Bacteriocin-Producing Enterococcus faecium OV3-6 as a Bio-Preservative Agent to Produce Fermented Houttuynia cordata Thunb. Beverages: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Strains Used in the Study
2.2. Plant Used in the Study
2.3. Antimicrobial Activity of E. faecium OV3-6
2.4. Assessment of E. faecium OV3-6 Growth in MRS Medium
2.5. Assessment of pH Changes during E. faecium OV3-6 Growth
2.6. Survival of E. faecium OV3-6 in the Fermented Beverage (FB) with and without H. cordata
2.7. Assessment of the Bio-Preservative Property of E. faecium OV3-6 in Simulated FHB
2.8. Statistics Analysis
3. Results
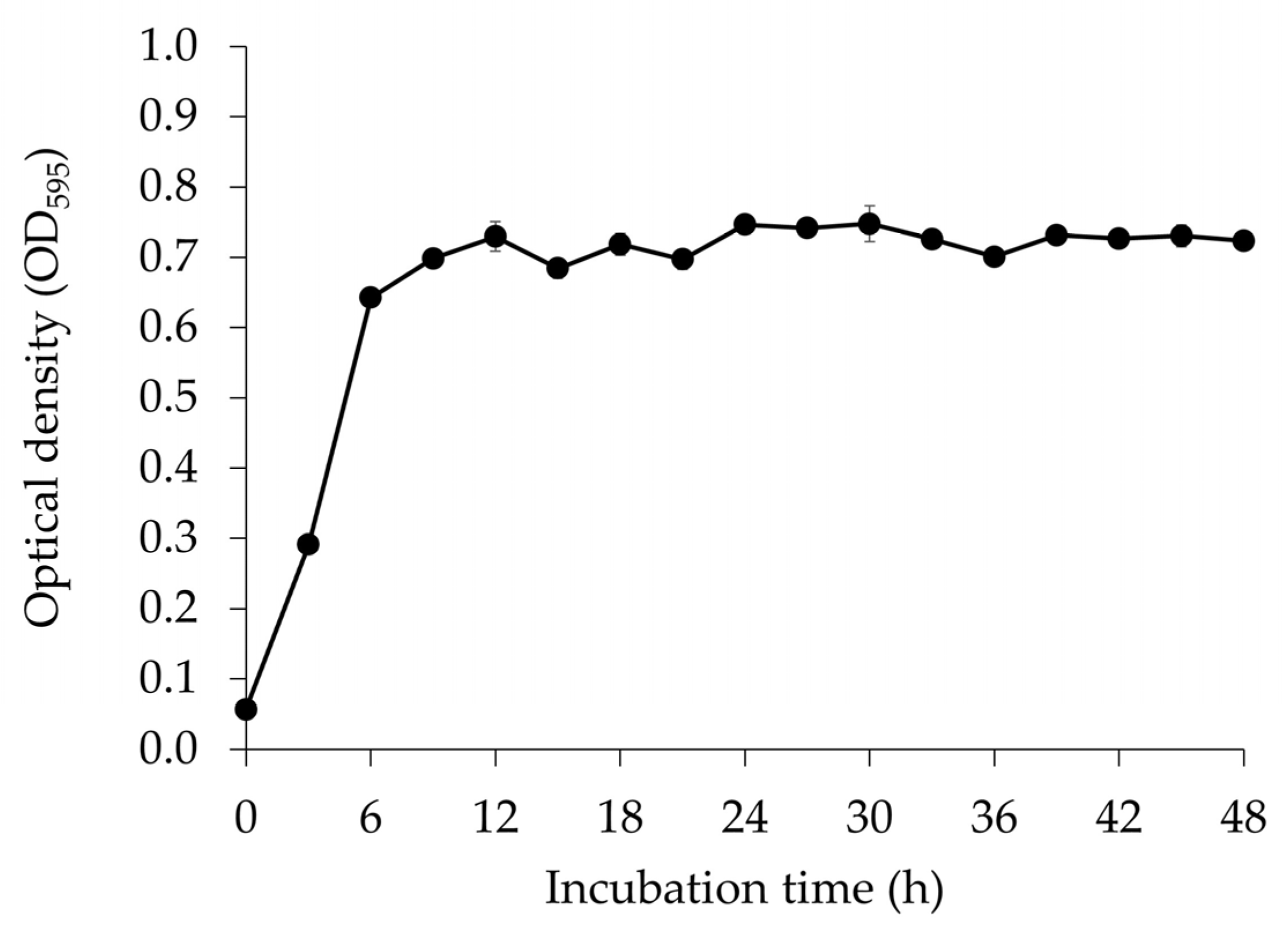
3.1. Microbial Growth of E. faecium OV3-6 Count in MRS Medium
3.2. pH Change during the Growth of E. faecium OV3-6 in MRS Medium
3.3. Antimicrobial Activity of E. faecium OV3-6
3.4. Cell Number of E. faecium OV3-6 in FB with and without H. cordata
3.5. Microbial Load in FHBs with Different Preservative Treatments
3.6. pH Changes in FHB with Different Preservative Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woraharn, S.; Lailerd, N.; Sivamaruthi, B.S.; Wangcharoen, W.; Sirisattha, S.; Peerajan, S.; Chaiyasut, C. Evaluation of factors that influence the L-glutamic and γ-aminobutyric acid production during Hericium erinaceus fermentation by lactic acid bacteria. CyTA-J. Food 2016, 14, 47–54. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Sivamaruthi, B.S.; Makhamrueang, N.; Peerajan, S.; Kesika, P. A survey of consumer’s opinion about consumption and health benefits of fermented plant beverages in Thailand. Food Sci. Technol. 2017, 38, 299–309. [Google Scholar] [CrossRef]
- Sõukand, R.; Pieroni, A.; Biró, M.; Dénes, A.; Dogan, Y.; Hajdari, A.; Kalle, R.; Reade, B.; Mustafa, B.; Nedelcheva, A. An ethnobotanical perspective on traditional fermented plant foods and beverages in Eastern Europe. J. Ethnopharmacol. 2015, 170, 284–296. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Impact of fermented foods on human cognitive function-A review of outcome of clinical trials. Sci. Pharm. 2018, 86, 22. [Google Scholar] [CrossRef]
- Das, G.; Paramithiotis, S.; Sivamaruthi, B.S.; Wijaya, C.H.; Suharta, S.; Sanlier, N.; Shin, H.S.; Patra, J.K. Traditional fermented foods with anti-aging effect: A concentric review. Food Res. Int. 2020, 134, 109269. [Google Scholar] [CrossRef]
- Senawong, T.; Khaopha, S.; Misuna, S.; Komaikul, J.; Senawong, G.; Wongphakham, P.; Yunchalard, S. Phenolic acid composition and anticancer activity against human cancer cell lines of the commercially available fermentation products of Houttuynia cordata. Sci. Asia 2014, 40, 420–427. [Google Scholar] [CrossRef]
- Satthakarn, S.; Chung, W.; Promsong, A.; Nittayananta, W. Houttuynia cordata modulates oral innate immune mediators: Potential role of herbal plant on oral health. Oral Dis. 2015, 21, 512–518. [Google Scholar] [CrossRef]
- Chiow, K.; Phoon, M.; Putti, T.; Tan, B.K.; Chow, V.T. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac. J. Trop. Med. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Řebíčková, K.; Bajer, T.; Šilha, D.; Houdková, M.; Ventura, K.; Bajerová, P. Chemical Composition and Determination of the Antibacterial Activity of Essential Oils in Liquid and Vapor Phases Extracted from Two Different Southeast Asian Herbs-Houttuynia cordata (Saururaceae) and Persicaria odorata (Polygonaceae). Molecules 2020, 25, 2432. [Google Scholar] [CrossRef]
