Integrated Transcriptomics and Metabolomics Reveal the Mechanism of Alliin in Improving Hyperlipidemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Animal Experimental Design and Sample Collection
2.3. Blood Lipids Detection
2.4. SOD, CAT, and MDA Detection in Mice Liver
2.5. Histological Analysis
2.6. Transcriptomics Analysis
2.7. Determination of mRNA Expression Leve ls by qRT-PCR in Liver
2.8. Metabolomics Analysis
2.9. Statistical Analysis
3. Results
3.1. Effects of Alliin on Body Weight, Feed Intake, Fat Weight, and Liver Weight in Mice
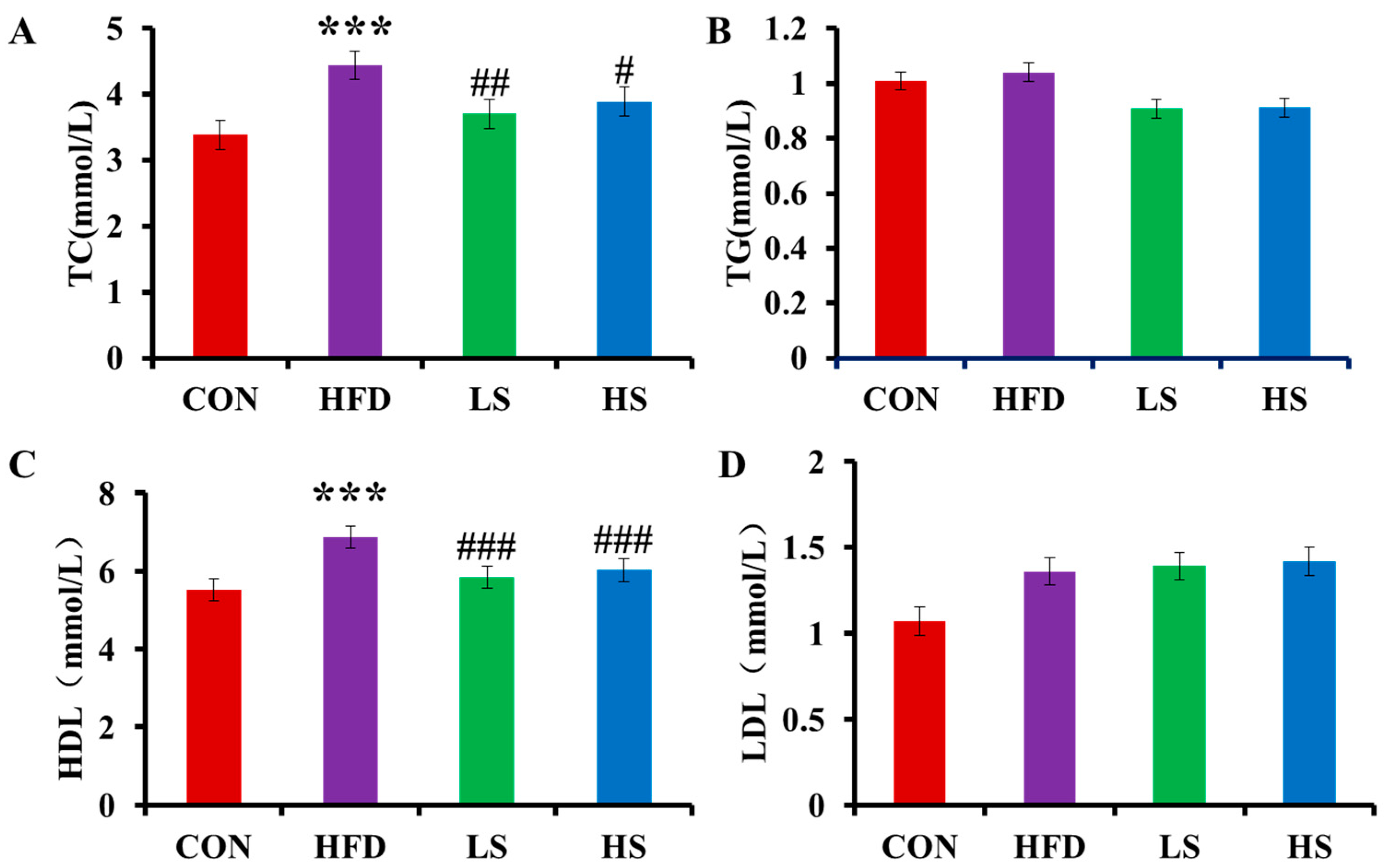
3.2. Effects of Alliin on Blood Lipids
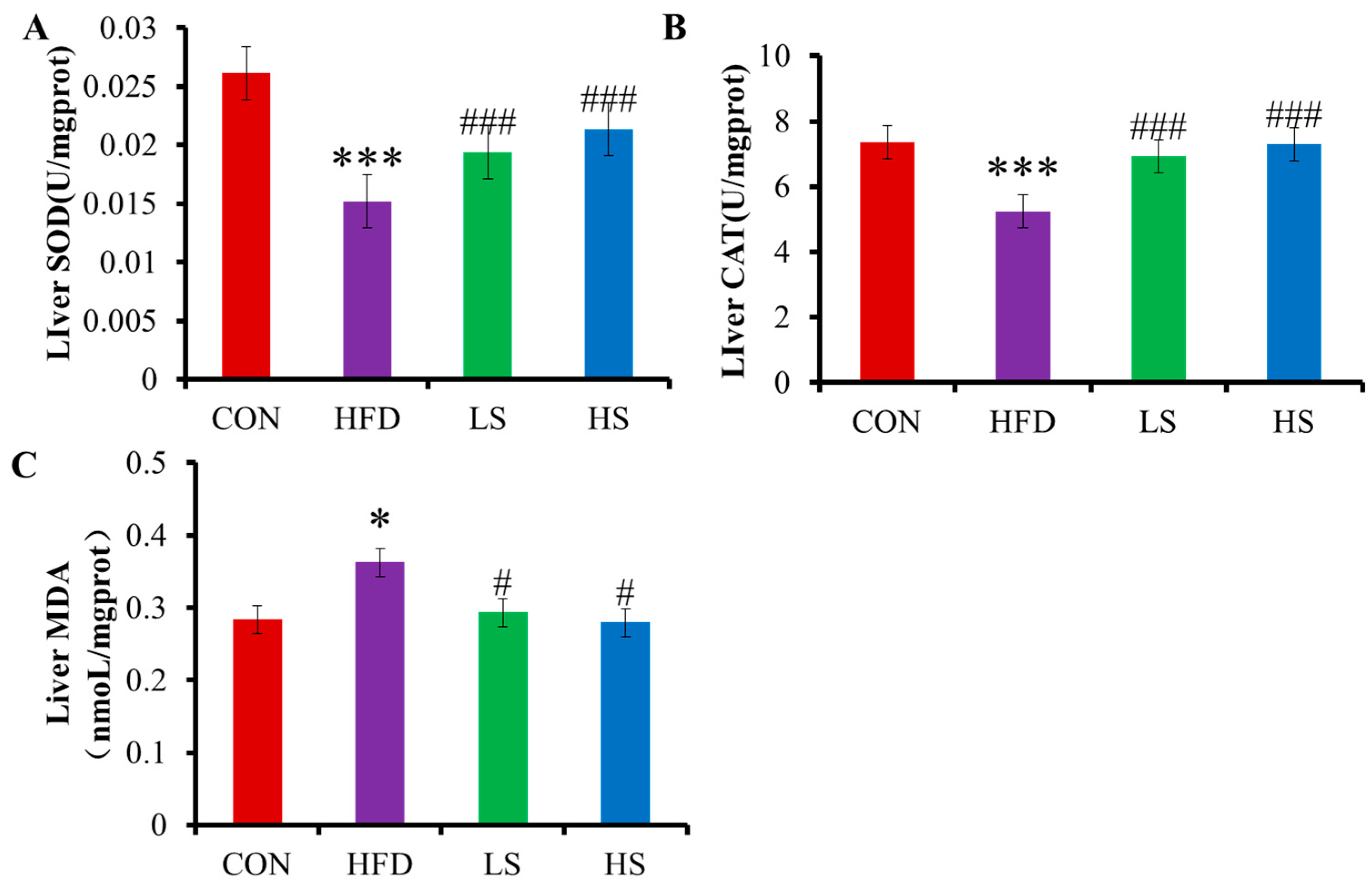
3.3. Effects of Alliin on SOD, CAT, and MDA in Mice Liver
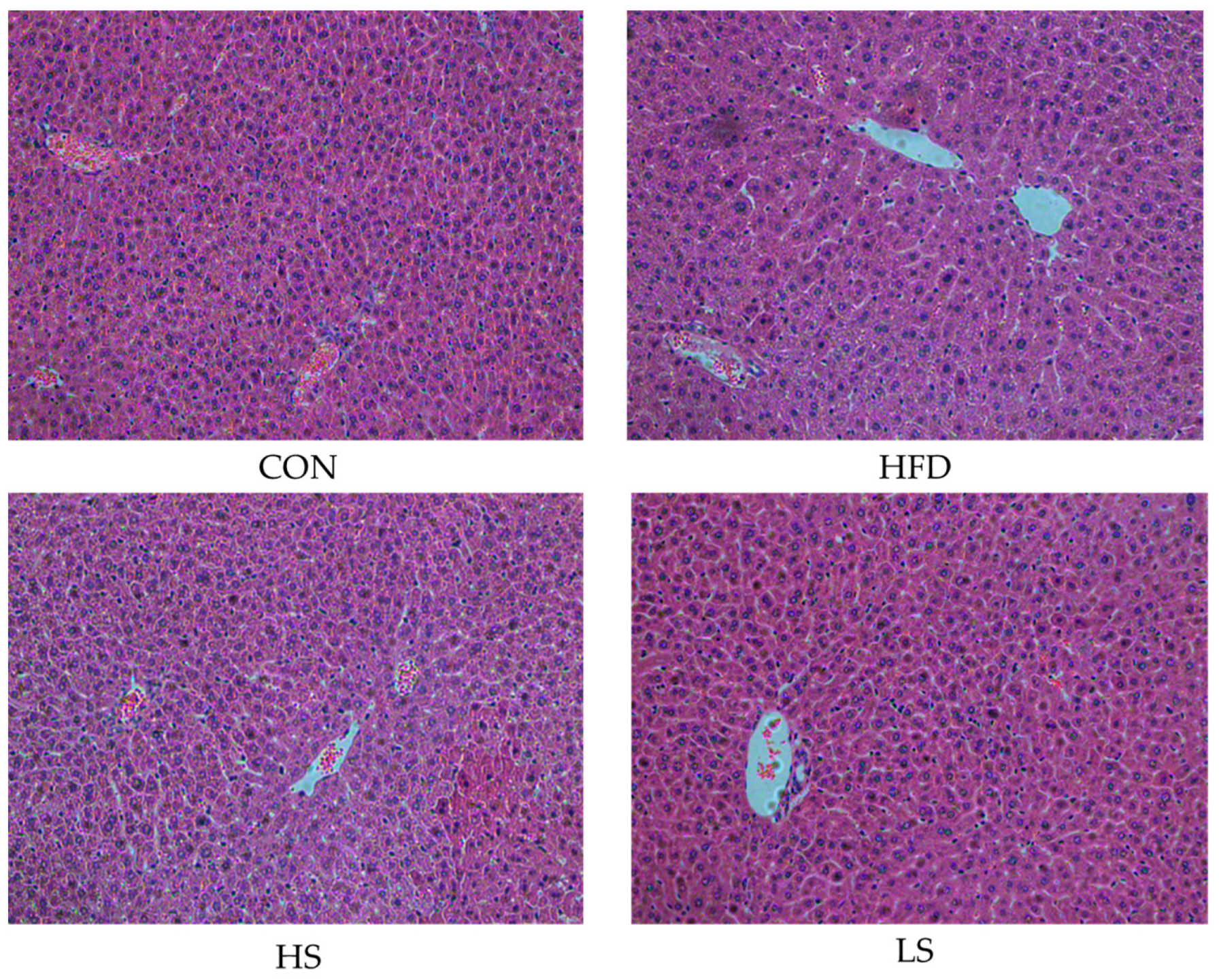
3.4. Effects of Alliin on Liver Histomorphology
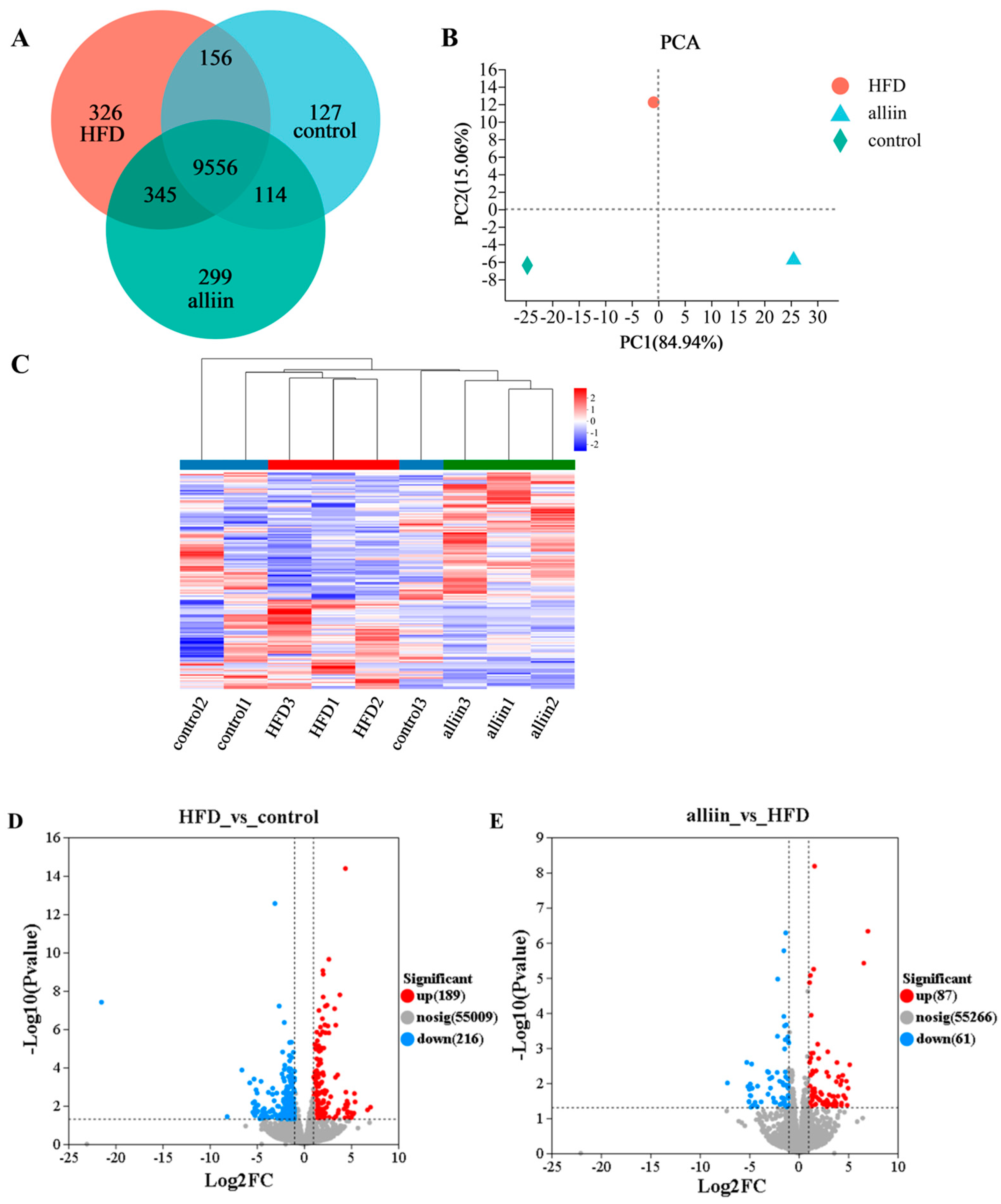
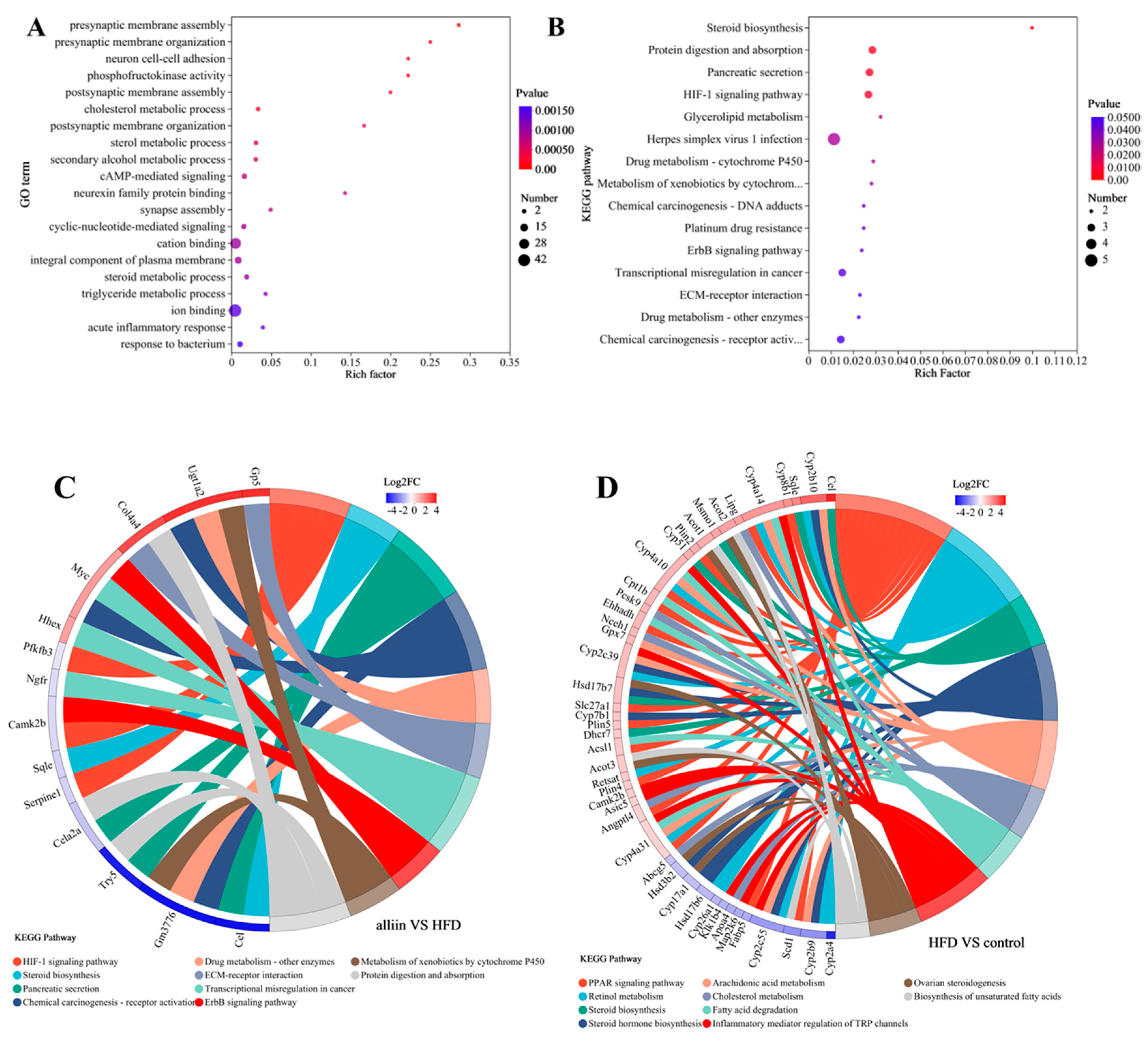
3.5. Transcriptomics Analysis
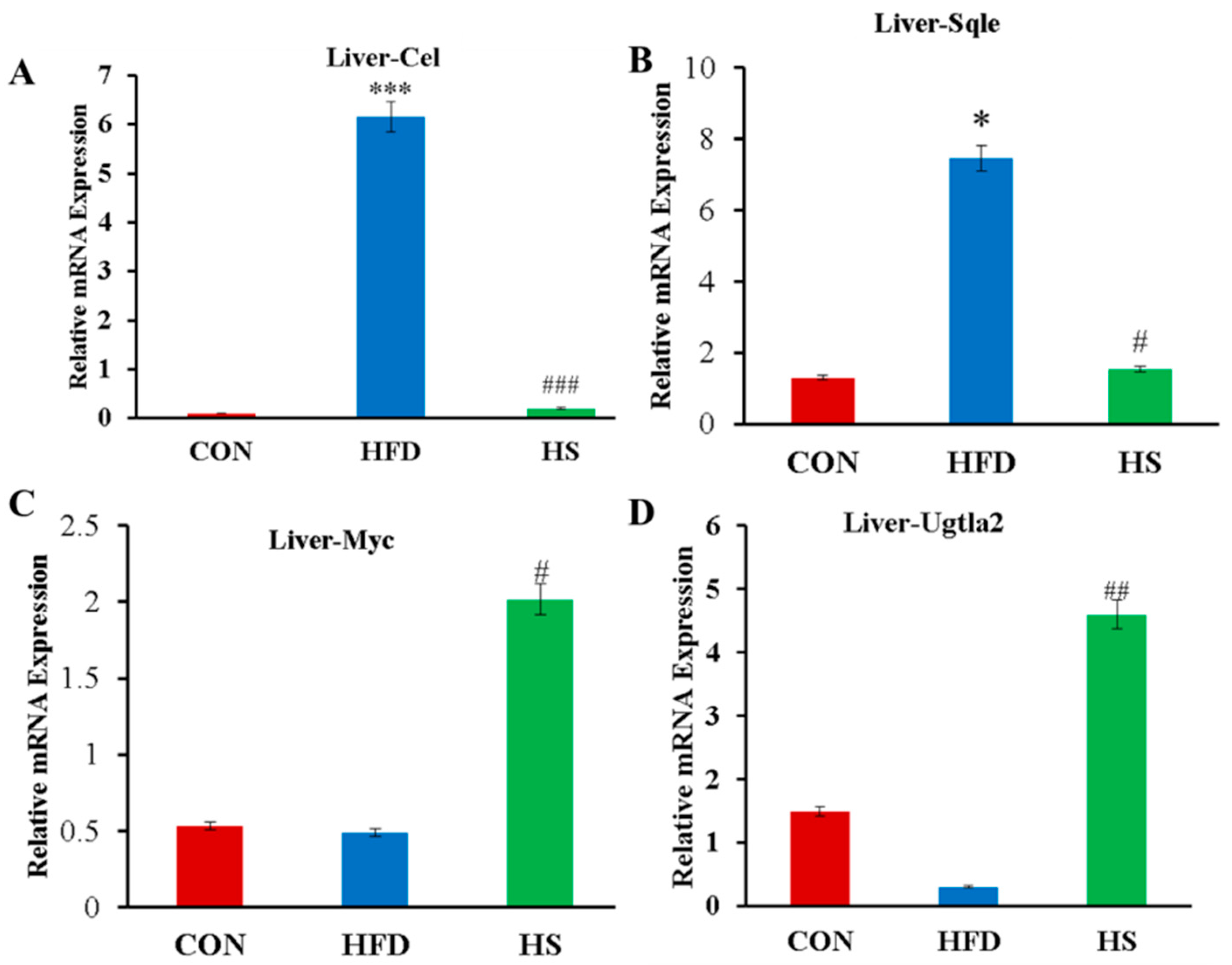
3.6. Confirmation of Differential Expression of Lipid Metabolism Genes by qRT-PCR Analysis
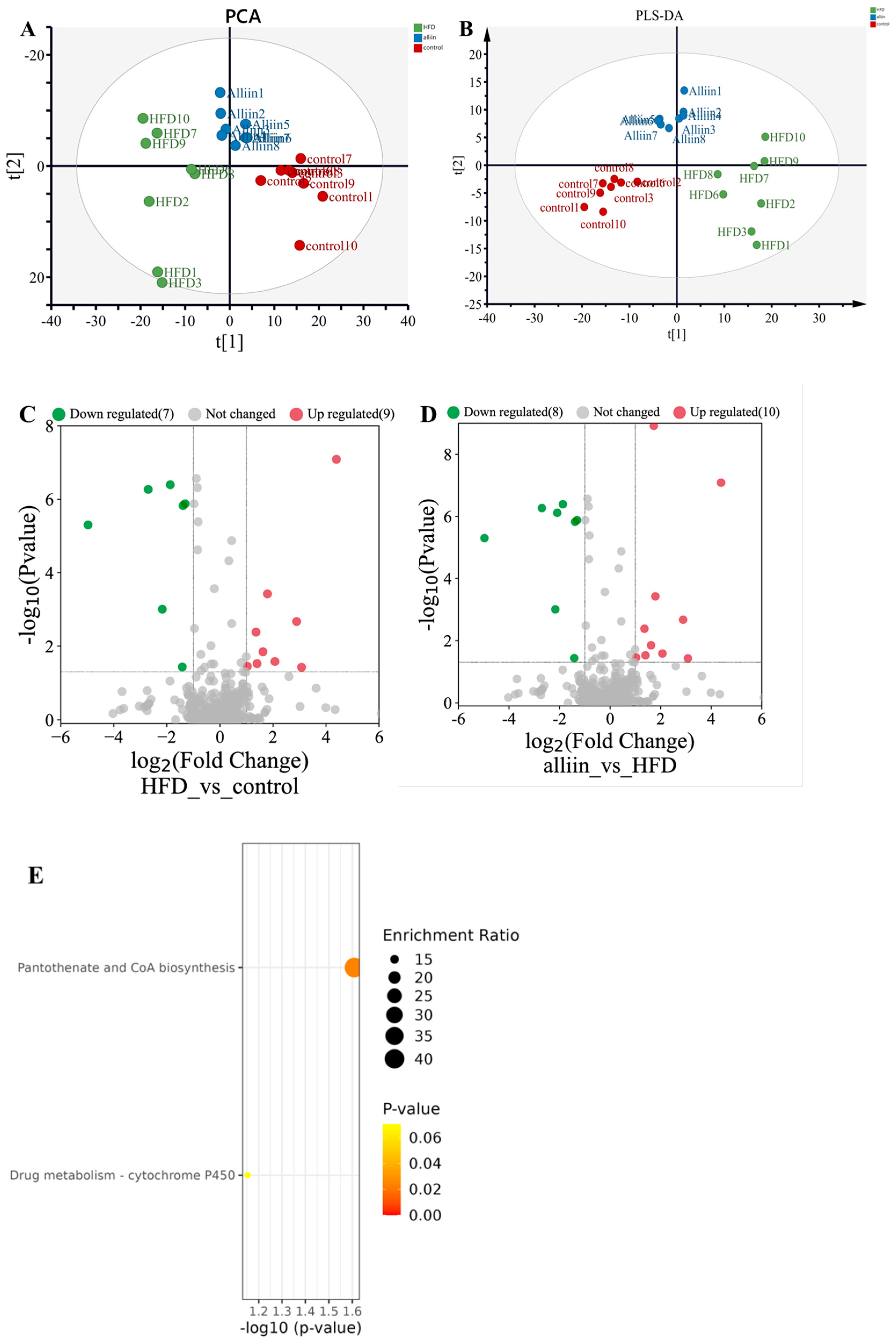
3.7. Metabolomics Analysis
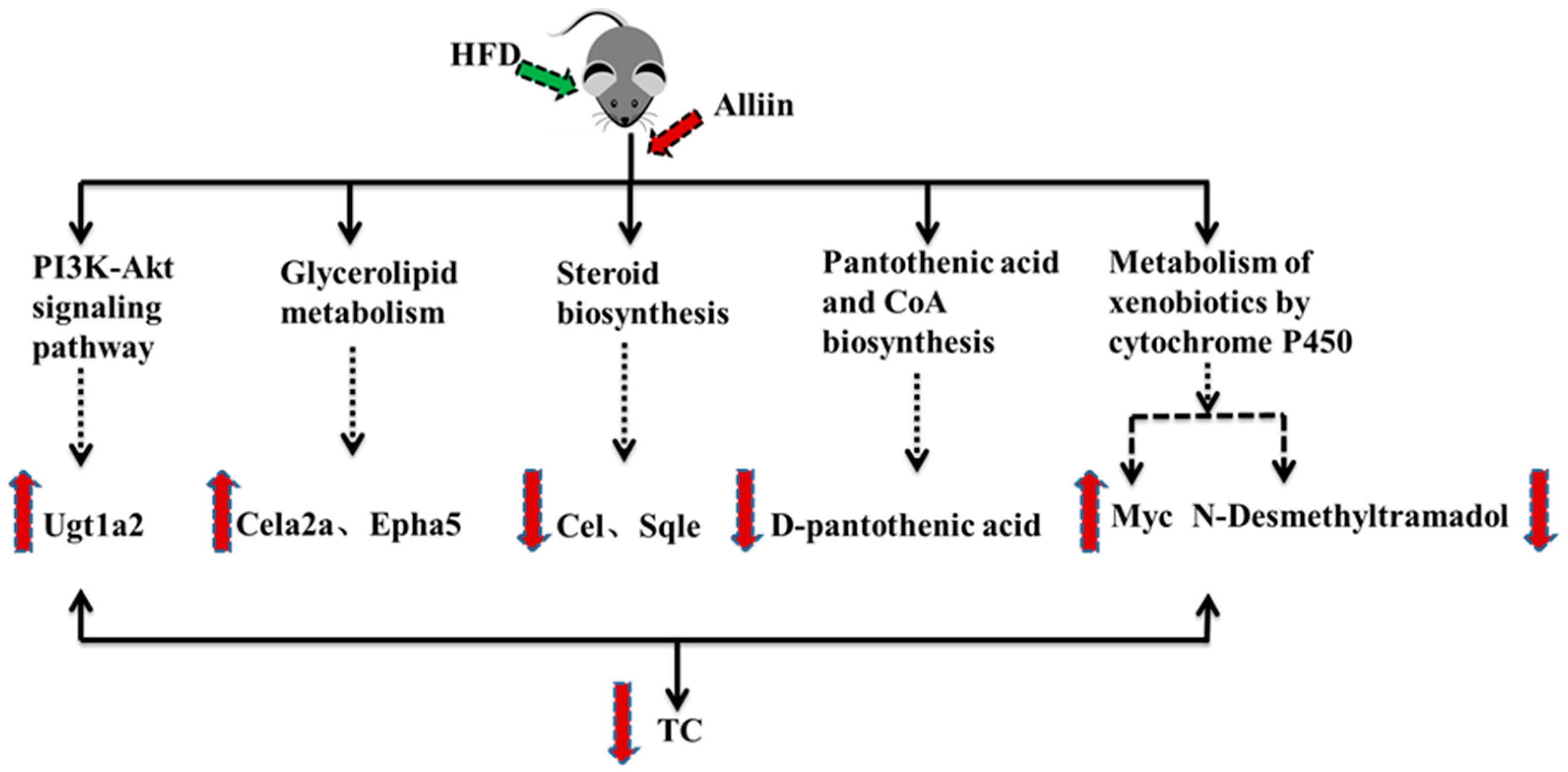
3.8. An Association Analysis of Differential Genes and Metabolites in Mice Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pittler, M.H.; Ernst, E. Clinical effectiveness of garlic (Allium sativum). Mol. Nutr. Food Res. 2007, 51, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Heidarian, E.; Jafari-dehkordi, E.; Seidkhani-nahal, A. Effect of garlic on liver phosphatidate phosphohydrolase and plasma lipid levels in hyperlipidemic rats. Food Chem. Toxicol. 2011, 49, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Zhang, C.; Sheng, Y.; Sheng, Y.; Zhao, C.; He, X.; Xu, W.; Huang, K.L.; Luo, Y.B. Hypoglycemic and hypolipidemic effect of s-allyl-cysteine sulfoxide (alliin) in dio mice. Sci. Rep. 2018, 8, 3527. [Google Scholar] [CrossRef] [PubMed]
