Abstract
Volatile compounds (VOCs) present in the oil extracted from yellow horn seeds were first analyzed using GC-IMS and GC-O-MS at varying roasting temperatures. A total of 97 VOCs were detected using GC-IMS, while 77 were tentatively identified using GC-O-MS. Moreover, both methods allowed the identification of 24 VOCs, of which the type of aldehydes is the most abundant. Combining the results of GC-IMS, GC-O-MS, OAVs, and VIP, it was concluded that hexanal, 2,5-dimethylpyrazine, heptanal, 2-pentylfuran, 1-hexanol, and 1-octen-3-ol were the key aroma compounds. The PLS-DA and OPLS-DA models have demonstrated the ability to discriminate between different oil roasting temperatures with high accuracy. The roasting temperature of 160 °C was found to yield the highest content of main aroma substances, indicating its optimality for yellow horn seed oil production. These findings will prove beneficial for optimizing industrial production and enhancing oil aroma control.
1. Introduction
Xanthoceras sorbifolia bunge (yellow horn), a deciduous tree widely distributed in central and northern China, represents the sole species within the genus Sapindaceae [1]. The oil content of yellow horn seed is up to 55–65% [2], being considered a high-quality functional edible oil with an unsaturated fatty acid content of 85–93% [3,4,5], including linoleic (41.2%), oleic (42.3%) and nervonic acids (2–5%) [6]. Nervonic acid is capable of enhancing cognitive function and memory, as well as repairing damage to the central nervous system [7]. The production and consumption of yellow horn seed oil have been on the rise in recent years, owing to the growing recognition of its nutritional and functional properties by consumers. The seeds are roasted at an optimal temperature and time before being pressed to extract the oil, which boasts a robust aroma that is highly valued by consumers [8].
In the current process of plant oil production, raw materials are typically subjected to elevated temperatures of roasting prior to pressing to increase oil yield and enhance aroma [9]. Properly executed high-temperature roasting can enrich oils with aromatic substances through a series of reactions such as the Maillard reaction, lipid oxidation, Strecker degradation and caramelization [10], which generate a variety of aroma substances, including aldehydes, alcohols, and pyrazines [11].
Aldehydes and heterocyclic compounds are considered pivotal compounds in oils, contributing to the distinctive fragrance [12]. It has been reported that 2-methylpyrazine, 2,5-dimethylpyrazine, 2-ethyl-3-methylpyrazine, and 2,6-dimethylpyrazine could be produced by heating virgin rapeseed oil at 100 °C and contributed toasted, nutty, woody, and burnt profiles [13]. The aroma of cold-pressed and hot-pressed walnut oil was studied using GC-IMS and GC×GC-O-MS. It was concluded that the key aroma active compounds in cold-pressed walnut oil were identified as 1-octene-3-ol, cyclohexanol, and benzaldehyde. Conversely, the main aroma components in hot-pressed walnut oil were 3-methylbutyraldehyde, (E, E)-2, 4-nonadienal, and nonanal [14].
Gas chromatography–ion mobility spectrometry (GC-IMS), gas chromatography-olfactometry-mass spectrometry (GC-O-MS), and electronic nose (E-nose) are commonly employed in food aroma research for the detection of volatile compounds (VOCs) due to their low limits of detection, high selectivity, robust operational stability, rapid analysis capabilities, and cost-effectiveness [15,16]. GC includes both a mobile phase and a stationary phase. The components of the sample are separated in the chromatographic column based on their different forces in the two-phase system. IMS is a method capable of detecting volatile and semi-volatile compounds at parts per billion (ppb) [17]. These compounds are distinguished based on the mobility of ions in the electric field of neutral buffer gas under atmospheric pressure [18]. GC-IMS not only exploits the high separation capacity of GC but also capitalizes on the high sensitivity and rapid response properties of IMS. Olfactometry (O) employs human sense of smell as a detector to identify aroma contributions in food [19]. Combined with the advantages of GC-O and GC-MS, GC-O-MS enables rapid and accurate identification of VOCs while also allowing for the recording of substances’ aroma through olfactometer export [20,21].
In recent years, the combination of multiple analytical methods to identify the key aroma compounds in oils has been used in various studies of oil flavor substances. The results obtained from these different methods are cross-verified to enhance accuracy, scientific rigor, and reliability. For example, He et al. [12] used both GC-IMS and GC-MS to analyze the aroma substances generated during different microwave treatment times of camellia seed oil and found that the primary odors varied with different treatments. According to a report [21], analyses using GC-MS and GC-O revealed that aldehydes and pyrazines were the primary aroma active compounds in roasted walnut oil.
Yellow horn seed oil has garnered increasing attention due to its valuable nutrients and distinct aroma, yet there is still a gap in the study of its aroma substances. Therefore, this study aims to identify unknown VOCs in yellow horn seed oil using GC-IMS and GC-O-MS, followed by determining the key aroma compounds by evaluating their contribution. Additionally, the impact of the roasting temperature on the aroma of the yellow horn seed oil is also discussed. The results obtained from this study will provide an analytical, theoretical, and technical foundation for further research into the aroma profile of yellow horn seed oil.
2. Materials and Methods
2.1. Materials and Chemicals
Yellow horn seeds were provided in September 2022 by Shandong Woqi Agricultural Development Co. Ltd. (Shandong, China). The shell of the seed was removed and the kernel remained intact. The dried seed kernels with a moisture content of 10% were stored at 4 °C. Divinylbenzene/carboxen/polydimethylsiloxane-fiber (DVB/CAR/PDMS, 50/30 μm, 1 cm) headspace glass vials were purchased from Sigma-Aldrich (St. Louis, MO, USA).
The 2-octanol standard was obtained from Shanghai Maclin Biochemical Technology Co., Ltd. (Shanghai, China). n-ketones C4–C9 were purchased from Sinopharm Chemical Reagent Beijing Co., Ltd. (Beijing, China). n-alkanes C7–C30 were purchased from TCI (Beijing, China).
2.2. Sample Preparation
Following the method reported in [22,23] with slight modifications, the preparation of yellow horn seed oil involved weighing 150 g of peeled yellow horn kernels and roasting them at six different temperatures (120 °C, 130 °C, 140 °C, 150 °C, 160 °C, and 170 °C) in an oven for a duration of 25 min. Subsequently, the roasted yellow horn kernels were transferred to an automatic oil press for extraction. The obtained samples of yellow horn seed oil were centrifuged, and the supernatant was sealed in a glass vial at 4 °C and stored away from light. Three replicate experiments were performed for each sample preparation.
2.3. HS-GC-IMS Analysis
HS-GC-IMS analysis was conducted using the GC-IMS FlavourSpec® Instrument (Gesellschaft für Analytische Sensorsysteme, Dortmund, Germany) equipped with an automatic sampler (CTC Analytics AG, Zwingen, Switzerland). GC is equipped with a DB-WAX metal capillary column (15 m × 0.53 mm, 1 μm). The detection method used in [12] was adapted with some modifications, whereby the initial flow rate was maintained at 2 mL/min for 5 min and increased to 100 mL/min for 20 min. Substances were eluted and separated in the column. Subsequently, the compounds were transferred and ionized in an ionization chamber with a 3H ionization source in the positive ion mode. The derived ions were driven into the drift tube (98 mm) at 45 °C with a linear voltage of 500 V/cm.
n-ketones C4–C9 were used as an external reference to calculate the retention index (RI) of VOCs detected under the same chromatographic conditions. VOCs were identified by comparing RI and drift time (DT) of standard compounds in the NIST 17 library and the GC-IMS database (G.A.S GmbH, Dortmund, Germany). LAV software (LAV, Dortmund, Germany) was used to quantitatively analyze the signal peak area of the samples detected using GC-IMS. The average peak areas were utilized to indicate the relative abundance of volatiles.
