Abstract
Polyphenols, as common components with various functional activities in plants, have become a research hotspot. However, researchers have found that the bioavailability and bioactivity of plant polyphenols is generally low because they are usually in the form of tannins, anthocyanins and glycosides. Polyphenol-rich fermented foods (PFFs) are reported to have better bioavailability and bioactivity than polyphenol-rich foods, because polyphenols are used as substrates during food fermentation and are hydrolyzed into smaller phenolic compounds (such as quercetin, kaempferol, gallic acid, ellagic acid, etc.) with higher bioactivity and bioavailability by polyphenol-associated enzymes (PAEs, e.g., tannases, esterases, phenolic acid decarboxylases and glycosidases). Biotransformation pathways of different polyphenols by PAEs secreted by different microorganisms are different. Meanwhile, polyphenols could also promote the growth of beneficial bacteria during the fermentation process while inhibiting the growth of pathogenic bacteria. Therefore, during the fermentation of PFFs, there must be an interactive relationship between polyphenols and microorganisms. The present study is an integration and analysis of the interaction mechanism between PFFs and microorganisms and is systematically elaborated. The present study will provide some new insights to explore the bioavailability and bioactivity of polyphenol-rich foods and greater exploitation of the availability of functional components (such as polyphenols) in plant-derived foods.
1. Introduction
Fermented food has been an essential part of the human diet for thousands of years. In the past, fermentation was commonly applied to extend the shelf life of foods and improve their flavor, etc. [1]. According to the origins of food materials, fermented foods could be divided into plant sources and animal sources. Plant fermented foods commonly include soy sauce, wine, vinegar, fruit, kimchi, etc., while animal fermented foods commonly include ham, sausage, cheese, yogurt, etc. In the past, researchers paid more attention to the flavor and texture of fermented foods. In the food industry, additionally, it is also expected to obtain fermented products with better flavors and more popular tastes [2]. But as the concept of a healthy diet has taken root, in addition to the flavor and preservation of fermented foods people are also paying attention to the functional activity and health benefits of fermented foods [3]. Many fermented foods are found to have great functional activities, such as hypoglycemic [4], antioxidant [5], hypolipidemic [6], and antihypertension effects [7], and they also improve the human intestinal environment [8], etc. This might be attributed to the possibility that the fermentation processes influences the characteristics of food components [9]. During the fermentation process, some functional microorganisms produce secondary metabolites with anti-pathogenic microbial activities [10]. Moreover, microorganisms could degrade proteins into bioactive peptides [11] and synthesize extracellular polysaccharides with strong biological activities [12,13]. In recent years, researchers have found that polyphenols in plant fermented foods may be the main components conferring their many functional activities [14]. Therefore, the research on polyphenols in plant fermented foods has become increasingly popular.
Polyphenols are a class of plant secondary metabolites with multiple biological activities that are synthesized by plants in the presence of harsh climatic environments or pathogens [15]. As shown in Figure 1, polyphenols are mainly divided into flavonoids and non-flavonoids. Up to now, more than 8000 kinds of phenolic substances have been identified, among which more than 4000 kinds of flavonoids have been identified. Flavonoids are compounds with a parent nucleus structure of two benzene rings connected by three carbon atoms, which can be divided into flavanols, flavones, chalcones, isoflavones, flavanols and flavanones according to their structure. Non-flavonoids include phenolic acids, tannins, astragalus and lignans, etc., mainly referring to a class of substances that must contain at least one aromatic nucleus and more than one hydroxyl group [16]. Phenolic acids are mainly divided into two categories according to their structures, namely hydroxybenzoic acid and hydroxycinnamic acid. Polyphenols are the main bioactive components in fermented plant foods, such as whole-grain fermented foods, vegetable fermented foods and fruit fermented foods. However, only a small amount (5–10%) of the polyphenols in food are absorbed into the body; the unabsorbed substances enter the colon and partially interact with intestinal microorganisms, thus playing a role in promoting the health of the host [17]. Rodríguez-Daza et al. [18] suggested that polyphenols could promote the growth of probiotic bacteria and inhibit the growth of harmful bacteria at the same time. A new term “duplibiotic” was proposed to describe the dual action of polyphenols. In addition, polyphenols promote the secretion of polyphenol-related enzymes (tannase, gallate decarboxylase, esterase, phenolic acid decarboxylase and glycosidase) by intestinal microorganisms to metabolize difficult-to-use phenolics into nutritional growth factors. In a similar way, microorganisms interact with polyphenols during food fermentation. Under the dual action of polyphenols, the quality of fermented foods will be changed. Meanwhile, the hydrolysis of polyphenols by microorganisms could improve the bioavailability of polyphenols in food materials and produce a series of bioactive components that are more favorable for human digestion, absorption and utilization [19].
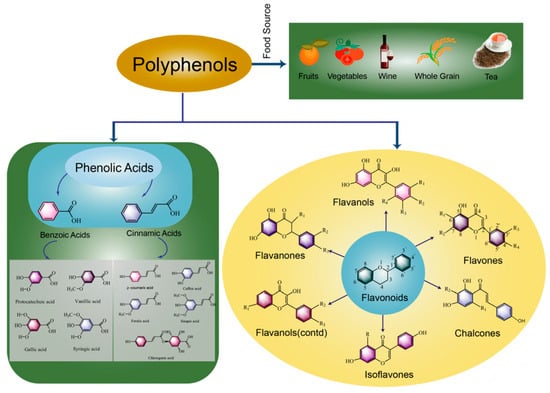
Figure 1.
Classification and food sources of polyphenols in fermented foods (polyphenols are classified into two main groups: flavonoids and phenolic acids).
Although plant foods are rich in polyphenols, there are not enough studies focusing on the overall variation of polyphenols and the changes in their functional activities during fermentation in plant foods. Based on the current research status, the aim of this review is to integrate and analyze the current research on the interaction between polyphenols and microorganisms during polyphenol-rich fermented food (PFF) fermentation. The mechanisms of polyphenol–microbial interactions during PFF fermentation were systematically described from four aspects: (1) changes of functional activity and polyphenols in food after fermentation, (2) polyphenol-associated enzymes (PAEs) secreted by microorganisms, (3) biotransformation pathways of different polyphenols and (4) effects of polyphenols on microorganisms affecting the quality of PFFs. The present study might provide theoretical support for further exploration of the functional activity and bioavailability of PFFs.
2. Effect of Microorganisms on Polyphenol Metabolism during PFF Fermentation
Although polyphenols show good biological activity, they still show the drawbacks of a short half-life and low bioavailability after oral administration [20,21]. This may be due to the interaction between the polyphenols and the polysaccharides combined and the formation of glycosides through coulombic interactions, hydrogen bonds, π-π interaction forces, etc., and then the biological activity of the glycosylated polyphenols is reduced. In addition, flavan-3-ol or flavan-3,4-diol form condensed tannins (proanthocyanidins), ellagic acid and gallic acid form hydrolysable tannins, ellagic acid combines with sugar molecules through lipid bonds to form ellagitannins, and gallic tannins and other phenolic polymers have more complex structures and larger molecular weights, which make them difficult to be absorbed after entering the human body, thus reducing their bioavailability. However, during the fermentation process of PFFs, the glycosidic bonds that are contained in large-molecular-weight polyphenols could be metabolized by microorganisms and thus degraded, and the degraded glucoside could be directly utilized by beneficial intestinal microorganisms in the human body as a direct carbon source, thus increasing the abundance of beneficial bacteria. Phenolic compounds can also be metabolized by microorganisms into other small-molecule phenols to enhance their biological activities. During PFF fermentation, key fermentable microorganisms can secrete a variety of PAEs (tannases, esterases, phenolic acid decarboxylases, glycosidases) to release the bound phenols that are contained in a food matrix. This biotransformation induces an increase in the amount and type of free phenols in fermented foods and thus enhances the bioactivity and bioavailability of PFFs. For the details, the mechanism of actions of microorganisms in metabolizing polyphenols during food fermentation to enhance the functional activity of foods are systematically summarized as follows.
2.1. Changes of Functional Activity and Polyphenols in PFFs after Fermentation
After spontaneous fermentation or probiotic fermentation, the nutrients contained in PFFs will be fully utilized by microorganisms as substrates, which will result in significant changes in the composition of nutrients contained in PFFs [22]. As one of the important components in PFFs, the type and content of polyphenols also changed significantly. As shown in Abbreviations, all the polyphenols contained in PFFs showed significant differences after fermentation. Generally, the change trend is a transition from large-molecule to small-molecule polyphenols. Macromolecular polyphenols mainly include proanthocyanidins, flavonoid glycosides, ellagitannins, etc. The molecular weight of these polyphenols is usually >500 kDa, and they will be hydrolyzed into small-molecule polyphenols such as aglycone, ellagic acid and catechin by microorganisms. For example, pomegranate, bayberry and other foods are rich in ellagic tannins [23]; during the fermentation, tannase secreted by microorganisms could degrade ellagic tannin to some intermediates (punicalin, gallagic acid), and eventually into ellagic acid. In addition, the content of rutin in food will decrease significantly during fermentation, and the corresponding content of quercetin will increase significantly. This is because rutin is a flavonoid glycoside formed by the combination of quercetin and rutinoside. Under the action of glycosidase secreted by microorganisms, the glycosidic bond contained in rutin is hydrolyzed and broken to form quercetin [24]. In addition, soy is rich in flavonoids, but usually in the form of glucosides. For example, after fermentation, daidzin, glycitin, and genistin are hydrolyzed by probiotics with β-glycosidase activity to form aglycones, such as genistein, daidzein, and glycitein [25]. Moreover, foods rich in proanthocyanidins include blueberry, strawberry, black wolfberry, black mulberry, sorghum, etc. The contents of proanthocyanidins in those food will be significantly reduced after fermentation [26]; this is because anthocyanins such as malvidin-3-glucosides, cyanindin-3-glucoside, peonidin-3-glucoside, and malvidin-3-galactoside could be metabolized to some free phenolic acids (e.g., syringic acid, gallic acid and protocatechuic acid) by probiotics [6]. In addition, foods such as blueberries, blackberries and green coffee beans are rich in chlorogenic acid, which is formed by a lipid bond between caffeic acid and quinic acid. Microorganisms capable of secreting cinnamic esterase can hydrolyze lipid bonds and metabolize chlorogenic acid to caffeic acid and quinic acid during fermentation.
During these fermentation processes, the composition and content of polyphenols in foods are usually significantly changed, and these changes also alter the functional activities of foods, as different polyphenols have different physiological activities or there is a strong or weak gap for the same physiological activity. In general, polyphenols with small molecular mass and unbound glycosides showed stronger physiological activity. However, food fermentation is the process of converting large-molecule polyphenols into small molecules. Therefore, foods normally have higher nutritional value after fermentation. For example, quercetin is a flavonol that easily combines with glycosides to form flavonoid aglycones, such as isoquercitrin and rutin. But in fact, quercetin shows stronger antioxidant, anti-inflammatory activity and an ability to protect the liver [27]. In the fermentation process of sourdough bread [24], black tartary buckwheat [28], jujubes [29] and other foods, rutin and isoquercitrin are all hydrolyzed to quercetin. Moreover, for example, glucosides such as glycitin, daidzin and genistin, which soybeans are rich in, decrease significantly after fermentation, and correspondingly, aglycones such as daidzein, glycitein and genistein increase significantly. Their molecular weights decreased from 416.378 kDa, 432.378 kDa, 446.404 kDa to 284.263 kDa, 254.238 kDa and 270.237 kDa, respectively. Meanwhile, fermented soybeans had higher significant antioxidant activity and better DNA damage protection [30]. In the same way, a similar transformation process was reported for tannins. For example, punicalagin is an ellagic tannin that is abundant in pomegranate, bayberry and other fruits. With a molecular weight of 1084.718 kDa, punicalagin can be bio-converted to ellagic acid with a molecular weight of 302.193 kDa and shows great biological activity. This transformation process usually occurs during the fermentation of fruit juices such as pomegranate juice, bayberry juice and raspberry juice [7]. Moreover, gallic acid (GA), with a molecular weight of 170.12 kDa, is a small-molecule polyphenol with strong antioxidant, anti-inflammatory, anti-hyperlipidemia and other functional activities, and also shows better bioavailability. However, in some foods, GA is usually linked in the form of lipid bonds to form hydrolyzed tannins in some foods, such as green tea, grape, mango, etc. After fermentation, GA could be enriched to enhance the functional activity of food [31]. For example, as a kind of fermented tea, pu’er tea has a better weight loss effect than other teas, and the content of GA is regarded as one of the important indicators to evaluate the quality of pu’er tea [32]. Based on the results of previous studies, more and more researchers are trying to screen microorganisms with better PAE activity to ferment food, to improve the nutritional value of food. As shown in Table 1, some lactic acid bacteria, Bifidobacteria and molds have been proved to show strong polyphenol hydrolase activity and were able to be used for the hydrolysis of macromolecular polyphenols.

