Synergistic Effect of Combined Treatment with Allicin and Antioxidant of Bamboo Leaves and Preservation of Bullfrogs (Lithobates catesbeiana) during Refrigeration Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Bullfrog Samples and Sample Treatment
2.3. Microbiological Analysis
2.4. Physicochemical Analysis
2.5. Protein Characteristic
2.5.1. Fluorescence Spectroscopy Analysis
2.5.2. Myofibril Fragmentation Index (MFI)
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
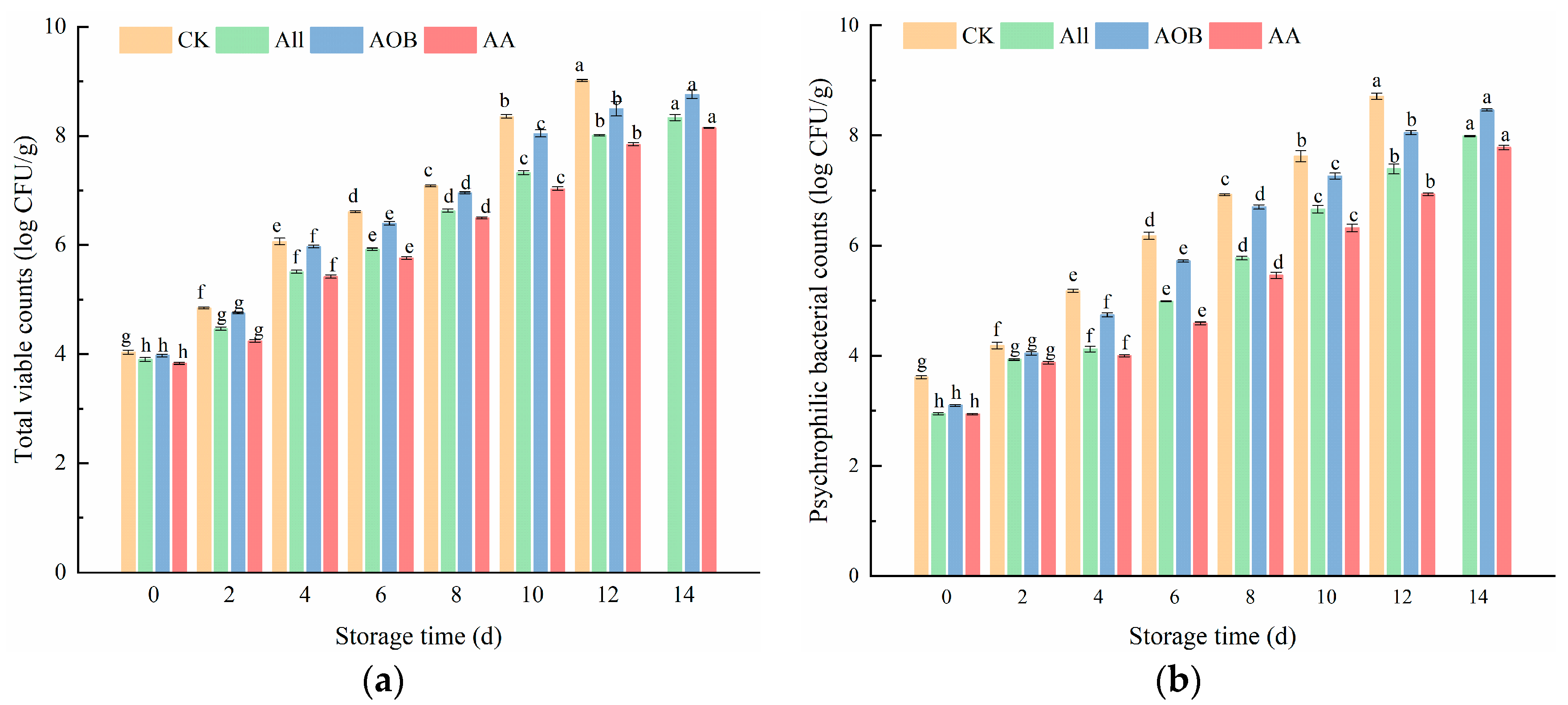
3.1. Microbiological Analysis
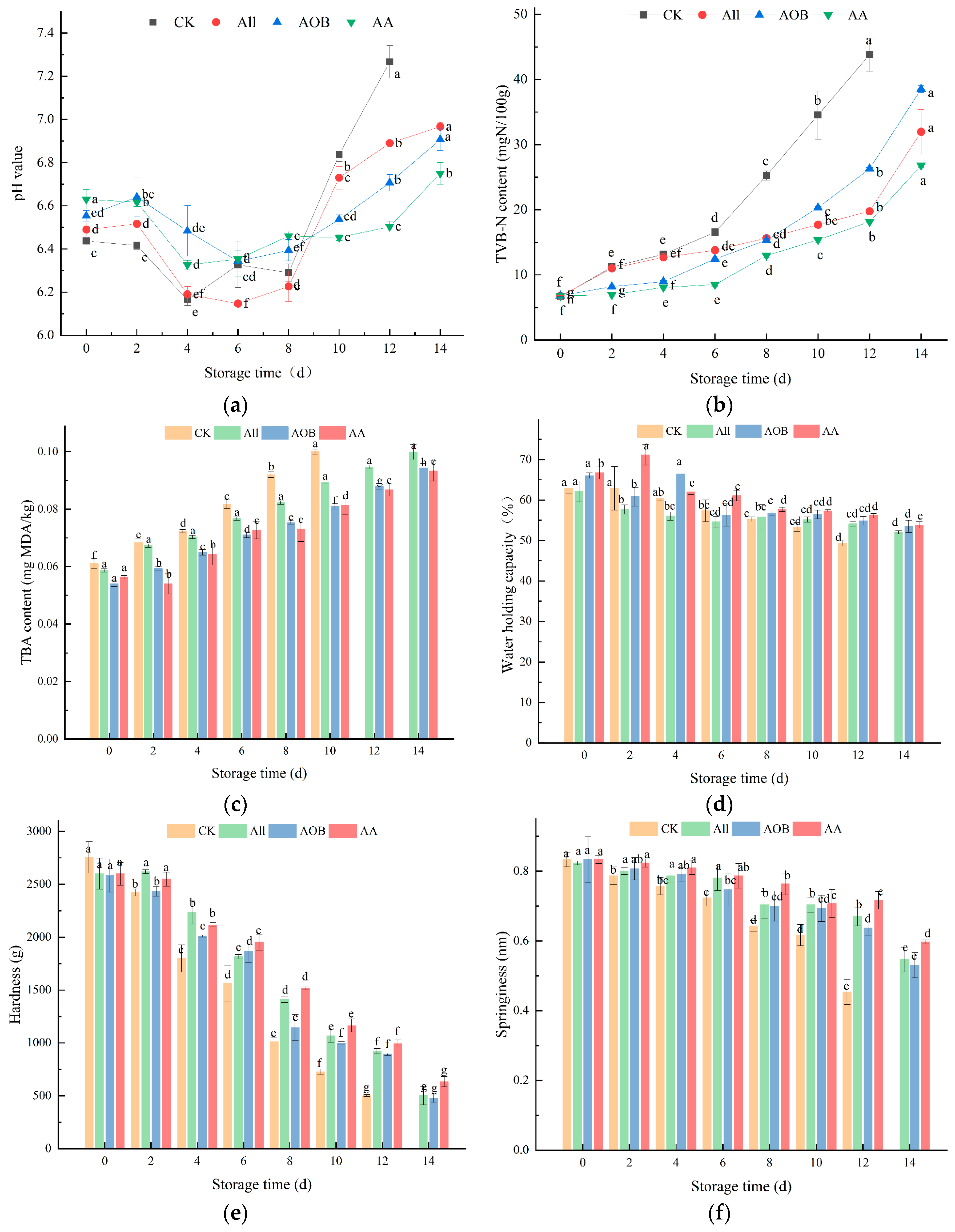
3.2. Physicochemical Analysis
3.2.1. Change in pH Value and TVB-N and TBA Contents
3.2.2. Change in WHC and TPA
3.2.3. Free Amino Acids
3.3. Protein Characteristics
3.3.1. Fluorescence Spectroscopy Analysis
3.3.2. Myofibril Fragmentation Index (MFI)
3.4. Sensory Evaluation
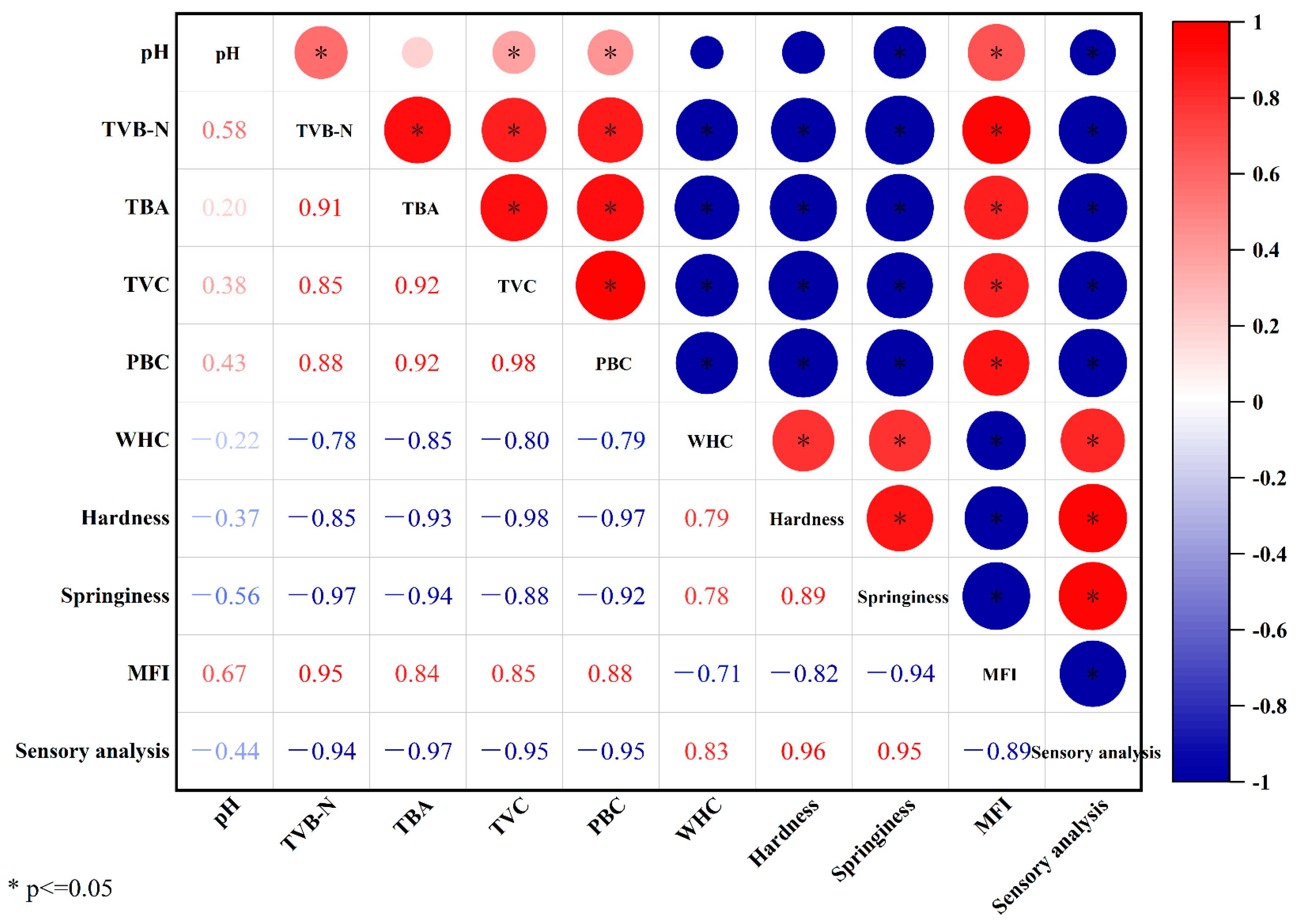
3.5. Correlation Analysis
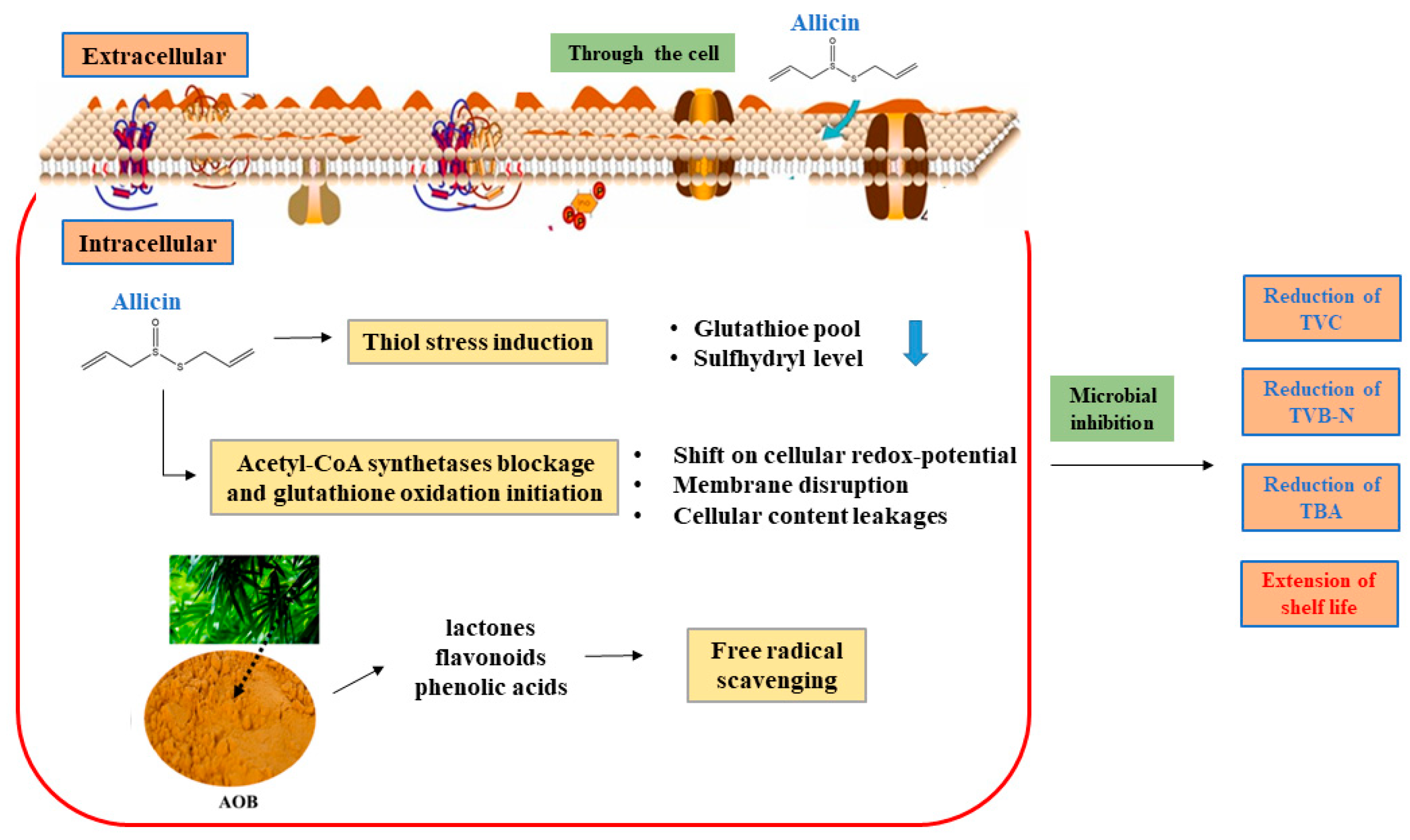
3.6. Diagram of Antibacterial Mechanism of Allicin and AOB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nóbrega, I.C.; Ataíde, C.S.; Moura, O.M.; Livera, A.V.; Menezes, P.H. Volatile constituents of cooked bullfrog (Rana catesbeiana) legs. Food Chem. 2007, 102, 186–191. [Google Scholar] [CrossRef]
- Gratwicke, B.; Evans, M.J.; Jenkins, P.T.; Kusrini, M.D.; Moore, R.D.; Sevin, J.; Wildt, D.E. Is the international frog legs trade a potential vector for deadly amphibian pathogens? J. Front. Ecol. Environ. 2010, 8, 438–442. [Google Scholar] [CrossRef]
- Silva, H.L.A.; Costa, M.P.; Frasao, B.S.; Mesquita, E.F.M.; Mello, S.C.R.P.; Conte-Junior, C.A.; Franco, R.M.; Miranda, Z.B. Efficacy of Ultraviolet-C Light to Eliminate Staphylococcus Aureus on Precooked Shredded Bullfrog Back Meat. J. Food Saf. 2015, 35, 318–323. [Google Scholar] [CrossRef]
