Application of GC–TOF/MS and GC×GC–TOF/MS to Discriminate Coffee Products in Three States (Bean, Powder, and Brew)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coffee Samples
2.2. Headspace Solid-Phase Microextraction
2.3. GC–TOF/MS Condition
2.4. GC×GC–TOF/MS Condition
2.5. Identification of Volatile Components
3. Results
3.1. Identification of VOCs in the Coffee Bean, Powder, and Brew
3.2. PCA Based on VOCs Identified by GC–TOF/MS and GC×GC–TOF/MS
3.3. Identification of Key VOCs in Coffee Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, S.-H.; Qing, P.; Hu, W.; Liu, Y. Using a Modified Payment Card Survey to Measure Chinese Consumers’ Willingness to Pay for Fair Trade Coffee: Considering Starting Points. Can. J. Agric. Econ. Rev. Can. D’agroecon. 2013, 61, 119–139. [Google Scholar] [CrossRef]
- Seninde, D.R.; Chambers, E. Coffee Flavor: A Review. Beverages 2020, 6, 44. [Google Scholar] [CrossRef]
- Spencer, M.; Sage, E.; Velez, M.; Guinard, J.X. Using Single Free Sorting and Multivariate Exploratory Methods to Design a New Coffee Taster’s Flavor Wheel. J. Food Sci. 2016, 81, S2997–S3005. [Google Scholar] [CrossRef] [PubMed]
- Sunarharum, W.B.; Williams, D.J.; Smyth, H.E. Complexity of coffee flavor: A compositional and sensory perspective. Food Res. Int. 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Błaszkiewicz, J.; Nowakowska-Bogdan, E.; Barabosz, K.; Kulesza, R.; Dresler, E.; Woszczyński, P.; Biłos, Ł.; Matuszek, D.B.; Szkutnik, K. Effect of green and roasted coffee storage conditions on selected characteristic quality parameters. Sci. Rep. 2023, 13, 6447. [Google Scholar] [CrossRef] [PubMed]
- Carcea, M.; Danesi, I.; De Gara, L.; Diretto, G.; Fanali, C.; Raffo, A.; Sinesio, F.; Della Posta, S.; Frusciante, S.; Moneta, E.; et al. Chemical composition and sensory profile of the Italian espresso coffee powder and beverage under different roasting conditions. Eur. Food Res. Technol. 2023, 249, 1287–1301. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, J.; Ed-Dra, A.; Li, X.; Peng, X.; Xia, L.; Guo, Q.; Yao, G.; Yue, M. Prevalence and genomic investigation of Salmonella isolates recovered from animal food-chain in Xinjiang, China. Food Res. Int. 2021, 142, 110198. [Google Scholar] [CrossRef]