Table 1.
Changes of polyphenols in PFFs after fermentation. (↑: Increased polyphenol content and functional activity of PPFs; ↓: The content of polyphenols was reduced).
2.2. PAEs Secreted by Microorganisms during Fermentation
2.2.1. Tannase
Tannins are large polyphenolic polymers that form complexes with proteins or other macromolecules (cellulose, starch), mostly composed of lipid bonds among gallic acids, catechins, ellagic acid and glucosidic bonds, with molar masses between 300–3000 Da. The complex structure of tannins gives them a wide range of biological activities, but they are difficult to hydrolyze and absorb by the body. It has been shown that the higher the molecular weight of tannins is, the lower is the bioactivity and bioavailability [46,47]. The anti-nutritional properties of tannins could be reduced by decreasing the tannin polymerization during food fermentation, and the hydrolysis of tannin components by tannases is a feasible method to enhance the digestibility and bioactivity of fermented foods. Tannase, also known as tannin acyl hydrolase, is a functional enzyme produced by microorganisms that, with the ability to hydrolyze hydrolysable tannins, condense tannins, pentosidine tannins and gallic acid lipids into small molecules of phenolics and glucose. Normally, tannase can be produced by fungi [48] and bacteria [49], i.e., yeast, Lactobacillus plantarum, Lactobacillus, Bacillus, and Aspergillus niger [50].
As typical condensed tannins, proanthocyanidins (PACs) are pigment components that are widely found in plants. It is a multimer (dimer, trimer up to decamer; below pentamer is called oligomeric PACs, above pentamer is called polymeric PACs) that are formed by the combination of different amounts of catechins and epicatechin [51]. Proanthocyanidins have been reported to have antioxidant [52], antimicrobial [53] and antihypertension properties [54]. However, PACs have low bioavailability after oral administration, and generally PACs with polymerization degree > 4 are not directly absorbed by the body, and small amounts of monomers or dimers could be detected in the plasma [55]. Unabsorbed PACs are eventually fermented and degraded by colonic microflora into small molecular phenolic acids such as hydroxyphenylacetic acid, hydroxyphenylpropionic acid, hydroxyphenylpentanoic acid and 3,4-dihydroxyphenylacetic acid [56]. For example, green tea is a food abundant in PACs, such as (−)-epigallocatechin-3-gallate (EGCG), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG), (−)-epicatechin (EC), gallocatechins (GA) and gallocatechin gallate [57]. Among them, EGCG is the main component, accounting for about 50–70% of the tea polyphenol content [58]. EGCG gives green tea great bioactivity, but the complexity of its structure leads to poor absorption, reduced bioavailability and bioactivity, DNA damage after oral administration [59], and may enhance the incidence of colon cancer [60]. Under the hydrolysis of tannases, EGCG could be hydrolyzed to ECG, EGC, EC and GA, which reduces the potential risk of green tea while increasing its bioavailability and antioxidant activity [61]. For example, Govindarajan et al. [62] investigated the biotransformation of tea samples by tannases produced by E. cloacae strain 41 and evaluated the antioxidant capacity of the biotransformed tea. They found that the quality and taste of the tea were improved after tannase treatment. Particularly, the content of ester molecules (EGCG and ECG) in tea liquor also decreased significantly after biotransformation, while the content of non-ester molecules (EGC, EC and GA) and antioxidant capacity increased significantly.
Moreover, blueberry is also a kind of fruit that is rich in PACs, and long-term intake of blueberries is beneficial in preventing cardiovascular disease, type II diabetes and neurological decline [63]. Similarly, grapes are abundant in PACs, and the content and structure of PACs are important factors in the quality of grapes. The fermentation of grapes and blueberries is commonly applied to make wine, but astringency is one of the key factors that affect the sensory characteristics of the wine [64]. The astringency is attributed to the binding of PACs to salivary proteins and oral epithelial proteins, and PACs with a higher degree of polymerization and gallic acyl content showed a stronger astringent taste [65], but gallic acid does not contribute to astringency [66]. This may be due to the more complex structural composition of highly polymerized PACs, which are more likely to bind to salivary proteins to form complexes and thus cause astringency [67], whereas the monomeric flavan-3-ol hardly reacts with salivary proteins [68]. For example, Kyraleou et al. [69] found a positive correlation between astringency and total phenolic concentration, with PACs playing the most influential role. EGCs, monomers and dimers are reduced in astringency because the B-ring is hydroxylated. Therefore, wine showed more astringency as the degree of polymerization of PAC increases.. This outcome is consistent with the studies of Sun et al. [70]. Therefore, adjusting the concentration of these water-soluble tannins during the wine-making process could improve the astringency of less vintage wines [71]. Tannases secreted by microorganisms have been found to reduce the astringency of wines and remove tannin content. For instance, González-Royo et al. [72] added three inactive dry yeast strains for wine fermentation and found a significant decrease in the astringency index as well as a significant decrease in the average degree of polymerization (mDP) of PACs. They found that dry yeast strains alleviated the astringency of the wine by eliminating PACs with high mDP.
2.2.2. Esterase
Hydroxycinnamic acid derivatives are the most abundant polyphenols in cereals. This group includes mainly ferulic acid, caffeic acid, erucic acid and p-coumaric acid, which usually exist in the bound state in cereals and are linked to proteins, lignin, cellulose and other cell wall structural components through lipid bonds, forming methyl ferulate, methyl p-caffeate, methyl p-coumarate, methyl erucic acid and other bound phenols [73]. The bound phenols are not easily digested and absorbed in the body [74]. Esterase is an enzyme with hydroxycinnamate hydrolytic activity secreted by bacteria or fungi (Lactobacillus gastricus, Lactobacillus acidophilus, Bifidobacterium bifidum, Aspergillus niger, Myceliophthora thermophila) [75]. Among them, feruloyl esterase (FE) is the most common type of esterase. As shown in Table 2, FEs are classified into four main types, which catalyze the hydrolysis of hydroxycinnamic acid esters in plant cell walls to release hydroxycinnamic acid alone, including ferulic acid [76]. Among the hydroxycinnamic acids that are contained in cereals, ferulic acid is the main and potentially therapeutic substance for treating diabetes, Alzheimer’s disease and other oxidation-related diseases [77]. Currently, strains with the ability to secrete FE are widely used in the solid-state fermentation of cereals and beverages (Miso, cake and Shochu). For example, Annél Smit et al. [78] found that the esterified bound phenols in grapes are released by cinnamoyl esterases into free phenolic acids (ferulic acid, p-coumarin, caffeic acid) during the wine-brewing process, which are then hydrolyzed by microbially secreted phenolic acid decarboxylases into volatile phenols such as 4-vinyl and 4-ethyl derivatives to enrich the aroma of the wine. Zhang et al. [79] used multiple bacteria (Klebsiella pneumoniae JZE, Bacillus mycoides JEF, Bacillus cereus JZ3, Bacillus velezensis G1, Siccibacter colletis G2, and Bacillus subtilis strain G6) in a synergistic manner to ferment waste wine lees (mainly composed of wheat bran and sorghum) left after brewing, which enhances the release of ferulic acid effectively, and the release of ferulic acid from the cell wall of grains by FE makes fermented grain foods more nutritious. In addition, chocolate is a food with a special flavor formed by fermentation of cocoa beans. Cocoa beans are rich in ferulic acid, which has obvious astringency and bitterness [80]. They cannot produce the chocolate flavor by direct baking without fermentation, but after fermentation, ferulic acid is released and the antioxidant activity of cocoa beans is significantly increased, the bitterness is significantly reduced and the flavor is more abundant [81]. The four types of FE (A [82], B [83], C [84,85], D [86]) are listed in Table 2. In addition, microorganisms capable of secreting different types of FE and polyphenols that FE is capable of hydrolyzing are listed in Table 2.

Table 2.
Classification of FE.
2.2.3. Phenolic acid Decarboxylase
Phenolic acid decarboxylase (PAD) is an enzyme secreted by microorganisms (yeast, lactic acid bacteria, Mycobacteria) that converts hydroxycinnamic acid into volatile phenols. PAD transfers protons from the active site to the nucleophilic C2 carbon of the substrate through deprotonation of the phenolic acid hydroxyl group to form intermediates, which promote electron transfer of the ester and lead to C-C bond breakage, resulting in the formation of volatile phenols such as 4-vinyl guaiacol (4-VG) [87]. There are many microorganisms with PAD activity during food fermentation [88]. For example, Svensson et al. [89] fermented four strains of Lactobacillus with culture mediums containing caffeic acid, ferulic acid and naringenin-7-O-glucoside, respectively. They found that ferulic acid, caffeic acid and naringenin-7-O-glucoside were all able to be metabolized by L. plantarum and L. ferumum to small-molecule metabolites (dihydroferulic acid, vinylcatechol, ethylcatechol and naringenin) by PADs. After fermentation, the phenolic acid esters contained in sorghum dough are metabolized to free phenolic acids, and further metabolized to additional metabolites with smaller molecular weights, and improve the nutritional value and taste of fermented sorghum food. A similar conclusion was also reported by Ripari et al. [90], who co-fermented whole wheat dough with Lactobacillus and yeast to release the bound ferulic acid and then converted the released ferulic acid into dihydroferulic acid and volatile metabolites; they also suggested that the texture of bread could be improved by targeted conversion of phenolic acids during the fermentation process. Moreover, Xu et al. [91] found that yeast could grow in culture medium containing ferulic acid (50 mg/L) and metabolize ferulic acid to 4-VG, which was an essential flavoring substance in wine, white wine and beer. Rosimi et al. [92] isolated six strains of lactic acid bacteria (Lactobacillus plantarum and Lactobacillus pentosus) with PAD activities from spontaneously fermented kimchi, and they were able to metabolize ferulic acid and p-coumaric acid to produce volatile phenols. Mycobacteria are also capable of secreting PADs. For example, Linke et al. [93] found that ascomycete Isaria farinose, used to ferment wheat bran and sugar beets, was able to secrete PADs to metabolize ferulic acid to produce 4-VG.
2.2.4. Glucosidase
Polyphenols can be decorated with monosaccharides and are usually combined with monosaccharides such as glucose, rhamnose, fructose, arabinose and galactose [94]. Glycosides are linked to phenolic hydroxyl groups by α-glycosidic bonds or β-glycosidic bonds to form O-glycosides or C-glycosides. O-glycosylation reduces the biological activity of flavonoids, while C-glycosylation enhances the benefits to humans [95]. The glycosylated polyphenols are more water-soluble; their polarity increases with increasing molecular weight, and the chemical structure and degree of hydrolyzability of these compounds determine their bioavailability [96]. Glycosides have more stable structures when combined with sugars to form glycosides, but for the bioavailability, the glycosides need to be hydrolyzed to release the biological activity. For example, C-glycosides show higher biological activity, but they are harder for the body to degrade and utilize than the O-glycosides [97]. And phenols with a single hydroxyl group show very low antioxidant activity when attached to glycosides, probably because the glycosidic bond replaces the hydroxyl group, thus leaving the phenol without a free hydroxyl group to neutralize free radicals [98]. In addition, glycosylation also leads to a reduction in the bioactivity and bioavailability of flavonoids (e.g., glycosylation reduces the antioxidant activity of catechin) [99,100]. Hostetler et al. [101] analyzed the anti-inflammatory activity of celery extracts and found that extracts rich in flavonoid aglycones were effective in reducing the production of inflammatory factors, while the extracts rich in glycosides were not. Additionally, they formulated diets containing glycosides or aglycones to feed mice separately and found that the absorption of aglycones was significantly higher than that of glycosides.