- Nasim, S.A.; Dhir, B.; Kapoor, R.; Fatima, S.; Mujib, A. Alliin obtained from leaf extract of garlic grown under in situ conditions possess higher therapeutic potency as analyzed in alloxan-induced diabetic rats. Pharm. Biol. 2011, 49, 416–421. [Google Scholar] [CrossRef]
- Chan, J.Y.; Yuen, A.C.; Chan, R.Y.; Chan, S.W. A review of the cardiovascular benefits and antioxidant properties of allicin. Phytother. Res. 2013, 27, 637–646. [Google Scholar] [CrossRef]
- Dorrigiv, M.; Zareiyan, A.; Hosseinzadeh, H. Garlic (Allium sativum) as an antidote or a protective agent against natural or chemical toxicities: A comprehensive update review. Phytother. Res. 2020, 34, 1770–1797. [Google Scholar] [CrossRef]
- Subramanian, M.S.; Ms, G.N.; Nordin, S.A.; Thilakavathy, K.; Joseph, N. Prevailing knowledge on the bioavailability and biological activities of sulphur compounds from alliums: A potential drug candidate. Molecules 2020, 25, 4111. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Yang, X.G.; Hemler, E.C.; Fang, Y.H.; Zhao, L.Y.; Zhang, J.; Yang, Z.Y.; Wang, Z.; He, L.; et al. The dietary transition and its association with cardiometabolic mortality among chinese adults, 1982-2012: A cross-sectional population-based study. Lancet Diabetes Endocrinol. 2019, 7, 540–548. [Google Scholar] [CrossRef]
- Su, Z.; Nie, Y.; Huang, X.; Huang, X.; Zhu, Y.; Zheng, G. Mitophagy in hepatic insulin resistance: Therapeutic potential and concerns. Front. Pharmacol. 2019, 10, 1193. [Google Scholar] [CrossRef]
- Teramoto, T.; Sasaki, J.; Ishibashi, S.; Birou, S.; Yokote, K. Comprehensive risk management for the prevention of cardiovascular disease. J. Atheroscler. Thromb. 2013, 20, 603. [Google Scholar] [CrossRef]
