Flavor Characterization of Traditional Fermented Soybean Pastes from Northeast China and Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Physicochemical Characteristics Analysis
2.3. E-Tongue Analysis
2.4. Volatile Compounds Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Color Properties of Soybean Paste Samples
3.2. pH, Titratable Acidity (TA), Moisture, Salinity, Reducing Sugar, and Amino Acid Nitrogen Content of Soybean Paste
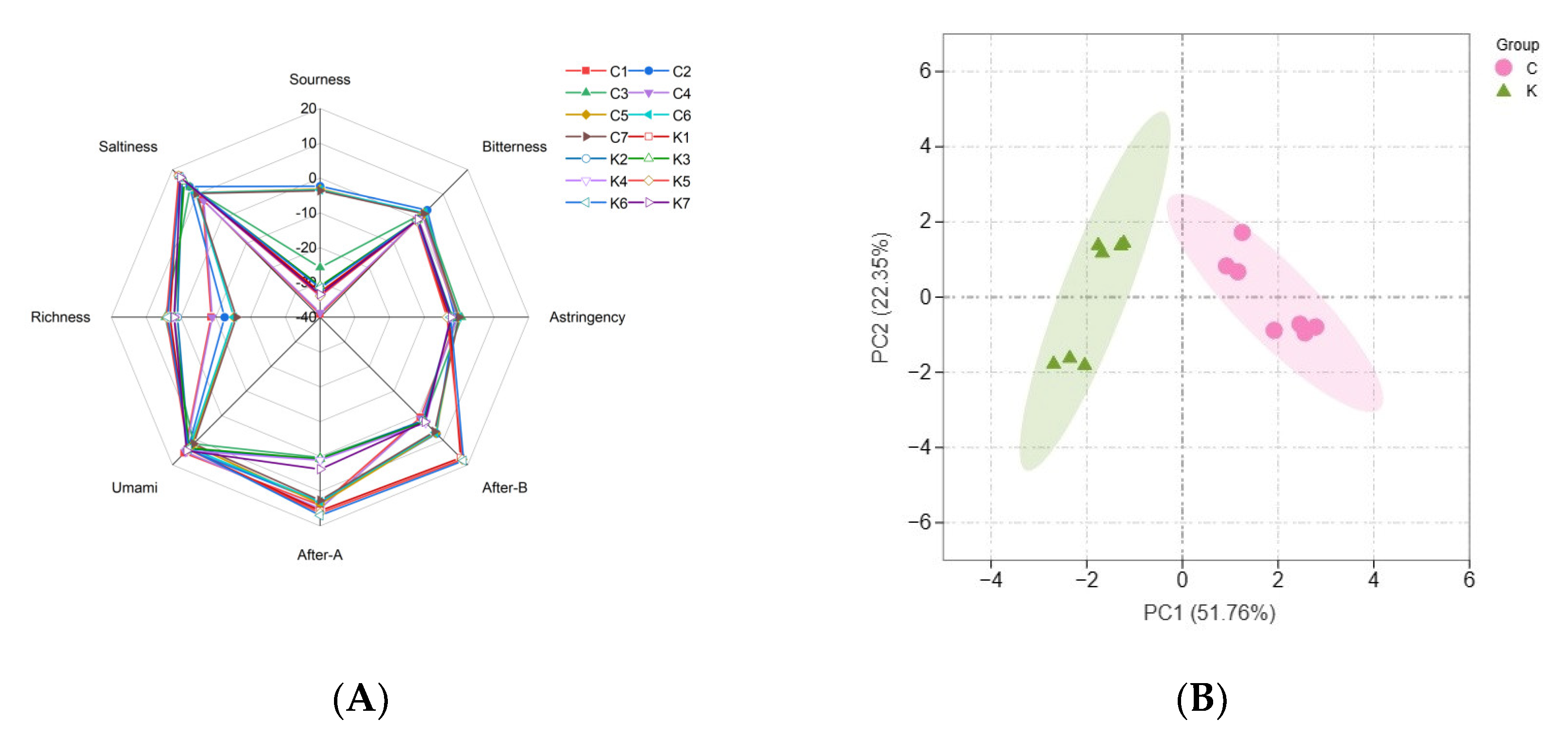
3.3. E-Tongue Analysis
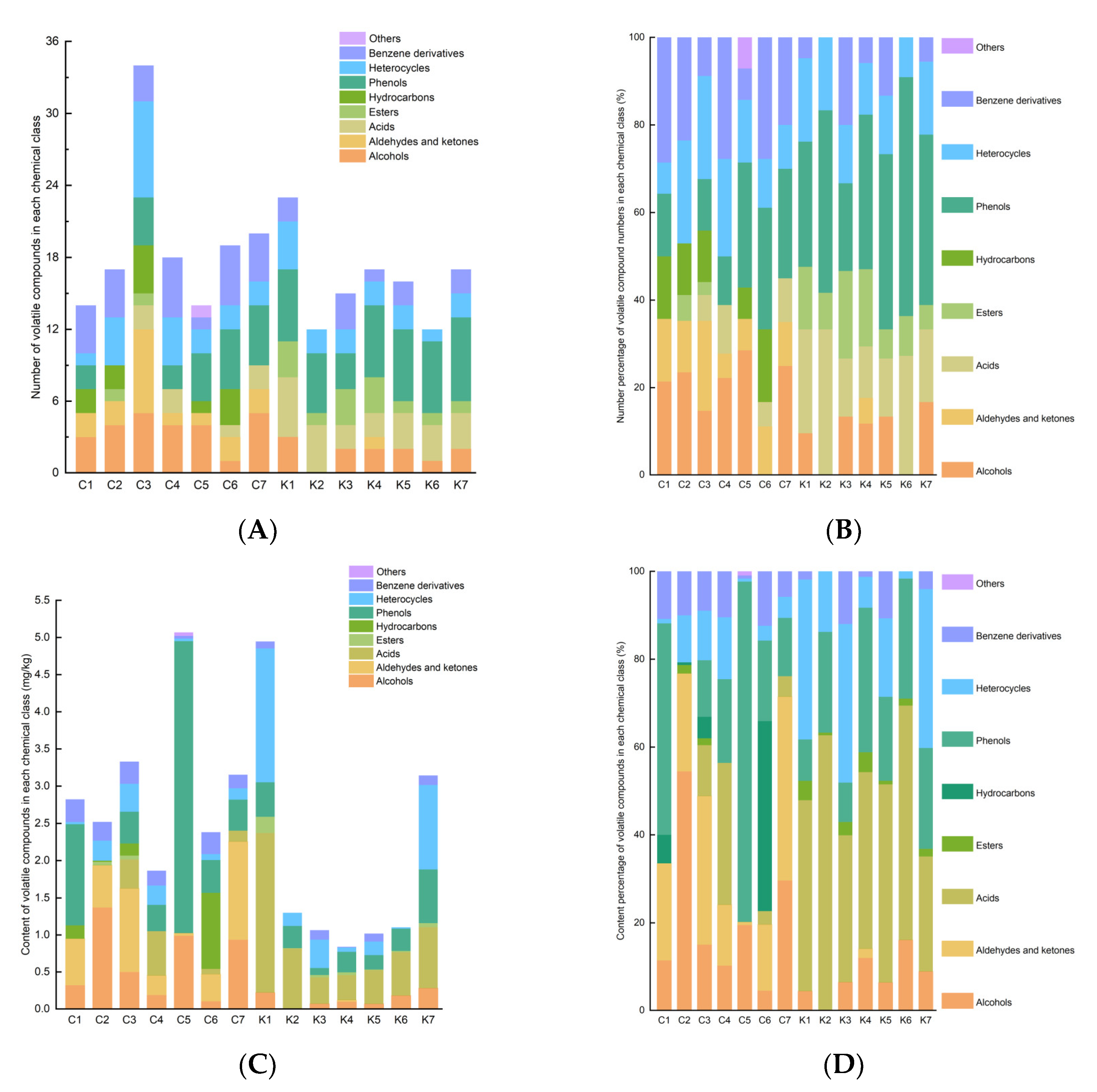
3.4. Comparison of Volatile Compound Profiles for Soybean Pastes
3.5. Distribution of Differential Flavor Compounds in Soybean Paste Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Zhang, M.; Feng, X.; Ding, T.; Zhao, Y.; Sun, C.; Zhou, S.; He, J.; Wang, C. Characterization of fragrant compounds in different types of high-salt liquid-state fermentation soy sauce from China. LWT 2022, 169, 113993. [Google Scholar] [CrossRef]
- Namgung, H.J.; Park, H.J.; Cho, I.H.; Choi, H.K.; Kwon, D.Y.; Shim, S.M.; Kim, Y.S. Metabolite profiling of doenjang, fermented soybean paste, during fermentation. J. Sci. Food Agric. 2010, 90, 1926–1935. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.H.; Green-Johnson, J.M.; Buckleyc, N.D.; Lind, Q.Y. Bioactivity of soy-based fermented foods: A review. Biotechnol. Adv. 2019, 37, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Seo, J.Y.; Cho, K.M.; Lee, C.K.; Kim, J.H.; Kim, J.S. Antioxidant and Neuroprotective Effects of Doenjang Prepared with Rhizopus, Pichia, and Bacillus. Prev. Nutr. Food Sci. 2016, 21, 221–226. [Google Scholar] [CrossRef]
- Park, S.M.; Oh, J.; Kim, J.E.; Kim, J.S. Effect of Drying Conditions on Nutritional Quality and In Vitro Antioxidant Activity of Traditional Doenjang. Prev. Nutr. Food Sci. 2018, 23, 144–151. [Google Scholar] [CrossRef]
- Sasaki, H.; Pham Thi Ngoc, D.; Nishikawa, M.; Kanauchi, M. Lipopolysaccharide neutralizing protein in Miso, Japanese fermented soybean paste. J. Food Sci. 2020, 85, 2498–2505. [Google Scholar] [CrossRef] [PubMed]
- Sim, I.; Koh, J.H.; Kim, D.J.; Gu, S.H.; Park, A.; Lim, Y.H. In vitro assessment of the gastrointestinal tolerance and immunomodulatory function of Bacillus methylotrophicus isolated from a traditional Korean fermented soybean food. J. Appl. Microbiol. 2015, 118, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Sran, K.S.; Bisht, B.; Mayilraj, S.; Choudhury, A.R. Structural characterization and antioxidant potential of a novel anionic exopolysaccharide produced by marine Microbacterium aurantiacum FSW-25. Int. J. Biol. Macromol. 2019, 131, 343–352. [Google Scholar] [CrossRef]