Glycosidase is an enzyme secreted by microorganisms that can degrade plant cell walls and break glycosidic bonds, and the metabolized glycosides can be converted into carbon sources for microbial growth. During PFF fermentation, glycosidases play a role in improving the flavor and increasing the nutritional function of PFFs. As shown in Table 3, naringin is one of the factors of excessive bitterness in citrus juice. Peng et al. [102] used glycosidase to treat citrus juice and reduced the concentration of naringin from 356.33 mg/L to 10.52 mg/L, which improved the flavor and increased the antioxidant activity. Quercetin, kaempferol and isorhamnetin in sea buckthorn are usually combined with glucose, rhamnose or rutinosek. Gu et al. [103] screened a fungal strain with high glycosidase activity to ferment sea buckthorn, and they found that the total phenolic content of fermented sea buckthorn was significantly increased due to the increased content of flavonol aglycones (quercetin, kaempferol, isorhamnetin). And the antioxidant activity of sea buckthorn was positively correlated with the aglycone content, suggesting that aglycones released by glycosidase hydrolysis of glycosides could increase the antioxidant activity of phenols. Baffi et al. [104] purified a strain of Bacillus sp. with glycosidase activity of 20.0 U/mg, which is highly tolerant to ethanol and glucose and could be used for brewing, and they found that the addition of Bacillus sp. during wine fermentation could hydrolyze glycosidic terpenes, release free terpenes and enhance the aroma of the wine. Moreover, Na Guo et al. [105] used Monascus to solidly ferment mulberry leaves, and found that the glycosides gradually decreased with increasing fermentation time and were almost completely consumed at the end, while at the same time the content of aglycones (quercetin and kaempferol) reached a maximum. They suggested that during the fermentation process, the highly active cellulase broke down the cell walls of mulberry leaves, dissociated the polyphenols in the cell walls of mulberry leaves, and then hydrolyzed the β-glycosidic bonds by β-glycosidase, thus converting the dissociated polyphenolic glycosides into aglycones. Other studies [106,107,108,109] that showed similar results are also summarized in Table 3.

Table 3.
Studies related to the hydrolysis of polyphenolic glycosides by glycosidases during PFF fermentation.
2.3. Biotransformation Pathways of Polyphenols during PFF Fermentation
2.3.1. Biotransformation Pathway of Tannin
The common tannins in food are hydrolysable tannins and condensed tannins, which have different biotransformation pathways. Hydrolysable tannins are composed of glycosidic and lipid bonds. Condensed tannins, also known as proanthocyanidins, are complex polymers with flavan-3-ol as the base structure [110]. As shown in Figure 2A, Ascacio et al. [111] studied the degradation process of prune tannin and found that prune tannin belonged to a polymer of ellagic acid (ellagitannin), in which, under the action of tannase secreted by Aspergillus niger, the lipid bond was broken to form punicalin, and under the action of β-glucosidase, the glycosidic bond was cleaved from polysaccharides to form gallagic acid, and finally an ellagic acid monomer was formed. Ellagitannin is also present in pomegranate [23], which could be biotransformed during the fermentation process to enhance its bioavailability [112]. For example, Valero-Cases et al. [113] fermented pomegranate juice with Lactobacillus and found a 40–70% reduction in ellagic tannins and a significant increase in ellagic acid content. As shown in Figure 2B, Chavez-Gonzalez et al. [114] used Aspergillus niger GH1 solid-state fermentation of tannic acid (pentagalloylglucose, a pentagalloyl tannin) and found that the degree of polymerization of tannic acid decreased sequentially from pentagalloylglucose to tetragalloylglucose, trigalloylglucose and digalloylglucose. This biotransformation is the result of tannic acid breaking the ether bond in the presence of tannase, releasing a gallic group and eventually forming a large amount of gallic acid. Moreover, the gallic acid is further cleaved into pyrogallol, piruvate, and resocinol by the action of descarboxylase, pyrogallol 1, 2 dioxigenase and 5-oxo-6 methyl-hexanoate.
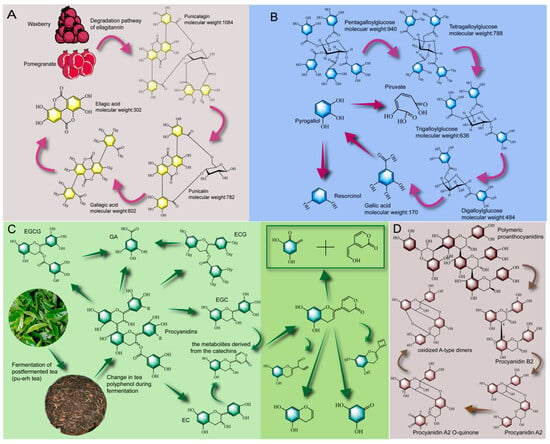
Figure 2.
Biotransformation pathways of tannin contained in PFFs: Biotransformation pathway of ellagic tannin rich in Myrica rubra and pomegranate (A), biotransformation pathway of tannic acid (B), biotransformation pathway of tea tannin (C), biotransformation pathway of proanthocyanidins (D).
Proanthocyanidins are commonly found in grapes, cocoa, tea and other raw food materials, whose structure is composed of two aromatic rings and a six-membered pyran ring connected by a carbon skeleton, and usually exist in the form of a degree of polymerization > 5 [115]. Its constituent unit is mainly flavan-3-ol and can be divided into type A and type B according to C4-C8 or C6-C8 interflavan linkages. Type A is usually produced by oxidation of type B [116]. As shown in Figure 2D, Wen et al. [117] found that the polymerized proanthocyanidins could be depolymerized into oligomeric proanthocyanidins (dimer, proanthocyanidin B2) during the fermentation process. De Taeye et al. [118] found that proanthocyanidins B2 (C4-C8) contained in cocoa could be converted into proanthocyanidins A2 (C2-O-C7) during baking and fermentation, further formed A2 open structure intermediates, and eventually formed oxidation products (oxidized A-type dimers). In addition, tea polyphenols normally exist in the form of anthocyanins before fermentation. After fermentation, the levels of EGCG and ECG in tea were significantly reduced [119]. In the fermentation of tea, aspergillus is the dominant species and can secrete polyphenol-related enzymes that metabolize tea polyphenols [120]. In the initial stage of fermentation, macromolecular compounds containing ester bonds, such as EGCG and ECG, were mainly present in tea. However, after long-term fermentation, the content of flavanols containing no ester linkage of gallic acid (i.e., EC, GA, and EGC) were gradually increased. Furthermore, under the longer fermentation, these compounds are further degraded into lower molecular substances with simple structures. Qin et al. [121] found that catechin produces an intermediate metabolite of catechin (3, 6-dihydro-6-oxo-2H-pyran moity) during the fermentation process, which is further cleaved into various small molecular substances (Figure 2C). In other words, in the early stage of fermentation, the degradation of gallate bonds was the main pathway for the metabolism of tea polyphenols, but in the later stage of fermentation, the small molecular substances produced by the B-ring biotransformation of flavanols were the reasons for the metabolism of catechins. A similar conclusion was also reported by An et al. [122]. In general, the increase in the biological activity of the fermented tea and the improvement in flavor were both caused by the hydrolysis of the ester bond of tea tannin and the degradation of flavonol glycosides, and finally the formation of GA and other small molecular metabolites, and the contribution of fungi (Aspergillus) was greater than that of bacteria [32]. In summary, tannin, as a polyphenol with high polymerization degree, exists widely in the PFFs. Under the action of tannase, polyphenol could be hydrolyzed into small molecular substances by means of lipid bond rupture, glycosidic bond rupture, ether bond rupture and oxidation, etc.
2.3.2. Biotransformation Pathway of Phenolic Acid
As shown in Figure 3A, Morata et al. [123] added hydroxycinnamic acid to produce wine by fermentation of grapes with Saccharomyces cerevisiae. It was found that the addition of caffeic acid produced two new vinyl phenol pigments (malvidin-3-O-glucoside-4-vinylcatechol and malvidin-3-O-glucoside-4-vinylguaiacol), which is formed by condensation of malvidin-3-O-glucoside and vinylguaiacol formed by decarboxylation of caffeic acid with the action of phenolic acid decarboxylase. Additionally, with the addition of p-coumaric acid, ferulic acid could produce five new vinylphenol compounds, and the content of malvidin-3-O-glucide-4-vinyl phenyl was significantly higher than that of other compounds. As the hydroxycinnamic acid in the grape was converted into the vinyl phenol pyran anthocyanin under the action of the esterase, the production of 4-ethylphenol (a negative flavor substance, the excessive content of which can affect the flavor of wine) in wine was reduced and the stability of the wine in the aging process was improved [124]. A similar conclusion was also reported by Vanbeneden Nele et al. [125], who found that 4-vinylguaiacol showed a high content in all kinds of beers (wheat beers, blond specialty beers and dark specialty beers), which was due to the conversion of hydroxycinnamic acid into ethyl derivatives by Saccharomyces cerevisiae. Hydroxycinnamic acid in malt is usually acylated with plant cell walls to form the conjugated phenols, and the fermentation process could promote the release of hydroxycinnamic acid and improve the phenolic aroma of wort. Moreover, Ricci et al. [126] found that phenolic acid decarboxylase and phenolic acid reductase secreted by Lactobacillus in elderberry juice could metabolize caffeic acid and protocatechuic acid to dihydrocaffeic acid and catechol. Dihydrocaffeic acid and catechol showed stronger antioxidant activity as hydrolysates of hydroxycinnamic acid [127]. As shown in Figure 3B, Sáyago-Ayerdi et al. [128] found that phenolic acids were mainly present in the form of hydroxycinnamates in Hibiscus sabdariffa L. calyces. Among them, 5-caffeoylquinic acid, p-coumaroylquinic acid and 5-feruloylquinic acid were the main compounds. With the extension of the fermentation time, hydroxycinnamates were hydrolyzed by esterase into hydroxycinnamic acid (caffeic acid, p-coumaric acid, and ferulic acid), further metabolized into dihydrohydroxycinnamic acid by phenolic acid reductase, and finally degraded into phenylacetic acid, benzoic acid and its derivatives under the action of decarboxylase. Similarly, after fermentation, hydroxycinnamic acid could form abundant metabolites such as 3-(ρ-hydroxycinnamic acid, 3-(3-hydroxycinnamic acid) and 4-hydroxycinnamic acid [129]. Moreover, as shown in Figure 3C, during the fermentation process, p-coumaric acid and ferulic acid were easily decarboxylated by phenolic acid decarboxylase, forming metabolites 4-vinylphenol (4-VP) [130] and 4-vinylguaiacol (4-VG) [131]. Further, Kitaoka et al. [132] found that 4-VP and 4-VG could be connected to glucose through ether bonds to form 4-Vinyl Phenyl β-D-glucide (4-VPG) and 4-Vinyl Guaiacol β-D-glucide (4-VGG), and the glucose moiety on 4-VPG and 4-VGG was then connected to xylose through ether bonds to form 4-4-vinylphenol β-primeveroside and 4-vinylguaiacol β-primeveroside (4-VGP), and this biotransformation pathway might be catalyzed by glycosyltransferases [133]. In summary, the bound phenolic acids presented in the food were predominantly hydroxycinnamates, which readily esterified with 1 L-3/4/5- quinic acid to form isomers or derivatives of chlorogenic acid [134]. They could be gradually transformed into ethyl derivatives or small molecular metabolites such as phenylpropionic acid, phenylacetic acid and catechol under the action of esterase, phenolic acid reductase and phenolic acid decarboxylase secreted by microorganisms.
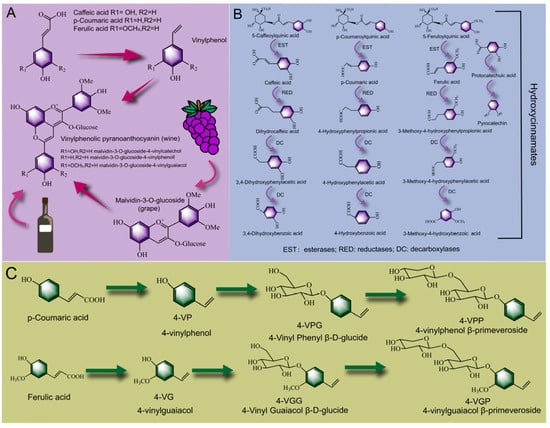
Figure 3.
Biotransformation pathways of phenolic acid in PFF fermentation process: the combination pathway of hydroxycinnamic acid and grape anthocyanin in the wine-brewing process (A), the biotransformation pathway of caffeic acid ester, ferulic acid ester and p-coumaric acid ester (B), the biotransformation process of p-coumaric acid and ferulic acid into volatile flavor substances (C).