- Chen, D.; Ci, M.; Dai, R.; Chen, R.; Li, T. Changes in the Microbial Communities of Tiger Frog (Rana tigrina) Meat during Refrigerated Storage. J. Food Prot. 2021, 84, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Zucca, P.; Orhan, I.E.; Azzini, E.; Adetunji, C.O.; Mohammed, S.A.; Banerjee, S.K.; Sharopov, F.; Rigano, D.; Sharifi-Rad, J.; et al. Allicin and health: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 502–516. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Sanches-Silva, A.; Daglia, M.; Nabavi, S.F.; Jafari, N.J.; Izadi, M.; Ajami, M.; Nabavi, S.M. Antifungal and antibacterial activities of allicin: A review. Trends Food Sci. Technol. 2016, 52, 49–56. [Google Scholar] [CrossRef]
- He, B.; Wang, W.; Song, Y.; Ou, Y.; Zhu, J. Structural and physical properties of carboxymethyl cellulose/gelatin films functionalized with antioxidant of bamboo leaves. Int. J. Biol. Macromol. 2020, 164, 1649–1656. [Google Scholar] [CrossRef]
- Nirmala, C.; Bisht, M.S.; Bajwa, H.K.; Santosh, O. Bamboo: A rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends Food Sci. Technol. 2018, 77, 91–99. [Google Scholar] [CrossRef]
- Xie, H.-K.; Zhou, D.-Y.; Liu, Z.-Y.; Li, D.-Y.; Tan, Z.-F.; Dong, X.-F.; Liu, X.-Y.; Shahidi, F.; Zhu, B.-W. Effects of natural phenolics on shelf life and lipid stability of freeze-dried scallop adductor muscle. Food Chem. 2019, 295, 423–431. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, G.; Yin, F.; Liu, Z.; Wang, J.; Qin, L.; Zhou, D.; Shahidi, F.; Zhu, B. Effects of roasting temperature and time on aldehyde formation derived from lipid oxidation in scallop (Patinopecten yessoensis) and the deterrent effect by antioxidants of bamboo leaves. Food Chem. 2022, 369, 130936. [Google Scholar] [CrossRef]