- Kumazawa, T.; Nishimura, A.; Asai, N.; Adachi, T. Isolation of immune-regulatory Tetragenococcus halophilus from miso. PLoS ONE 2018, 13, e0208821. [Google Scholar] [CrossRef]
- Yue, X.; Li, M.; Liu, Y.; Zhang, X.; Zheng, Y. Microbial diversity and function of soybean paste in East Asia: What we know and what we don’t. Curr. Opin. Food Sci. 2021, 37, 145–152. [Google Scholar] [CrossRef]
- Allwood, J.G.; Wakeling, L.T.; Bean, D.C. Fermentation and the microbial community of Japanese koji and miso: A review. J. Food Sci. 2021, 86, 2194–2207. [Google Scholar] [CrossRef] [PubMed]
- Han, D.M.; Chun, B.H.; Kim, H.M.; Jeon, C.O. Characterization and correlation of microbial communities and metabolite and volatile compounds in doenjang fermentation. Food Res. Int. 2021, 148, 110645. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Yun, J.H.; Lee, E.; Hong, S.P. Untargeted Metabolomics reveals Doenjang metabolites affected by manufacturing process and microorganisms. Food Res. Int. 2022, 157, 111422. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Lee, S.; Lee, S.H.; Kim, H.J.; Lee, C.H. Comparative Evaluation of Six Traditional Fermented Soybean Products in East Asia: A Metabolomics Approach. Metabolites 2019, 9, 183. [Google Scholar] [CrossRef]
- Hansen, E.B. Redox reactions in food fermentations. Curr. Opin. Food Sci. 2018, 19, 98–103. [Google Scholar] [CrossRef]
- Ben Akacha, N.; Gargouri, M. Microbial and enzymatic technologies used for the production of natural aroma compounds: Synthesis, recovery modeling, and bioprocesses. Food Bioprod. Process. 2015, 94, 675–706. [Google Scholar] [CrossRef]
- Zhu, L.; He, S.; Lu, Y.; Gan, J.; Tao, N.; Wang, X.; Jiang, Z.; Hong, Y.; Xu, C. Metabolomics mechanism of traditional soy sauce associated with fermentation time. Food Sci. Hum. Wellness 2022, 11, 297–304. [Google Scholar] [CrossRef]
- Jo, Y.; Lee, J.; Kim, M.K. Physicochemical and sensory characteristics of commercially available rice-based Doenjang and their correlation to consumer acceptability for Korean young adults. Food Sci. Biotechnol. 2023, 32, 949–957. [Google Scholar] [CrossRef]
- Yu, S.; Huang, X.; Wang, L.; Ren, Y.; Zhang, X.; Wang, Y. Characterization of selected Chinese soybean paste based on flavor profiles using HS-SPME-GC/MS, E-nose and E-tongue combined with chemometrics. Food Chem. 2022, 375, 131840. [Google Scholar] [CrossRef]