2.4. HS-SPME/GC-O-MS Analysis
2.4.1. Extraction of Volatile Compounds
Following the method reported in [14] with minor modifications, VOCs in yellow horn seed oil treated at different temperatures were isolated and analyzed using HS-SPME/GC-O-MS. The yellow horn seed oil (3.0 g) and 3 μL 2-octanol (25 mg/L methanol), which was an internal standard (IS), were added to a headspace vial. The SPME fibers (DVB/CAR/PDMS) were thoroughly exposed to the top of the headspace vial (20 mL) and extracted at 80 °C for 20 min. After extraction, the fibers with extracts were inserted into the injection port of the GC system in the splitless mode and desorbed at 250 °C for 10 min [24]—three times in parallel for each sample. As decripted in Figure 1.
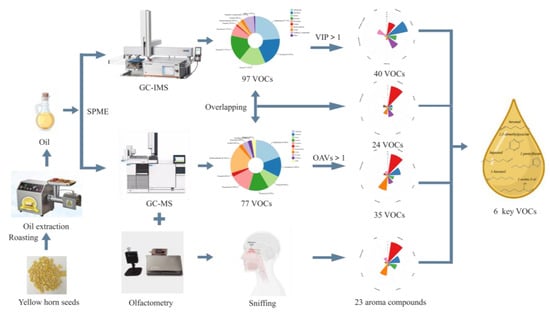
Figure 1.
A scheme of the overall process to achieve the key aroma compounds.
2.4.2. GC-O-MS Analysis of Volatile Compounds in Yellow Horn Seed Oil
A combination of 7890B-GC (Agilent Technologies, Inc., Santa Clara, CA, USA), olfactometer (Sniffer 9000, Brechbuhler, Schlieren, Switzerland), and 5977A-MS (Agilent Technologies, Inc., Santa Clara, CA, USA) was applied. VOCs were separated using a DB-5MS capillary column (30 m × 250 μm, 0.25 μm, Agilent Technologies Inc., Santa Clara, CA, USA). The chromatographic column temperature was set to 40 °C for 2 min, increased to 220 °C at a rate of 6 °C/min, then ramped up to 280 °C at a rate of 20 °C/min, and held for 10 min. The carrier gas was helium (purity ≥ 99.999%) at a flow rate of 3.0 mL/min. Ionization was carried out in the electron ionization mode at 70 eV, and the resulting mass spectrum obtained was in the 30–330 m/z range. The MS source temperature was kept at 230 °C. Three experienced panelists (two females and one male, aged between 22 and 25) were recruited to perform a sniff test on the olfactory output to identify aroma active compounds. Wet air (high-purity nitrogen and distilled water) was used for ventilation to improve the comfort of panelists. The terminal effluent from the capillary flowed into the MS and olfactometer with a split ratio of 1:1, respectively. Panelists were asked to record the perceived aroma, intensity, and time during sniffing.
MS characterization, odor description (O), and retention index (RI) were used for quantitative analysis [25]. MS characterization required that target compounds with a matching degree greater than 800 were screened from the NIST17 library based on the MS results. Odor description (O) was used to record the time and fragrance of smell on the sniffer port. The retention index of each compound was determined using the retention time of the n-alkanes C7-C30 by linear interpolation and compared to the RI of standard compounds in the NIST17 library [26]. The semi-quantization of the VOCs in the GC-O-MS sample was based on a linear relation between the peak area and the concentration of the IS and the concentration of the VOCs. The concentration was determined using the following equation.
: correction factor of compound; : peak area of unknown compound; : peak area of internal standard; : concentration of internal standard
2.5. Odor Active Values (OAVs)
The OAV is calculated based on the ratio of the concentration of the detected compound to its odor detection threshold in water [27], which is used as a visualization tool to determine the extent to which a compound contributes to food aroma [28]. VOCs with OAVs ≥ 1 are considered to contribute significantly to the aroma of yellow horn seed oil.
: concentration of detected compound; : odor threshold of corresponding compounds.
2.6. Statistical Analysis
The samples were collected in triplicate, and the resulting data were presented as mean ± standard deviation. Statistical analysis of the obtained data was performed using IBM SPSS 26 software (SPSS Inc., Chicago, IL, USA) and Origin Pro 2018 (OriginLab, Northampton, MA, USA). Data were subjected to one-way analysis of variance (ANOVA) using SPSS 26 software, and the multiple comparisons between the samples were performed via the Duncan test (p < 0.05). Partial least squares–discriminate analysis (PLS-DA) and orthogonal PLS-DA (OPLS-DA) were performed using SIMCA14.1 software (Umetrics, Umea, Sweden). The heat map was produced through the use of TBtools software (version v1.113).
3. Results
3.1. GC-IMS Analysis
3.1.1. GC-IMS Topographic Plots and Fingerprints
GC-IMS utilizes ion mobility in the buffer to effectively separate and identify aroma compounds. Moreover, it enables rapid and accurate acquisition and visualization of IMS data for VOCs present in yellow horn seed oil. Figure 2A shows the 3D topographic of the VOCs in yellow horn seed oil roasted at different temperatures, with the red color indicating a higher content. Although the VOCs in yellow horn seed oil roasted at different temperatures exhibit similarities, there are slight variations in their signal intensity. As depicted in Figure 2A(a), an increase in roasting temperature leads to a rise in the majority of aroma substances. Additionally, certain constituents exhibit an inverse relationship with temperature, as depicted in Figure 2A(b). This finding is consistent with He et al.’s [12] study on a microwave treatment applied to rapeseed oil. The kinds and concentrations of VOCs were compared precisely in the range of 120–170 °C in a 2D topographic (Figure 2B). The reference spectrum at 120 °C was utilized, and the spectra of other temperatures were obtained via subtraction. The red area in the figure indicates a higher VOC content than the reference, while the blue indicates a lower concentration. As depicted in Figure 2B, the proportion of red compound area gradually increases with roasting temperature.
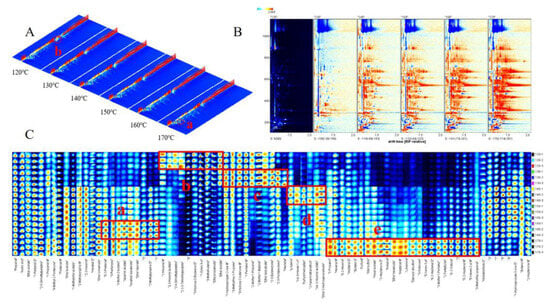
Figure 2.
GC-IMS topographic plots and fingerprints of the yellow horn seed oil. (A) 3D topographic. (B) 2D topographic subtraction plots of the VOCs. (C) VOC fingerprint comparisons of the yellow horn seed oil.