- Caprioli, G.; Cortese, M.; Cristalli, G.; Maggi, F.; Odello, L.; Ricciutelli, M.; Sagratini, G.; Sirocchi, V.; Tomassoni, G.; Vittori, S. Optimization of espresso machine parameters through the analysis of coffee odorants by HS-SPME–GC/MS. Food Chem. 2012, 135, 1127–1133. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Q.; Linforth, R.; Fisk, I.D.; Yang, N. Modifying Robusta coffee aroma by green bean chemical pre-treatment. Food Chem. 2019, 272, 251–257. [Google Scholar] [CrossRef]
- Bressanello, D.; Liberto, E.; Cordero, C.; Rubiolo, P.; Pellegrino, G.; Ruosi, M.R.; Bicchi, C. Coffee aroma: Chemometric comparison of the chemical information provided by three different samplings combined with GC-MS to describe the sensory properties in cup. Food Chem. 2017, 214, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Dulsat-Serra, N.; Quintanilla-Casas, B.; Vichi, S. Volatile thiols in coffee: A review on their formation, degradation, assessment and influence on coffee sensory quality. Food Res. Int. 2016, 89, 982–988. [Google Scholar] [CrossRef]
- Gruczyńska, E.; Kowalska, D.; Kozłowska, M.; Majewska, E.; Tarnowska, K. Furan in roasted, ground and brewed coffee. Rocz. Panstw. Zakl. Hig. 2018, 69, 111–118. [Google Scholar]
- Zapata, J.; Londoño, V.; Naranjo, M.; Osorio, J.; Lopez, C.; Quintero, M. Characterization of aroma compounds present in an industrial recovery concentrate of coffee flavour. CYTA J. Food 2018, 16, 367–372. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Cui, D.; Fang, X.; Gao, J.; Liu, Y. Fast and Non-Destructive Profiling of Commercial Coffee Aroma under Three Conditions (Beans, Powder, and Brews) Using GC-IMS. Molecules 2022, 27, 6262. [Google Scholar] [CrossRef] [PubMed]
- Tranchida, P.Q.; Franchina, F.A.; Dugo, P.; Mondello, L. Comprehensive two-dimensional gas chromatography-mass spectrometry: Recent evolution and current trends. Mass Spectrom. Rev. 2016, 35, 524–534. [Google Scholar] [CrossRef]