- Li, C.; Liu, H.; Yang, J.; Mu, J.; Wang, R.; Zhao, X. Effect of soybean milk fermented with lactobacillus plantarum hfy01 isolated from yak yogurt on weight loss and lipid reduction in mice with obesity induced by a high-fat diet. RSC Adv. 2020, 10, 34276–34289. [Google Scholar] [CrossRef] [PubMed]
- Michos, E.D.; Mcevoy, J.W.; Blumenthal, R.S. Lipid Management for the Prevention of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2019, 381, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Kim, H.R.; Kim, J.H.; Om, A.S. Beneficial effects of allium sativum L. stem extract on lipid metabolism and antioxidant status in obese mice fed a high-fat diet. J. Sci. Food Agric. 2013, 93, 2749–2757. [Google Scholar] [CrossRef]
- Zhu, Y.; Anand, R.; Geng, X.; Ding, Y. A mini review: Garlic extract and vascular diseases. Neurol. Res. 2018, 40, 421–425. [Google Scholar] [CrossRef]
- Ashraf, R.; Aamir, K.; Shaikh, A.R.; Ahmed, T. Effects of garlic on dyslipidemia in patients with type 2 diabetes mellitus. JAMC 2005, 17, 60–64. [Google Scholar]
- Yamaguchi, Y.; Kumagai, H. Characteristics, biosynthesis, decomposition, metabolism and functions of the garlic odour precursor, s-allyl-l-cysteine sulfoxide. Exp. Ther. Med. 2020, 19, 1528–1535. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, J.; Li, X.; Luo, J.; Huang, X.; Liu, L.; Peng, X. Alliin alters gut microbiota and gene expression of colonic epithelial tissues. J. Food Biochem. 2019, 43, 236–243. [Google Scholar] [CrossRef]
- Xiao, X.; Tang, J.J.; Peng, C.; Wang, Y.; Fu, L.; Qiu, Z.P.; Xiong, Y.; Yang, L.F.; Cui, H.W.; He, X.L. Cholesterol modification of smoothened is required for hedgehog signaling. Mol. Cell 2017, 66, 154–162. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, B.; Meng, Z.; Fang, B.; Li, T.; Sun, M.; Liu, M.; Guan, S. Alliin attenuates 1, 3-dichloro-2-propanol-induced lipogenesis in hepg2 cells through activation of the amp-activated protein kinase-dependent pathway. Life Sci. 2018, 195, 19–24. [Google Scholar] [CrossRef]