- Liu, J.; Lan, W.; Sun, X.; Xie, J. Effects of chitosan grafted phenolic acid coating on microbiological, physicochemical and protein changes of sea bass (Lateolabrax japonicus) during refrigerated storage. J. Food Sci. 2020, 85, 2506–2515. [Google Scholar] [CrossRef]
- Li, T.; Hu, W.; Li, J.; Zhang, X.; Zhu, J.; Li, X. Coating effects of tea polyphenol and rosemary extract combined with chitosan on the storage quality of large yellow croaker (Pseudosciaena crocea). Food Control 2012, 25, 101–106. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Jiménez-Escrig, A.; Saura-Calixto, F.; Borderías, A. Antioxidant protection of white grape pomace on restructured fish products during frozen storage. LWT 2008, 41, 42–50. [Google Scholar] [CrossRef]
- Zhao, X.; Lan, W.; Zhai, Y.; Xie, J. Multi-frequency ultrasound:A potential method to improve the effects of surface decontamination and structural characteristics on large yellow croaker (Pseudosciaena crocea) during refrigerated storage. Ultrason. Sonochem. 2021, 79, 105787. [Google Scholar] [CrossRef] [PubMed]
- Merlo, T.C.; Contreras-Castillo, C.J.; Saldaña, E.; Barancelli, G.V.; Dargelio, M.D.B.; Yoshida, C.M.P.; Junior, E.E.R.; Massarioli, A.; Venturini, A.C. Incorporation of pink pepper residue extract into chitosan film combined with a modified atmosphere packaging: Effects on the shelf life of salmon fillets. Food Res. Int. 2019, 125, 108633. [Google Scholar] [CrossRef]