2.3.3. Biotransformation Pathway of Flavonoids
Common flavonoids in plants are mainly divided into four types: flavanol, flavone, flavnone and isoflavone. As shown in Figure 4A, flavonoids (kaempferol, quercetin, myricetin, isorhamnetin, etc.) normally combine with sugar groups (glucose, galactose, rhamnose, xylose, arabinose, etc.) to form glycosides, and the binding mode is that aglycones replace the hydroxyl groups at C-3, C-5, C-7, C-3′, C-4′ and C-5′ of flavonoids to connect with flavonoid aglycones. In particular, the ligands are most easily attached to the C-3 position of flavonoid skeletons to form O-glycosides [135]. Hundreds of glycosides are formed by the connection between the glycoconjugates and flavonoids found in plants. As shown in Figure 4B, catechin is a monomeric flavonoid present in plants, which is often linked to gallic acid by an ester bond to form catechin gallate and is widely found in tea and cocoa [136]. Under the action of tannase and esterase, catechin gallate could be converted into catechin and gallic acid [137], thus improving the absorption capacity and lipid metabolism of the human body [138]. Flavanone is characterized by no double bond in the C2-C3 position of its skeleton structure, like naringenin is the representative. Glucose is easily linked to the C7 position of naringenin, forming naringenin 7-O-glucoside, which could be metabolized by Lactobacillus plantarum into naringenin during fermentation [139]. In addition, baicalin, a representative of flavone, is formed by linking glucuronide at the C7 position of baicalein, and a high content of baicalin was found in Scutellaria baicalensisis, which has been widely used as a traditional Chinese herbal medicine [140]. Chen et al. [141] used a Lactobacillus brevis RO1 with β-glucosidase activity to ferment milk containing Scutellaria baicalensisis extract and found that the content of baicalein in fermented milk was significantly increased. This indicated that baicalin could be hydrolyzed by fermentation to form baicalein. Moreover, isoflavones (such as genistin, glycitin and daidzin) are widely found in legumes. And it has been proved that isoflavone aglycone can be absorbed by the human body more quickly after taking the same dose of isoflavone glycoside and isoflavone aglycone [142]. During the fermentation process, many microorganisms could secrete esterase, α-glucosidase, β-galactosidase and β-glucosidase to convert isoflavone from glycoside to aglycone [143]. For example, Chen et al. [144] found that the content of isoflavone 7-O-glucoside (genistin, glycitin and daidzin) was significantly decreased while the content of isoflavones (genistein, glycitein, daidzein) was significantly increased in soybean after being fermented by fungi, and the antioxidant capacity of fermented soybean food was significantly improved. Di Gioia et al. [145] also showed a similar conclusion that Bifidobacterium could effectively bio-convert bean flavonoids to aglycons in fermented soybean and bean milk. As shown in Figure 4 C, as one of the most common flavanols in plants, rutin is a glycosidic compound formed by connecting quercetin with a glucoside and a rhamnoside, which could be hydrolyzed to a variety of free flavonoid aglycones. For example, Gu et al. [103] isolated a fungus with glycosidase activity from fermented tea and used it to ferment Hippophae rhamnoides leaves and found that the content of rutin was significantly reduced after fermentation, while the content of flavanols such as kaempferol, quercetin and isorhamnetin significantly increased. The above results showed that rutin could be bio-transformed into free aglycones through deglycosylation, dihydroxylation, and O-methylation, etc. Rutin in litchi peel could also be deglycosylated by Aspergillus awamori fermentation, and this process could be divided into two pathways. In the first, rutin was hydrolyzed to quercetin-3-glucoside and quercetin, and in the second, rutin was further deglycosylated to form kaempferol-3-glucoside and kaempferol after being dehydroxylated to form kaempferol-3-rutinoside [146]. Moreover, Song et al. [147] adopted lactic acid bacteria and cellulase combined to ferment the cactus flavonoid extract (the main flavanols were isorhamnetin-3-O-rutinoside, isorhamnetin-3-O-glucoside, isorhamnetin and quercetin). After fermentation for 24 h, the content of isorhamnetin-3-O-rutinoside decreased to an undetectable level. The content of isorhamnetin-3-O-glucoside increased at the first 12 h and then rapidly decreased but could not be detected after 60 h of fermentation. On the contrary, the contents of quercetin and isorhamnetin were positively correlated with the fermentation duration. The above results indicated that isorhamnetin-3-O-rutinoside could be effectively converted into free aglycones by lactic acid bacteria. In addition, Duckstein, Lorenz and Stintzing [148] pointed out that quercetin and kaempferol were preferentially converted to quercetin and kaempferol; with the prolongation of fermentation time, the C-ring cleavage in the skeletal structures of quercetin and kaempferol, respectively, derived their respective B-ring fission products (protocatechuic acid and 4-hydroxybenzoic acid). Moreover, both quercetin and kaempferol could generate the A-ring fission product phloroglucinol after the C-ring cleavage. Similar results were found in another study by Wang et al. [149] on the transformation pathway of polyphenol contained in fermented red jujube; the authors found that during the fermentation process, the degradation pathway of rutin was as follows: rutin→quercetin-3-O-glucoside→quercetin→taxifolin→3,4-dihydroxyphenylacetic acid→protocatechuic acid→4- hydroxybenzoic acid. In summary, the metabolic pathway of flavonoids in the fermentation process was mainly the conversion from glycosides to aglycones, and this process was mainly catalyzed by the related glycosidases secreted by functional microorganisms. In addition, similar structures of flavonoids may undergo metabolic processes such as dehydroxylation, dihydroxylation and O- methylation.
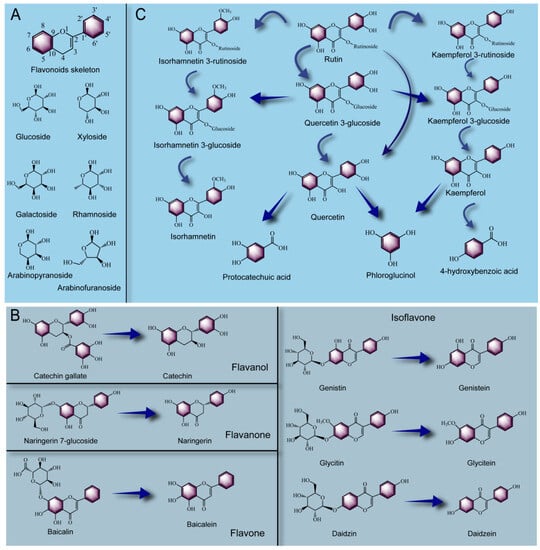
Figure 4.
Flavonoid skeleton and its easily linked glycosyl structure producing glycosides (A), biotransformation pathway of flavonoids with several different structures (B), biotransformation pathway of rutin (C).
3. Effects of Polyphenols Contained in PFFs on Microorganism
During the PFF fermentation process, microorganisms and polyphenols are in an interactive relationship. Microorganisms metabolize polyphenols into free small molecular substances, and polyphenols selectively promote the growth of certain beneficial bacteria (through hydrolysis of ester bonds and rupture of glycosidic bonds to provide a carbon source for functional microorganisms (lactic acid bacteria, yeast, and bifidobacterium) to promote their growth). In addition, polyphenols could inhibit the growth of a variety of pathogenic bacteria (e.g., E. coli, Staphylococcus aureuswine, etc.), and the inhibition might be achieved by causing the leakage of protein and nucleic acid from pathogenic cells, reducing intracellular ATP concentration, destroying cell walls or cell membranes [150]. In addition, PFFs could profoundly affect the intestinal microflora of the human body after consumption, thereby exerting a positive effect on human health.
3.1. Effects of Polyphenols on Microbial Growth in PFF Fermentation Process
As discussed in the previous sections, many beneficial bacteria (yeast, lactic acid bacteria, and Bifidobacterium) can metabolize polyphenols such as tannin, hydroxycinnamic acid, and flavonoid glycoside into small molecular products during the PFF fermentation process. Meanwhile, polyphenols can promote the growth of beneficial microorganisms in the fermentation process and inhibit the growth of pathogenic bacteria, thereby increasing the functional activity and safety of the fermented food [147]. As shown in Table 4, spontaneously fermented sausages are popular with consumers because of their special flavor. However, because there is no leavening agent, the special flavors entirely depend on the microorganisms existing in nature, and the biogenic amine will exceed the limited standard [148]. Zhang et al. [151] fermented rose polyphenol extract with sausage and found that rose polyphenol extract could prevent lipid oxidation of sausages and inhibit the formation of biogenic amines. In addition, rose polyphenol extract could significantly promote the growth of lactic acid bacteria while reducing the total number and diversity of bacteria, thereby improving the safety of fermented sausages effectively. Ahmed et al. [152] adopted a clove extract rich in polyphenol to improve the preservation period of beef burgers and found that clove polyphenol showed high antibacterial activity, inhibited the growth of pathogenic bacteria (disrupting the cell wall and cell membranes of pathogenic bacteria and releasing the contents of the cytoplasm) significantly, and had a broad-spectrum antibacterial effect. Clove polyphenols can also improve the lipid stability of beef burgers during storage. Moreover, Budryn et al. [153] used lactic acid bacteria to ferment legume sprouts to increase isoflavone content. After fermentation, the isoflavone content was increased from 1.1 g/100 g dw to 5.5 g/100 g dw, the number of lactic acid bacteria was also increased by 2 log 10 CFU/mL, and various pathogenic bacteria either did not appear or the content was significantly decreased. Abbasi-Parizad et al. [154] spontaneously fermented the tomato polyphenol extract and found that in the presence of polyphenol, lactic acid bacteria became the dominant species in the natural microflora and accumulated in large amounts, while other pathogenic bacteria died because of the large amount of lactic acid that was secreted by lactic acid bacteria. Li et al. [44] adopted Lactobacillus plantarum strains and Lactobacillus fermentum strains as the leavening agents for the fermentation of blueberry juice and reported that the number of viable counts of microorganism increased by 35% compared to the control. This is mainly owing to a large accumulation of Lactobacillus plantarum and Lactobacillus fermentum. Meanwhile, the total phenol content in the blueberry juice was significantly increased, but the proanthocyanidin content was significantly decreased, while the contents of gallic acid, protocatechuic acid and myricetin were significantly increased. Moreover, the antioxidant capacity of blueberry juice was significantly improved. Similar studies [155,156,157,158,159] are also summarized in Table 4. According to those results, we could conclude that polyphenols have a significant promoting effect on the growth of lactic acid bacteria, which could assist lactic acid bacteria to become the dominant species in the microflora. At the same time, lactic acid bacteria could degrade polyphenols such as flavonoid glycosides, proanthocyanidins and hydroxycinnamic acid into free aglycones and small-molecule phenols to increase the total phenol content and biological activity of the PFFs. It is an interactive relationship, which can endow the PFFs with more nutritional functions. Meanwhile, the PFFs could inhibit the growth of pathogenic bacteria in the fermentation process, prevent the accumulation of toxic and harmful microbial secondary metabolites and improve the safety of the fermented food.

Table 4.
Effects of Polyphenols on Microbes in PFF Fermentation.
3.2. Effects of PFFs on Human Intestinal Microorganisms
Humans are hosts to a large number of diverse microflora, and the distribution of intestinal microflora has a profound impact on human health [159]. The biomass of intestinal microbiota could be as high as 1.5 kg, and more than 1000 species of microbiota have been identified, mainly including Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria [160]. According to their functions, gut microbes can be divided into beneficial bacteria, neutral bacteria and harmful bacteria. Beneficial bacteria mainly include Lactobacillus and Bifidobacterium, which can inhibit the growth of pathogenic bacteria and decompose, metabolize, absorb and digest nutrients such as polyphenols and active peptides [161]. Harmful bacteria are Staphylococcus aureuswine, Salmonella, Campylobacter and so on. If the balance of intestinal flora is broken, the large growth of harmful bacteria will affect the immune system and lead to a variety of diseases [162]. Neutral bacteria refer to bacteria that are beneficial under normal conditions but can induce diseases once they proliferate in large quantities, such as Escherichia coli and Enterococcus. A growing number of studies have demonstrated that PFFs can increase the abundance of beneficial gut microbiota. For example, black tartary buckwheat is rich in rutin. Ren et al. [28] used Bacillus sp. DU-106 to ferment black tartary buckwheat. The content of rutin in the fermented black tartary buckwheat was significantly reduced, and the contents of total phenol, quercetin and kaonferol were significantly increased. The relative abundance of Lactobacillus, Faecalibaculum and Allobaculum in the gut of black tartary buckwheat-fed mice was significantly increased. In addition, Zhou et al. [163] obtained total polyphenol extracts from a fermented tea (Fu brick tea) to study the improvement of intestinal microflora, and found that total polyphenol extracts from a fermented tea could improve intestinal oxidative stress, intestinal inflammation and repair intestinal barrier function in mice. The extracts also improved intestinal microbiota dysbiosis (increasing the abundance of important intestinal microorganisms such as Akkermansia muciniphila, Alloprevotella, Bacteroides, and Faecalibaculum). A decrease in these beneficial microorganisms will lead to a decrease in intestinal barrier function and an increase in intestinal endotoxins. Therefore, the restorative effect of PFFs on intestinal barrier functions might be achieved by increasing the number of these beneficial microorganisms. For example, Zhao et al. [164] used unfermented celery juice and probiotic fermented celery juice to conduct dietary intervention in high-fat mice. Compared with unfermented celery juice, probiotic fermented celery juice significantly changed the composition of gut microbiota and hyperglycemic symptoms in mice (increased the relative abundance of Lactobacillus, Faecalibaculum, Ruminococcaceae UCG-014 and Blautia). Those results suggested that fermented PFFs were more effective in improving human gut microbiota, which was associated with significant changes in polyphenols in PFFs.