- Yu, Y.; Nie, Y.; Chen, S.; Xu, Y. Characterization of the dynamic retronasal aroma perception and oral aroma release of Baijiu by progressive profiling and an intra-oral SPME combined with GC × GC-TOFMS method. Food Chem. 2023, 405, 134854. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Y.P.; Blank, I.; Li, F.; Li, C.; Liu, Y. GC × GC-ToF-MS and GC-IMS based volatile profile characterization of the Chinese dry-cured hams from different regions. Food Res. Int. 2021, 142, 110222. [Google Scholar] [CrossRef]
- Eggermont, D.; Spadafora, N.D.; Aspromonte, J.; Purcaro, G. Unraveling the impact of the capsule material on the aroma of brewed coffee by headspace analysis using a HiSorb probe followed by reverse fill/flush flow modulation GC×GC-MS. Anal. Bioanal. Chem. 2023, 415, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Novaes, F.J.M.; Silva Junior, A.I.d.; Kulsing, C.; Nolvachai, Y.; Bizzo, H.R.; Aquino Neto, F.R.d.; Rezende, C.M.; Marriott, P.J. New approaches to monitor semi-volatile organic compounds released during coffee roasting using flow-through/active sampling and comprehensive two-dimensional gas chromatography. Food Res. Int. 2019, 119, 349–358. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef]
- Mehdinia, A.; Aziz-Zanjani, M.O. Advances for sensitive, rapid and selective extraction in different configurations of solid-phase microextraction. TrAC Trends Anal. Chem. 2013, 51, 13–22. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Y.P.; Deng, S.; Li, C.; Xu, X.; Zhou, G.; Liu, Y. Application of sensory evaluation, GC-ToF-MS, and E-nose to discriminate the flavor differences among five distinct parts of the Chinese blanched chicken. Food Res. Int. 2020, 137, 109669. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Cui, C.; Fisk, I.D. Variability of single bean coffee volatile compounds of Arabica and robusta roasted coffees analysed by SPME-GC-MS. Food Res. Int. 2018, 108, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Franca, A.S.; Oliveira, L.S.; Oliveira, R.C.S.; Agresti, P.C.M.; Augusti, R. A preliminary evaluation of the effect of processing temperature on coffee roasting degree assessment. J. Food Eng. 2009, 92, 345–352. [Google Scholar] [CrossRef]
- Echavarría, A.P.; Pagán, J.; Ibarz, A. Melanoidins Formed by Maillard Reaction in Food and Their Biological Activity. Food Eng. Rev. 2012, 4, 203–223. [Google Scholar] [CrossRef]