- Yoshinori, I.; Kikuko, I.; Sukenari, S.; Motonori, F. Effect of S-methylcysteine sulfoxide, S-allylcysteine sulfoxide and related sulfur-containing amino acids on lipid metabolism of experimental hypercholesterolemic rats. J. Nutr. 1973, 103, 88–92. [Google Scholar] [CrossRef]
- Sangeetha, T.; Darlin, Q.S. Preventive effect of s-allyl cysteine sulfoxide (alliin) on cardiac marker enzymes and lipids in isoproterenol-induced myocardial injury. J. Pharm. Pharmacol. 2006, 58, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. Stringtie enables improved reconstruction of a transcriptome from rna-seq reads. Nat. Biotechnol. 2015, 33, 290. [Google Scholar] [CrossRef] [PubMed]
- Daehwan, K.; Ben, L.; LS, S. Hisat: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 121–357. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for rna-seq data with deseq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Park, H.; Jeong, J.; Hyun, H.; Kim, J.; Kim, H.; Oh, H.; Choi, J.; Hwang, H.; Oh, D.; Kim, J. Effects of a hot-water extract of allium hookeri roots on bone formation in human osteoblast-like mg-63 cells in vitro and in rats in vivo. Planta Medica 2016, 82, 1410–1415. [Google Scholar] [CrossRef]
- Zou, X.; Deng, J.; Wang, Z.Y.; Zhang, M.; Sun, Y.; Li, M. Gut microbiota plays a predominant role in affecting hypolipidemic effect of deacetylated konjac glucomannan (da-kgm). Int. J. Biol. Macromol. 2022, 208, 858–868. [Google Scholar] [CrossRef]
- Rana, S.V.; Pal, R.; Vaiphei, K.; Sharma, S.K.; Ola, R.P. Garlic in health and disease. Nutr. Res. Rev. 2011, 24, 60–71. [Google Scholar] [CrossRef]
- Harunobu, B.L.; Amagase, P.H.; Itakura, A.Y. Recent advances on the nutritional effects associated with the use of garlic as a supplement. J. Nutr. 2001, 131, 951–1123. [Google Scholar]