- Li, P.; Peng, Y.; Mei, J.; Xie, J. Effects of microencapsulated eugenol emulsions on microbiological, chemical and organoleptic qualities of farmed Japanese sea bass (Lateolabrax japonicus) during cold storage. LWT 2020, 118, 108831. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, B.; Liu, L.; Pu, T.; Zhou, Y.; Xie, J. Slightly acidic electrolyzed water-slurry ice: Shelf-life extension and quality maintenance of mackerel (Pneumatophorus japonicus) during chilled storage. J. Sci. Food Agric. 2023, 103, 3787–3798. [Google Scholar] [CrossRef] [PubMed]
- Büyükdeveci, M.E.; Boga, E.K.; Ozyurt, G. Gamma-irradiation induced effects on biogenic amine formation and quality of frog legs (Rana esculenta) during storage. LWT 2019, 99, 379–386. [Google Scholar] [CrossRef]
- Muela, E.; Sañudo, C.; Campo, M.M.; Medel, I.; Beltrán, J.A. Effect of freezing method and frozen storage duration on instrumental quality of lamb throughout display. Meat Sci. 2010, 84, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Manju, S.; Jose, L.; Gopal, T.S.; Ravishankar, C.; Lalitha, K. Effects of sodium acetate dip treatment and vacuum-packaging on chemical, microbiological, textural and sensory changes of Pearlspot (Etroplus suratensis) during chill storage. Food Chem. 2007, 102, 27–35. [Google Scholar] [CrossRef]
- Lang, K.J.A.F.L. Der fluchtige Basenstickstoff (TVB-N) bei im Binnenland in der Verkehr gebrachten frischen Seefischen. II. Mitteilung. Archiv fur Lebensmittelhygiene 1979, 34, 7–10. [Google Scholar]
- Sohrab, M.; Reza, T.; Vali, H.S.; Mohammad, R.; Zoya, T.; Xesús, F.; Fereidoon, A. Effect of gamma radiation on the quality and shelf life of refrigerated rainbow trout (Oncorhynchus mykiss) fillets. J. Food Prot. 2009, 72, 1419–1426. [Google Scholar]