- Nie, S.; Huang, J.; Hu, J.; Zhang, Y.; Wang, S.; Li, C.; Marcone, M.; Xie, M. Effect of pH, temperature and heating time on the formation of furan in sugar–glycine model systems. Food Sci. Hum. Wellness 2013, 2, 87–92. [Google Scholar] [CrossRef]
- Bi, K.H.; Zhang, L.; Qiao, X.G.; Xu, Z.X. Tea Polyphenols as Inhibitors of Furan Formed in the Maillard Model System and Canned Coffee Model. J. Food Sci. 2017, 82, 1271–1277. [Google Scholar] [CrossRef]
- Baggenstoss, J.; Poisson, L.; Kaegi, R.; Perren, R.; Escher, F. Coffee Roasting and Aroma Formation: Application of Different Time−Temperature Conditions. J. Agric. Food Chem. 2008, 56, 5836–5846. [Google Scholar] [CrossRef]
- Steen, I.; Waehrens, S.S.; Petersen, M.A.; Munchow, M.; Bredie, W.L.P. Influence of serving temperature on flavour perception and release of Bourbon Caturra coffee. Food Chem. 2017, 219, 61–68. [Google Scholar] [CrossRef]
- Cui, D.D.; Liu, Y.; Chen, Y.P.; Feng, X.; Lu, Y.; Yu, B. Application of SPME-GC-TOFMS, E-nose, and sensory evaluation to investigate the flavor characteristics of Chinese Yunnan coffee at three different conditions (beans, ground powder, and brewed coffee). Flavour Fragr. J. 2020, 35, 541–560. [Google Scholar] [CrossRef]
- Moon, J.K.; Shibamoto, T. Role of roasting conditions in the profile of volatile flavor chemicals formed from coffee beans. J. Agric. Food Chem. 2009, 57, 5823–5831. [Google Scholar] [CrossRef]
- Maria, C.A.B.D.; Trugo, L.C.; Neto, F.R.A.; Moreira, B.R.F.A.; Alviano, C.S. Composition of green coffee water-soluble fractions and identification of volatiles formed during roasting. Food Chem. 1996, 55, 203–207. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, Q.; Zhu, P.; Hu, W.; Jia, Y.; Yang, S.; Huang, Y.; Yang, Z.; Chai, Z.; Zhai, T.; et al. High throughput screening of key functional strains based on improving tobacco quality and mixed fermentation. Front. Bioeng. Biotechnol. 2023, 11, 1108766. [Google Scholar] [CrossRef]
- Lee, J.-C. A Study of Flavor Compounds and Quality Characteristics in Espresso of Coffee Shop Franchises. Culin. Sci. Hosp. Res. 2019, 25, 67–75. [Google Scholar]
