholar] [CrossRef]
- Suiryanrayna, M.V.; Ramana, J. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar] [CrossRef]
- Government-of-Canada. Antimicrobial Resistance and Use in Canada. A Federal Framework for Action; Government-of-Canada: Ottawa, ON, Canada, 2014. [Google Scholar]
- Lagacé, L.; Camara, M.; Martin, N.; Ali, F.; Houde, J.; Corriveau, S.; Sadiki, M. Effect of the new high vacuum technology on the chemical composition of maple sap and syrup. Heliyon 2019, 5, 35. [Google Scholar] [CrossRef]
- Davis, D.R.; Gallander, J.F.; Hacskaylo, J.; Gould, W.A. The chemical composition of maple sugar sand. J. Food Sci. 1963, 28, 182–190. [Google Scholar] [CrossRef]
| Analysis | Standard Syrup | Ropy-1 | Ropy-2 | Ropy-3 | Buddy-1 | Buddy-2 |
|---|---|---|---|---|---|---|
| General | ||||||
| Brix (%) | 66.3 | 68.2 | 67.7 | 67.1 | 65.5 | 65.2 |
| Viscosity (Pa·s) | 0.16 | 0.52 | 0.75 | 0.49 | 0.17 | 0.15 |
| pH | 6.35 | 6.25 | 5.80 | 6.25 | 6.84 | 6.69 |
| Transmittance (% at 560 nm) | 30.60 | 30.47 | 47.51 | 44.75 | 30.60 | 26.47 |
| Color analysis ‡ | ||||||
| L | 56.89 | 58.34 | 19.17 | 30.91 | 62.87 | 47.89 |
| a | 33.50 | 26.28 | 24.82 | 36.45 | 30.81 | 15.36 |
| b | 92.16 | 70.43 | 32.34 | 52.55 | 82.03 | 51.97 |
| C*ab | 98.06 | 75.17 | 40.77 | 63.96 | 87.63 | 54.19 |
| Carbohydrates (mg/g) † | ||||||
| Total carbohydrate | 728.17 ± 29.01 | 601.45 ± 5.98 | 563.67 ± 5.55 | 609.94 ± 18.54 | 588.78 ± 31.54 | 551.48 ± 3.70 |
| Sucrose | 678.87 ± 5.21 | 533.25 ± 4.24 | 494.08 ± 2.21 | 544 ± 4.21 | 522.14 ± 3.65 | 382.3 ± 4.20 |
| Glucose | 8.9 ± 1.01 | 10 ± 0.87 | 24.17 ± 0.72 | 10.75 ± 1.10 | 23 ± 1.33 | 57.4 ± 2.22 |
| Fructose | 4.92 ± 0.05 | 8.3 ± 0.03 | 21.57 ± 1.20 | 10.8 ± 0.98 | 20.08 ± 1.51 | 31.2 ± 1.68 |
| Organic acids (mg/g) † | ||||||
| Malic | 6.12 ± 0.13 | 7.63 ± 0.18 | 7.01 ± 0.05 | 6.53 ± 0.31 | 9.13 ± 0.05 | 8.45 ± 0.24 |
| Citric | n.d | n.d | n.d | n.d | n.d | n.d |
| Fumaric | 0.1 ± 0.01 | 0.2 ± 0.01 | 0.22 ± 0.00 | 0.32 ± 0.01 | 0.15 ± 0.01 | 0.18 ± 0.00 |
| Total phenolics (µg/g) † | 230.89 ± 1.29 | 189.93 ± 1.08 | 338.67 ± 9.94 | 315.62 ± 25.26 | 133.13 ± 3.78 | 149.38 ± 11.54 |
| Minerals (ppm) † | ||||||
| Calcium | 1144.88 ± 235.88 | 1938.50 ± 227.64 | 1379.53 ± 235.37 | 1488.33 ± 228.79 | 1271.58 ± 32.20 | 1215.90 ± 57.10 |
| Iron | 10.56 ± 3.98 | 5.45 ± 1.95 | 9.83 ± 3.80 | 8.01 ± 2.80 | 4.74 ± 0.66 | 9.45 ± 1.49 |
