Yeast-Hydrolysate-Derived 1-Methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic Acid Inhibits Fat Accumulation during Adipocyte Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Yeast Hydrolysate
2.2. Cell Culture
2.3. WST-1 Assay
2.4. Oil Red O (ORO) Staining
2.5. Real-Time PCR
2.6. Western Blot Analysis
2.7. Identification of Active Compounds
2.8. Statistical Analysis
3. Results
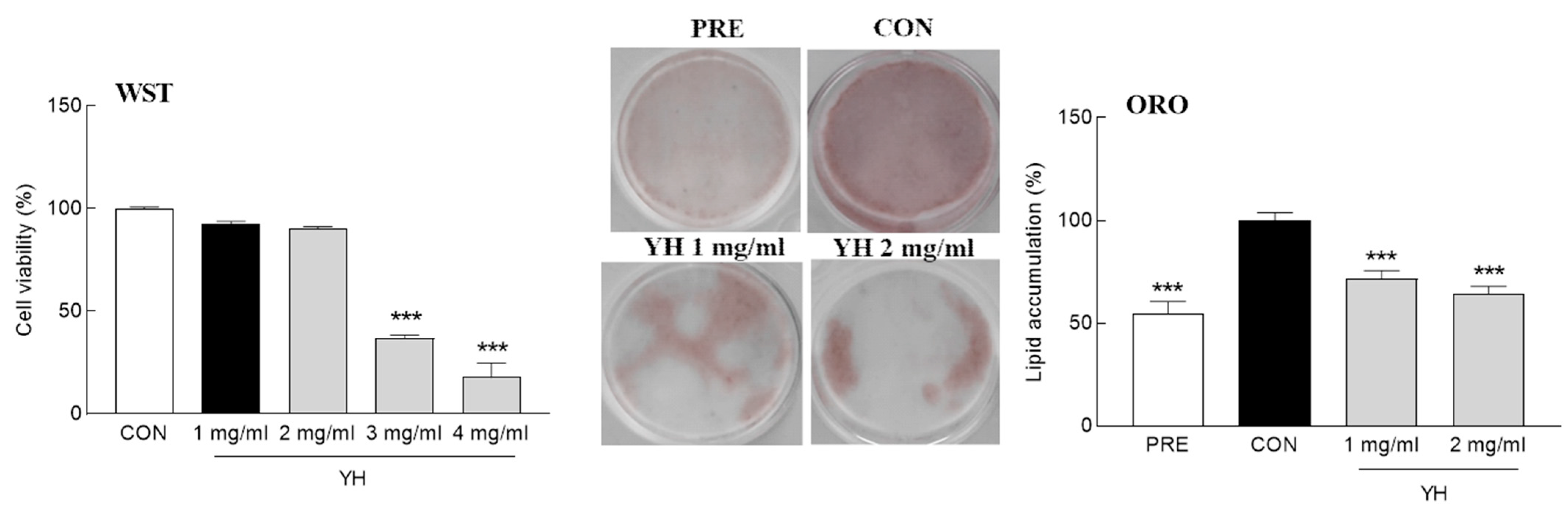
3.1. Effect of YH on Fat Storage
3.2. Effect of YH on the mRNA Expression of Adipogenic Factors
3.3. Effect of YH on the Expression of Fatty Acid/Cholesterol Synthetic Genes
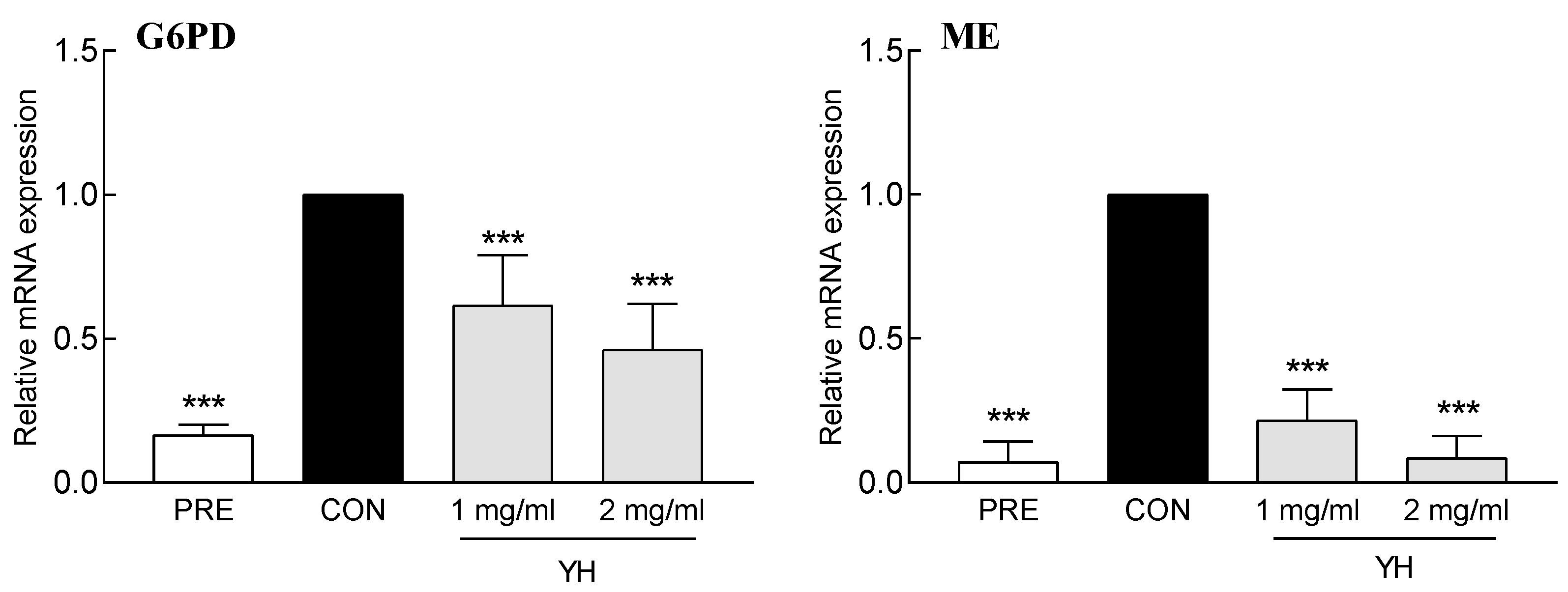
3.4. Effect of YH on the Expression of Lipogenic Cofactors
3.5. Isolation and Identification of the Active Compound in YH
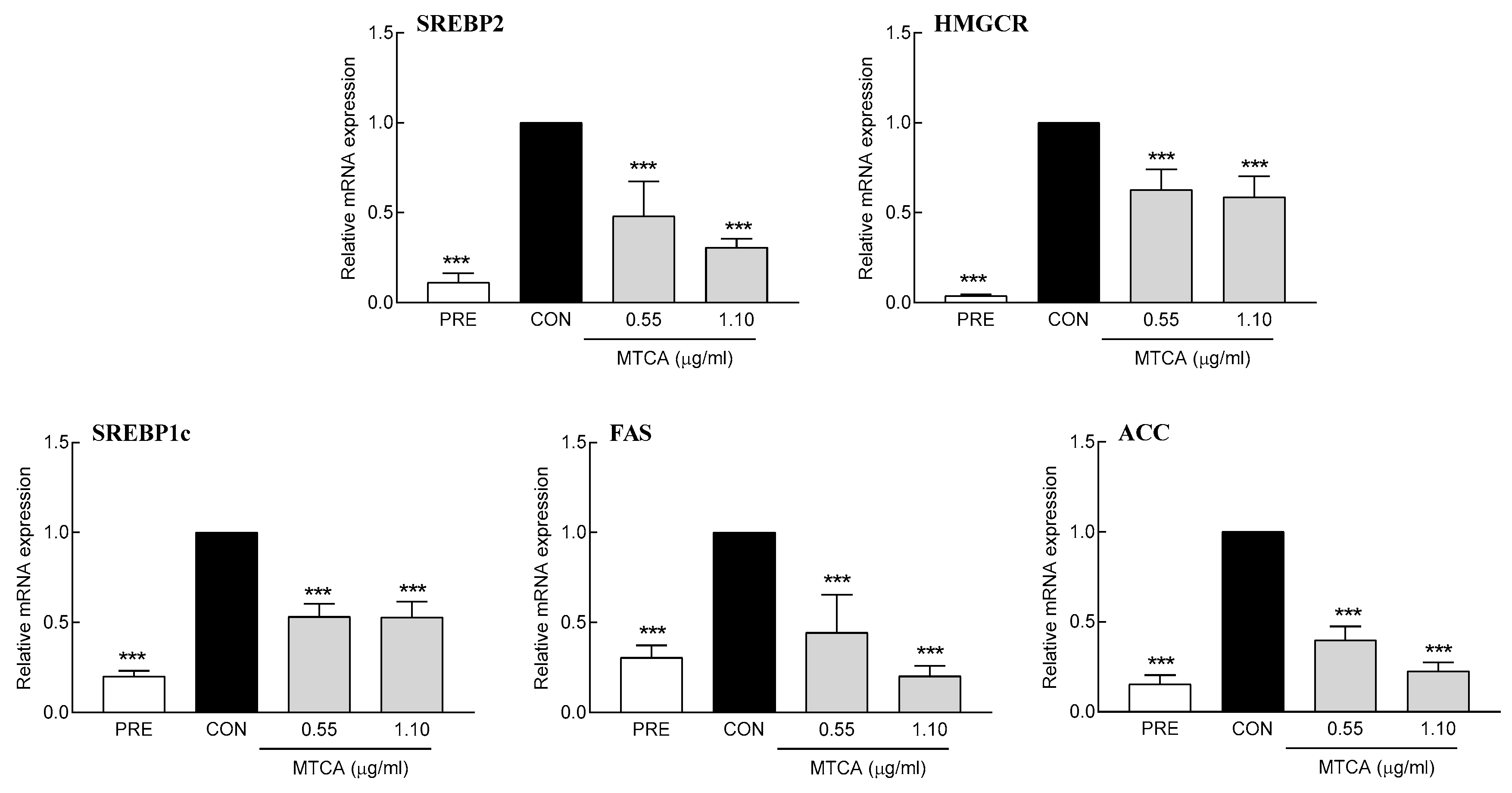
3.6. Effect of MTCA on the Expression of Lipid Synthetic Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Basu, T.; Selman, A.; Reddy, A.P.; Reddy, P.H. Current status of obesity: Protective role of catechins. Antioxidants 2023, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H.; Collier, D.; Kassotis, C.; Roepke, T.A.; Kim, M.J.; Blanc, E.; Barouki, R.; Bansal, A.; Cave, M.C.; Chatterjee, S. Obesity I: Overview and molecular and biochemical mechanisms. Biochem. Pharmacol. 2022, 199, 115012. [Google Scholar] [CrossRef]
- Rahman, H.A.; Saari, N.; Abas, F.; Ismail, A.; Mumtaz, M.W.; Hamid, A.A. Anti-obesity and antioxidant activities of selected medicinal plants and phytochemical profiling of bioactive compounds. Int. J. Food Prop. 2017, 20, 2616–2629. [Google Scholar] [CrossRef]
- Rahman, H.A.; Sahib, N.G.; Saari, N.; Abas, F.; Ismail, A.; Mumtaz, M.W.; Hamid, A.A. Anti-obesity effect of ethanolic extract from Cosmos caudatus Kunth leaf in lean rats fed a high fat diet. BMC Complement. Altern. Med. 2017, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Haththotuwa, R.N.; Wijeyaratne, C.N.; Senarath, U. Chapter 1—Worldwide Epidemic of Obesity. Obesity and Obstetrics, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–8. ISBN 9780128179215. [Google Scholar]