- Andueza, S.; De Peña, M.P.; Cid, C. Chemical and Sensorial Characteristics of Espresso Coffee as Affected by Grinding and Torrefacto Roast. J Agric. Food Chem. 2003, 51, 7034–7039. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, N.; Yang, Q.; Ayed, C.; Linforth, R.; Fisk, I.D. Enhancing Robusta coffee aroma by modifying flavour precursors in the green coffee bean. Food Chem. 2019, 281, 8–17. [Google Scholar] [CrossRef]
- Shibamoto, T.; Bernhard, R.A. Investigation of Pyrazine Formation Pathways in Sugar-Ammonia Model Systems. J. Agric. Food Chem. 1977, 25, 609–614. [Google Scholar] [CrossRef]
- Xia, X.; Zhai, Y.; Cui, H.; Zhang, H.; Hayat, K.; Zhang, X.; Ho, C.T. Structural diversity and concentration dependence of pyrazine formation: Exogenous amino substrates and reaction parameters during thermal processing of l-alanyl-l-glutamine Amadori compound. Food Chem. 2022, 390, 133144. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Liu, C.; Liu, X.; Degn, T.K.; Munchow, M.; Fisk, I. Determination of volatile marker compounds of common coffee roast defects. Food Chem. 2016, 211, 206–214, reprinted in Food Chem. 2020, 315, 126331. [Google Scholar] [CrossRef]
- Serra Bonvehí, J.; Ventura Coll, F. Factors Affecting the Formation of Alkylpyrazines during Roasting Treatment in Natural and Alkalinized Cocoa Powder. J Agric. Food Chem. 2002, 50, 3743–3750. [Google Scholar] [CrossRef]
- Hurtado-Benavides, A.; Dorado, A.D.; Sánchez-Camargo, A.d.P. Study of the fatty acid profile and the aroma composition of oil obtained from roasted Colombian coffee beans by supercritical fluid extraction. J. Supercrit. Fluids 2016, 113, 44–52. [Google Scholar] [CrossRef]
- Wang, X.; Ma, L.; Yan, S.; Chen, X.; Growe, A. Trade for Food Security: The Stability of Global Agricultural Trade Networks. Foods 2023, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wu, H.; Viejo, C.G.; Dunshea, F.R.; Suleria, H.A.R. Effects of postharvest processing on aroma formation in roasted coffee—A review. Int. J. Food Sci. Technol. 2023, 58, 1007–1027. [Google Scholar] [CrossRef]