- Zhao, C.J.; Schieber, A.; Gänzle, M.G. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations—A review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef]
- Liu, P.; Xiang, Q.; Chen, G.; Liu, Y.; Zhai, G.; Lu, S.; Che, Z. Consumer preference of Chinese traditional fermented fava pastes. Int. J. Food Prop. 2018, 21, 2469–2490. [Google Scholar] [CrossRef]
- Yu, S.; Huang, X.; Wang, L.; Chang, X.; Ren, Y.; Zhang, X.; Wang, Y. Qualitative and quantitative assessment of flavor quality of Chinese soybean paste using multiple sensor technologies combined with chemometrics and a data fusion strategy. Food Chem. 2023, 405, 134859. [Google Scholar] [CrossRef]
- Zhao, C.; Tian, Z.; Yi, J.; Shi, Y.; Zhu, J.; Ji, Z.; Chen, S.; Kang, Q.; Lu, J. Characterization and correlation of bacterial community and volatile flavor compounds in xiguajiang, a Chinese traditional fermented condiment. Food Res. Int. 2022, 162, 111904. [Google Scholar] [CrossRef]
- Cai, J.; Han, Y.; Wu, W.; Wu, X.; Mu, D.; Jiang, S.; Li, X. Correlation Analysis of Microbiota and Volatile Flavor Compounds of Caishiji Soybean Paste. Fermentation 2022, 8, 196. [Google Scholar] [CrossRef]
- Yılmaz Oral, Z.F.; Kaban, G. Effects of autochthonous strains on volatile compounds and technological properties of heat-treated sucuk. Food Biosci. 2021, 43, 101140. [Google Scholar] [CrossRef]
- Chen, X.; Lin, L.; Cai, H.; Gao, X. Identification and Analysis of Metabolites That Contribute to the Formation of Distinctive Flavour Components of Laoxianghuang. Foods 2023, 12, 425. [Google Scholar] [CrossRef]
- Ma, S.; Shang, Z.; Chen, J.; Shen, Y.; Li, Z.; Huang, D.; Luo, H. Differences in structure, volatile metabolites, and functions of microbial communities in Nongxiangxing daqu from different production areas. LWT 2022, 166, 113784. [Google Scholar] [CrossRef]
- Yang, C.; You, L.; Kwok, L.-Y.; Jin, H.; Peng, J.; Zhao, Z.; Sun, Z. Strain-level multiomics analysis reveals significant variation in cheeses from different regions. LWT 2021, 151, 112043. [Google Scholar] [CrossRef]
- Lu, K.; Liu, L.; Xu, Z.; Xie, W. The analysis of volatile compounds through flavoromics and machine learning to identify the origin of traditional Chinese fermented shrimp paste from different regions. LWT 2022, 171, 114096. [Google Scholar] [CrossRef]