- Lim, W.Q.; Cheam, J.Y.; Law, J.W. Role of garlic in chronic diseases: Focusing on gut microbiota modulation. Prog. Microbes Mol. Biol. 2022, 5, 12. [Google Scholar] [CrossRef]
- Chao, Y.; Li, L.; Yang, L.; Hui, L.; Wang, S.; Sun, G. Anti-obesity and hypolipidemic effects of garlic oil and onion oil in rats fed a high-fat diet. Nutr. Metab. 2018, 15, 43. [Google Scholar] [CrossRef]
- Gilham, D.; Labonté, E.D.; Rojas, J.C.; Jandacek, R.J.; Howles, P.N.; Hui, D.Y. Carboxyl ester lipase deficiency exacerbates dietary lipid absorption abnormalities and resistance to diet-induced obesity in pancreatic triglyceride lipase knockout mice. J. Biol. Chem. 2007, 282, 24642–24649. [Google Scholar] [CrossRef]
- Bengtsson-Ellmark, S.H.; Nilsson, J.; Orho-Melander, M.; Dahlenborg, K.; Groop, L.; Bjursell, G. Association between a polymorphism in the carboxyl ester lipase gene and serum cholesterol profile. Eur. J. Hum. Genet. 2004, 12, 627–632. [Google Scholar] [CrossRef]
- Hong, C.; Tontonoz, P. Liver x receptors in lipid metabolism: Opportunities for drug discovery. Nat. Rev. Drug Discov. 2014, 13, 433–444. [Google Scholar] [CrossRef]
- Jia, G.; Qiong, Z.; Yong-Hua, W. Health effects of omega-3 polyunsaturated fatty acids in common diseases. Int. Food Res. J. 2021, 28, 1098–1108. [Google Scholar] [CrossRef]
- Dang, C.V. Myc on the path to cancer. Cell 2012, 149, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, T.; Shimano, H.; Yahagi, N.; Kato, T.; Atsumi, A.; Yamamoto, T.; Inoue, N.; Ishikawa, M.; Okada, S.; Ishigaki, N.; et al. Crucial role of a long-chain fatty acid elongase, elovl6, in obesity-induced insulin resistance. Nat. Med. 2007, 13, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Meng, T.; Zuo, L.; Bei, Y.; Zhang, Q.; Su, Z.; Huang, Y.; Pang, J.; Xiang, Q.; Yang, H. Xyloketal b attenuates fatty acid-induced lipid accumulation via the srebp-1c pathway in nafld models. Mar. Drugs 2017, 15, 163. [Google Scholar] [CrossRef]