- Poisson, L.; Schaerer, A.; Spreng, S.; Mestdagh, F.; Blank, I.; Davidek, T. Generation of alpha-Diketones and 4-Hydroxy-2,5-dimethyl-3(2H)-furanone upon Coffee Roasting-Impact of Roast Degree on Reaction Pathways. J Agric. Food Chem. 2019, 67, 13829–13839. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhong, Y.; Tang, H.; Ou, C.; Zhao, H.; Yu, X. Analysis of Volatile Compounds of Meat Flavor Essence Through Two Comprehensive Dimensional Gas Chromatography-time of Flight Mass Spectrometry. J. Nucl. Agric. Sci. 2016, 30, 1331–1341. [Google Scholar]
- Giacalone, D.; Degn, T.K.; Yang, N.; Liu, C.; Fisk, I.; Münchow, M. Common roasting defects in coffee: Aroma composition, sensory characterization and consumer perception. Food Qual. Prefer. 2019, 71, 463–474. [Google Scholar] [CrossRef]
- Majcher, M.A.; Klensporf-Pawlik, D.; Dziadas, M.; Jelen, H.H. Identification of Aroma Active Compounds of Cereal Coffee Brew and Its Roasted Ingredients. J. Agric. Food Chem. 2013, 61, 2648–2654. [Google Scholar] [CrossRef]
- Gonzalez-Rios, O.; Suarez-Quiroz, M.L.; Boulanger, R.; Barel, M.; Guyot, B.; Guiraud, J.-P.; Schorr-Galindo, S. Impact of “ecological” post-harvest processing on coffee aroma: II. Roasted coffee. J. Food Compos. Anal. 2007, 20, 297–307. [Google Scholar] [CrossRef]
- Genovese, A.; De Vivo, A.; Aprea, A.; Cristina Tricarico, M.; Sacchi, R.; Sarghini, F. Particle size and variety of coffee used as variables in mitigation of furan and 2-methylfuran content in espresso coffee. Food Chem. 2021, 361, 130037. [Google Scholar] [CrossRef]
- Chen, D.; Sheng, M.; Wang, S.; Chen, X.; Leng, A.; Lin, S. Dynamic changes and formation of key contributing odorants with amino acids and reducing sugars as precursors in shiitake mushrooms during hot air drying. Food Chem. 2023, 424, 136409. [Google Scholar] [CrossRef]
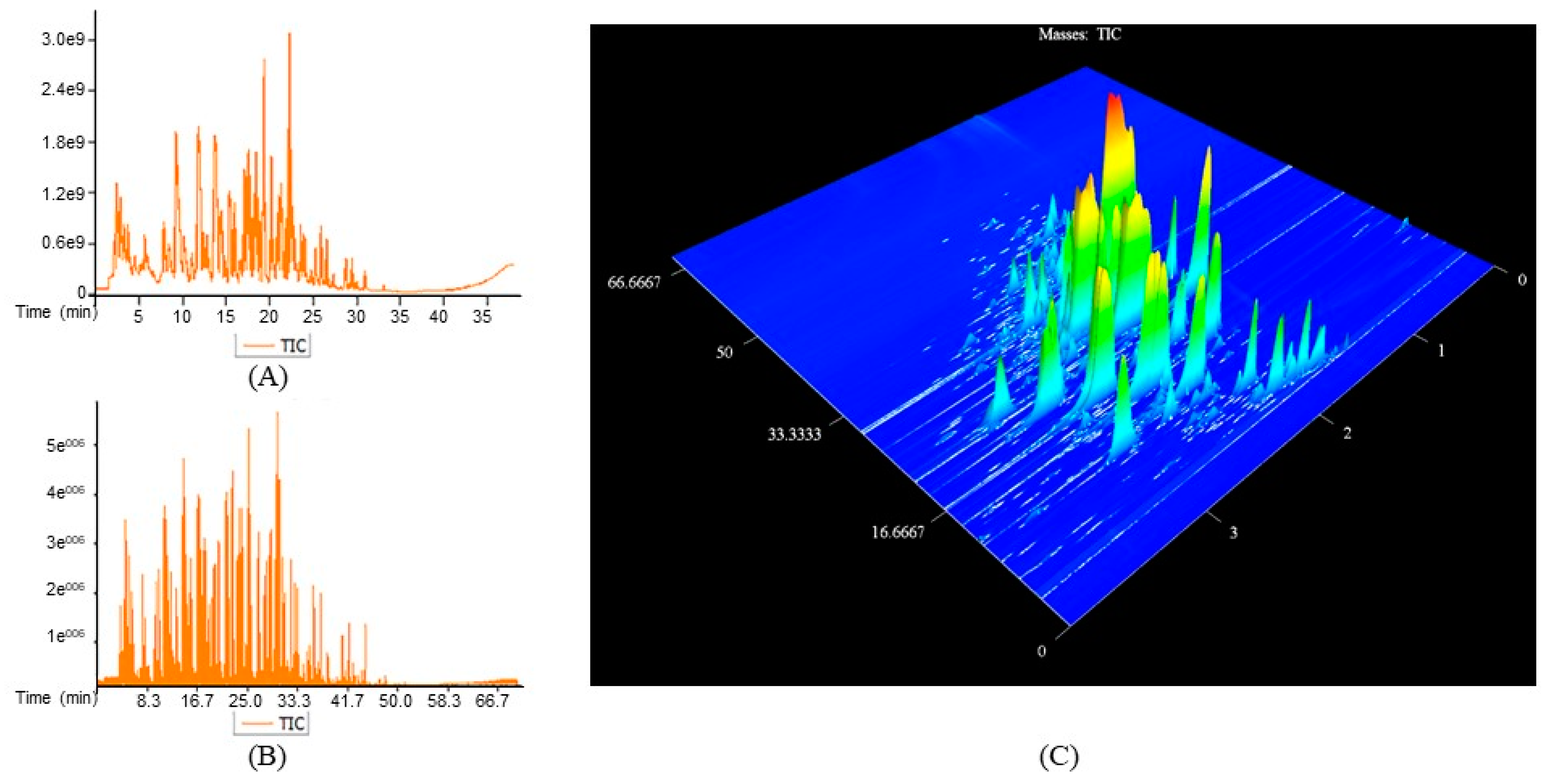
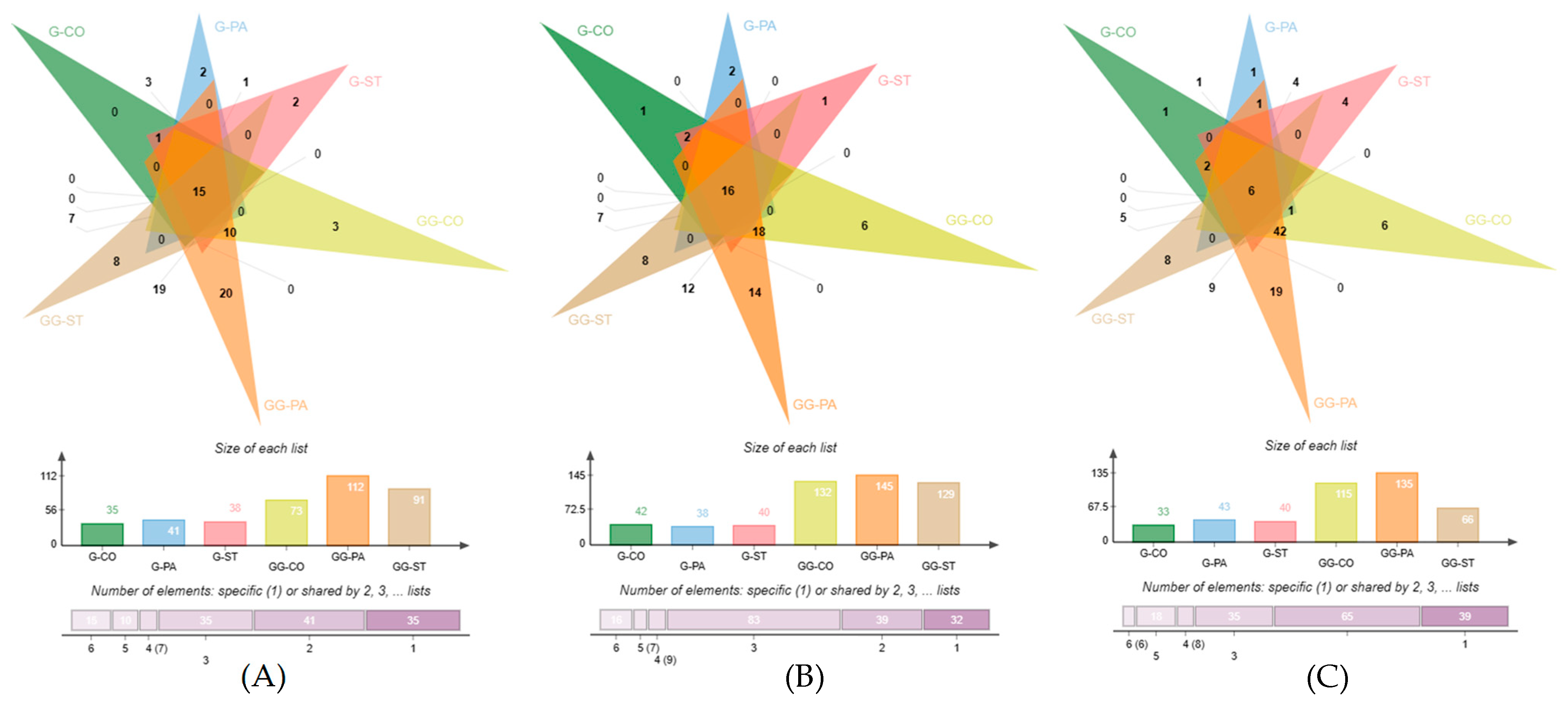

| GC–TOF/MS | GC×GC–TOF/MS | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beans | Powder | Brew | Beans | Powder | Brew | |||||||||||||
| CO | PA | ST | CO | PA | ST | CO | PA | ST | CO | PA | ST | CO | PA | ST | CO | PA | ST | |
| Hydrocarbons | 6 | 8 | 8 | 6 | 6 | 4 | 8 | 5 | 13 | 21 | 28 | 23 | 28 | 29 | 39 | 13 | 31 | 33 |
| Aldehydes | 4 | 5 | 3 | 5 | 8 | 7 | 10 | 10 | 10 | 21 | 25 | 13 | 26 | 28 | 26 | 17 | 28 | 18 |
| Ketones | 13 | 14 | 15 | 14 | 13 | 15 | 8 | 7 | 10 | 69 | 83 | 41 | 88 | 95 | 95 | 51 | 63 | 50 |