The VOC fingerprint of yellow horn seed oil was constructed to accurately represent the variations in VOC content resulting from different roasting temperatures (Figure 2C). Each row represents the signal peaks for different samples, while each column represents a distinct volatile compound. The brightness of the color is proportional to the VOC content, with higher concentrations resulting in a more intense red hue and lower concentrations, producing a brighter blue shade. Five areas (a, b, c, d, e) marked with wireframe exhibit elevated content of VOCs in the different samples of the yellow horn seed oil treated at 120–170 °C. The concentration of volatile components in a-region reached the highest in the sample of 160 °C, with nine VOCs present, including 2-pentenal (isomer), ethyl valerate and 2-methylpyrazine. The b-region exhibited the highest concentration of VOCs in the 120 °C sample, including 3-hydroxy-2-butanone ethyl acetate, 1-octen-3-ol and additional ten VOCs. Meanwhile, the c-zone was dominated by ten VOCs, such as 2,3-butanedione, 2,3-pentanedione and heptanal in the 130 °C sample. The d-region contained six VOCs, with the highest concentration observed in the 140 °C sample. These compounds included furfuryl mercaptan, 3-hexenyl acetate (isomer), 3-hexen-1-ol (isomer), etc. In contrast, the e-zone was dominated by 2-heptenal (isomer), 2-pentylfuran, and 2-octenal (isomer), among other VOCs. The 170 °C sample had the highest concentration of these aroma substances. The results above suggest that the volatile components in yellow horn seed oil samples roasted at different temperatures vary significantly (p < 0.05), indicating a significant impact of roasting temperature on the aroma profile of the oil.
3.1.2. Volatile Compounds Analysis
GC-IMS is a novel separation and detection technology with the advantages of sensitivity, accuracy, and rapidity, which can be applied to the determination of aroma compounds in yellow horn seed oil [29]. A total of 178 chromatographic signals have been detected by using GC-IMS, leading to the identification of 97 aroma compounds in the GC-IMS database. These compounds consisted of 23 aldehydes, 19 alcohols, 17 ketones, two acids, 17 esters, seven sulfur-containing compounds, one furan heterocyclic compounds, three hydrocarbons, seven pyrazine heterocyclic compounds, and one other compound (Figure S1A). Meanwhile, some compounds exhibit multiple signals (monomer and dimer) due to the addition of ions and neutral molecules in GC-IMS [30]. These compounds have similar retention times but different drift times. Further details regarding the VOCs are presented in Table S1.
Aldehydes, which contribute to the roasted and fatty aroma, are key compounds found in plant raw materials such as peanuts and canola oil [31]. In yellow horn seed oil, aldehydes were the most abundant VOCs detected via GC-IMS, accounting for 23.71% of the total, followed by alcohols (19.59%) and ketones (17.53%) (Figure 3A). From Figure 3B, it can be seen that the content of aldehydes increased significantly with the increase in roasting temperature within a certain temperature range, such as 2-octenal (isomer), 2-heptenal (isomer), and nonanal (p < 0.05). The formation of these compounds is related to lipid oxidation. For instance, nonanal, octanal, and decanal are produced via the auto-oxidation of oleic acid (C1:18), generating 10-hydroperoxides and 11-hydroperoxides, and/or secondary formation of 8-hydroperoxide [32]. The findings suggest that the heating process of yellow horn seed leads to lipid oxidation, which leads to the formation of these aroma compounds. Alcohols could be produced via the oxidation of polyunsaturated fatty acids catalyzed by lipoxygenase [33,34]. Meanwhile, the amino acid derivation pathway was also an essential source of some alcohols [35]. For instance, phenylethyl alcohol could be synthesized from phenylalanine by the action of aromatic amino acid aminotransferase (AAAAT) [36]. Phenylethyl alcohol was found in low content. In contrast, the concentration of 1-octen-3-ol was higher, indicating that the alcohol was produced, presumably, via fat oxidation or aldehyde reduction in yellow horn seed oil roasted at different temperatures. This result was consistent with Xu et al.’s [14] opinion of the results in their study concerning walnut oil. Ketones are typically produced through the decomposition of amino acids and thermal oxidative degradation of polyunsaturated fatty acids [14]. As shown in Figure 3B, the ketone content tended to increase and then decrease with increasing temperature. The sample at 150 °C had the highest content, while that at 120 °C had the lowest. Seven sulfur-containing compounds, including dimethyl disulfide, dimethyl trisulfide, and diethyl disulfide, have been detected in yellow horn seed oil. These findings were consistent with previous reports, which identified dimethyl trisulfide as one of the important aroma compounds in their analysis of fragrant rapeseed oil [10].
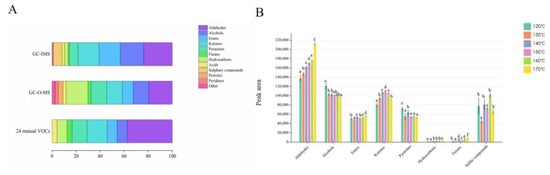
Figure 3.
GC-IMS and GC-MS data diagram in the yellow horn seed oil at different temperatures. (A) Comparison of stacked bar for extraction of VOCs. (B) The peak area of each component in oil was measured via GC-IMS. Different lowercase letters (a–f) mean statistically significant differences (p < 0.05).
3.1.3. Multivariate Statistical Analysis
The OPLS-DA model is capable of effectively highlighting inter-group sample differences, thereby enabling the classification information to be focused primarily on a single principal component, which enhances the analytical capability and validity of the model. When model parameters R2Y and Q2 are between 0.5 and 1, the model is considered optimal [37]. In the present study, the OPLS-DA model was developed using GC-IMS data from yellow horn seed oil. The parameters of the OPLS-DA model, R2Y = 0.915 and Q2 = 0.908, illustrated that the results had a good interpretation rate and predictability (Figure 4A). Additionally, 200 iterations of the permutation test (permutation test R2 = 0.432, Q2 = −0.606) fitted well without over-fitting, achieving rapid discrimination of the yellow horn seed oil at different temperatures (Figure 4B). The overall distribution of the six samples was scattered. The 120 °C and 130 °C samples were distributed in the fourth quadrant. Both the 150 °C and 160 °C samples were distributed close to each other in the second quadrant, indicating that their VOCs were relatively similar. The 140 °C samples were distributed in the first quadrant alone, and the 170 °C samples were distributed in the third quadrant alone, indicating that their VOCs differed greatly in similarity.
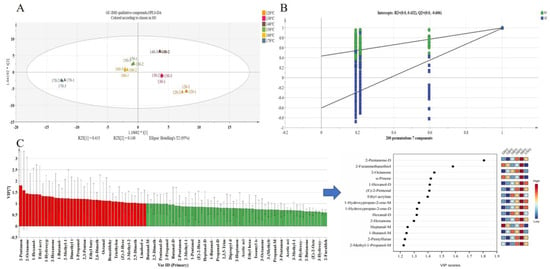
Figure 4.
Analysis results of OPLS-DA model of GC-IMS data in the yellow horn seed oil roasted at different temperatures. (A) OPLS-DA model (R2Y = 0.915, Q2 = 0.908). (B) Cross-validation plot produced via 200 permutation tests (R2 = 0.432, Q2 = −0.606). (C) VIP values of VOCs (red represents 40 key compounds with VIP > 1).
Variable importance in projection (VIP) describes the overall contribution of each variable to the model. Therefore, VIP served as a means to evaluate the strength and explanatory power of individual variables with respect to classification and discrimination [38]. In this study, a screening criterion of VIP > 1 was employed to identify compounds that significantly contributed to the sample classification results. As shown in Table 1, there were 40 key VOCs with VIP > 1. VOCs with VIP > 1 were highlighted in red in Figure 4C, including seven aldehydes, 11 alcohols, two pyrazine heterocyclic compounds, five esters, seven ketones, five sulfur-containing compounds, and one other compound. The shift in the content of these VOCs resulted in a change in the classification of the yellow horn seed oil produced at 120–170 °C.