- Chen, G.; Liang, G.; Ou, J.; Goldstein, J.L.; Brown, M.S. Central role for liver x receptor in insulin-mediated activation of srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc. Natl. Acad. Sci. USA 2004, 101, 11245–11250. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Wu, M.S.; Lin, Y.F.; Chen, C.R.; Peng, C.C. Nifedipine modulates renal lipogenesis via the ampk-srebp transcriptional pathway element-binding proteins 1/2 (srebp1/2). Int. J. Mol. Sci. 2019, 20, 1570. [Google Scholar] [CrossRef]
- Ma, F.; Luo, L.; Gao, X. Metabolite and transcriptome analyses revealed the modulation of fructo-oligosaccharide on ileum metabolism of taiping chickens. J. Appl. Microbiol. 2022, 132, 2249–2261. [Google Scholar] [CrossRef] [PubMed]
- Maksymchuk, O.; Kashuba, V. Dietary lipids and environmental xenobiotics as risk factors for prostate cancer: The role of cytochrome p450. Pharmacol. Rep. 2019, 71, 826–832. [Google Scholar] [CrossRef]
- Wang, H.; Tan, H.; Zhan, W.; Song, L.; Zhang, D.; Chen, X.; Lin, Z.; Wang, W.; Yang, Y.; Wang, L. Molecular mechanism of fufang zhenzhu tiaozhi capsule in the treatment of type 2 diabetes mellitus with nonalcoholic fatty liver disease based on network pharmacology and validation in minipigs. J. Ethnopharmacol. 2021, 274, 114056. [Google Scholar] [CrossRef]
- Garcia, M.; Leonardi, R.; Zhang, Y.M.; Rehg, J.E.; Jackowski, S. Germline deletion of pantothenate kinases 1 and 2 reveals the key roles for coa in postnatal metabolism. PLoS ONE 2012, 7, e40871. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Bao, T.; Yu, H.; Lisha, A.; Li, H.F.; Ba, G.; Cho, S. Metabolomics investigation on antiobesity effects of corydalis bungeana on high-fat high-sugar diet-induced obese rats. Chin. Herb. Med. 2022, 14, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Zangar, R.C.; Davydov, D.R.; Verma, S. Mechanisms that regulate production of reactive oxygen species by cytochrome p450. Toxicol. Appl. Pharmacol. 2004, 199, 316–331. [Google Scholar] [CrossRef]
- Jaroenporn, S.; Yamamoto, T.; Itabashi, A.; Nakamura, K.; Azumano, I.; Watanabe, G.; Taya, K. Effects of pantothenic acid supplementation on adrenal steroid secretion from male rats. Biol. Pharm. Bull. 2008, 31, 1205–1208. [Google Scholar] [CrossRef]
- Ombrone, D.; Salvatore, F.; Ruoppolo, M. Quantitative liquid chromatography coupled with tandem mass spectrometry analysis of urinary acylglycines: Application to the diagnosis of inborn errors of metabolism. Anal. Biochem. 2011, 417, 122–128. [Google Scholar] [CrossRef]
- JE, P. In vivo studies on the metabolism of hexanedioic acid. Clin. Chim. Acta 1975, 58, 43–50. [Google Scholar]
- Yamaguchi, Y.; Honma, R.; Yazaki, T.; Shibuya, T.; Kumagai, H. Sulfuric odor precursor s-allyl-l-cysteine sulfoxide in garlic induces detoxifying enzymes and prevents hepatic injury. Antioxidants 2019, 8, 385. [Google Scholar] [CrossRef]
- Li, J.F. Study on the Pharmacokinetics of Garlic Sulfur-Containing Compounds and Correlation with H_2S Action. Master’s Thesis, Xinjiang Medical University, Xinjiang, China, 2016. (In Chinese). [Google Scholar]
- Jiang, M.T.; Huang, X.S. Current status of research on the biological activity of alliin. Food Ferment. Ind. 2020, 46, 264–269. (In Chinese) [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B. Mechanisms and regulation of cholesterol homeostasis. Nature reviews. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- You, W.Q.; Ke, J.; Chen, Y.; Cai, Z.; Huang, Z.; Hu, P.; Wu, X. Sqle, a key enzyme in cholesterol metabolism, correlates with tumor immune infiltration and immunotherapy outcome of pancreatic adenocarcinoma. Front. Immunol. 