- Liu, L.; Lan, W.; Pu, T.; Zhou, Y.; Xie, J. Combining slightly acidic electrolyzed water and slurry ice to prolong the shelf-life of mackerel (Pneumatophorus japonicus). J. Food Process. Preserv. 2021, 45, e15762. [Google Scholar] [CrossRef]
- Zhao, J.; Li, J.; Wang, J.; Lv, W. Applying Different Methods to Evaluate the Freshness of Large Yellow Croacker (Pseudosciaena crocea) Fillets during Chilled Storage. J. Agric. Food Chem. 2012, 60, 11387–11394. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Tang, X.; Tang, S.; You, H.; Shi, H.; Gu, R. Combined effect of electrolyzed oxidizing water and chitosan on the microbiological, physicochemical, and sensory attributes of American shad (Alosa sapidissima) during refrigerated storage. Food Control. 2014, 46, 397–402. [Google Scholar] [CrossRef]
- Yuqing, S.; Weiqing, L.; Shucheng, L.; Yuan, G.; Shengyun, Z.; Jing, X. Preparation of chitosan grafted caffeic acid coating and its effect on pompano (Trachinotus ovatus) preservation. J. Sci. Food Agric. 2022, 102, 2835–2845. [Google Scholar]
- Da Silva-Buzanello, R.A.; Schuch, A.F.; Nogues, D.R.N.; de Melo, P.F.; Gasparin, A.W.; Torquato, A.S.; Canan, C.; Soares, A.L. Physicochemical and biochemical parameters of chicken breast meat influenced by stunning methods. Poult. Sci. 2018, 97, 3786–3792. [Google Scholar] [CrossRef]
- Xuan, X.-T.; Fan, Y.-F.; Ling, J.-G.; Hu, Y.Q.; Liu, D.-H.; Chen, S.G.; Ye, X.-Q.; Ding, T. Preservation of squid by slightly acidic electrolyzed water ice. Food Control. 2017, 73, 1483–1489. [Google Scholar] [CrossRef]
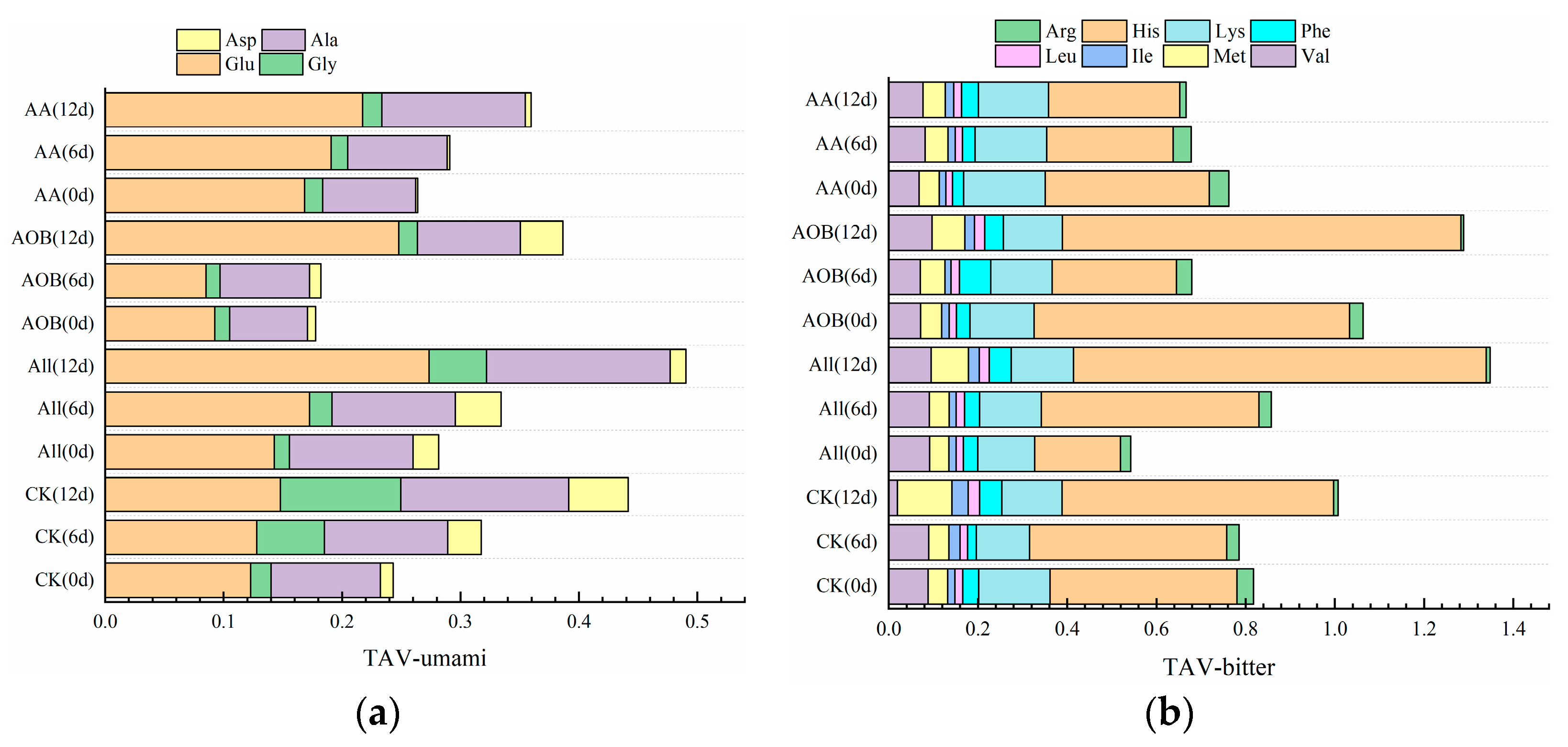


| Samples | Asp | Thr | Ser | Glu | Gly | Ala | Val | Met | Ile |
| CK-0 | 1.09 ± 0.16 c | 3.08 ± 0.05 a | 2.63 ± 0.00 b | 3.68 ± 0.03 b | 2.24 ± 0.01 c | 5.54 ± 0.04 c | 3.54 ± 0.40 a | 1.31 ± 0.39 b | 1.47 ± 0.02 c |