| Potassium | 522.32 ± 153.01 | 397.40 ± 219.69 | 435.41 ± 194.83 | 517.35 ± 181.42 | 420.26 ± 77.67 | 456.33 ± 148.20 |
| Magnesium | 230.93 ± 30.84 | 255.25 ± 13.21 | 243.97 ± 15.57 | 250.20 ± 15.07 | 207.23 ± 20.74 | 218.08 ± 28.34 |
| Manganese | 12.08 ± 5.84 | 34.43 ± 6.28 | 20.14 ± 6.96 | 23.28 ± 6.72 | 12.79 ± 3.52 | 13.49 ± 4.84 |
| Sodium | 551.41 ± 144.27 | 314.88 ± 201.26 | 341.62 ± 197.14 | 315.34 ± 184.60 | 478.62 ± 118.16 | 538.48 ± 144.17 |
| Phosphorus | 363.45 ± 607.13 | 46.48 ± 27.99 | 272.82 ± 541.10 | 64.88 ± 341.98 | 12.68 ± 0.73 | 343.69 ± 5.16 |
| Zinc | 16.15 ± 12.64 | 17.71 ± 7.71 | 17.20 ± 11.52 | 17.63 ± 9.35 | 24.00 ± 1.09 | 22.18 ± 2.31 |
| Lead | 0.16 ± 0.07 | 0.15 ± 0.09 | 0.11 ± 0.91 | 0.14 ± 0.08 | 0.20 ± 0.03 | 0.12 ± 0.03 |
| Sample | A1 | A2 | A3 | A4 | B1 | B2 | B3 | B4 | C1 | C2 | C3 | D1 | D2 | D3 | D4 | D5 | E1 | E2 | E3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture (%) | 66.73 | 70.54 | 67.35 | 70.14 | 57.77 | 57.06 | 57.08 | 75.21 | 39.19 | 39.8 | 47.27 | 41.32 | 39.935 | 65.56 | 51.85 | 53.236 | 54.87 | 35.30 | 25.32 |
| Carbohydrates (mg/g) | |||||||||||||||||||
| Total | 88.30 ± 0.95 | 36.03 ± 0.62 | 75.58 ± 2.08 | 44.36 ± 1.78 | 117.33 ± 12.18 | 5.01 ± 0.17 | 404.19 ± 26.81 | 244.26 ± 11.72 | 445.04 ± 3.68 | 450.66 ± 26.34 | 514.28 ± 9.44 | 579.28 ± 18.76 | 461.22 ± 17.31 | 407.50 ± 20.16 | 483.21 ± 16.75 | 252.84 ± 3.04 | 424.93 ± 5.48 | 585.18 ± 7.95 | 610.87 ± 11.52 |
| Sucrose | 89.49 ± 1.39 | 35.05 ± 2.22 | 64.21 ± 2.09 | 44.06 ± 1.88 | 95.63 ± 1.71 | 1.23 ± 0.17 | 393.52 ± 5.16 | 232.49 ± 4.30 | 405.85 ± 4.48 | 440.68 ± 5.43 | 468.72 ± 5.93 | 554.52 ± 7.13 | 448.13 ± 5.26 | 374.52 ± 4.58 | 442.69 ± 6.19 | 232.45 ± 4.24 | 405.86 ± 4.30 | 534.67 ± 5.71 | 570.80 ± 7.70 |
| Glucose | n.d | n.d | 4.00 ± 0.13 | n.d | n.d | 0.15 ± 0.02 | n.d | n.d | n.d | n.d | 6.98 ± 0.34 | n.d | 7.40 ± 0.04 | 6.93 ± 0.27 | 9.57 ± 0.91 | 5.09 ± 0.00 | 6.75 ± 0.57 | n.d | n.d |
| Fructose | n.d | n.d | 0.90 ± 0.04 | n.d | n.d | 4.29 ± 0.17 | 2.81 ± 0.23 | 2.12 ± 0.14 | n.d | n.d | 4.01 ± 0.11 | n.d | 5.22 ± 0.17 | 4.79 ± 0.37 | 6.67 ± 0.05 | 4.10 ± 0.01 | 4.33 ± 0.03 | n.d | n.d |
| Organic acids (mg/g) | |||||||||||||||||||
| Malic | 69.05 ± 1.89 | 71.12 ± 1.19 | 75.73 ± 2.71 | 80.72 ± 2.63 | 72.04 ± 1.46 | 98.87 ± 2.63 | 90.94 ± 1.91 | 93.14 ± 2.59 | 73.68 ± 1.80 | 77.38 ± 2.28 | 74.88 ± 1.06 | 75.84 ± 2.72 | 79.78 ± 2.69 | 88.78 ± 2.21 | 91.34 ± 1.78 | 103.86 ± 2.64 | 74.96 ± 2.71 | 67.89 ± 2.27 | 80.45 ± 2.26 |