- Sekita, Y.; Murakami, K.; Yumoto, H.; Mizuguchi, H.; Amoh, T.; Ogino, S.; Matsuo, T.; Miyake, Y.; Fukui, H.; Kashiwada, Y. Anti-bacterial and anti-inflammatory effects of ethanol extract from Houttuynia cordata poultice. Biosci. Biotechnol. Biochem. 2016, 80, 1205–1213. [Google Scholar] [CrossRef]
- Verma, R.S.; Joshi, N.; Padalia, R.C.; Singh, V.R.; Goswami, P.; Kumar, A.; Iqbal, H.; Verma, R.K.; Chanda, D.; Chauhan, A. Chemical Composition and Allelopathic, Antibacterial, Antifungal, and Antiacetylcholinesterase Activity of Fish-mint (Houttuynia cordata Thunb.) from India. Chem. Biodivers. 2017, 14, e1700189. [Google Scholar] [CrossRef]
- Pakyntein, C.L.; Syiem, D.; Thabah, D.; Sunn, S.E. Antioxidant, anti-inflammatory and anti-hyperglycemic activity of aqueous and methanolic extract of Houttuynia cordata: An in vitro and in vivo study. GSC Biol. Pharm. Sci. 2021, 16, 145–154. [Google Scholar] [CrossRef]
- Woranam, K.; Senawong, G.; Utaiwat, S.; Yunchalard, S.; Sattayasai, J.; Senawong, T. Anti-inflammatory activity of the dietary supplement Houttuynia cordata fermentation product in RAW264. 7 cells and Wistar rats. PLoS ONE 2020, 15, e0230645. [Google Scholar] [CrossRef]
- Pinto, T.; Vilela, A.; Cosme, F. Chemical and Sensory Characteristics of Fruit Juice and Fruit Fermented Beverages and Their Consumer Acceptance. Beverages 2022, 8, 33. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Elsadek, M.F.; Mohamed, A.S.; Taha, A.E.; Ahmed, B.M.; Saad, A.M. Effects of chemical and natural additives on cucumber juice’s quality, shelf life, and safety. Foods 2020, 9, 639. [Google Scholar] [CrossRef]
- Silva, M.M.; Lidon, F. Food preservatives—An overview on applications and side effects. Emir. J. Food Agric. 2016, 28, 366–373. [Google Scholar] [CrossRef]
- Raj, T.; Chandrasekhar, K.; Kumar, A.N.; Kim, S.H. Recent biotechnological trends in lactic acid bacterial fermentation for food processing industries. Syst. Microbiol. Biomanuf. 2022, 2, 14–40. [Google Scholar] [CrossRef]
- Aspri, M.; O’Connor, P.M.; Field, D.; Cotter, P.D.; Ross, P.; Hill, C.; Papademas, P. Application of bacteriocin-producing Enterococcus faecium isolated from donkey milk, in the bio-control of Listeria monocytogenes in fresh whey cheese. Int. Dairy J. 2017, 73, 1–9. [Google Scholar] [CrossRef]
- Nascimento, M.S.; Moreno, I.; Kuaye, A.Y. Applicability of bacteriocin-producing Lactobacillus plantarum, Enterococcus faecium and Lactococcus lactis ssp. lactis as adjunct starter in Minas Frescal cheesemaking. Int. J. Dairy Technol. 2008, 61, 352–357. [Google Scholar] [CrossRef]
- Prezzi, L.E.; Lee, S.H.; Nunes, V.M.; Corassin, C.H.; Pimentel, T.C.; Rocha, R.S.; Ramos, G.L.; Guimarães, J.T.; Balthazar, C.F.; Duarte, M.C.K. Effect of Lactobacillus rhamnosus on growth of Listeria monocytogenes and Staphylococcus aureus in a probiotic Minas Frescal cheese. Food Microbiol. 2020, 92, 103557. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, Y.; Wang, Y.; Huang, Y.; Li, P.; Li, P. Lactobacillus plantarum LPL-1, a bacteriocin producing strain, changed the bacterial community composition and improved the safety of low-salt fermented sausages. LWT 2020, 128, 109385. [Google Scholar] [CrossRef]
- Obadina, A.; Oyewole, O.; Sanni, L.O.; Tomlins, K.I. Bio-preservative activities of Lactobacillus plantarum strains in fermenting Casssava ‘fufu’. Afr. J. Biotechnol. 2006, 5, 620–623. [Google Scholar]