- Zhao, Q.; Xi, J.; Xu, D.; Jin, Y.; Wu, F.; Tong, Q.; Yin, Y.; Xu, X. A comparative HS-SPME/GC-MS-based metabolomics approach for discriminating selected japonica rice varieties from different regions of China in raw and cooked form. Food Chem. 2022, 385, 132701. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, P.; Wu, J.; Tao, D.; Wu, R. Effects of Leuconostoc mesenteroides on physicochemical and microbial succession characterization of soybean paste, Da-jiang. LWT 2019, 115, 108028. [Google Scholar] [CrossRef]
- Zhang, P.; Li, H.; Zhao, W.; Xiong, K.; Wen, H.; Yang, H.; Wang, X. Dynamic analysis of physicochemical characteristics and microbial communities of Aspergillus-type douchi during fermentation. Food Res. Int. 2022, 153, 110932. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Niu, C.T.; Zheng, F.Y.; Liu, C.F.; Wang, J.J.; Lu, Z.M.; Xu, Z.H.; Li, Q. Development of a defined autochthonous starter through dissecting the seasonal microbiome of broad bean paste. Food Chem. 2021, 357, 129625. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Niu, C.T.; Lu, Z.M.; Zhang, X.J.; Chai, L.J.; Shi, J.S.; Xu, Z.H.; Li, Q. A Bottom-up Approach to Develop a Synthetic Microbial Community Model: Application for Efficient Reduced-Salt Broad Bean Paste Fermentation. Appl. Environ. Microbiol. 2020, 86, e00306-20. [Google Scholar] [CrossRef]
- Feng, Y.; Cheng, H.; Cheng, Y.; Zhao, J.; He, J.; Li, N.; Wang, J.; Guan, J. Chinese Traditional Pear Paste: Physicochemical Properties, Antioxidant Activities and Quality Evaluation. Foods 2023, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Arihara, K.; Yokoyama, I.; Ohata, M. Bioactivities generated from meat proteins by enzymatic hydrolysis and the Maillard reaction. Meat Sci. 2021, 180, 108561. [Google Scholar] [CrossRef]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef]
- Aburto, N.J.; Ziolkovska, A.; Hooper, L.; Elliott, P.; Cappuccio, F.P.; Meerpohl, J.J. Effect of lower sodium intake on health: Systematic review and meta-analyses. BMJ 2013, 346, f1326. [Google Scholar] [CrossRef]
- Kloss, L.; Meyer, J.D.; Graeve, L.; Vetter, W. Sodium intake and its reduction by food reformulation in the European Union—A review. NFS J. 2015, 1, 9–19. [Google Scholar] [CrossRef]
- Chun, B.H.; Kim, K.H.; Jeong, S.E.; Jeon, C.O. The effect of salt concentrations on the fermentation of doenjang, a traditional Korean fermented soybean paste. Food Microbiol. 2020, 86, 103329. [Google Scholar] [CrossRef]
- Kim, M.K.; Chung, H.-J.; Bang, W.-S. Correlating physiochemical quality characteristics to consumer hedonic perception of traditional Doenjang (fermented soybean paste) in Korea. J. Sens. Stud. 2018, 33, e12462. [Google Scholar] [CrossRef]