| CK-6 | 2.82 ± 0.02 b | 1.78 ± 0.02 b | 6.10 ± 0.03 a | 3.84 ± 0.03 b | 7.39 ± 0.05 b | 6.25 ± 0.00 b | 3.59 ± 0.00 a | 1.35 ± 0.02 b | 2.26 ± 0.09 b |
| CK-12 | 5.02 ± 0.38 a | 1.29 ± 0.06 c | 6.39 ± 0.36 a | 4.43 ± 0.08 a | 13.23 ± 0.39 a | 8.50 ± 0.13 a | 0.79 ± 0.02 b | 3.65 ± 0.16 a | 3.27 ± 0.20 a |
| All-0 | 2.17 ± 0.03 c | 2.04 ± 0.05 b | 1.51 ± 0.03 c | 4.28 ± 0.15 c | 1.64 ± 0.01 c | 6.27 ± 0.03 b | 3.67 ± 0.57 a | 1.30 ± 0.06 b | 1.44 ± 0.11 b |
| All-6 | 3.86 ± 0.13 a | 1.89 ± 0.04 b | 3.16 ± 0.08 a | 5.17 ± 0.23 b | 2.47 ± 0.02 b | 6.25 ± 0.00 b | 3.63 ± 0.22 a | 1.34 ± 0.01 b | 1.39 ± 0.35 b |
| All-12 | 1.33 ± 0.02 b | 2.46 ± 0.07 a | 1.74 ± 0.05 b | 8.20 ± 0.21 a | 6.30 ± 0.04 a | 9.32 ± 0.05 a | 3.80 ± 0.03 a | 2.51 ± 0.01 a | 2.20 ± 0.08 a |
| AOB-0 | 0.67 ± 0.02 b | 0.67 ± 0.02 c | 1.76 ± 0.09 b | 2.78 ± 0.33 b | 1.62 ± 0.01 b | 3.95 ± 0.05 c | 2.86 ± 0.02 b | 1.41 ± 0.02 b | 1.49 ± 0.01 b |
| AOB-6 | 0.96 ± 0.08 b | 1.45 ± 0.02 b | 1.74 ± 0.01 b | 2.55 ± 0.12 b | 1.53 ± 0.05 b | 4.54 ± 0.23 b | 2.83 ± 0.05 b | 1.66 ± 0.03 b | 1.22 ± 0.12 b |
| AOB-12 | 3.60 ± 0.81 a | 2.19 ± 0.06 a | 3.50 ± 0.17 a | 7.43 ± 0.12 a | 2.07 ± 0.03 a | 5.21 ± 0.20 a | 3.87 ± 0.36 a | 2.20 ± 0.17 a | 1.99 ± 0.10 a |
| AA-0 | 0.17 ± 0.01 c | 0.17 ± 0.01 c | 1.62 ± 0.24 a | 5.05 ± 0.83 a | 1.97 ± 0.27 a | 4.71 ± 0.34 b | 2.72 ± 0.04 a | 1.37 ± 0.37 a | 1.31 ± 0.03 c |
| AA-6 | 0.22 ± 0.01 b | 2.36 ± 0.07 a | 1.61 ± 0.06 a | 5.72 ± 0.23 a | 1.84 ± 0.00 a | 5.03 ± 0.01 b | 3.25 ± 0.12 a | 1.54 ± 0.16 a | 1.51 ± 0.00 b |
| AA-12 | 0.50 ± 0.00 a | 1.98 ± 0.00 b | 1.25 ± 0.02 a | 6.52 ± 0.02 a | 2.11 ± 0.01 a | 7.27 ± 0.04 a | 3.07 ± 0.46 a | 1.50 ± 0.59 a | 1.67 ± 0.01 a |