- Siroli, L.; Patrignani, F.; D’Alessandro, M.; Salvetti, E.; Torriani, S.; Lanciotti, R. Suitability of the Nisin Z-producer Lactococcus lactis subsp. lactis CBM 21 to be Used as an Adjunct Culture for Squacquerone Cheese Production. Animals 2020, 10, 782. [Google Scholar] [CrossRef]
- Hassan, H.; St-Gelais, D.; Gomaa, A.; Fliss, I. Impact of Nisin and Nisin-Producing Lactococcus lactis ssp. lactis on Clostridium tyrobutyricum and Bacterial Ecosystem of Cheese Matrices. Foods 2021, 10, 898. [Google Scholar] [CrossRef]
- Chang, J.Y.; Chang, H.C. Growth inhibition of foodborne pathogens by kimchi prepared with bacteriocin-producing starter culture. J. Food Sci. 2011, 76, M72–M78. [Google Scholar] [CrossRef]
- Zinoviadou, K.G.; Gougouli, M.; Biliaderis, C.G. Innovative Biobased Materials for Packaging Sustainability. In Innovation Strategies in the Food Industry: Tools for Implementation, 1st ed.; Galanakis, C.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 167–189. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipic, M.; Frutos, M.J.; Galtier, P.; Gundert-Remy, U. Safety of nisin (E 234) as a food additive in the light of new toxicological data and the proposed extension of use. EFSA J. 2017, 15, e05063. [Google Scholar]
- Khelissa, S.; Chihib, N.E.; Gharsallaoui, A. Conditions of nisin production by Lactococcus lactis subsp. lactis and its main uses as a food preservative. Arch. Microbiol. 2021, 203, 465–480. [Google Scholar] [CrossRef]
- Müller-Auffermann, K.; Grijalva, F.; Jacob, F.; Hutzler, M. Nisin and its usage in breweries: A review and discussion. J. Inst. Brew. 2015, 121, 309–319. [Google Scholar] [CrossRef]
- Özel, B.; Şimşek, Ö.; Akçelik, M.; Saris, P.E. Innovative approaches to nisin production. Appl. Microbiol. Biotechnol. 2018, 102, 6299–6307. [Google Scholar] [CrossRef]
- Davies, E.; Bevis, H.; Potter, R.; Harris, J.; Williams, G.; Delves-Broughton, J. Research note: The effect of pH on the stability of nisin solution during autoclaving. Lett. Appl. Microbiol. 1998, 27, 186–187. [Google Scholar] [CrossRef]
- Choeisoongnern, T.; Sirilun, S.; Waditee-Sirisattha, R.; Pintha, K.; Peerajan, S.; Chaiyasut, C. Potential Probiotic Enterococcus faecium OV3-6 and Its Bioactive Peptide as Alternative Bio-Preservation. Foods 2021, 10, 2264. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.S.; Anis, A.; Elbawab, R.H.; Mohammed, A.A.; Orabi, S.H.; Fathalla, S.I. Distinctive antagonistic role of new Enterococcus faecium ER-3M strain and its bacteriocin effect against Staphylococcus aureus Pneumonia. Rend. Lincei. Sci. Fis. Nat. 2018, 29, 675–690. [Google Scholar] [CrossRef]
- Valledor, S.J.D.; Bucheli, J.E.V.; Holzapfel, W.H.; Todorov, S.D. Exploring beneficial properties of the bacteriocinogenic Enterococcus faecium ST10Bz strain isolated from boza, a Bulgarian cereal-based beverage. Microorganisms 2020, 8, 1474. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasut, C.; Kusirisin, W.; Lailerd, N.; Lerttrakarnnon, P.; Suttajit, M.; Srichairatanakool, S. Effects of phenolic compounds of fermented Thai indigenous plants on oxidative stress in streptozotocin-induced diabetic rats. Evid. -Based Complement. Altern. Med. 2011, 2011, 749307. [Google Scholar] [CrossRef]