- Ohman, M.K.; Luo, W.; Abdallah, W.; Guo, C.A.; Russo, H.M.; Eitzman, D.T. Perivascular visceral adipose tissue transplantation triggers local atherothrombosis in ApoE-/- mice. Obesity 2010, 18, S72. [Google Scholar] [CrossRef]
- Jannat Ali Pour, N.; Zabihi-Mahmoudabadi, H.; Ebrahimi, R.; Yekaninejad, M.S.; Hashemnia, S.M.R.; Meshkani, R.; Emamgholipour, S. Principal component analysis of adipose tissue gene expression of lipogenic and adipogenic factors in obesity. BMC Endocrinol. Disord. 2023, 23, 94. [Google Scholar] [CrossRef] [PubMed]
- Suryaningtyas, I.T.; Je, J.-Y. Bioactive peptides from food proteins as potential anti-obesity agents: Mechanisms of action and future perspectives. Trends Food Sci. Technol. 2023, 138, 141–152. [Google Scholar] [CrossRef]
- DeBose-Boyd, R.A.; Ye, J. SREBPs in lipid metabolism, insulin signaling, and beyond. Trends Biochem. Sci. 2018, 43, 358–368. [Google Scholar] [CrossRef]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nature Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, H.; Zhang, Y.; Chen, L.; Tian, C.; Huang, B.; Chen, Y.; Ma, L. Associations of dietary antioxidant micronutrients with the prevalence of obesity in adults. Front. Nutr. 2023, 10, 1098761. [Google Scholar] [CrossRef]
- Wen, Q.; Zhang, L.; Zhao, F.; Chen, Y.; Su, Y.; Zhang, X.; Chen, P.; Zheng, T. Production technology and functionality of bioactive peptides. Curr. Pharm. Des. 2023, 29, 652–674. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Chen, P.; Chen, X. Bioactive peptides derived from fermented foods: Preparation and biological activities. J. Funct. Foods 2023, 101, 105422. [Google Scholar] [CrossRef]
- Mirzaei, M.; Shavandi, A.; Mirdamadi, S.; Soleymanzadeh, N.; Motahari, P.; Mirdamadi, N.; Moser, M.; Subra, G.; Alimoradi, H.; Goriely, S. Bioactive peptides from yeast: A comparative review on production methods, bioactivity, structure-function relationship, and stability. Trends Food Sci. Technol. 2021, 118, 297–315. [Google Scholar] [CrossRef]
- Xie, Z.; Cao, N.; Wang, C. A review on β-carboline alkaloids and their distribution in foodstuffs: A class of potential functional components or not? Food Chem. 2021, 348, 129067. [Google Scholar] [CrossRef] [PubMed]