4. Conclusions
Natural plant-derived foods are rich in polyphenols, but the bioavailability is generally low. The fermented food formed by spontaneous fermentation or probiotic fermentation often shows better flavor, better functional activity and higher bioavailability. On the one hand, during fermentation, beneficial microorganisms could secrete PAEs such as tannase, esterase, phenolic decarboxylase, glycosidase, and metabolize macromolecular-bound phenols such as tannic acid, proanthocyanidins, gallic acid esters, flavonoid glycosides, etc., into free phenols such as quercetin, phloroglucinol, kaempferol, gallic acid, etc. Those small-molecule free phenols showed higher biological activities and bioavailability than the macromolecular-bound phenols. On the other hand, due to the double action of the polyphenols, the growth of pathogenic bacteria is inhibited by the polyphenols during the PFF fermentation process, and functional microorganisms such as lactic acid bacteria, Bifidobacterium and yeast, which are selectively promoted to grow, become the dominant strains in the microflora, thereby ensuring the functional activity and safety of the PFFs. After oral administration, some of the PFFs are absorbed by human digestion and metabolism, and some of the PFFs enter the intestinal tract and are used by the intestinal microbial community, thus improving the structure of the intestinal microbial community. Through a series of pathways, PFFs finally showed a variety of functional activities beneficial to human health, such as promoting digestion, resisting oxidation, and lowering blood sugar and blood lipids.
In summary, the current review performed a systematic discussion on the current work and achievements of PFFs from different aspects. According to the existing results, PFFs have good biological activity and bioavailability. However, there are still few studies on the interaction between microorganisms and polyphenols in the PFF fermentation process, and most of the studies mainly focused on the interaction between polyphenols and intestinal microorganisms. In the future, functional microorganisms with high-activity PAEs should be further screened for fermentation of PFFs. Moreover, non-targeted metabolomics and targeted metabolomics should be applied to track the polyphenol cleavage pathway, analyze the changes in phenolic species and content before and after fermentation, and further compare the changes of PFFs biological activity and bioavailability before and after fermentation. In addition, the total phenol content in PFFs did not always increase with the fermentation time. Therefore, we need to pay more attention to the change of polyphenol content during the whole fermentation process to achieve the optimal fermentation time. Meanwhile, we also should optimize the fermentation parameters, so as to realize the maximum use of polyphenols. The exploration of these mechanisms will provide an experimental basis and theoretical support for in-depth exploration of the biological activity of polyphenols and the development of more nutritious fermented foods.
Author Contributions
Conceptualization: X.Y. and L.W.; methodology: S.C.; software: F.Y. and C.C.; validation: D.N. and Y.Y.; formal analysis: Y.L.; investigation: F.Y.; resources: Y.Y.; writing—original draft preparation: F.Y. and C.C.; writing—review and editing: F.Y., X.Y. and J.T.; visualization: Chao Chen; supervision: L.W.; project administration: Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Author Li Wang is employed by the company Kweichow Moutai Group; authors Fan Yang, Chao Chen, Derang Ni, Yubo Yang and Yuanyi Li are employed by the company Kweichow Moutai Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
| PFFs | Polyphenol-rich fermented foods |
| PAEs | Polyphenol-associated enzymes |
| 4-VP | 4-Vinylphenol |
| 4-VPG | 4-Vinyl Phenyl β-D-glucide |
| 4-VPP | 4-Vinylphenol β-primeveroside |
| 4-VG | 4-Vinylguaiacol |
| 4-VGG | 4-Vinyl Guaiacol β-D-glucide |
| 4-VGP | 4-Vinylguaiacol β-primeveroside |
| PAD | Phenolic acid decarboxylase |
| PPE | Pomegranate peel extracts |
| FE | Feruloyl esterase |
| PACs | Proanthocyanidins |
References
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M. The periodic table of fermented foods: Limitations and opportunities. Appl. Microbiol. Biotechnol. 2022, 106, 2815–2826. [Google Scholar] [CrossRef] [PubMed]
- Marti-Quijal, F.J.; Remize, F.; Meca, G.; Ferrer, E.; Ruiz, M.-J.; Barba, F.J. Fermentation in fish and by-products processing: An overview of current research and future prospects. Curr. Opin. Food Sci. 2020, 31, 9–16. [Google Scholar] [CrossRef]
- Long, X.-S.; Liao, S.-T.; Li, E.-N.; Pang, D.-R.; Li, Q.; Liu, S.-C.; Hu, T.-G.; Zou, Y.-X. The hypoglycemic effect of freeze-dried fermented mulberry mixed with soybean on type 2 diabetes mellitus. Food Sci. Nutr. 2021, 9, 3641–3654. [Google Scholar] [CrossRef] [PubMed]
- Tonolo, F.; Moretto, L.; Folda, A.; Scalcon, V.; Bindoli, A.; Bellamio, M.; Feller, E.; Rigobello, M.P. Antioxidant Properties of Fermented Soy during Shelf Life. Plant Foods Hum. Nutr. 2019, 74, 287–292. [Google Scholar] [CrossRef]
- Yue, Q.; Wang, Z.; Tang, X.; Zhao, C.; Li, K.; Su, L.; Zhang, S.; Sun, X.; Liu, X.; Zhao, L. Hypolipidemic Effects of Fermented Seaweed Extracts by Saccharomyces cerevisiae and Lactiplantibacillus plantarum. Front. Microbiol. 2021, 12, 772585. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Shu, G.; Yuan, J.; Zhang, J.; Qin, S.; Li, J. Enhanced antihypertensive potential of fermented pomegranate juice: The contribution of phenolic compounds biotransformation and the resultant angiotensin-I-converting enzyme inhibition mechanism. Food Chem. 2023, 404, 134745. [Google Scholar] [CrossRef]
- Yu, C.; Liu, Y.; Xuemei, Z.; Ma, A.; Jianxin, T.; Yiling, T. Fermented Carrot Pulp Regulates the Dysfunction of Murine Intestinal Microbiota. Oxidative Med. Cell. Longev. 2022, 2022, 2479956. [Google Scholar] [CrossRef]
- Cai, W.; Tang, F.; Zhao, X.; Guo, Z.; Zhang, Z.; Dong, Y.; Shan, C. Different lactic acid bacteria strains affecting the flavor profile of fermented jujube juice. J. Food Process. Preserv. 2019, 43, e14095. [Google Scholar] [CrossRef]
- Ashokbhai, J.K.; Basaiawmoit, B.; Sakure, A.; Das, S.; Patil, G.B.; Mankad, M.; Hati, S. Purification and characterization of antioxidative and antimicrobial peptides from lactic-fermented sheep milk. J. Food Sci. Technol. 2022, 59, 4262–4272. [Google Scholar] [CrossRef]
- Taha, S.; El Abd, M.; De Gobba, C.; Abdel-Hamid, M.; Khalil, E.; Hassan, D. Antioxidant and antibacterial activities of bioactive peptides in buffalo’s yoghurt fermented with different starter cultures. Food Sci. Biotechnol. 2017, 26, 1325–1332. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, J.; Xu, Y.; Xiao, G.; Zou, B. The effect of litchi juice on exopolysaccharide production in milk fermented by Lactobacillus casei. Int. J. Food Sci. Technol. 2018, 53, 2730–2737. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Liu, Y.; Nan, J.; Park, H.J.; Chen, Y.; Yang, L. The chemical structure and immunomodulatory activity of an exopolysaccharide produced by Morchella esculenta under submerged fermentation. Food Funct. 2021, 12, 9327–9338. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw Terefe, N.; Augustin, M.A. Fermentation for tailoring the technological and health related functionality of food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 2887–2913. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Garcia-Alonso, A.; Sánchez-Paniagua López, M.; Manzanares-Palenzuela, C.L.; Redondo-Cuenca, A.; López-Ruíz, B. Edible plant by-products as source of polyphenols: Prebiotic effect and analytical methods. Crit. Rev. Food Sci. Nutr. 2022, 1–22. [Google Scholar] [CrossRef]
- Estrela, J.M.; Mena, S.; Obrador, E.; Benlloch, M.; Castellano, G.; Salvador, R.; Dellinger, R.W. Polyphenolic Phytochemicals in Cancer Prevention and Therapy: Bioavailability versus Bioefficacy. J. Med. Chem. 2017, 60, 9413–9436. [Google Scholar] [CrossRef]
- Siemińska-Kuczer, A.; Szymańska-Chargot, M.; Zdunek, A. Recent advances in interactions between polyphenols and plant cell wall polysaccharides as studied using an adsorption technique. Food Chem. 2022, 373, 131487. [Google Scholar] [CrossRef] [PubMed]
- Castellone, V.; Bancalari, E.; Rubert, J.; Gatti, M.; Neviani, E.; Bottari, B. Eating Fermented: Health Benefits of LAB-Fermented Foods. Foods 2021, 10, 2639. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Tomás-Barberán, F.A.; García-Villalba, R. Pomegranate Fruit and Juice (cv. Mollar), Rich in Ellagitannins and Anthocyanins, Also Provide a Significant Content of a Wide Range of Proanthocyanidins. J. Agric. Food Chem. 2019, 67, 9160–9167. [Google Scholar] [CrossRef] [PubMed]
- Lukšič, L.; Bonafaccia, G.; Timoracka, M.; Vollmannova, A.; Trček, J.; Nyambe, T.K.; Melini, V.; Acquistucci, R.; Germ, M.; Kreft, I. Rutin and quercetin transformation during preparation of buckwheat sourdough bread. J. Cereal Sci. 2016, 69, 71–76. [Google Scholar] [CrossRef]
- Li, S.; Jin, Z.; Hu, D.; Yang, W.; Yan, Y.; Nie, X.; Lin, J.; Zhang, Q.; Gai, D.; Ji, Y.; et al. Effect of solid-state fermentation with Lactobacillus casei on the nutritional value, isoflavones, phenolic acids and antioxidant activity of whole soybean flour. LWT 2020, 125, 109264. [Google Scholar] [CrossRef]
- Nie, Q.; Feng, L.; Hu, J.; Wang, S.; Chen, H.; Huang, X.; Nie, S.; Xiong, T.; Xie, M. Effect of fermentation and sterilization on anthocyanins in blueberry. J. Sci. Food Agric. 2017, 97, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kim, Y.; Lee, J. Comparison of antioxidant and anti-inflammatory activity of quercetin, isoquercitrin and rutin against alcohol-induced liver injury in HepG2 Cells. FASEB J. 2018, 32, 670.60. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, S.; Xia, Y.; Huang, J.; Ye, J.; Xuan, Z.; Li, P.; Du, B. Probiotic-fermented black tartary buckwheat alleviates hyperlipidemia and gut microbiota dysbiosis in rats fed with a high-fat diet. Food Funct. 2021, 12, 6045–6057. [Google Scholar] [CrossRef]
- Ren, W.; Ma, Y.; Liu, D.; Liang, P.; Du, J.; Yang, S.; Tang, L.; Wu, Y. Chemical composition analysis, antioxidant activity, and target cell-based screening of the potential active components in jujube and its fermented product. J. Food Sci. 2022, 87, 664–685. [Google Scholar] [CrossRef]
- Xiao, Y.; Fan, J.; Chen, Y.; Rui, X.; Zhang, Q.; Dong, M. Enhanced total phenolic and isoflavone aglycone content, antioxidant activity and DNA damage protection of soybeans processed by solid state fermentation with Rhizopus oligosporus RT-3. RSC Adv. 2016, 6, 29741–29756. [Google Scholar] [CrossRef]