- Peerajan, S.; Chaiyasut, C.; Sirilun, S.; Chaiyasut, K.; Kesika, P.; Sivamaruthi, B.S. Enrichment of nutritional value of Phyllanthus emblica fruit juice using the probiotic bacterium, Lactobacillus paracasei HII01 mediated fermentation. Food Sci. Technol. 2016, 36, 116–123. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Shi, J.; Yi, H.; Zhang, Y.; Zhang, S.; Gao, W.; Du, M.; Han, X.; Yu, X. Assessment of the safety and applications of bacteriocinogenic Enterococcus faecium Y31 as an adjunct culture in North-eastern Chinese traditional fermentation paocai. Food Control 2015, 50, 637–644. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- American Public Health Association. Compendium of Methods for the Microbiological Examination of Foods, 2nd ed.; APHA: Washington, DC, USA, 1984. [Google Scholar]
- Wang, C.L.; Shi, D.J.; Gong, G.L. Microorganisms in Daqu: A starter culture of Chinese Maotai-flavor liquor. World J. Microbiol. Biotechnol. 2008, 24, 2183–2190. [Google Scholar] [CrossRef]
- Lancetti, R.; Sciarini, L.; Pérez, G.T.; Salvucci, E. Technological performance and selection of lactic acid bacteria isolated from Argentinian grains as starters for wheat sourdough. Curr. Microbiol. 2021, 78, 255–264. [Google Scholar] [CrossRef]
- Sun, W.; Liu, J.; Xu, H.; Li, W.; Zhang, J. L-Lactic acid fermentation by Enterococcus faecium: A new isolate from bovine rumen. Biotechnol. Lett. 2015, 37, 1379–1383. [Google Scholar] [CrossRef]
- Guan, X.; Xu, Q.; Zheng, Y.; Qian, L.; Lin, B. Screening and characterization of lactic acid bacterial strains that produce fermented milk and reduce cholesterol levels. Braz. J. Microbiol. 2017, 48, 730–739. [Google Scholar] [CrossRef]
- García-Núñez, I.M.; Santacruz, A.; Serna-Saldívar, S.O.; Hernandez, S.L.; Guerra, C.A. Assessment of Potential Probiotic and Synbiotic Properties of Lactic Acid Bacteria Grown In Vitro with Starch-Based Soluble Corn Fiber or Inulin. Foods 2022, 11, 4020. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Paula, A.; Casarotti, S.; Penna, A. Lactic acid bacteria antimicrobial compounds: Characteristics and applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- Lambert, R.; Stratford, M. Weak-acid preservatives: Modelling microbial inhibition and response. J. Appl. Microbiol. 1999, 86, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; Wachsman, M.; Tomé, E.; Dousset, X.; Destro, M.T.; Dicks, L.M.T.; de Melo Franco, B.D.G.; Vaz-Velho, M.; Drider, D. Characterisation of an antiviral pediocin-like bacteriocin produced by Enterococcus faecium. Food Microbiol. 2010, 27, 869–879. [Google Scholar] [CrossRef]
- Hadji-Sfaxi, I.; El-Ghaish, S.; Ahmadova, A.; Batdorj, B.; Le Blay-Laliberté, G.; Barbier, G.; Haertlé, T.; Chobert, J.M. Antimicrobial activity and safety of use of Enterococcus faecium PC4. 1 isolated from Mongol yogurt. Food Control 2011, 22, 2020–2027. [Google Scholar] [CrossRef]
- McAuliffe, O.; Ross, R.P.; Hill, C. Lantibiotics: Structure, biosynthesis and mode of action. FEMS Microbiol. Rev. 2001, 25, 285–308. [Google Scholar] [CrossRef]
- Wiedemann, I.; Böttiger, T.; Bonelli, R.R.; Wiese, A.; Hagge, S.O.; Gutsmann, T.; Seydel, U.; Deegan, L.; Hill, C.; Ross, P.; et al. The mode of action of the lantibiotic lacticin 3147—A complex mechanism involving specific interaction of two peptides and the cell wall precursor lipid II. Mol. Microbiol. 2006, 61, 285–296. [Google Scholar] [CrossRef]
- Castiglione, F.; Lazzarini, A.; Carrano, L.; Corti, E.; Ciciliato, I.; Gastaldo, L.; Candiani, P.; Losi, D.; Marinelli, F.; Selva, E.; et al. Determining the Structure and Mode of Action of Microbisporicin, a Potent Lantibiotic Active Against Multi resistant Pathogens. Chem. Biol. 2008, 15, 22–31. [Google Scholar] [CrossRef]