- Piechowska, P.; Zawirska-Wojtasiak, R.; Mildner-Szkudlarz, S. Bioactive β-carbolines in food: A Review. Nutrients 2019, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, S.; Zhu, S.; Luo, J.; Zhang, Y.; Weng, Q. Synthesis and fungicidal activity of β-Carboline alkaloids and their derivatives. Molecules 2015, 20, 13941–13957. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; Pena, A.; Mateo, H.; Herraiz, M.; Salgado, A. Formation, characterization, and occurrence of beta-carboline alkaloids derived from alpha-dicarbonyl compounds and L-tryptophan. J. Agric. Food Chem. 2022, 70, 9143–9153. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.C.; Nam, K.H.; Yi, S.A.; Jo, M.S.; Lee, K.H.; Lee, Y.H.; Lee, J.; Kim, K.H. Anti-adipogenic effect of beta-carboline alkaloids from garlic (Allium sativum). Foods 2019, 8, 673. [Google Scholar] [CrossRef]
- Herraiz, T.; Peña, A.; Salgado, A. Identification, formation, and occurrence of perlolyrine: A β-carboline alkaloid with a furan moiety in foods. J. Agric. Food Chem. 2023, 71, 13451–13461. [Google Scholar] [CrossRef]
- Ribeiro, P.V.M.; Tavares, J.F.; Costa, M.A.C.; Mattar, J.B.; Alfenas, R.C.G. Effect of reducing dietary advanced glycation end products on obesity-associated complications: A systematic review. Nutr. Rev. 2019, 77, 725–734. [Google Scholar] [CrossRef]
- Turki, J.A.; Alameri, A.A.; Iqbal Doewes, R.; El-Sehrawy, A.A.; Ahmad, I.; Ramaiah, P.; Kadhim, M.M.; Kzar, H.H. Sivaraman R, Romero-Parra RM, Ansari MJ, Fakri Mustafa Y. Circulating and dietary advanced glycation end products and obesity in an adult population: A paradox of their detrimental effects in obesity. Front. Endocrinol. 2022, 13, 966590. [Google Scholar] [CrossRef] [PubMed]
- Nya, E.; Etukudo, O. Industrial potentials of Saccharomyces cerevisiae. Br. J. Multidiscip. Adv. Stud. 2023, 4, 23–46. [Google Scholar] [CrossRef]
- Jung, E.Y.; Cho, M.K.; Hong, Y.H.; Kim, J.H.; Park, Y.; Chang, U.J.; Suh, H.J. Yeast hydrolysate can reduce body weight and abdominal fat accumulation in obese adults. Nutrition 2014, 30, 25–32. [Google Scholar] [CrossRef]