Table 1.
Volatile compounds identified of VIP > 1 via GC-IMS in yellow horn seed oil.
3.2. GC-O-MS Analysis
3.2.1. Volatile Compounds Analysis
By integrating instrumental and sensory analysis, GC-O-MS is now capable of a more comprehensive analysis of the compounds. Meanwhile, the human nose is also used as an aroma detector, which was combined with sensory analysis to realize the transformation from chemical composition analysis to aroma analysis [39]. A total of 77 VOCs were detected via GC-O-MS, consisting of fifteen aldehydes, fourteen hydrocarbons, ten alcohols, ten ketones, two acids, seven esters, ten pyrazine heterocyclic compounds, two furan heterocyclic compounds, and seven other compounds (Figure S1C). Aldehydes were the most diverse, accounting for 19.48% of the total compounds. This was followed by hydrocarbons (18.18%), pyrazines (12.99%), ketones (12.99%), and alcohols (12.99%). As illustrated in Figure S1D, the difference in the VOCs determined in the oil via GC-O-MS and GC-IMS can be related to the affinity of the VOCs to the rest phase of these two GC columns.
Based on the odor recordings, the time of the occurrence of the aromas was combined with the peak time of the VOCs, and the olfactory outcomes were obtained by matching the sniffed compounds. In conjunction with sensory evaluation results, a total of 23 aroma substances were identified, including seven aldehydes, four alcohols, three esters, one ketone, four pyrazine heterocyclic compounds, one furan heterocyclic compound, one hydrocarbon, and two other compounds (Table 2). The main aroma characteristics included nutty, oily, green, grassy, earthy, and mushroom profiles. Among aroma compounds, aldehydes [40] contribute to a fresh and fatty profile, while pyrazine and furan impart a nutty aroma [41]. In addition, grassy and fruity profiles were predominantly provided by alcohols and esters.

Table 2.
Volatile compounds identified of OAV ≥ 1 via GC-O-MS in yellow horn seed oil.
The overall aroma of a food product is influenced by its aroma active compounds, which are determined by their concentration and odor threshold. OAVs can be used to identify the key contributors to the aroma profile of food products, making it a valuable screening tool in aroma studies [42]. OAVs ≥ 1 indicate that the compound contributes significantly to the overall aroma profile of food. The higher the OAV, the more prominent its contribution to food aroma [43]. The concentration of VOCs was determined via semi-quantization. The OAV of each compound was calculated as the ratio between its concentration and the corresponding odor threshold, and the results were summarized in Table S2. OAVs ≥ 1 were used as the screening criteria for the aroma distribution of the yellow horn seed oil in this study, and 35 compounds were screened (Table 2). Possible synergistic or additional complex interactive effects may not be explained accurately since only considering OAVs > 1. However, OAVs > 1 can predict the overall aroma characteristics of roasting yellow horn seed oil at different temperatures [44].
Aldehydes: The yellow horn seed oil exhibited the highest concentration of volatile aldehydes, as detected via GC-O-MS at 160 °C, with a concentration of 2.7649 mg/kg (Figure 5A). A total of 10 aldehydes with OAVs ≥ 1 were identified, including isobutyraldehyde (fresh, aldehydic, floral, and green), 3-methylbutanal (ethereal, chocolate, peach, and fatty), pentanal (fermented, bready, fruity, nutty, and berry), hexanal (fresh, green, fatty, grass, and fruity), heptanal (fresh, green, fatty, fruity, and sweaty), octanal (green, herbal, fresh, and fatty), (E)-2-octenal (fresh, cucumber, fatty, and green), nonanal (fresh, orange, peel, and fatty), (E)-2,4-decadienal (fried), (E,E)-2,4-decadienal (earthy, fried, and oily), providing fatty, nutty, grass, fruity and green for the roasted yellow horn seed oil. Among the aldehydes, the OAV of isobutyraldehyde was the highest in the 160 °C sample, up to 757.60. Previous studies have indicated that isobutyraldehyde was mainly produced via the Strecker degradation of isoleucine, leucine, and valine and provided maltiness to the aroma distribution of the oil [45,46]. Nonanal, octanal and hexanal have been reported to provide grass and fatty in rapeseed oil [45]. Benzeneacetaldehyde, which has a sweet and rose-like aroma, is a vital product of the shikimate pathway [47]. According to the above results, it is believed that the detected aldehydes contribute significantly to the aroma profile of the oil.
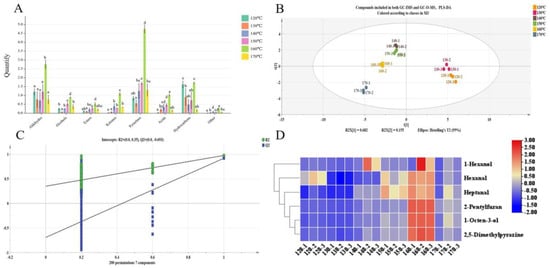
Figure 5.
PLS-DA analysis results of GC-O-MS and GC-IMS in the yellow horn seed oil roasted at different temperatures and Heat map of 6 key aroma compounds. (A)The peak area of each component in oil was measured via GC-IMS. (B) PLS-DA model (R2Y = 0.979, Q2 = 0.904). (C) Cross-validation plot by 200 permutation tests (R2 = 0.35, Q2 = −0.693). (D) Heat map of 6 key aroma compounds. Different lowercase letters (a–e) mean statistically significant differences (p < 0.05).
Alcohols: Alcohols can be generated through either lipid oxidation or aldehyde reduction, as previously reported [48]. The highest alcohol content of 1.0126 mg/kg was observed in the sample heated at 160 °C. The VOCs with OAVs ≥ 1 included 1-hexanol (ethereal, oil, fruity, sweet, green) and 1-octene-3-ol (mushroom, earthy). 1-hexanol (1.79–15.81%) and 1-octene-3-ol (2.09–4.95%) were also found to be important alcohol compounds in cold-pressed peanut oil [40]. Aroma compounds were determined by comparing the combined time of olfactometry-based aroma detection with the peak retention times of the GC-O-MS-detected compounds. Distinct scents of grass, mushrooms, and earthy notes are perceptible during the peak production period of these aroma compounds.
Esters: Studies have reported that most esters were associated with fruity and sweet aromas [49]. Volatile esters were formed via the esterification of various alcohols and carboxylic acids and/or esterification, which was catalyzed by using alcohol acyltransferases [50]. The content of ester components showed the maximum value (0.51 mg/kg) in the 160 °C sample and the minimum value (0.099 mg/kg) in the 130 °C sample. Among the yellow horn seed oil, three ester compounds with OVAs ≥ 1 were identified, including butyl acetate (ethereal, solvent, fruity, and banana), ethyl 2-methylbutyrate (fruity) and isoamyl acetate (sweet, fruity, banana, and solvent). Combined with olfactory analysis, it was determined that fruity aromas are distinctly detectable during their peak period.