- Nishie, M.; Nagao, J.-I.; Sonomoto, K. Antibacterial Peptides “Bacteriocins”: An Overview of Their Diverse Characteristics and Applications. Biocontrol Sci. 2012, 17, 1–16. [Google Scholar] [CrossRef]
- Cavera, V.L.; Arthur, T.D.; Kashtanov, D.; Chikindas, M.L. Bacteriocins and their position in the next wave of conventional antibiotics. Int. J. Antimicrob. Agents 2015, 46, 494–501. [Google Scholar] [CrossRef]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, antimicrobial peptides from bacterial origin: Overview of their biology and their impact against multidrug-resistant bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef]
- Duquesne, S.; Destoumieux-Garzón, D.; Peduzzi, J.; Rebuffat, S. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat. Prod. Rep. 2007, 24, 708–734. [Google Scholar] [CrossRef] [PubMed]
- Mathavan, I.; Beis, K. The role of bacterial membrane proteins in the internalization of microcin MccJ25 and MccB17. Biochem. Soc. Trans. 2012, 40, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Valledor, S.J.D.; Dioso, C.M.; Bucheli, J.E.V.; Park, Y.J.; Suh, D.H.; Jung, E.S.; Kim, B.; Holzapfel, W.H.; Todorov, S.D. Characterization and safety evaluation of two beneficial, enterocin-producing Enterococcus faecium strains isolated from kimchi, a Korean fermented cabbage. Food Microbiol. 2022, 102, 103886. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Ghosh, R.; Mandal, N.C. Production optimization of broad spectrum bacteriocin of three strains of Lactococcus lactis isolated from homemade buttermilk. Ann. Agrar. Sci. 2018, 16, 286–296. [Google Scholar] [CrossRef]
- Sansinenea, E.; Ortiz, A. Secondary metabolites of soil Bacillus spp. Biotechnol. Lett. 2011, 33, 1523–1538. [Google Scholar] [CrossRef]
- Zangeneh, M.; Khorrami, S.; Khaleghi, M. Bacteriostatic activity and partial characterization of the bacteriocin produced by L. plantarum sp. isolated from traditional sourdough. Food Sci. Nutr. 2020, 8, 6023–6030. [Google Scholar] [CrossRef]
- Favaro, L.; Basaglia, M.; Casella, S.; Hue, I.; Dousset, X.; de Melo Franco, B.D.G.; Todorov, S.D. Bacteriocinogenic potential and safety evaluation of non-starter Enterococcus faecium strains isolated from homemade white brine cheese. Food Microbiol. 2014, 38, 228–239. [Google Scholar] [CrossRef]
- Nešuta, O.; Buděšínský, M.; Hadravová, R.; Monincová, L.; Humpoličková, J.; Čeřovský, V. How proteases from Enterococcus faecalis contribute to its resistance to short α-helical antimicrobial peptides. Pathog. Dis. 2017, 75, ftx091. [Google Scholar] [CrossRef]
- Herranz, C.; Martinez, J.; Rodriguez, J.; Hernandez, P.; Cintas, L. Optimization of enterocin P production by batch fermentation of Enterococcus faecium P13 at constant pH. Appl. Microbiol. Biotechnol. 2001, 56, 378–383. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Calderon, M.; Loiseau, G.; Guyot, J.P. Nutritional requirements and simplified cultivation medium to study growth and energetics of a sourdough lactic acid bacterium Lactobacillus fermentum Ogi E1 during heterolactic fermentation of starch. J. Appl. Microbiol. 2001, 90, 508–516. [Google Scholar] [CrossRef]