- Jung, E.Y.; Hong, Y.H.; Kim, J.H.; Park, Y.; Bae, S.H.; Chang, U.J.; Suh, H.J. Effects of yeast hydrolysate on hepatic lipid metabolism in high-fat-diet-induced obese mice: Yeast hydrolysate suppresses body fat accumulation by attenuating fatty acid synthesis. Ann. Nutr. Metabol. 2012, 61, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.Y.; Kim, S.Y.; Bae, S.H.; Chang, U.J.; Choi, J.W.; Suh, H.J. Weight reduction effects of yeast hydrolysate below 10 kda on obese young women. J. Food Biochem. 2011, 35, 337–350. [Google Scholar] [CrossRef]
- Kim, H.; Lim, J.J.; Shin, H.Y.; Suh, H.J.; Choi, H.S. Lactobacillus plantarum K8-based paraprobiotics suppress lipid accumulation during adipogenesis by the regulation of JAK/STAT and AMPK signaling pathways. J. Funct. Foods 2021, 87, 104824. [Google Scholar] [CrossRef]
- Harshitha, R.; Arunraj, D.R. Real-time quantitative PCR: A tool for absolute and relative quantification. Biochem. Mol. Biol. Edu. 2021, 49, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, X.; Liu, P. Lipid droplet proteins and metabolic diseases. Biochim. Biophys. Acta-Mol. Basis Dis. 2018, 1864, 1968–1983. [Google Scholar] [CrossRef]
- Welte, M.A.; Gould, A.P. Lipid droplet functions beyond energy storage. Biochim. Biophys. Acta-Mol. Cell Biol. Lpids 2017, 1862, 1260–1272. [Google Scholar] [CrossRef]
- Zadoorian, A.; Du, X.; Yang, H. Lipid droplet biogenesis and functions in health and disease. Nature Rev. Endocrinol. 2023, 19, 443–459. [Google Scholar] [CrossRef]
- Heendeniya, S.N.; Keerthirathna, L.R.; Manawadu, C.K.; Dissanayake, I.H.; Ali, R.; Mashhour, A.; Alzahrani, H.; Godakumbura, P.; Boudjelal, M.; Peiris, D.C. Therapeutic efficacy of nyctanthes arbor-tristis flowers to inhibit proliferation of acute and chronic primary human leukemia cells, with adipocyte differentiation and in silico analysis of interactions between survivin protein and selected secondary metabolites. Biomolecules 2020, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Fos-Domenech, J.; Serra, D.; Herrero, L.; Sánchez-Infantes, D. White adipose tissue dysfunction in obesity and aging. Biochem. Pharmacol. 