Pyrazines: With increasing roasting temperature, the content of pyrazine compounds in yellow horn seed oil VOCs significantly changed (p < 0.05), reaching a peak (4.76 mg/kg) in the sample roasted at 160 °C. The VOCs with OAVs ≥ 1 contributed significantly to food aroma distribution. Seven Pyrazine compounds with OAVs ≥ 1 were screened from the yellow horn seed oil, including 2,5-dimethylpyrazine (roasted, nutty, popcorn), 2-mthyl-6-methylpyrazine (roasted, baked potato), 2,3,5-trimethylpyrazine (roasted, nutty), 3-ethyl-2,5-dimethylpyrazine (roasted, nutty), 2-ethyl-3,5-dimethylpyrazine (nutty, roasted, sweet), 2,6-diethylpyrazine (roasted, nutty, sweet), and 2,3-diethyl-5-methylpyrazine (raw peanut, potato). 2, 6-diethylpyrazine, an important product of the Maillard reaction [51], has been identified as a key aroma compound in sesame oil [11] and virgin rapeseed oil [52], providing nutty and toasty profiles. The results suggest that the presence of these aroma compounds contributes to the development of a nuanced roasted and nutty aroma in yellow horn seed oil.
Ketones: Ketones are typically generated through amino acid decomposition and the thermal oxidative degradation of polyunsaturated fatty acids [14]. Ketones with carbon numbers below seven were VOCs derived from lipids, contribute to fatty, herbal, flowery, and sweet aromas in samples [53]. The contents of ketones were the highest in the 160 °C sample (1.0949 mg/kg) in the aroma assay of the yellow horn seed oil roasted at different temperatures. There were three aroma substances with OAVs ≥ 1, including acetoin (sweet, buttery, and creamy), 4-hydroxy-2, 5-dimethyl-3 (2H) -furanone (sweet, soap, and bread), and 2-decanone (soap). Acetoin, as a short-chain ketone, could be derived from pyruvate conversion and citrate metabolism and/or conversion of diacetyl by diacetyl reductase enzyme [54]. Meanwhile, He et al. [12] confirmed that acetoin was a key aroma compound, providing a buttery odor to camellia seed oil. 4-hydroxy-2, 5-dimethyl-3 (2H)-furanone was a product of the Maillard reaction [55], providing fruitiness and sweetness profiles to yellow horn seed oil. 2-decanone possesses a soap aroma in yellow horn seed oil, mainly formed from the degradation of linoleic acid [56].
Hydrocarbons: Hydrocarbons were mainly derived from the decomposition of fatty acids alkoxy groups [14]. The total hydrocarbon content was the highest in the 160 °C sample, with a content of 1.7149 mg/kg in yellow horn seed oil. The OAVs of the unsaturated and saturated hydrocarbons in yellow horn seed oil are mostly low due to their high odor threshold, which limits their contribution to food aroma. Only the OAV (3.64) of styrene (sweet, balsamic, floral, and plastic) exceeds 1 in the 120 °C sample, imparting an herbal and citrus aroma to the oil.
3.2.2. Olfactometry Results
According to the combination of olfactory time and gas chromatography–retention time analysis, 31 compounds were screened out, including five alcohols (16.13%), three esters (9.68%), eight aldehydes (25.81%), two ketones (6.45%), six pyrazines heterocyclic compounds (19.35%), one furan heterocyclic compounds (3.23%), two hydrocarbons (6.45%), and four others (12.90%) (Table S3). Among them, 23 aroma compounds exhibited an OAV greater than 1 (Table 2). These compounds, which possess distinct aromas, are considered as significantly contributing to the overall aroma profile of yellow horn seed oil. Strong oily, fatty, and nutty odors are provided by the aroma compounds, such as 2-octenal, nonanal, and 2,4-decadienal in yellow horn seed oil. The grassy, green, and fruity aromas are mainly provided by aroma compounds, such as hexanol, 1-octen-3-ol, hexanal, 2-pentylfuran, and ethyl 2-methylbutyrate. Studies have reported that 2,4-decadienal provided a pleasant oily fragrance in fragrant rapeseed oil [57]. Similarly, nonanal is an oxidation product of oleic acid and contributes an oily aroma to food, as reported in [32,58]. (E)-2-octenal, which is a product of the oxidation of linoleic acid, was detected in nut oil [59]. (E)-2-octenal presented elevated content and low odor threshold, causing the nut oil to have its distinctive fatty aroma. 1-octen-3-ol and 2-pentylfuran were also detected in walnut oil and provided a green aroma in relation to walnut oil [60]. In this study, the synergistic effect of these aroma compounds contributed to the development of a robust aroma profile in hot-pressed yellow horn seed oil.
3.3. Combined Analysis of HS-SPME× GC-O-MS and HS-GC-IMS
3.3.1. Results of GC-O-MS and GC-IMS Combined Analysis
Through the analysis of GC-O-MS and GC-IMS data, it was found that 24 compounds were detected through the use of both methods (Figure S2A), including nine aldehydes (37.50%), four ketones (16.67%), three pyrazine heterocyclic compounds (12.50%), two esters (8.33%), two alcohols (8.33%), two hydrocarbons (8.33%), one furan heterocyclic compound (4.17%), and one acid (4.17%). As depicted in Figure S2B, the six groups of samples exhibited discernible discrepancies. Notably, the abundances of p-Xylene and Isoamyl acetate were higher in the 120 °C sample than those in other groups. However, the overall abundance was highest in the 160 °C samples. These results suggest that treatment at 160 °C is optimal for achieving maximum volatile compound abundance.
The PLS-DA model was established using data from 24 compounds detected via GC-IMS and GC-O-MS, as illustrated in Figure 5B. The separation of six samples is clearly observed in the PLS-DA model. The model parameters were R2Y = 0.979 and Q2 = 0.904, which meant the PLS-DA model was reliable. Meanwhile, as shown in Figure 5C, the results of 200 permutation tests indicated a good model fit with no evidence of overfitting (R2 = 0.35, Q2 = −0.693). Specifically, the 120 °C sample and 130 °C sample were distributed in the fourth quadrant, indicating their VOCs were relatively similar. The 140 °C, 150 °C, and 160 °C samples were distributed in the third quadrant, suggesting that their VOCs were relatively similar. In contrast, the 170 °C sample was distributed in the third quadrant alone, which indicates that the content of the 170 °C sample was significantly different from other samples. The six samples could be divided into three categories: the 120 °C and 130 °C samples belong to one group, while the 140 °C, 150 °C, and 160 °C samples from another group. The remaining sample is categorized separately at a temperature of 170 °C. The results were consistent with the OPLS-DA model established via GC-IMS.
3.3.2. Determination of the Key Aroma Compounds in Yellow Horn Seed Oil
Forty kinds of VOCs with VIP > 1 in GC-IMS and 23 kinds of main aroma compounds with combined OAVs ≥ 1 and sniffing in GC-O-MS were selected as screening criteria. Six VOCs were screened, including hexanal, 2,5-dimethylpyazine, heptanal, 2-pentylfuran, 1-hexanol and 1-octen-3-ol. They were considered to be the key aroma compounds in yellow horn seed oil. Hexanal in flaxseed oil contributes fatty, oily, sweet, nutty, and green odors [61]. It was reported that 2,5-dimethylpyrazine was a key aroma compound in oils such as canola oil [62], nut oil [59], and olive oil [13] and provided roasted and nutty aromas. In a roasted peanut oil study conducted by Yang et al. [63], it has been confirmed that heptanol is the product of fatty-acid oxidation and one of the important aroma compounds, providing fatty and oily aromas for roasted peanut oil. 2-pentylfuran is formed via the cyclization of peroxidation free radicals in the oxidation process of linoleic acid [64], which was considered as an important index of lipid oxidation. 2-pentylfuran can impart fruity and green aromas to the yellow horn seed oil. Through thermal oxidation studies on different varieties of olive oil, Kiralan et al. [65] reported that 2-pentylfuran was found in thyme-flavored olive oil during thermal oxidation. Yang et al. [66] reported that 1-hexanol provided a typical fresh odor for raw flaxseed powders, giving green-like, fresh-like, mint-like aromas via the aroma description of a microwave treatment of flaxseed meal. It was reported that linoleic acid (C2:10)-derived 10-L(S)-hydroperoxy-cis-9 and trans-11-octadecadieuoic acid via enzymatic oxidation, which then converted to 1-octen-3-ol [32]. Meanwhile, 1-octen-3-ol mainly contributed to mushroom and sweet odors to food. As shown in Figure 5D, the abundances of hexanal and heptanal in the 150 °C and 160 °C samples were significantly different from the other groups (p < 0.05). Moreover, 2-pentylfuran, 1-octen-3-ol, and 2,5-dimethylpyrazine in the 160 °C sample were significantly more abundant than in the other groups. To sum up, these six VOCs were important aroma substances that provided key aromas to yellow horn seed oil.