| Samples | Leu | Tyr | Phe | Lys | His | Arg | Pro | Total | |
| CK-0 | 3.35 ± 0.09 b | 2.18 ± 0.02 c | 3.20 ± 0.09 ab | 7.99 ± 0.00 a | 8.38 ± 0.09 b | 1.86 ± 0.15 c | 2.47 ± 0.31 a | 54.01 ± 1.87 | |
| CK-6 | 3.17 ± 0.26 b | 4.40 ± 0.15 b | 1.75 ± 0.20 b | 5.97 ± 0.41 b | 8.85 ± 0.02 b | 1.38 ± 0.02 b | 2.91 ± 0.12 a | 63.82 ± 1.44 | |
| CK-12 | 4.85 ± 0.05 a | 6.95 ± 0.29 a | 4.52 ± 0.90 a | 6.74 ± 0.22 b | 12.17 ± 0.71 a | 0.50 ± 0.03 a | 3.72 ± 0.67 a | 86.03 ± 4.67 | |
| All-0 | 3.02 ± 0.05 c | 2.20 ± 0.18 c | 2.96 ± 0.11 a | 6.37 ± 0.24 a | 3.84 ± 0.04 c | 1.17 ± 0.01 b | 2.85 ± 0.10 b | 46.73 ± 1.80 | |
| All-6 | 3.63 ± 0.09 b | 4.33 ± 0.07 b | 3.02 ± 1.54 a | 6.93 ± 0.23 a | 9.75 ± 0.07 b | 1.41 ± 0.02 a | 20.04 ± 0.00 c | 60.22 ± 3.16 | |
| All-12 | 4.19 ± 0.08 a | 4.86 ± 0.03 a | 4.45 ± 0.06 a | 6.96 ± 0.09 a | 18.51 ± 0.05 a | 0.42 ± 0.00 c | 4.12 ± 0.16 a | 81.38 ± 1.04 | |
| AOB-0 | 3.11 ± 0.02 a | 3.33 ± 0.02 b | 2.72 ± 0.02 c | 7.19 ± 0.01 a | 14.15 ± 0.08 b | 1.50 ± 0.03 b | 2.39 ± 0.76 a | 51.60 ± 1.53 | |
| AOB-6 | 3.57 ± 0.13 a | 2.84 ± 0.19 b | 6.32 ± 0.14 a | 6.89 ± 0.24 ab | 5.57 ± 0.21 c | 1.73 ± 0.04 a | 2.47 ± 0.01 a | 47.87 ± 1.68 | |
| AOB-12 | 4.33 ± 0.71 a | 4.49 ± 0.39 a | 3.76 ± 0.16 b | 6.63 ± 0.00 b | 17.86 ± 0.01 a | 0.33 ± 0.01 c | 3.09 ± 0.05 a | 72.56 ± 3.35 | |
| AA-0 | 2.81 ± 0.01 c | 3.30 ± 0.00 b | 2.27 ± 0.01 c | 9.12 ± 0.10 a | 7.36 ± 0.13 a | 2.20 ± 0.03 a | 2.05 ± 0.24 b | 48.20 ± 2.66 | |
| AA-6 | 3.06 ± 0.02 b | 3.43 ± 0.02 b | 2.56 ± 0.02 b | 8.02 ± 0.47 a | 5.67 ± 0.92 a | 20.03 ± 0.00 b | 2.66 ± 0.05 a | 50.48 ± 2.20 | |
| AA-12 | 3.41 ± 0.06 a | 4.04 ± 0.11 a | 3.35 ± 0.12 a | 7.88 ± 0.51 a | 5.89 ± 0.03 a | 0.71 ± 0.00 c | 2.73 ± 0.12 a | 53.87 ± 2.10 |