2021, 192, 114723. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and obesity: Role and clinical implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Audano, M.; Pedretti, S.; Caruso, D.; Crestani, M.; De Fabiani, E.; Mitro, N. Regulatory mechanisms of the early phase of white adipocyte differentiation: An overview. Cell. Mol. Life Sci. 2022, 79, 139. [Google Scholar] [CrossRef] [PubMed]
- Kou, H.; Deng, J.; Gao, D.; Song, A.; Han, Z.; Wei, J.; Jin, X.; Ma, R.; Zheng, Q. Relationship among adiponectin, insulin resistance and atherosclerosis in non-diabetic hypertensive patients and healthy adults. Clin. Exp. Hypertens. 2018, 40, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.S.; Siersbaek, R.; Boergesen, M.; Nielsen, R.; Mandrup, S. Peroxisome proliferator-activated receptor gamma and C/EBPalpha synergistically activate key metabolic adipocyte genes by assisted loading. Mol. Cell Biol. 2014, 34, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peng, W.Q.; Guo, Y.Y.; Liu, Y.; Tang, Q.Q.; Guo, L. Krüppel-like factor 10 (KLF10) is transactivated by the transcription factor C/EBPβ and involved in early 3T3-L1 preadipocyte differentiation. J. Biol. Chem. 2018, 293, 14012–14021. [Google Scholar] [CrossRef]
- Madison, B.B. Srebp2: A master regulator of sterol and fatty acid synthesis. J. Lipid Res. 2016, 57, 333–335. [Google Scholar] [CrossRef]
- Jemai, R.; Drira, R.; Makni, M.; Fetoui, H.; Sakamoto, K. Colocynth (Citrullus colocynthis) seed extracts attenuate adipogenesis by down-regulating PPARγ/ SREBP-1c and C/EBPα in 3T3-L1 cells. Food Biosci. 2020, 33, 100491. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Lee, K.-H. Expressional Evaluation of C/EBP Family, SREBP1, and Steroid Hormone Receptors in the Epididiymal Fat of Postnatally Developing Mouse. Develop. Reprod. 2022, 26, 49–58. [Google Scholar] [CrossRef]