4. Conclusions
In this study, the aroma composition of hot-pressed yellow horn seed oil was assayed by using the seed of yellow horn roasted at different temperatures. VOCs were identified using HS-GC-IMS and HS-SPME/GC-O-MS. GC-IMS allowed for the determination of 97 VOCs, while GC-O-MS allowed for the determination of 77 VOCs, and both methods allowed for the identification of 24 common VOCs. In addition, the OAV values were calculated, and 35 VOCs were screened by OAVs ≥ 1 as the standard. Subsequently, twenty-three aroma compounds were confirmed through the use of olfactory probe recordings.
2,5-dimethylpyrazine, hexanal, heptanal, 2-pentylfuran, hexanol and 1-octen-3-ol were determined as the key aroma compounds in yellow horn seed oil by VOCs with VIP > 1 in GC-IMS and aroma compounds with combined OAVs ≥ 1 and olfactometry in GC-O-MS. Combining the result of OPLS-DA and PLS-DA, it was concluded that the aroma compounds of the samples were similar at 120 °C and 130 °C, while those of the samples were similar at 140 °C, 150 °C and 160 °C. According to the results of GC-O-MS, the VOC content of the sample at 160 °C is higher. The results of the present study supplemented the understanding of the composition and aroma of VOCs in yellow horn seed oil.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods12173174/s1, Figure S1: Rose and pie chart of VOCs detected by GC-IMS (A), (B) and GC-O-MS(C), (D) in yellow horn seed oil; Figure S2: Pie chart(A), heat map (B), and VIP map (C) of 24 VOCs detected jointly by GC-MS and GC-IMS; Table S1: The VOCs identified by GC-IMS in yellow horn seed oil; Table S2: The VOCs identified by GC-O-MS in yellow horn seed oil; Table S3: The VOCs identified by the combination of olfactory time and GC-MS retention time.
Author Contributions
Conceptualization, H.G. and J.S.; methodology, H.G.; software, H.G., M.L., H.L. and S.Z.; validation, H.G., M.L. and X.C.; formal analysis, H.G.; investigation, L.Z., Z.Z. and T.Z.; resources, J.S.; data curation, H.G. and S.L.; writing, original draft preparation, H.G. and M.L.; writing, review and editing, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Project supported by Qingdao Natural Science Foundation: 23-2-1-180-zyyd-jch; Key R&D Program of Shandong Province, China: 2021TZXD010; The Two Hundred Talents project of Yantai City in 2020; Innovation Ability Improvement Project of Science and Technology smes in Shandong Province: 2022TSGC2520, 2023TSGC0892; Qingdao people’s Livelihood Science and Technology Plan Project: 23-2-8-xdny-6-nsh, 23-3-8-xdny-1-nsh.
Data Availability Statement
The data used to support the findings of this study can be made available by the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, Q.Y.; Liu, G.S. The embryology of Xanthoceras and its phylogenetic implications. Plant Syst. Evol. 2012, 298, 457–468. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Ao, Y.; Saunders, M.R.; Wang, X. Diversity of seed and seed oil physicochemical traits of Xanthoceras sorbifolium Bunge. J. Food Compos. Anal. 2021, 96, 103705. [Google Scholar] [CrossRef]
- Li, J.; Zu, Y.G.; Luo, M.; Gu, C.B.; Zhao, C.J.; Efferth, T.; Fu, Y.J. Aqueous enzymatic process assisted by microwave extraction of oil from yellow horn (Xanthoceras sorbifolia Bunge.) seed kernels and its quality evaluation. Food Chem. 2013, 138, 2152–2158. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Fan, S.; Bi, Q.; Wang, S.; Hu, X.; Chen, M.; Wang, L. Seed morphology, oil content and fatty acid composition variability assessment in yellow horn (Xanthoceras sorbifolium Bunge) germplasm for optimum biodiesel production. Ind. Crops Prod. 2017, 97, 425–430. [Google Scholar] [CrossRef]
- Gu, L.-B.; Zhang, G.-J.; Du, L.; Du, J.; Qi, K.; Zhu, X.-L.; Zhang, X.-Y.; Jiang, Z.-H. Comparative study on the extraction of Xanthoceras sorbifolia Bunge (yellow horn) seed oil using subcritical n-butane, supercritical CO2, and the Soxhlet method. LWT 2019, 111, 548–554. [Google Scholar] [CrossRef]
- Liang, Q.; Fang, H.; Liu, J.; Zhang, B.; Bao, Y.; Hou, W.; Yang, K.Q. Analysis of the nutritional components in the kernels of yellowhorn (Xanthoceras sorbifolium Bunge) accessions. J. Food Compos. Anal. 2021, 100, 103925. [Google Scholar] [CrossRef]
- Liu, F.; Wu, R.; Ma, X.; Su, E. The Advancements and Prospects of Nervonic Acid Production. J. Agric. Food. Chem. 2022, 70, 12772–12783. [Google Scholar] [CrossRef]
- Matthäus, B.; Bonte, A.; Sinning, B.; Charrouf, Z. Aroma-Relevant Volatile Compounds as Markers for the Sensory Quality of Argan Oil. Eur. J. Lipid Sci. Technol. 2019, 121, 1900279. [Google Scholar] [CrossRef]
- Ji, J.; Liu, Y.; Shi, L.; Wang, N.; Wang, X. Effect of roasting treatment on the chemical composition of sesame oil. LWT 2019, 101, 191–200. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhen, C.; Zhao, B.; Zhou, S.; Jiang, Y.; Wang, X.; Jin, Q.; Zhang, Y. Comparative characterization of key odorants and aroma profiles of fragrant rapeseed oil under different roasting conditions. Food Res. Int. 2023, 163, 112195. [Google Scholar] [CrossRef]
- Ma, G.; He, S.; Liu, S.; Zhang, Z.; Zhang, T.; Wang, L.; Ma, Y.; Sun, H. Application of Maillard Reaction Products Derived Only from Enzymatically Hydrolyzed Sesame Meal to Enhance the Flavor and Oxidative Stability of Sesame Oil. Molecules 2022, 27, 8857. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wu, H.; Yu, Z. Microwave pretreatment of camellia (Camellia oleifera Abel.) seeds: Effect on oil flavor. Food Chem. 2021, 364, 130388. [Google Scholar] [CrossRef] [PubMed]
- Kraljić, K.; Stjepanović, T.; Obranović, M.; Pospišil, M.; Balbino, S.; Škevin, D. Influence of Conditioning Temperature on the Quality, Nutritional Properties and Volatile Profile of Virgin Rapeseed Oil. Food Technol. Biotechnol. 2018, 56, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bi, S.; Niu, X.; Chen, Y.; Liu, Y.; Zhou, Q. Comparison of aroma active compounds in cold- and hot-pressed walnut oil by comprehensive two-dimensional gas chromatography-olfactory-mass spectrometry and headspace-gas chromatography-ion mobility spectrometry. Food Res. Int. 2023, 163, 112208. [Google Scholar] [CrossRef]