| FAA | Taste | Threshold (mg/100 mL) |
|---|---|---|
| Asp | Umami | 100 |
| Glu | Umami | 30 |
| Ser | Sweet | 150 |
| His | Bitter | 20 |
| Gly | Umami/Sweet | 130 |
| Thr | Sweet | 260 |
| Arg | Sweet/Bitter | 50 |
| Ala | Umami/Sweet | 60 |
| Val | Sweet/Bitter | 40 |
| Met | Sweet/Bitter | 30 |
| Phe | Bitter | 90 |
| Ile | Bitter | 90 |
| Leu | Bitter | 190 |
| Lys | Sweet/Bitter | 50 |
| Pro | Sweet/Bitter | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, W.; Zhang, B.; Du, J.; Zhu, S.; Xu, X.; Xie, J. Synergistic Effect of Combined Treatment with Allicin and Antioxidant of Bamboo Leaves and Preservation of Bullfrogs (Lithobates catesbeiana) during Refrigeration Storage. Foods 2023, 12, 3467. https://doi.org/10.3390/foods12183467
Lan W, Zhang B, Du J, Zhu S, Xu X, Xie J. Synergistic Effect of Combined Treatment with Allicin and Antioxidant of Bamboo Leaves and Preservation of Bullfrogs (Lithobates catesbeiana) during Refrigeration Storage. Foods. 2023; 12(18):3467. https://doi.org/10.3390/foods12183467
Chicago/Turabian StyleLan, Weiqing, Bingjie Zhang, Jintao Du, Shengyun Zhu, Xiao Xu, and Jing Xie. 2023. "Synergistic Effect of Combined Treatment with Allicin and Antioxidant of Bamboo Leaves and Preservation of Bullfrogs (Lithobates catesbeiana) during Refrigeration Storage" Foods 12, no. 18: 3467. https://doi.org/10.3390/foods12183467
APA StyleLan, W., Zhang, B., Du, J., Zhu, S., Xu, X., & Xie, J. (2023). Synergistic Effect of Combined Treatment with Allicin and Antioxidant of Bamboo Leaves and Preservation of Bullfrogs (Lithobates catesbeiana) during Refrigeration Storage. Foods, 12(18), 3467. https://doi.org/10.3390/foods12183467