- Liu, M.; Xie, H.; Ma, Y.; Li, H.; Li, C.; Chen, L.; Jiang, B.; Nian, B.; Guo, T.; Zhang, Z.; et al. High Performance Liquid Chromatography and Metabolomics Analysis of Tannase Metabolism of Gallic Acid and Gallates in Tea Leaves. J. Agric. Food Chem. 2020, 68, 4946–4954. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, S.; Yu, Q.; Wang, J.; Deng, Y.; Hua, J.; Zhou, Q.; Yuan, H.; Jiang, Y. Chemical profile of a novel ripened Pu-erh tea and its metabolic conversion during pile fermentation. Food Chem. 2022, 378, 132126. [Google Scholar] [CrossRef] [PubMed]
- D’Antuono, I.; Bruno, A.; Linsalata, V.; Minervini, F.; Garbetta, A.; Tufariello, M.; Mita, G.; Logrieco, A.F.; Bleve, G.; Cardinali, A. Fermented Apulian table olives: Effect of selected microbial starters on polyphenols composition, antioxidant activities and bioaccessibility. Food Chem. 2018, 248, 137–145. [Google Scholar] [CrossRef]
- Zulkawi, N.; Ng, K.H.; Zamberi, R.; Yeap, S.K.; Jaganath, I.B.; Satharasinghe, D.; Yeah Yong, C.; Jamaluddin, A.B.; Tan, S.W.; Ho, W.Y.; et al. The in vivo hepato-recovery effects of the polyphenol-rich fermented food Xeniji™ on ethanol-induced liver damage. RSC Adv. 2017, 7, 38287–38299. [Google Scholar] [CrossRef]
- Chan, C.-L.; Gan, R.-y.; Shah, N.P.; Corke, H. Enhancing antioxidant capacity of Lactobacillus acidophilus-fermented milk fortified with pomegranate peel extracts. Food Biosci. 2018, 26, 185–192. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, H.; Xu, J. Characterization, Antioxidant and Anti-Inflammation Capacities of Fermented Flammulina velutipes Polyphenols. Molecules 2021, 26, 6205. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Yu, H.; Qi, M.; Yuan, X.; Ruan, Z.; Hu, C.; Xiao, M.; Xue, Y.; Yao, Y.; Liu, Q. Biotransformation of citrus fruits phenolic profiles by mixed probiotics in vitro anaerobic fermentation. LWT 2022, 160, 113087. [Google Scholar] [CrossRef]
- Gao, Q.; Song, Y.; Liang, Y.; Li, Y.; Chang, Y.; Ma, R.; Cao, X.; Wang, S. Dynamics of Physicochemical Properties, Functional Compounds and Antioxidant Capacity during Spontaneous Fermentation of Lycium ruthenicum Murr. (Qinghai–Tibet Plateau) Natural Vinegar. Foods 2022, 11, 1344. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Chen, J.; Li, X.-L.; Yi, K.; Ye, Y.; Liu, G.; Wang, S.-F.; Hu, H.-L.; Zou, L.; Wang, Z.-G. Dynamic changes in antioxidant activity and biochemical composition of tartary buckwheat leaves during Aspergillus niger fermentation. J. Funct. Foods 2017, 32, 375–381. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, M.; Cui, C.; Huang, M.; Lin, L.; Feng, Y. A new sight on soy isoflavones during the whole soy sauce fermentation process by UPLC-MS/MS. LWT 2021, 152, 112249. [Google Scholar] [CrossRef]
- Piao, Y.-Z.; Eun, J.-B. Physicochemical characteristics and isoflavones content during manufacture of short-time fermented soybean product (cheonggukjang). J. Food Sci. Technol. 2020, 57, 2190–2197. [Google Scholar] [CrossRef]
- Choi, I.; Kim, Y.; Park, Y.; Seog, H.; Choi, H. Anti-obesity activities of fermented soygerm isoflavones by Bif idobacterium breve. BioFactors 2007, 29, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Pan, M.; El-Nezami, H.S.; Wan, J.M.F.; Wang, M.F.; Habimana, O.; Lee, J.C.Y.; Louie, J.C.Y.; Shah, N.P. Effects of Lactic Acid Bacteria-Fermented Soymilk on Isoflavone Metabolites and Short-Chain Fatty Acids Excretion and Their Modulating Effects on Gut Microbiota. J. Food Sci. 2019, 84, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tao, Y.; Li, D.; Wen, G.; Zhou, J.; Manickam, S.; Han, Y.; Chai, W.S. Fermentation of blueberry juices using autochthonous lactic acid bacteria isolated from fruit environment: Fermentation characteristics and evolution of phenolic profiles. Chemosphere 2021, 276, 130090. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Wang, J.; Wang, Y.; Xu, Z.; Li, B.; Meng, X.; Sun, X.; Zhu, J. Influence of fermentation by lactic acid bacteria and in vitro digestion on the biotransformations of blueberry juice phenolics. Food Control. 2022, 133, 108603. [Google Scholar] [CrossRef]
- Jia, B.; Wei, Z.; Kong, X.; Xia, S.; Gan, L.; Han, S. Antioxidant Properties of Larch Tannins with Different Mean Polymerization Degrees: Controlled Degradation Based on Hydroxyl Radical Degradation. J. Agric. Food Chem. 2022, 70, 9367–9376. [Google Scholar] [CrossRef]
- Zhong, H.; Xue, Y.; Lu, X.; Shao, Q.; Cao, Y.; Wu, Z.; Chen, G. The Effects of Different Degrees of Procyanidin Polymerization on the Nutrient Absorption and Digestive Enzyme Activity in Mice. Molecules 2018, 23, 2916. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Q.; McKinstry, W.J.; Ren, B. Characterization of a tannin acyl hydrolase from Streptomyces sviceus with substrate preference for digalloyl ester bonds. Appl. Microbiol. Biotechnol. 2015, 99, 2663–2672. [Google Scholar] [CrossRef]
- Aguilar, C.N.; Rodríguez, R.; Gutiérrez-Sánchez, G.; Augur, C.; Favela-Torres, E.; Prado-Barragan, L.A.; Ramírez-Coronel, A.; Contreras-Esquivel, J.C. Microbial tannases: Advances and perspectives. Appl. Microbiol. Biotechnol. 2007, 76, 47–59. [Google Scholar] [CrossRef]
- Lekshmi, R.; Arif Nisha, S.; Thirumalai Vasan, P.; Kaleeswaran, B. A comprehensive review on tannase: Microbes associated production of tannase exploiting tannin rich agro-industrial wastes with special reference to its potential environmental and industrial applications. Environ. Res. 2021, 201, 111625. [Google Scholar] [CrossRef]
- de la Iglesia, R.; Milagro, F.I.; Campión, J.; Boqué, N.; Martínez, J.A. Healthy properties of proanthocyanidins. BioFactors 2010, 36, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-X.; Feng, H.-L.; Ding, Y.-M.; Chai, W.-M.; Xiang, Z.-H.; Shi, Y.; Chen, Q.-X. Structure characterization of proanthocyanidins from Caryota ochlandra Hance and their bioactivities. Food Chem. 2014, 155, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Mo, M.; Zhang, K.; Bi, Y.; Kong, F. Preparation, characterization and biological activity of proanthocyanidin-chitosan nanoparticles. Int. J. Biol. Macromol. 2021, 188, 43–51. [Google Scholar] [CrossRef]
- Fan, W.; Zong, H.; Zhao, T.; Deng, J.; Yang, H. Bioactivities and mechanisms of dietary proanthocyanidins on blood pressure lowering: A critical review of in vivo and clinical studies. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Li, D.; Ho, C.-T.; Li, J.; Wan, X. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016, 7, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F.; Pérez-Jiménez, J.; Touriño, S.; Serrano, J.; Fuguet, E.; Torres, J.L.; Goñi, I. Proanthocyanidin metabolites associated with dietary fibre from in vitro colonic fermentation and proanthocyanidin metabolites in human plasma. Mol. Nutr. Food Res. 2010, 54, 939–946. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Tea Catechins and Polyphenols: Health Effects, Metabolism, and Antioxidant Functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef]
- Bertram, B.; Bollow, U.; Rajaee-Behbahani, N.; Bürkle, A.; Schmezer, P. Induction of poly(ADP-ribosyl)ation and DNA damage in human peripheral lymphocytes after treatment with (−)-epigallocatechin-gallate. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003, 534, 77–84. [Google Scholar] [CrossRef]
- Malik, A.; Azam, S.; Hadi, N.; Hadi, S.M. DNA degradation by water extract of green tea in the presence of copper ions: Implications for anticancer properties. Phytother. Res. 2003, 17, 358–363. [Google Scholar] [CrossRef]
- Macedo, J.A.; Ferreira, L.R.; Camara, L.E.; Santos, J.C.; Gambero, A.; Macedo, G.A.; Ribeiro, M.L. Chemopreventive potential of the tannase-mediated biotransformation of green tea. Food Chem. 2012, 133, 358–365. [Google Scholar] [CrossRef]
- Govindarajan, R.K.; Khanongnuch, C.; Mathivanan, K.; Shyu, D.J.H.; Sharma, K.P.; De Mandal, S. In-vitro biotransformation of tea using tannase produced by Enterobacter cloacae 41. J. Food Sci. Technol. 2021, 58, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ju, Y.; Ma, T.; Zhang, J.; Fang, Y.; Sun, X. New perspectives on the biosynthesis, transportation, astringency perception and detection methods of grape proanthocyanidins. Crit. Rev. Food Sci. Nutr. 2021, 61, 2372–2398. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Wollmann, N.; Schieberle, P.; Hofmann, T. Reconstitution of the Flavor Signature of Dornfelder Red Wine on the Basis of the Natural Concentrations of Its Key Aroma and Taste Compounds. J. Agric. Food Chem. 2011, 59, 8866–8874. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Brandão, E.; Mateus, N.; de Freitas, V. Sensorial properties of red wine polyphenols: Astringency and bitterness. Crit. Rev. Food Sci. Nutr. 2017, 57, 937–948. [Google Scholar] [CrossRef]
- Ramos-Pineda, A.M.; García-Estévez, I.; Brás, N.F.; Martín del Valle, E.M.; Dueñas, M.; Escribano Bailón, M.T. Molecular Approach to the Synergistic Effect on Astringency Elicited by Mixtures of Flavanols. J. Agric. Food Chem. 2017, 65, 6425–6433. [Google Scholar] [CrossRef]
- Morzel, M.; Canon, F.; Guyot, S. Interactions between Salivary Proteins and Dietary Polyphenols: Potential Consequences on Gastrointestinal Digestive Events. J. Agric. Food Chem. 2022, 70, 6317–6327. [Google Scholar] [CrossRef]
- Kyraleou, M.; Kotseridis, Y.; Koundouras, S.; Chira, K.; Teissedre, P.-L.; Kallithraka, S. Effect of irrigation regime on perceived astringency and proanthocyanidin composition of skins and seeds of Vitis vinifera L. cv. Syrah grapes under semiarid conditions. Food Chem. 2016, 203, 292–300. [Google Scholar] [CrossRef]
- Sun, B.; Sá, M.d.; Leandro, C.; Caldeira, I.; Duarte, F.L.; Spranger, I. Reactivity of Polymeric Proanthocyanidins toward Salivary Proteins and Their Contribution to Young Red Wine Astringency. J. Agric. Food Chem. 2013, 61, 939–946. [Google Scholar] [CrossRef]
- McRae, J.M.; Schulkin, A.; Kassara, S.; Holt, H.E.; Smith, P.A. Sensory Properties of Wine Tannin Fractions: Implications for In-Mouth Sensory Properties. J. Agric. Food Chem. 2013, 61, 719–727. [Google Scholar] [CrossRef] [PubMed]
- González-Royo, E.; Esteruelas, M.; Kontoudakis, N.; Fort, F.; Canals, J.M.; Zamora, F. The effect of supplementation with three commercial inactive dry yeasts on the colour, phenolic compounds, polysaccharides and astringency of a model wine solution and red wine. J. Sci. Food Agric. 2017, 97, 172–181. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, X.; Liu, Y.; Zhang, M.; Zhang, R.; Abbasi, A.M.; You, L.; Li, T.; Liu, R.H. Comparative assessment of phytochemical profile, antioxidant capacity and anti-proliferative activity in different varieties of brown rice (Oryza sativa L.). LWT 2018, 96, 19–25. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef] [PubMed]