- Kobayashi, M.; Fujii, N.; Narita, T.; Higami, Y. SREBP-1c-Dependent Metabolic Remodeling of White Adipose Tissue by Caloric Restriction. Int. J. Mol. Sci. 2018, 19, 3335. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Goldstein, J.L.; Brown, M.S. Insulin induction of SREBP-1c in rodent liver requires LXRα-C/EBPβ complex. Proc. Natl. Acad. Sci. USA 2016, 113, 8182–8187. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Liu, W.D.; Pellicane, C.; Sahyoun, C.; Joseph, B.K.; Gallo-Ebert, C.; Donigan, M.; Pandya, D.; Giordano, C.; Bata, A.; et al. Identification of miR-185 as a regulator of de novo cholesterol biosynthesis and low density lipoprotein uptake. J. Lipid Res. 2014, 55, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Sarna, L.K.; Hwang, S.Y.; Zhu, Q.J.; Wang, P.Q.; Siow, Y.L.; Karmin, O. Activation of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase during high fat diet feeding. Biochim. Biophys. Acta-Mol. Basis Dis. 2013, 1832, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Radhakrishnan, A.; Goldstein, J.L. Retrospective on cholesterol homeostasis: The central role of scap. Ann. Rev. Biochem. 2018, 87, 783–807. [Google Scholar] [CrossRef] [PubMed]
- Gaspar-Pintiliescu, A.; Oancea, A.; Cotarlet, M.; Vasile, A.M.; Bahrim, G.E.; Shaposhnikov, S.; Craciunescu, O. Angiotensin-converting enzyme inhibition, antioxidant activity and cytotoxicity of bioactive peptides from fermented bovine colostrum. Int. J. Dairy Technol. 2020, 73, 108–116. [Google Scholar] [CrossRef]
- Rai, A.K.; Kumari, R.; Sanjukta, S.; Sahoo, D. Production of bioactive protein hydrolysate using the yeasts isolated from soft chhurpi. Bioresour. Technol. 2016, 219, 239–245. [Google Scholar] [CrossRef]
- Herraiz, T. β-Carboline Alkaloids in Soy Sauce and Inhibition of Monoamine Oxidase (MAO). Molecules 2023, 28, 2723. [Google Scholar] [CrossRef]
- Backhaus, K.; Ludwig-Radtke, L.; Xie, X.; Li, S.M. Manipulation of the Precursor Supply in Yeast Significantly Enhances the Accumulation of Prenylated β-Carbolines. ACS Syn. Biol. 2017, 6, 1056–1064. [Google Scholar] [CrossRef]




| Gene | Primer | Primer Sequence (5’-3’) |
|---|---|---|
| GAPDH | F | CATCACTGCCACCCAGAAGACTG |
| R | ATGCCAGTGAGCTTCCCGTTCAG | |
| FAS | F | TGCTTGCTGGCTCACAGTTA |