- Capitain, C.; Weller, P. Non-Targeted Screening Approaches for Profiling of Volatile Organic Compounds Based on Gas Chromatography-Ion Mobility Spectroscopy (GC-IMS) and Machine Learning. Molecules 2021, 26, 5457. [Google Scholar] [CrossRef]
- Christmann, J.; Rohn, S.; Weller, P. Finding features—Variable extraction strategies for dimensionality reduction and marker compounds identification in GC-IMS data. Food Res. Int. 2022, 161, 111779. [Google Scholar] [CrossRef]
- Joscha, C.; Sascha, R.; Philipp, W. gc-ims-tools—A new Python package for chemometric analysis of GC–IMS data. Food Chem. 2022, 394, 133476. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Yu, M.; Yang, P.; Song, H.; Guan, X. Research progress in comprehensive two-dimensional gas chromatography-mass spectrometry and its combination with olfactometry systems in the flavor analysis field. J. Food Compos. Anal. 2022, 114, 104790. [Google Scholar] [CrossRef]
- Song, H.; Liu, J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar] [CrossRef]
- Bi, S.; Niu, X.; Yang, F.; Xu, Y.; Dai, Y.; Liu, Y.; Zhou, Q. Roasting pretreatment of walnut (Juglans regia L.) kernels: Improvement of the oil flavor profile and correlation with the chemical composition. Food Funct. 2022, 13, 10956–10969. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Ahmed, I.A.M.; Ozcan, M.M.; Al-Juhaimi, F.Y.; Babiker, E.E.; Azmi, I.U. An evaluation of bioactive compounds, fatty acid composition and oil quality of chia (Salvia hispanica L.) seed roasted at different temperatures. Food Chem. 2020, 333, 127531. [Google Scholar] [CrossRef] [PubMed]
- Potočnik, T.; Košir, I.J. Influence of roasting temperature of pumpkin seed on PAH and aroma formation. Eur. J. Lipid Sci. Technol. 2016, 119, 1500593. [Google Scholar] [CrossRef]
- Ismail, B.B.; Huang, R.; Liu, D.; Ye, X.; Guo, M. Potential valorisation of baobab (Adansonia digitata) seeds as a coffee substitute: Insights and comparisons on the effect of roasting on quality, sensory profiles, and characterisation of volatile aroma compounds by HS-SPME/GC–MS. Food Chem. 2022, 394, 133475. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, H.; Hou, Y.; Li, J.; Zou, T.; Zhang, D.; Wen, R.; Li, H.; Song, H. Characterization of Key Odor-Active Off-Flavor Compounds in Aged Pasteurized Yogurt by Sensory-Directed Flavor Analysis. J. Agric. Food Chem. 2022, 70, 14439–14447. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y.; Wang, B.; Song, H.; Zou, T. Screening of the volatile compounds in fresh and thermally treated watermelon juice via headspace-gas chromatography-ion mobility spectrometry and comprehensive two-dimensional gas chromatography-olfactory-mass spectrometry analysis. LWT 2021, 137, 110478. [Google Scholar] [CrossRef]
- Gemert, L. Compilations of Odour Threshold Values in Air, Water and Other Media; BACIS (Boelens Aroma Chemical Information Service): Huizen, The Netherlands, 2003. [Google Scholar]
- Yang, Y.; Yu, P.; Sun, J.; Jia, Y.; Wan, C.; Zhou, Q.; Huang, F. Investigation of volatile thiol contributions to rapeseed oil by odor active value measurement and perceptual interactions. Food Chem. 2022, 373, 131607. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, H.; Chen, J.; Xie, J.; Shen, S.; Deng, Y.; Zhu, J.; Yuan, H.; Jiang, Y. Characterization of the key aroma compounds in black teas with different aroma types by using gas chromatography electronic nose, gas chromatography-ion mobility spectrometry, and odor activity value analysis. LWT 2022, 163, 113492. [Google Scholar] [CrossRef]
- Li, M.; Yang, R.; Zhang, H.; Wang, S.; Chen, D.; Lin, S. Development of a flavor fingerprint by HS-GC-IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef]
- Kocadagli, T.; Methven, L.; Kant, A.; Parker, J.K. Targeted precursor addition to increase baked flavour in a low-acrylamide potato-based matrix. Food Chem. 2021, 339, 128024. [Google Scholar] [CrossRef]
- Ding, A.; Zhu, M.; Qian, X.; Shi, L.; Huang, H.; Xiong, G.; Wang, J.; Wang, L. Effect of fatty acids on the flavor formation of fish sauce. LWT 2020, 134, 110259. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, X.; Liu, S.Q. Aroma modulation of vegetable oils—A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1538–1551. [Google Scholar] [CrossRef]
- Sabatini, N.; Mucciarella, M.R.; Marsilio, V. Volatile compounds in uninoculated and inoculated table olives with Lactobacillus plantarum (Olea europaea L., cv. Moresca and Kalamata). LWT 2008, 41, 2017–2022. [Google Scholar] [CrossRef]
- Wu, S.; Peng, Y.; Xi, J.; Zhao, Q.; Xu, D.; Jin, Z.; Xu, X. Effect of sourdough fermented with corn oil and lactic acid bacteria on bread flavor. LWT 2022, 155, 112935. [Google Scholar] [CrossRef]
- Wang, M.; Maeda, H.A. Aromatic amino acid aminotransferases in plants. Phytochem. Rev. 2017, 17, 131–159. [Google Scholar] [CrossRef]
- Wu, W.; Wang, X.; Hu, P.; Zhang, Y.; Li, J.; Jiang, J.; Zheng, R.; Zhang, L. Research on flavor characteristics of beef cooked in tomato sour soup by gas chromatography-ion mobility spectrometry and electronic nose. LWT 2023, 179, 114646. [Google Scholar] [CrossRef]
- Rong, Y.; Xie, J.; Yuan, H.; Wang, L.; Liu, F.; Deng, Y.; Jiang, Y.; Yang, Y. Characterization of volatile metabolites in Pu-erh teas with different storage years by combining GC-E-Nose, GC–MS, and GC-IMS. Food Chem. X 2023, 18, 100693. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G. A review of advances and new developments in the analysis of biological volatile organic compounds. Microchem. J. 2010, 95, 127–139. [Google Scholar] [CrossRef]
- Dun, Q.; Yao, L.; Deng, Z.; Li, H.; Li, J.; Fan, Y.; Zhang, B. Effects of hot and cold-pressed processes on volatile compounds of peanut oil and corresponding analysis of characteristic flavor components. LWT 2019, 112, 107648. [Google Scholar] [CrossRef]
- Jia, W.; Fan, Z.; Du, A.; Li, Y.; Zhang, R.; Shi, Q.; Shi, L.; Chu, X. Recent advances in Baijiu analysis by chromatography based technology—A review. Food Chem. 2020, 324, 126899. [Google Scholar] [CrossRef]