- Byeon, Y.S.; Heo, J.; Park, K.; Chin, Y.W.; Hong, S.P.; Lim, S.D.; Kim, S.S. Consumer Preference of Traditional Korean Soy Sauce (Ganjang) and Its Relationship with Sensory Attributes and Physicochemical Properties. Foods 2023, 12, 2361. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Ameer, K.; Shi, Y.; Yi, J.; Zhu, J.; Kang, Q.; Lu, J.; Zhao, C. Characterization of physicochemical properties, microbial diversity and volatile compounds of traditional fermented soybean paste in Henan province of China. Food Biosci. 2022, 50, 102045. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, T.; Jia, H.; Guo, C.; Wang, Z.; Yue, T.; Yuan, Y. Comparative evaluation of the effects of natural and artificial inoculation on soybean paste fermentation. LWT 2022, 155, 112936. [Google Scholar] [CrossRef]
- Suezawa, Y.; Suzuki, M. Bioconversion of ferulic acid to 4-vinylguaiacol and 4-ethylguaiacol and of 4-vinylguaiacol to 4-ethylguaiacol by halotolerant yeasts belonging to the genus Candida. Biosci. Biotechnol. Biochem. 2007, 71, 1058–1062. [Google Scholar] [CrossRef]
- Flores, M.; Durá, M.A.; Marco, A.; Toldrá, F. Effect of Debaryomyces spp. on aroma formation and sensory quality of dry-fermented sausages. Meat Sci. 2004, 68, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, W.; Lu, S.; Zhang, H.; Yin, L. Metabolic Engineering of Shikimic Acid Biosynthesis Pathway for the Production of Shikimic Acid and Its Branched Products in Microorganisms: Advances and Prospects. Molecules 2022, 27, 4779. [Google Scholar] [CrossRef]
- Wright, J.D. Fungal degradation of benzoic acid and related compounds. World J. Microbiol. Biotechnol. 1993, 9, 9–16. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, Y.; Jin, Y.; Liu, Y.; Xia, B.; Sun, Q. Dynamics of microbial community during the extremely long-term fermentation process of a traditional soy sauce. J. Sci. Food Agric. 2017, 97, 3220–3227. [Google Scholar] [CrossRef]
- Koné, M.K.; Guéhi, S.T.; Durand, N.; Ban-Koffi, L.; Berthiot, L.; Tachon, A.F.; Brou, K.; Boulanger, R.; Montet, D. Contribution of predominant yeasts to the occurrence of aroma compounds during cocoa bean fermentation. Food Res. Int. 2016, 89, 910–917. [Google Scholar] [CrossRef]




| Sample | Raw Materials | Origin | Latitude (°) | longitude (°) |
|---|---|---|---|---|
| C1 | Soybean, water, salt | Shenyang, Liaoning | 123.41 | 41.80 |
| C2 | Soybean, water, salt | Shenyang, Liaoning | 123.41 | 41.80 |
| C3 | Soybean, water, salt | Shenyang, Liaoning | 123.41 | 41.80 |
| C4 | Soybean, water, salt | Shenyang, Liaoning | 123.41 | 41.80 |