2022, 13, 864244. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.J.; Zhou, Y.X.; Wu, L.K.; Wang, Y.C.; Teng, X.S.; Yao, J. Circ_0000182 promotes cholesterol synthesis and proliferation of stomach adenocarcinoma cells by targeting mir-579-3p/sqle axis. Discov. Oncol. 2023, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- David, C.J.; Chen, M.; Assanah, M.; Canoll, P.; Manley, J.L. Hnrnp proteins controlled by c-myc deregulate pyruvate kinase mrna splicing in cancer. Nature 2010, 463, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.A.; Sherman, M.; Kalman, D.; Morgan, E.T. Expression of udp-glucuronosyltransferase isoform mrnas during inflammation and infection in mouse liver and kidney. Drug Metab. Dispos. 2006, 34, 351–353. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zou, X.; Du, Y.; Pan, Z.; He, F.; Sun, Y.; Li, M. Integrated Transcriptomics and Metabolomics Reveal the Mechanism of Alliin in Improving Hyperlipidemia. Foods 2023, 12, 3407. https://doi.org/10.3390/foods12183407
Zhang M, Zou X, Du Y, Pan Z, He F, Sun Y, Li M. Integrated Transcriptomics and Metabolomics Reveal the Mechanism of Alliin in Improving Hyperlipidemia. Foods. 2023; 12(18):3407. https://doi.org/10.3390/foods12183407
Chicago/Turabian StyleZhang, Min, Xiaoying Zou, Yixuan Du, Zhuangguang Pan, Fangqing He, Yuanming Sun, and Meiying Li. 2023. "Integrated Transcriptomics and Metabolomics Reveal the Mechanism of Alliin in Improving Hyperlipidemia" Foods 12, no. 18: 3407. https://doi.org/10.3390/foods12183407
APA StyleZhang, M., Zou, X., Du, Y., Pan, Z., He, F., Sun, Y., & Li, M. (2023). Integrated Transcriptomics and Metabolomics Reveal the Mechanism of Alliin in Improving Hyperlipidemia. Foods, 12(18), 3407. https://doi.org/10.3390/foods12183407