- Gou, M.; Bi, J.; Chen, Q.; Wu, X.; Fauconnier, M.-L.; Qiao, Y. Advances and Perspectives in Fruits and Vegetables Flavor Based on Molecular Sensory Science. Food Rev. Int. 2021, 39, 3066–3079. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, J.; Zhou, X.; Chen, R.; Liu, D.; Ye, X. Advances in identification and biosynthetic pathway of key aroma in fruits. J. Chin. Inst. Food Sci. Technol. 2016, 16, 211–218. [Google Scholar] [CrossRef]
- Petronilho, S.; Lopez, R.; Ferreira, V.; Coimbra, M.A.; Rocha, S.M. Revealing the Usefulness of Aroma Networks to Explain Wine Aroma Properties: A Case Study of Portuguese Wines. Molecules 2020, 25, 272. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Zhao, X.; Huyan, Z.; Liu, T.; Yu, X. Relationship of Glucosinolate Thermal Degradation and Roasted Rapeseed Oil Volatile Odor. J. Agric. Food Chem. 2019, 67, 11187–11197. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Jia, X.; Yao, Y.Z.; Wang, B.; Wei, C.Q.; Zhang, M.; Huang, F. Characterization of the Aroma-Active Compounds in Commercial Fragrant Rapeseed Oils via Monolithic Material Sorptive Extraction. J. Agric. Food Chem. 2019, 67, 11454–11463. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, D.; Jiang, H.; Sun, H.; Zhang, C.; Zhao, H.; Li, X.; Yan, F.; Chen, C.; Xu, Z. Aroma characterization of Hanzhong black tea (Camellia sinensis) using solid phase extraction coupled with gas chromatography–mass spectrometry and olfactometry and sensory analysis. Food Chem. 2019, 274, 130–136. [Google Scholar] [CrossRef]
- Farooq, A.; Rahman, Q.; Ali, A. Chapter 44—Cold pressed walnut (Juglans regia L.) oil. In Cold Pressed Oils; Academic Press: Cambridge, MA, USA, 2020; pp. 491–495. [Google Scholar] [CrossRef]
- Kelebek, H.; Kesen, S.; Sonmezdag, A.S.; Cetiner, B.; Kola, O.; Selli, S. Characterization of the key aroma compounds in tomato pastes as affected by hot and cold break process. J. Food Meas. Charact. 2018, 12, 2461–2474. [Google Scholar] [CrossRef]
- Goulet, C.; Kamiyoshihara, Y.; Lam, N.B.; Richard, T.; Taylor, M.G.; Tieman, D.M.; Klee, H.J. Divergence in the enzymatic activities of a tomato and Solanum pennellii alcohol acyltransferase impacts fruit volatile ester composition. Mol. Plant 2015, 8, 153–162. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, M.; Xie, J.; Zhao, M.; Hou, L.; Liang, J.; Wang, S.; Cheng, J. Volatile flavor constituents in the pork broth of black-pig. Food Chem. 2017, 226, 51–60. [Google Scholar] [CrossRef]
- Anna, G.; Henryk, H.J.; Małgorzata, M.; Aleksander, S.; Anna, K. Flavoromics approach in monitoring changes in volatile compounds of virgin rapeseed oil caused by seed roasting. J. Chromatogr. A 2016, 1428, 292–304. [Google Scholar] [CrossRef]
- Selli, S.; Kelebek, H.; Ayseli, M.T.; Tokbas, H. Characterization of the most aroma-active compounds in cherry tomato by application of the aroma extract dilution analysis. Food Chem. 2014, 165, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Mallia, S.; Fernández-García, E.; Bosset, J.O. Comparison of purge and trap and solid phase microextraction techniques for studying the volatile aroma compounds of three European PDO hard cheeses. Int. Dairy J. 2005, 15, 741–758. [Google Scholar] [CrossRef]
- Poisson, L.; Schaerer, A.; Spreng, S.; Mestdagh, F.; Blank, I.; Davidek, T. Generation of alpha-Diketones and 4-Hydroxy-2,5-dimethyl-3(2H)-furanone upon Coffee Roasting-Impact of Roast Degree on Reaction Pathways. J. Agric. Food Chem. 2019, 67, 13829–13839. [Google Scholar] [CrossRef]
- Zhang, Z.; Zang, M.; Zhang, K.; Li, D.; Wang, S.; Li, X.; Zhou, H.; Zhang, X. Changes in volatile profiles of a refrigerated-reheated xylose-cysteine-lecithin reaction model analyzed by GC×GC-MS and E-nose. J. Food Sci. 2022, 87, 1069–1081. [Google Scholar] [CrossRef]
- Cao, L.; Jia, P.; Liu, H.; Kang, S.; Jiang, S.; Pang, M. Effects of High-Canolol Phenolic Extracts on Fragrant Rapeseed Oil Quality and Flavor Compounds during Frying. Foods 2023, 12, 827. [Google Scholar] [CrossRef]
- Ba, H.; Ryu, K.; Lan, N.; Hwang, I. Influence of particular breed on meat quality parameters, sensory characteristics and volatile compounds. Food Sci. Biotechnol. 2013, 22, 651–658. [Google Scholar] [CrossRef]
- Leal, A.R.; Dionisio, A.P.; Abreu, F.A.P.; Oliveira, G.F.; Araujo, I.; Magalhaes, H.C.R.; Leite, A.B.; Silva, E.; Nascimento, R.F.D.; Nascimento, H.O.D.; et al. Impact of different kernel grades on volatile compounds profile, fatty acids and oxidative quality of cashew nut oil. Food Res. Int. 2023, 165, 112526. [Google Scholar] [CrossRef]
- Xu, Y.; Bi, S.; Xiong, C.; Dai, Y.; Zhou, Q.; Liu, Y. Identification of aroma active compounds in walnut oil by monolithic material adsorption extraction of RSC18 combined with gas chromatography-olfactory-mass spectrometry. Food Chem. 2023, 402, 134303. [Google Scholar] [CrossRef]
- Wei, C.; Xi, W.; Nie, X.; Liu, W.; Wang, Q.; Yang, B.; Cao, D. Aroma characterization of flaxseed oils using headspace solid-phase microextraction and gas chromatography-olfactometry. Eur. J. Lipid Sci. Technol. 2013, 115, 1032–1042. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Chen, J.; Jing, B.; Zhang, L.; Yu, X. Characterization of Differences in Flavor in Virgin Rapeseed Oils by Using Gas Chromatography–Mass Spectrometry, Electronic Nose, and Sensory Analysis. Eur. J. Lipid Sci. Technol. 2019, 122, 1900205. [Google Scholar] [CrossRef]
- Yang, K.M.; Chao, L.K.; Wu, C.S.; Ye, Z.S.; Chen, H.C. Headspace Solid-Phase Microextraction Analysis of Volatile Components in Peanut Oil. Molecules 2021, 26, 3306. [Google Scholar] [CrossRef] [PubMed]
- Elmore, J.S.; Mottram, D.S.; Enser, M.; Wood, J.D. Effect of the polyunsaturated fatty acid composition of beef muscle on the profile of aroma volatiles. J. Agric. Food Chem. 1999, 47, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Kiralan, S.S.; Karagoz, S.G.; Ozkan, G.; Kiralan, M.; Ketenoglu, O. Changes in Volatile Compounds of Virgin Olive Oil Flavored with Essential Oils During Thermal and Photo-Oxidation. Food Anal. Methods 2021, 14, 883–896. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, Q.; Jia, X.; Shi, J.; Wan, C.; Zhou, Q.; Wang, Q. Characterization of key odorants in peeled and unpeeled flaxseed powders using solvent-assisted flavor evaporation and odor activity value calculation. LWT 2021, 138, 110724. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).