| C5 | Soybean, water, salt | Shenyang, Liaoning | 123.48 | 41.85 |
| C6 | Soybean, water, salt | Shenyang, Liaoning | 123.48 | 41.85 |
| C7 | Soybean, water, salt | Shenyang, Liaoning | 123.48 | 41.85 |
| K1 | Soybean, water, salt | Yanji, Jilin | 129.51 | 42.91 |
| K2 | Soybean, water, salt | Yanji, Jilin | 129.51 | 42.91 |
| K3 | Soybean, water, salt | Yanji, Jilin | 129.51 | 42.91 |
| K4 | Soybean, water, salt | Yanji, Jilin | 129.51 | 42.91 |
| K5 | Soybean, water, salt | Yanji, Jilin | 129.51 | 42.91 |
| K6 | Soybean, water, salt | Yanji, Jilin | 129.51 | 42.91 |
| K7 | Soybean, water, salt | Yanji, Jilin | 129.51 | 42.90 |
| Sample | L* | a* | b* |
|---|---|---|---|
| C1 | 37.3 ± 0.04 cd | 1.89 ± 0.05 h | 14.02 ± 0.10 e |
| C2 | 33.63 ± 0.04 i | 2.92 ± 0.20 f | 12.25 ± 0.19 h |
| C3 | 32.26 ± 0.04 j | 1.82 ± 0.08 h | 10.05 ± 0.11 i |
| C4 | 37.7 ± 0.15 cd | 3.80 ± 0.27 d | 16.93 ± 0.37 a |
| C5 | 40.34 ± 0.23 a | 3.42 ± 0.12 e | 17.08 ± 0.40 a |
| C6 | 35.43 ± 0.16 h | 2.97 ± 0.14 f | 13.99 ± 0.43 e |
| C7 | 37.35 ± 0.14 de | 2.57 ± 0.06 g | 14.42 ± 0.18 de |
| K1 | 39.50 ± 0.45 b | 5.39 ± 0.09 a | 16.70 ± 0.18 a |
| K2 | 37.83 ± 0.63 cd | 4.70 ± 0.06 b | 15.01 ± 0.21 bc |
| K3 | 36.86 ± 0.28 de | 4.44 ± 0.05 c | 14.80 ± 0.38 cd |
| K4 | 36.63 ± 0.38 f | 4.01 ± 0.09 d | 13.41 ± 0.32 f |
| K5 | 38.23 ± 0.16 c | 4.76 ± 0.03 b | 15.31 ± 0.03 b |
| K6 | 36.34 ± 0.56 fg | 3.79 ± 0.12 d | 12.88 ± 0.09 g |
| K7 | 35.83 ± 0.26 gh | 3.79 ± 0.07 d | 12.36 ± 0.23 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Sai, Y.; Liu, W.; Zhao, H.; Bai, X.; Song, W.; Zheng, Y.; Yue, X. Flavor Characterization of Traditional Fermented Soybean Pastes from Northeast China and Korea. Foods 2023, 12, 3294. https://doi.org/10.3390/foods12173294
Zhao S, Sai Y, Liu W, Zhao H, Bai X, Song W, Zheng Y, Yue X. Flavor Characterization of Traditional Fermented Soybean Pastes from Northeast China and Korea. Foods. 2023; 12(17):3294. https://doi.org/10.3390/foods12173294
Chicago/Turabian StyleZhao, Shanshan, Yuhang Sai, Wanting Liu, Huiwen Zhao, Xue Bai, Wanying Song, Yan Zheng, and Xiqing Yue. 2023. "Flavor Characterization of Traditional Fermented Soybean Pastes from Northeast China and Korea" Foods 12, no. 17: 3294. https://doi.org/10.3390/foods12173294
APA StyleZhao, S., Sai, Y., Liu, W., Zhao, H., Bai, X., Song, W., Zheng, Y., & Yue, X. (2023). Flavor Characterization of Traditional Fermented Soybean Pastes from Northeast China and Korea. Foods, 12(17), 3294. https://doi.org/10.3390/foods12173294