| Citric | 1.20 ± 0.02 | 1.36 ± 0.11 | 1.75 ± 0.14 | 2.76 ± 0.17 | 1.56 ± 0.07 | 3.77 ± 0.18 | 2.97 ± 0.07 | 8.00 ± 0.28 | 1.36 ± 0.04 | 1.60 ± 0.05 | 1.66 ± 0.14 | 1.68 ± 0.14 | 1.81 ± 0.11 | 1.95 ± 0.16 | 2.33 ± 0.04 | 3.59 ± 0.28 | 1.66 ± 0.14 | 1.83 ± 0.18 | 3.04 ± 0.28 |
| Fumaric | 0.10 ± 0.00 | 0.15 ± 0.01 | 0.24 ± 0.01 | 0.32 ± 0.03 | 0.06 ± 0.00 | 0.69 ± 0.06 | 0.94 ± 0.05 | 1.15 ± 0.10 | 0.15 ± 0.01 | 0.21 ± 0.02 | 0.26 ± 0.00 | 0.24 ± 0.02 | 0.34 ± 0.02 | 0.43 ± 0.02 | 0.22 ± 0.02 | 0.15 ± 0.01 | 0.32 ± 0.01 | 0.20 ± 0.00 | 0.24 ± 0.00 |
| Total phenolic content (µg/g) | 11.30 ± 1.35 | 17.16 ± 1.35 | 17.12 ± 1.78 | 31.12 ± 2.43 | 24.53 ± 1.17 | 57.56 ± 7.00 | 62.58 ± 6.02 | 100.50 ± 6.07 | 59.43 ± 6.64 | 72.98 ± 4.09 | 96.75 ± 5.83 | 97.81 ± 10.52 | 84.55 ± 3.76 | 55.84 ± 3.14 | 85.66 ± 10.94 | 71.88 ± 4.18 | 79.61 ± 6.62 | 118.67 ± 10.13 | 120.95 ± 12.32 |
| Mineral | Low Sugar Content | Intermediate Sugar | High Sugar Content |
|---|---|---|---|
| Calcium | 113,577 ± 9121 | 66,329 ± 6401 | 55,451 ± 4090 |
| Iron | 18.60 ± 3.37 | 16.73 ± 2.56 | 18.13 ± 0.75 |
| Potassium | 574 ± 17 | 1180 ± 98 | 2266 ± 115 |
| Magnesium | 3098 ± 186 | 1877 ± 185 | 3243 ± 45 |
| Manganese | 813 ± 70 | 676 ± 145 | 583 ± 68 |
| Sodium | 335 ± 140 | 173 ± 39 | 4467 ± 202 |
| Phosphorus | 2345 ± 131 | 1142 ± 459 | 1173 ± 30 |
| Zinc | 117 ± 8 | 76 ± 1 | 108 ± 4 |
| Lead | 0.50 ± 0.00 | 0.58 ± 0.07 | 0.50 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decabooter, G.; Aspirault, C.; Filteau, M.; Fliss, I. The Physicochemical Characterization and In Vitro Digestibility of Maple Sugar Sand and Downgraded Maple Syrups. Foods 2023, 12, 3528. https://doi.org/10.3390/foods12193528
Decabooter G, Aspirault C, Filteau M, Fliss I. The Physicochemical Characterization and In Vitro Digestibility of Maple Sugar Sand and Downgraded Maple Syrups. Foods. 2023; 12(19):3528. https://doi.org/10.3390/foods12193528
Chicago/Turabian StyleDecabooter, Gautier, Claudie Aspirault, Marie Filteau, and Ismail Fliss. 2023. "The Physicochemical Characterization and In Vitro Digestibility of Maple Sugar Sand and Downgraded Maple Syrups" Foods 12, no. 19: 3528. https://doi.org/10.3390/foods12193528
APA StyleDecabooter, G., Aspirault, C., Filteau, M., & Fliss, I. (2023). The Physicochemical Characterization and In Vitro Digestibility of Maple Sugar Sand and Downgraded Maple Syrups. Foods, 12(19), 3528. https://doi.org/10.3390/foods12193528