- Schär, A.; Sprecher, I.; Topakas, E.; Faulds, C.B.; Nyström, L. Hydrolysis of Nonpolar n-Alkyl Ferulates by Feruloyl Esterases. J. Agric. Food Chem. 2016, 64, 8549–8554. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Soomro, L.; Wei, X.; Yuan, X.; Gu, T.; Li, Z.; Wang, Y.; Bao, Y.; Wang, F.; Wen, B.; et al. Directed evolution of feruloyl esterase from Lactobacillus acidophilus and its application for ferulic acid production. Bioresour. Technol. 2021, 332, 124967. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef]
- Smit, A.; Cordero Otero, R.R.; Lambrechts, M.G.; Pretorius, I.S.; van Rensburg, P. Enhancing Volatile Phenol Concentrations in Wine by Expressing Various Phenolic Acid Decarboxylase Genes in Saccharomyces cerevisiae. J. Agric. Food Chem. 2003, 51, 4909–4915. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.; Li, Y.; Feng, Z.; Zhang, X.; Zhou, R.; Liu, C.; Yang, L. The Combined Cultivation of Feruloyl Esterase-Producing Strains with CMCase and Xylanase-Producing Strains Increases the Release of Ferulic Acid. Microorganisms 2022, 10, 1889. [Google Scholar] [CrossRef]
- Kongor, J.E.; Hinneh, M.; de Walle, D.V.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—A review. Food Res. Int. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- Melo, T.S.; Pires, T.C.; Engelmann, J.V.P.; Monteiro, A.L.O.; Maciel, L.F.; Bispo, E.d.S. Evaluation of the content of bioactive compounds in cocoa beans during the fermentation process. J. Food Sci. Technol. 2021, 58, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.P.; vanKuyk, P.A.; Kester, H.C.M.; Visser, J. The Aspergillus niger faeB gene encodes a second feruloyl esterase involved in pectin and xylan degradation and is specifically induced in the presence of aromatic compounds. Biochem. J. 2002, 363, 377–386. [Google Scholar] [CrossRef]
- Crepin, V.F.; Faulds, C.B.; Connerton, I.F. A non-modular type B feruloyl esterase from Neurospora crassa exhibits concentration-dependent substrate inhibition. Biochem. J. 2003, 370, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Conesa, M.-T.; Crepin, V.F.; Goldson, A.J.; Williamson, G.; Cummings, N.J.; Connerton, I.F.; Faulds, C.B.; Kroon, P.A. The feruloyl esterase system of Talaromyces stipitatus: Production of three discrete feruloyl esterases, including a novel enzyme, TsFaeC, with a broad substrate specificity. J. Biotechnol. 2004, 108, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Moukouli, M.; Topakas, E.; Christakopoulos, P. Cloning, characterization and functional expression of an alkalitolerant type C feruloyl esterase from Fusarium oxysporum. Appl. Microbiol. Biotechnol. 2008, 79, 245–254. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Wood, T.M.; Williamson, G.; Faulds, C.; Hazlewood, G.P.; Black, G.W.; Gilbert, H.J. A modular esterase from Pseudomonas fluorescens subsp. cellulosa contains a non-catalytic cellulose-binding domain. Biochem. J. 1993, 294, 349–355. [Google Scholar] [CrossRef]
- Sheng, X.; Lind, M.E.S.; Himo, F. Theoretical study of the reaction mechanism of phenolic acid decarboxylase. FEBS J. 2015, 282, 4703–4713. [Google Scholar] [CrossRef]
- Nyachoti, C.M.; Atkinson, J.L.; Leeson, S. Sorghum tannins: A review. World’s Poult. Sci. J. 1997, 53, 5–21. [Google Scholar] [CrossRef]
- Svensson, L.; Sekwati-Monang, B.; Lutz, D.L.; Schieber, A.; Gänzle, M.G. Phenolic Acids and Flavonoids in Nonfermented and Fermented Red Sorghum (Sorghum bicolor (L.) Moench). J. Agric. Food Chem. 2010, 58, 9214–9220. [Google Scholar] [CrossRef]
- Ripari, V.; Bai, Y.; Gänzle, M.G. Metabolism of phenolic acids in whole wheat and rye malt sourdoughs. Food Microbiol. 2019, 77, 43–51. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Cui, Y.; Zeng, D.; Bao, X. Increasing the level of 4-vinylguaiacol in top-fermented wheat beer by secretory expression of ferulic acid decarboxylase from Bacillus pumilus in brewer’s yeast. Biotechnol. Lett. 2020, 42, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Rosimin, A.A.; Kim, K.-S. Production of volatile phenols by kimchi Lactobacillus plantarum isolates and factors influencing their phenolic acid decarboxylase gene expression profiles. Food Res. Int. 2015, 78, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Linke, D.; Riemer, S.J.L.; Schimanski, S.; Nieter, A.; Krings, U.; Berger, R.G. Cold generation of smoke flavour by the first phenolic acid decarboxylase from a filamentous ascomycete—Isaria farinosa. Fungal Biol. 2017, 121, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Kytidou, K.; Artola, M.; Overkleeft, H.S.; Aerts, J.M.F.G. Plant Glycosides and Glycosidases: A Treasure-Trove for Therapeutics. Front. Plant Sci. 2020, 11, 357. [Google Scholar] [CrossRef]
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef]
- Johnson, J.B.; Mani, J.S.; Broszczak, D.; Prasad, S.S.; Ekanayake, C.P.; Strappe, P.; Valeris, P.; Naiker, M. Hitting the sweet spot: A systematic review of the bioactivity and health benefits of phenolic glycosides from medicinally used plants. Phytother. Res. 2021, 35, 3484–3508. [Google Scholar] [CrossRef]
- Goh, L.M.L.; Barlow, P.J. Flavonoid recovery and stability from Ginkgo biloba subjected to a simulated digestion process. Food Chem. 2004, 86, 195–202. [Google Scholar] [CrossRef]
- Zhang, X.; Thuong, P.T.; Min, B.-S.; Ngoc, T.M.; Hung, T.M.; Lee, I.S.; Na, M.; Seong, Y.-H.; Song, K.-S.; Bae, K. Phenolic Glycosides with Antioxidant Activity from the Stem Bark of Populus davidiana. J. Nat. Prod. 2006, 69, 1370–1373. [Google Scholar] [CrossRef]
- Plumb, G.W.; De Pascual-Teresa, S.; Santos-Buelga, C.; Cheynier, V.; Williamson, G. Antioxidant properties of catechins and proanthocyanidins: Effect of polymerisation, galloylation and glycosylation. Free. Radic. Res. 1998, 29, 351–358. [Google Scholar] [CrossRef]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the Flavonoid C-glycosides and Health Benefits. Crit. Rev. Food Sci. Nutr. 2016, 56, S29–S45. [Google Scholar] [CrossRef]
- Hostetler, G.; Riedl, K.; Cardenas, H.; Diosa-Toro, M.; Arango, D.; Schwartz, S.; Doseff, A.I. Flavone deglycosylation increases their anti-inflammatory activity and absorption. Mol. Nutr. Food Res. 2012, 56, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Li, R.; Ni, H.; Li, L.J.; Li, Q.B. The effects of α-L-rhamnosidase, β-D-glucosidase, and their combination on the quality of orange juice. J. Food Process. Preserv. 2021, 45, e15604. [Google Scholar] [CrossRef]
- Gu, Q.; Duan, G.; Yu, X. Bioconversion of Flavonoid Glycosides from Hippophae rhamnoides Leaves into Flavonoid Aglycones by Eurotium amstelodami. Microorganisms 2019, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Baffi, M.A.; Martin, N.; Tobal, T.M.; Ferrarezi, A.L.; Lago, J.H.G.; Boscolo, M.; Gomes, E.; Da-Silva, R. Purification and Characterization of an Ethanol-Tolerant β-Glucosidase from Sporidiobolus pararoseus and Its Potential for Hydrolysis of Wine Aroma Precursors. Appl. Biochem. Biotechnol. 2013, 171, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zhu, Y.-W.; Jiang, Y.-W.; Li, H.-K.; Liu, Z.-M.; Wang, W.; Shan, C.-H.; Fu, Y.-J. Improvement of flavonoid aglycone and biological activity of mulberry leaves by solid-state fermentation. Ind. Crops Prod. 2020, 148, 112287. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Wu, Y.; Liu, Y.; Wu, Z. Fermentation and complex enzyme hydrolysis for improving the total soluble phenolic contents, flavonoid aglycones contents and bio-activities of guava leaves tea. Food Chem. 2018, 264, 189–198. [Google Scholar] [CrossRef]
- Santos, A.C.d.; Biluca, F.C.; Braghini, F.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Phenolic composition and biological activities of stingless bee honey: An overview based on its aglycone and glycoside compounds. Food Res. Int. 2021, 147, 110553. [Google Scholar] [CrossRef]
- Khosravi, A.; Razavi, S.H. Therapeutic effects of polyphenols in fermented soybean and black soybean products. J. Funct. Foods 2021, 81, 104467. [Google Scholar] [CrossRef]
- Lodha, D.; Das, S.; Hati, S. Antioxidant activity, total phenolic content and biotransformation of isoflavones during soy lactic-fermentations. J. Food Process. Preserv. 2021, 45, e15583. [Google Scholar] [CrossRef]
- Jian, X.; Zhang, J. Component and Structure of Aspergillus flavipes sp.-Biodegraded Bayberry Tannins: A Potential Routine for Condensed Tannin Cleaner Degradation and Disposal. ACS Omega 2022, 7, 5809–5816. [Google Scholar] [CrossRef]
- Ascacio-Valdés, J.A.; Aguilera-Carbó, A.F.; Buenrostro, J.J.; Prado-Barragán, A.; Rodríguez-Herrera, R.; Aguilar, C.N. The complete biodegradation pathway of ellagitannins by Aspergillus niger in solid-state fermentation. J. Basic Microbiol. 2016, 56, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.; Lee, R.; Hardy, M.; Heber, D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Sep. Purif. Technol. 2005, 41, 49–55. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Nuncio-Jáuregui, N.; Frutos, M.J. Influence of Fermentation with Different Lactic Acid Bacteria and in Vitro Digestion on the Biotransformation of Phenolic Compounds in Fermented Pomegranate Juices. J. Agric. Food Chem. 2017, 65, 6488–6496. [Google Scholar] [CrossRef] [PubMed]
- Chávez-González, M.L.; Guyot, S.; Rodríguez-Herrera, R.; Prado-Barragán, A.; Aguilar, C.N. Production profiles of phenolics from fungal tannic acid biodegradation in submerged and solid-state fermentation. Process Biochem. 2014, 49, 541–546. [Google Scholar] [CrossRef]
- Li, C.; Leverence, R.; Trombley, J.D.; Xu, S.; Yang, J.; Tian, Y.; Reed, J.D.; Hagerman, A.E. High Molecular Weight Persimmon (Diospyros kaki L.) Proanthocyanidin: A Highly Galloylated, A-Linked Tannin with an Unusual Flavonol Terminal Unit, Myricetin. J. Agric. Food Chem. 2010, 58, 9033–9042. [Google Scholar] [CrossRef]
- Poupard, P.; Sanoner, P.; Baron, A.; Renard, C.M.G.C.; Guyot, S. Characterization of procyanidin B2 oxidation products in an apple juice model solution and confirmation of their presence in apple juice by high-performance liquid chromatography coupled to electrospray ion trap mass spectrometry. J. Mass Spectrom. 2011, 46, 1186–1197. [Google Scholar] [CrossRef]
- Wen, K.-S.; Ruan, X.; Wang, J.; Yang, L.; Wei, F.; Zhao, Y.-X.; Wang, Q. Optimizing Nucleophilic Depolymerization of Proanthocyanidins in Grape Seeds to Dimeric Proanthocyanidin B1 or B2. J. Agric. Food Chem. 2019, 67, 5978–5988. [Google Scholar] [CrossRef]
- De Taeye, C.; Caullet, G.; Eyamo Evina, V.J.; Collin, S. Procyanidin A2 and Its Degradation Products in Raw, Fermented, and Roasted Cocoa. J. Agric. Food Chem. 2017, 65, 1715–1723. [Google Scholar] [CrossRef]
- Zuo, Y.; Chen, H.; Deng, Y. Simultaneous determination of catechins, caffeine and gallic acids in green, Oolong, black and pu-erh teas using HPLC with a photodiode array detector. Talanta 2002, 57, 307–316. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Tong, H.R.; Zhou, L.; Wang, E.X.; Liu, Q.J. Fungal colonization of Pu-Erh tea in yunnan. J. Food Saf. 2010, 30, 769–784. [Google Scholar] [CrossRef]