| Acids | 3 | 5 | 10 | 10 | 6 | 7 | 9 | 6 | 8 | 13 | 13 | 5 | 13 | 14 | 16 | 10 | 7 | 6 |
| Phenols | 5 | 7 | 7 | 8 | 8 | 8 | 8 | 10 | 9 | 11 | 10 | 5 | 11 | 8 | 10 | 6 | 10 | 7 |
| Alcohols | 4 | 9 | 8 | 8 | 6 | 7 | 5 | 6 | 5 | 26 | 36 | 19 | 32 | 40 | 30 | 19 | 25 | 19 |
| Esters | 8 | 8 | 7 | 5 | 5 | 6 | 1 | 3 | 7 | 25 | 37 | 15 | 31 | 41 | 36 | 14 | 25 | 19 |
| Pyridines | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 0 | 7 | 8 | 3 | 7 | 10 | 6 | 2 | 5 | 4 |
| Pyrroles | 2 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 4 | 2 | 7 | 7 | 5 | 5 | 5 | 5 |
| Pyrazines | 8 | 9 | 6 | 8 | 8 | 8 | 6 | 5 | 7 | 27 | 31 | 10 | 25 | 23 | 28 | 14 | 17 | 16 |
| Furans | 9 | 10 | 10 | 11 | 11 | 11 | 11 | 14 | 12 | 20 | 23 | 13 | 27 | 28 | 26 | 15 | 26 | 25 |
| Sulfur-containing compounds | 3 | 3 | 4 | 4 | 2 | 4 | 3 | 5 | 4 | 19 | 22 | 13 | 23 | 29 | 24 | 12 | 24 | 15 |
| Other compounds | 6 | 6 | 6 | 6 | 5 | 10 | 2 | 5 | 8 | 24 | 24 | 13 | 24 | 32 | 33 | 8 | 20 | 12 |
| Total | 73 | 89 | 89 | 88 | 81 | 89 | 73 | 77 | 94 | 285 | 345 | 175 | 342 | 384 | 374 | 186 | 286 | 229 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Chen, Y.; Gao, J.; Run, Z.; Chen, H.; Shi, R.; Li, Y.; Zhang, H.; Liu, Y. Application of GC–TOF/MS and GC×GC–TOF/MS to Discriminate Coffee Products in Three States (Bean, Powder, and Brew). Foods 2023, 12, 3123. https://doi.org/10.3390/foods12163123
Fang X, Chen Y, Gao J, Run Z, Chen H, Shi R, Li Y, Zhang H, Liu Y. Application of GC–TOF/MS and GC×GC–TOF/MS to Discriminate Coffee Products in Three States (Bean, Powder, and Brew). Foods. 2023; 12(16):3123. https://doi.org/10.3390/foods12163123
Chicago/Turabian StyleFang, Xiaolei, Yanping Chen, Jie Gao, Zimu Run, He Chen, Ruoqi Shi, Yingqiu Li, Haihua Zhang, and Yuan Liu. 2023. "Application of GC–TOF/MS and GC×GC–TOF/MS to Discriminate Coffee Products in Three States (Bean, Powder, and Brew)" Foods 12, no. 16: 3123. https://doi.org/10.3390/foods12163123
APA StyleFang, X., Chen, Y., Gao, J., Run, Z., Chen, H., Shi, R., Li, Y., Zhang, H., & Liu, Y. (2023). Application of GC–TOF/MS and GC×GC–TOF/MS to Discriminate Coffee Products in Three States (Bean, Powder, and Brew). Foods, 12(16), 3123. https://doi.org/10.3390/foods12163123