- Rai, M.; Pandit, R.; Gaikwad, S.; Kövics, G. Antimicrobial peptides as natural bio-preservative to enhance the shelf-life of food. J. Food Sci. Technol. 2016, 53, 3381–3394. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic acid bacteria as antibacterial agents to extend the shelf life of fresh and minimally processed fruits and vegetables: Quality and safety aspects. Microorganisms 2020, 8, 952. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic acid bacteria as antimicrobial agents: Food safety and microbial food spoilage prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef]
- Vesković-Moračanin, S.M.; Đukić, D.A.; Memiši, N.R. Bacteriocins produced by lactic acid bacteria: A review. Acta Period. Technol. 2014, 45, 271–283. [Google Scholar] [CrossRef]
- Woraprayote, W.; Malila, Y.; Sorapukdee, S.; Swetwiwathana, A.; Benjakul, S.; Visessanguan, W. Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci. 2016, 120, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Serrazanetti, D.I.; Gottardi, D.; Montanari, C.; Gianotti, A. Dynamic stresses of lactic acid bacteria associated to fermentation processes. In Lactic Acid Bacteria-R & D for Food, Health and Livestock Purposes, 1st ed.; Kongo, J.M., Ed.; IntechOpen: London, UK, 2013; pp. 539–570. [Google Scholar]
- Syrokou, M.K.; Themeli, C.; Paramithiotis, S.; Mataragas, M.; Bosnea, L.; Argyri, A.A.; Chorianopoulos, N.G.; Skandamis, P.N.; Drosinos, E.H. Microbial ecology of Greek wheat sourdoughs, identified by a culture-dependent and a culture-independent approach. Foods 2020, 9, 1603. [Google Scholar] [CrossRef]
- Sadiq, M.B. Lactic acid bacteria as potential probiotics. In Probiotics, Prebiotics and Synbiotics: Technological Advancements towards Safety and Industrial Applications, 1st ed.; Panesar, P.S., Anal, A.K., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 57–72. [Google Scholar]
- Waheed, S.; Rasool, M.H.; Aslam, B.; Muzammil, S.; Waseem, M.; Shahid, M.; Saqib, M.; Hayat, S.; Naeem, M.; Taj, Z.; et al. Antagonistic Potential of Dairy Origin Enterococcus faecium Against Multidrug-Resistant Foodborne Pathogens. Rom. Biotechnol. Lett. 2021, 26, 2406–2415. [Google Scholar] [CrossRef]
- Anyogu, A.; Awamaria, B.; Sutherland, J.; Ouoba, L. Molecular characterisation and antimicrobial activity of bacteria associated with submerged lactic acid cassava fermentation. Food Control 2014, 39, 119–127. [Google Scholar] [CrossRef]
- Héchard, Y.; Sahl, H.G. Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie 2002, 84, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins- A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, T.J. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef] [PubMed]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of specialized biomolecules against gram-positive and gram-negative bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef]
- Palombo, E.A.; Semple, S.J. Antibacterial activity of traditional Australian medicinal plants. J. Ethnopharmacol. 2001, 77, 151–157. [Google Scholar] [CrossRef]
- Nostro, A.; Germano, M.; D’angelo, V.; Marino, A.; Cannatelli, M. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett. Appl. Microbiol. 2000, 30, 379–384. [Google Scholar] [CrossRef]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef]
- Li, Q.; Montalban-Lopez, M.; Kuipers, O.P. Increasing the Antimicrobial Activity of Nisin-Based Lantibiotics against Gram-Negative Pathogens. Appl. Environ. Microbiol. 2018, 84, e00052-18. [Google Scholar] [CrossRef]
- TISI. Thai Community Products Standard (TCPS) No. 481/2004. Available online: https://www.tisi.go.th/home/en (accessed on 7 July 2022).