- Qin, J.-H.; Li, N.; Tu, P.-F.; Ma, Z.-Z.; Zhang, L. Change in Tea Polyphenol and Purine Alkaloid Composition during Solid-State Fungal Fermentation of Postfermented Tea. J. Agric. Food Chem. 2012, 60, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Chen, M.; Zu, Z.; Chen, Q.; Lu, H.; Yue, P.; Gao, X. Untargeted and targeted metabolomics reveal changes in the chemical constituents of instant dark tea during liquid-state fermentation by Eurotium cristatum. Food Res. Int. 2021, 148, 110623. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; González, C.; Suárez-Lepe, J.A. Formation of vinylphenolic pyranoanthocyanins by selected yeasts fermenting red grape musts supplemented with hydroxycinnamic acids. Int. J. Food Microbiol. 2007, 116, 144–152. [Google Scholar] [CrossRef]
- Morata, A.; Vejarano, R.; Ridolfi, G.; Benito, S.; Palomero, F.; Uthurry, C.; Tesfaye, W.; González, C.; Suárez-Lepe, J.A. Reduction of 4-ethylphenol production in red wines using HCDC+ yeasts and cinnamyl esterases. Enzym. Microb. Technol. 2013, 52, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Vanbeneden, N.; Gils, F.; Delvaux, F.; Delvaux, F.R. Formation of 4-vinyl and 4-ethyl derivatives from hydroxycinnamic acids: Occurrence of volatile phenolic flavour compounds in beer and distribution of Pad1-activity among brewing yeasts. Food Chem. 2008, 107, 221–230. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Calani, L.; Bernini, V.; Neviani, E.; Del Rio, D.; Galaverna, G.; Lazzi, C. In vitro metabolism of elderberry juice polyphenols by lactic acid bacteria. Food Chem. 2019, 276, 692–699. [Google Scholar] [CrossRef]
- Huang, J.; de Paulis, T.; May, J.M. Antioxidant effects of dihydrocaffeic acid in human EA.hy926 endothelial cells. J. Nutr. Biochem. 2004, 15, 722–729. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Venema, K.; Tabernero, M.; Sarriá, B.; Bravo, L.; Mateos, R. Bioconversion of polyphenols and organic acids by gut microbiota of predigested Hibiscus sabdariffa L. calyces and Agave (A. tequilana Weber) fructans assessed in a dynamic in vitro model (TIM-2) of the human colon. Food Res. Int. 2021, 143, 110301. [Google Scholar] [CrossRef]
- Cárdenas-Castro, A.P.; Venema, K.; Sarriá, B.; Bravo, L.; Sáyago-Ayerdi, S.G.; Mateos, R. Study of the impact of a dynamic in vitro model of the colon (TIM-2) in the phenolic composition of two Mexican sauces. Food Res. Int. 2021, 139, 109917. [Google Scholar] [CrossRef]
- Rodriguez, A.; Meadows, J.A.; Sun, N.; Simmons, B.A.; Gladden, J.M. Evaluation of bacterial hosts for conversion of lignin-derived p-coumaric acid to 4-vinylphenol. Microb. Cell Factories 2021, 20, 181. [Google Scholar] [CrossRef]
- Sun, L.-H.; Lv, S.-W.; Yu, F.; Li, S.-N.; He, L.-Y. Biosynthesis of 4-vinylguaiacol from crude ferulic acid by Bacillus licheniformis DLF-17056. J. Biotechnol. 2018, 281, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, N.; Nomura, T.; Ogita, S.; Kato, Y. Bioproduction of 4-Vinylphenol and 4-Vinylguaiacol β-Primeverosides Using Transformed Bamboo Cells Expressing Bacterial Phenolic Acid Decarboxylase. Appl. Biochem. Biotechnol. 2021, 193, 2061–2075. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, F.; De Paepe, B.; Maertens, J.; Beauprez, J.; De Cocker, P.; Mincke, S.; Stevens, C.; De Mey, M. Development of an in vivo glucosylation platform by coupling production to growth: Production of phenolic glucosides by a glycosyltransferase of Vitis vinifera. Biotechnol. Bioeng. 2015, 112, 1594–1603. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hydroxycinnamic acids on gut microbiota and health. Compr. Rev. Food Sci. Food Saf. 2021, 20, 710–737. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, N.; Mandal, A.K.A. Pharmacokinetic, toxicokinetic, and bioavailability studies of epigallocatechin-3-gallate loaded solid lipid nanoparticle in rat model. Drug Dev. Ind. Pharm. 2019, 45, 1506–1514. [Google Scholar] [CrossRef]
- Kim, H.-S.; Jeon, D.Y.; Javaid, H.M.A.; Sahar, N.E.; Lee, H.-N.; Hong, S.-J.; Huh, J.Y.; Kim, Y.-M. Bio-transformation of green tea infusion with tannase and its improvement on adipocyte metabolism. Enzym. Microb. Technol. 2020, 135, 109496. [Google Scholar] [CrossRef]
- Roberto, B.S.; Macedo, G.A.; Macedo, J.A.; Martins, I.M.; Nakajima, V.M.; Allwood, J.W.; Stewart, D.; McDougall, G.J. Immobilized tannase treatment alters polyphenolic composition in teas and their potential anti-obesity and hypoglycemic activities in vitro. Food Funct. 2016, 7, 3920–3932. [Google Scholar] [CrossRef]
- María Landete, J.; Hernández, T.; Robredo, S.; Dueñas, M.; de las Rivas, B.; Estrella, I.; Muñoz, R. Effect of soaking and fermentation on content of phenolic compounds of soybean (Glycine max cv. Merit) and mung beans (Vigna radiata [L] Wilczek). Int. J. Food Sci. Nutr. 2015, 66, 203–209. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Xu, C.; Ji, G.E. Bioconversion of Flavones During Fermentation in Milk Containing Scutellaria baicalensis Extract by Lactobacillus brevis. J. Microbiol. Biotechnol. 2013, 23, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Izumi, T.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kikuchi, M.; Piskula, M.K.; Kubota, Y. Soy Isoflavone Aglycones Are Absorbed Faster and in Higher Amounts than Their Glucosides in Humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Lim, B.; Kim, H.Y.; Kwon, S.-J.; Eom, S.H. Deglycosylation patterns of isoflavones in soybean extracts inoculated with two enzymatically different strains of lactobacillus species. Enzym. Microb. Technol. 2020, 132, 109394. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Chen, J.; Tang, H.; Wang, C.; Li, Z.; Xiao, Y. Bioprocessing of soybeans (Glycine max L.) by solid-state fermentation with Eurotium cristatum YL-1 improves total phenolic content, isoflavone aglycones, and antioxidant activity. RSC Adv. 2020, 10, 16928–16941. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, D.; Strahsburger, E.; Lopez de Lacey, A.M.; Bregola, V.; Marotti, I.; Aloisio, I.; Biavati, B.; Dinelli, G. Flavonoid bioconversion in Bifidobacterium pseudocatenulatum B7003: A potential probiotic strain for functional food development. J. Funct. Foods 2014, 7, 671–679. [Google Scholar] [CrossRef]
- Lin, S.; Zhu, Q.; Wen, L.; Yang, B.; Jiang, G.; Gao, H.; Chen, F.; Jiang, Y. Production of quercetin, kaempferol and their glycosidic derivatives from the aqueous-organic extracted residue of litchi pericarp with Aspergillus awamori. Food Chem. 2014, 145, 220–227. [Google Scholar] [CrossRef]
- Song, W.; Lagmay, V.; Jeong, B.-G.; Jung, J.; Chun, J. Changes in physicochemical and functional properties of Opuntia humifusa during fermentation with cellulolytic enzyme and lactic acid bacteria. LWT 2022, 159, 113192. [Google Scholar] [CrossRef]
- Duckstein, S.M.; Lorenz, P.; Stintzing, F.C. Conversion of Phenolic Constituents in Aqueous Hamamelis virginiana Leaf Extracts During Fermentation. Phytochem. Anal. 2012, 23, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, P.; Zhang, B.; Yu, X.; Li, X.; Han, G.; Ren, Y.; Zhang, J. Combining Transcriptomics and Polyphenol Profiling to Provide Insights into Phenolics Transformation of the Fermented Chinese Jujube. Foods 2022, 11, 2546. [Google Scholar] [CrossRef]
- Jia, R.; Ge, S.; Ren, S.; Luo, Y.; Xiu, L.; Sanabil; Liu, H.; Cai, D. Antibacterial mechanism of adzuki bean seed coat polyphenols and their potential application in preservation of fresh raw beef. Int. J. Food Sci. Technol. 2021, 56, 5025–5039. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Jiang, M.; Rui, X.; Li, W.; Chen, X.H.; Dong, M.S. Effect of rose polyphenols on oxidation, biogenic amines and microbial diversity in naturally dry fermented sausages. Food Control. 2017, 78, 324–330. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; Babiker, E.E.; Al-Juhaimi, F.Y.; Bekhit, A.E. Clove Polyphenolic Compounds Improve the Microbiological Status, Lipid Stability, and Sensory Attributes of Beef Burgers during Cold Storage. Antioxidants 2022, 11, 1354. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Klewicka, E.; Grzelczyk, J.; Gałązka-Czarnecka, I.; Mostowski, R. Lactic acid fermentation of legume seed sprouts as a method of increasing the content of isoflavones and reducing microbial contamination. Food Chem. 2019, 285, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Parizad, P.; De Nisi, P.; Adani, F.; Pepé Sciarria, T.; Squillace, P.; Scarafoni, A.; Iametti, S.; Scaglia, B. Antioxidant and Anti-Inflammatory Activities of the Crude Extracts of Raw and Fermented Tomato Pomace and Their Correlations with Aglycate-Polyphenols. Antioxidants 2020, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Milutinović, M.; Dimitrijević-Branković, S.; Rajilić-Stojanović, M. Plant Extracts Rich in Polyphenols as Potent Modulators in the Growth of Probiotic and Pathogenic Intestinal Microorganisms. Front. Nutr. 2021, 8, 688843. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.L.; Wen, G.; Zhou, J. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, Y.; Chen, J.; Cheng, Y.; Zhang, H.; Gao, T.; Xu, F.; Pan, S.; Tao, Y.; Lu, J. Fermentation of ginkgo biloba kernel juice using Lactobacillus plantarum Y2 from the ginkgo peel: Fermentation characteristics and evolution of phenolic profiles, antioxidant activities in vitro, and volatile flavor compounds. Front. Nutr. 2022, 9, 1025080. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Z.; Zhang, L. Optimization of lactic acid fermentation conditions for fermented tofu whey beverage with high-isoflavone aglycones. LWT 2019, 111, 211–217. [Google Scholar] [CrossRef]
- Lalouckova, K.; Mala, L.; Marsik, P.; Skrivanova, E. In Vitro Antibacterial Effect of the Methanolic Extract of the Korean Soybean Fermented Product Doenjang against Staphylococcus aureus. Animals 2021, 11, 2319. [Google Scholar] [CrossRef]
- Kuziel, G.A.; Rakoff-Nahoum, S. The gut microbiome. Curr. Biol. 2022, 32, R257–R264. [Google Scholar] [CrossRef]
- Linares, D.M.; Ross, P.; Stanton, C. Beneficial Microbes: The pharmacy in the gut. Bioengineered 2016, 7, 11–20. [Google Scholar] [CrossRef]
- Gou, H.-Z.; Zhang, Y.-L.; Ren, L.-F.; Li, Z.-J.; Zhang, L. How do intestinal probiotics restore the intestinal barrier? Front. Microbiol. 2022, 13, 929346. [Google Scholar] [CrossRef]
- Zhou, F.; Li, Y.-L.; Zhang, X.; Wang, K.-B.; Huang, J.-A.; Liu, Z.-H.; Zhu, M.-Z. Polyphenols from Fu Brick Tea Reduce Obesity via Modulation of Gut Microbiota and Gut Microbiota-Related Intestinal Oxidative Stress and Barrier Function. J. Agric. Food Chem. 2021, 69, 14530–14543. [Google Scholar] [CrossRef]
- Zhao, D.; Cao, J.; Jin, H.; Shan, Y.; Fang, J.; Liu, F. Beneficial impacts of fermented celery (Apium graveolens L.) juice on obesity prevention and gut microbiota modulation in high-fat diet fed mice. Food Funct. 2021, 12, 9151–9164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).