| R | ATCAGTTTCACGAACCCGCC | |
| G6PD | F | GACCAAGAAGCCTGGCATGTTC |
| R | AGACATCCAGGATGAGGCGTTC | |
| SREBP2 | F | TGTCGCACTGCAAAGGGAG |
| R | GCTCCCTAGTCTGTACCCGA | |
| HMGCR | F | GCTCGTCTACAGAAACTCCACG |
| R | GCTTCAGCAGTGCTTTCTCCGT | |
| SREBP1-C | F | GGGGAACTTTTCCTTAACGTGG |
| R | TCCAGTTCGCACATCTCGG | |
| ME | F | AGAGCAGTGCTACAAGGTGACC |
| R | CCAAGAGCAACTCCAGGGAACA | |
| c/EBPβ | F | CAAGATGCGCAACCTGGAGA |
| R | GACAGCTGCTCCACCTTCTT | |
| KLF2 | F | CTCTCCATGGGATTGGACGG |
| R | TCCGGGTAGTAGAAGGCAGG | |
| PPARΥ | F | TTCGATCCGTAGAAGCCGTG |
| R | TGGACACCATACTTGAGCAGA | |
| FABP4 | F | TCACCATCCGGTCAGAGAGTA |
| R | TGTCGTCTGCGGTGATTTCAT | |
| c/EBPα | F | GGGAGAACTCTAACTCCCCCA |
| R | GGAGGTGACTGCTCATCGG | |
| ACC | F | TGGACCTAGAAGAGAAGGAGGG |
| R | GCCAGAGATCCCCAAATCAGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.; Lee, S.; Jung, E.-J.; Jung, E.Y.; Chang, U.-J.; Jin, C.-M.; Suh, H.J.; Choi, H.-S. Yeast-Hydrolysate-Derived 1-Methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic Acid Inhibits Fat Accumulation during Adipocyte Differentiation. Foods 2023, 12, 3466. https://doi.org/10.3390/foods12183466
Kim N, Lee S, Jung E-J, Jung EY, Chang U-J, Jin C-M, Suh HJ, Choi H-S. Yeast-Hydrolysate-Derived 1-Methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic Acid Inhibits Fat Accumulation during Adipocyte Differentiation. Foods. 2023; 12(18):3466. https://doi.org/10.3390/foods12183466
Chicago/Turabian StyleKim, Nari, Sekyung Lee, Eun-Jin Jung, Eun Young Jung, Un-Jae Chang, Cheng-Min Jin, Hyung Joo Suh, and Hyeon-Son Choi. 2023. "Yeast-Hydrolysate-Derived 1-Methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic Acid Inhibits Fat Accumulation during Adipocyte Differentiation" Foods 12, no. 18: 3466. https://doi.org/10.3390/foods12183466
APA StyleKim, N., Lee, S., Jung, E.-J., Jung, E. Y., Chang, U.-J., Jin, C.-M., Suh, H. J., & Choi, H.-S. (2023). Yeast-Hydrolysate-Derived 1-Methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic Acid Inhibits Fat Accumulation during Adipocyte Differentiation. Foods, 12(18), 3466. https://doi.org/10.3390/foods12183466