- Jiang, F.G.; Cheng, H.J.; Liu, D.; Wei, C.; An, W.J.; Wang, Y.F.; Sun, H.T.; Song, E.L. Treatment of Whole-Plant Corn Silage With Lactic Acid Bacteria and Organic Acid Enhances Quality by Elevating Acid Content, Reducing pH, and Inhibiting Undesirable Microorganisms. Front. Microbiol. 2020, 11, 593088. [Google Scholar] [CrossRef]
- Choi, Y.J.; Yong, S.; Lee, M.J.; Park, S.J.; Yun, Y.R.; Park, S.H.; Lee, M.A. Changes in volatile and non-volatile compounds of model kimchi through fermentation by lactic acid bacteria. LWT 2019, 105, 118–126. [Google Scholar] [CrossRef]
- Mariam, S.H.; Zegeye, N.; Tariku, T.; Andargie, E.; Endalafer, N.; Aseffa, A. Potential of cell-free supernatants from cultures of selected lactic acid bacteria and yeast obtained from local fermented foods as inhibitors of Listeria monocytogenes, Salmonella spp. and Staphylococcus aureus. BMC Res. Notes 2014, 7, 606. [Google Scholar] [CrossRef] [PubMed]
- Ballén, V.; Ratia, C.; Cepas, V.; Soto, S.M. Enterococcus faecalis inhibits Klebsiella pneumoniae growth in polymicrobial biofilms in a glucose-enriched medium. Biofouling 2020, 36, 846–861. [Google Scholar] [CrossRef] [PubMed]
- Hasan, F.B.; Reza, M.; Al Masud, H.A.; Uddin, M.K.; Uddin, M.S. Preliminary characterization and inhibitory activity of bacteriocin like substances from Lactobacillus casei against multi-drug resistant bacteria. Bangladesh J. Microbiol. 2019, 36, 1–6. [Google Scholar] [CrossRef]
- Nongkhlaw, F.M.W.; Joshi, S. Distribution pattern analysis of epiphytic bacteria on ethnomedicinal plant surfaces: A micrographical and molecular approach. J. Microsc. Ultrastruct. 2014, 2, 34–40. [Google Scholar]
- Nout, M.; Rombouts, F. Fermentative preservation of plant foods. J. Appl. Microbiol. 1992, 73, 136S–147S. [Google Scholar] [CrossRef]
- War Nongkhlaw, F.M.; Joshi, S. Epiphytic and endophytic bacteria that promote growth of ethnomedicinal plants in the subtropical forests of Meghalaya, India. Rev. Biol. Trop. 2014, 62, 1295–1308. [Google Scholar] [CrossRef]





| Treatment Numbers | Factors | |
|---|---|---|
| Bio-Preservatives | Pathogens * | |
| 1 | No preservative | - |
| 2 | No preservative | + |
| 3 | E. faecium OV3-6 | - |
| 4 | E. faecium OV3-6 | + |
| 5 | CFS of E. faecium OV3-6 | - |
| 6 | CFS of E. faecium OV3-6 | + |
| 7 | Nisin | - |
| 8 | Nisin | + |
| Treatments | E. coli (Log CFU/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 Day | 0.5 Day | 1 Day | 2 Days | 3 Days | 5 Days | 7 Days | 10 Days | 15 Days | |
| 2 | 5.17 ± 0.35 a | 7.69 ± 0.08 a | 7.43 ± 0.01 a | 7.30 ± 0.15 b | 6.91 ± 0.13 b | 6.03 ± 0.40 a | 4.85 ± 0.17 b | ND | ND |
| 4 | 5.57 ± 0.06 a | ND | ND | ND | ND | ND | ND | ND | ND |
| 6 | 5.12 ± 0.42 a | ND | ND | ND | ND | ND | ND | ND | ND |
| 8 | 5.07 ± 0.10 a | 8.02 ± 0.14 b | 7.81 ± 0.18 b | 6.37 ± 0.39 a | 6.03 ± 0.02 a | 5.72 ± 0.01 a | 4.31 ± 0.39 a | ND | ND |
| Total Salmonella (Log CFU/mL) | |||||||||
| 2 | 5.18 ± 0.15 a | 8.47 ± 0.31 b | 8.22 ± 0.01 a | 6.95 ± 0.10 a | 6.59 ± 0.15 a | 6.38 ± 0.29 a | 6.30 ± 0.15 b | 5.95 ± 0.05 a | 2.97 ± 0.07 a |
| 4 | 5.57 ± 0.06 a, b | ND | ND | ND | ND | ND | ND | ND | ND |
| 6 | 5.10 ± 0.44 a | ND | ND | ND | ND | ND | ND | ND | ND |
| 8 | 5.85 ± 0.10 b | 8.12 ± 0.14 a | 8.23 ± 0.18 a | 6.96 ± 0.04 a | 6.72 ± 0.24 a | 6.93 ± 0.01 b | 5.55 ± 0.10 a | 6.00 ± 0.03 a | 3.04 ± 0.04 a |
| S. aureus (Log CFU/mL) | |||||||||
| 2 | 5.47 ± 0.26 b | ND | ND | ND | ND | ND | ND | ND | ND |
| 4 | 5.06 ± 0.09 a | ND | ND | ND | ND | ND | ND | ND | ND |
| 6 | 5.55 ± 0.07 b | ND | ND | ND | ND | ND | ND | ND | ND |
| 8 | 5.32 ± 0.22 a, b | ND | ND | ND | ND | ND | ND | ND | ND |
| B. cereus (Log CFU/mL) | |||||||||
| 2 | 5.30 ± 0.06 a | ND | ND | ND | ND | ND | ND | ND | ND |
| 4 | 5.17 ± 0.20 a | ND | ND | ND | ND | ND | ND | ND | ND |
| 6 | 5.09 ± 0.27 a | ND | ND | ND | ND | ND | ND | ND | ND |
| 8 | 5.25 ± 0.21 a | ND | ND | ND | ND | ND | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choeisoongnern, T.; Chaiyasut, C.; Sivamaruthi, B.S.; Makhamrueang, N.; Peerajan, S.; Sirilun, S.; Sittiprapaporn, P. Bacteriocin-Producing Enterococcus faecium OV3-6 as a Bio-Preservative Agent to Produce Fermented Houttuynia cordata Thunb. Beverages: A Preliminary Study. Foods 2023, 12, 3520. https://doi.org/10.3390/foods12193520
Choeisoongnern T, Chaiyasut C, Sivamaruthi BS, Makhamrueang N, Peerajan S, Sirilun S, Sittiprapaporn P. Bacteriocin-Producing Enterococcus faecium OV3-6 as a Bio-Preservative Agent to Produce Fermented Houttuynia cordata Thunb. Beverages: A Preliminary Study. Foods. 2023; 12(19):3520. https://doi.org/10.3390/foods12193520
Chicago/Turabian StyleChoeisoongnern, Thiwanya, Chaiyavat Chaiyasut, Bhagavathi Sundaram Sivamaruthi, Netnapa Makhamrueang, Sartjin Peerajan, Sasithorn Sirilun, and Phakkharawat Sittiprapaporn. 2023. "Bacteriocin-Producing Enterococcus faecium OV3-6 as a Bio-Preservative Agent to Produce Fermented Houttuynia cordata Thunb. Beverages: A Preliminary Study" Foods 12, no. 19: 3520. https://doi.org/10.3390/foods12193520
APA StyleChoeisoongnern, T., Chaiyasut, C., Sivamaruthi, B. S., Makhamrueang, N., Peerajan, S., Sirilun, S., & Sittiprapaporn, P. (2023). Bacteriocin-Producing Enterococcus faecium OV3-6 as a Bio-Preservative Agent to Produce Fermented Houttuynia cordata Thunb. Beverages: A Preliminary Study. Foods, 12(19), 3520. https://doi.org/10.3390/foods12193520








