Prevalence of Listeria monocytogenes in RTE Meat Products of Quevedo (Ecuador)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Detection of Listeria monocytogenes in Meat Products
2.3. Listeria monocytogenes Enumeration in Meat Products
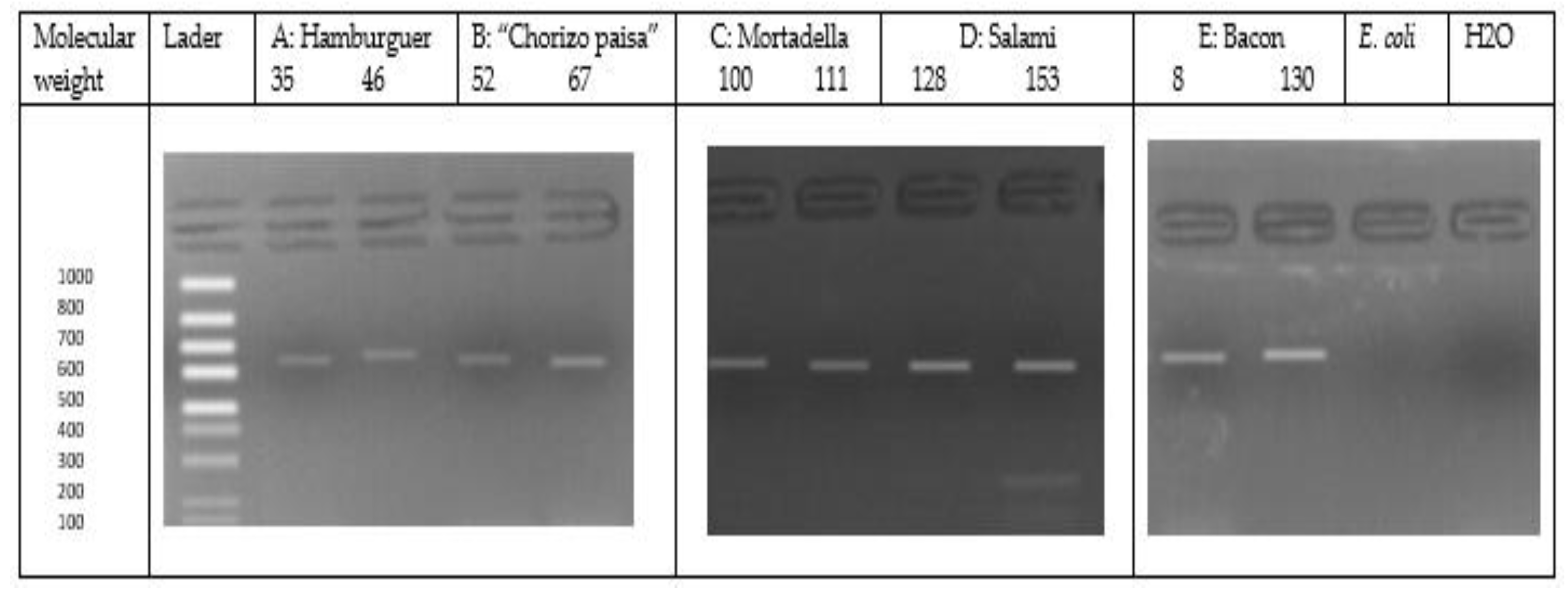
2.4. Identification of Listeria monocytogenes by Amplification of the Iap Gene
2.4.1. Extraction of DNA
2.4.2. Identification of Listeria monocytogenes by PCR Assay
2.5. Statistical Analysis
3. Results
3.1. Detection of Listeria monocytogenes in RTE Meat Products
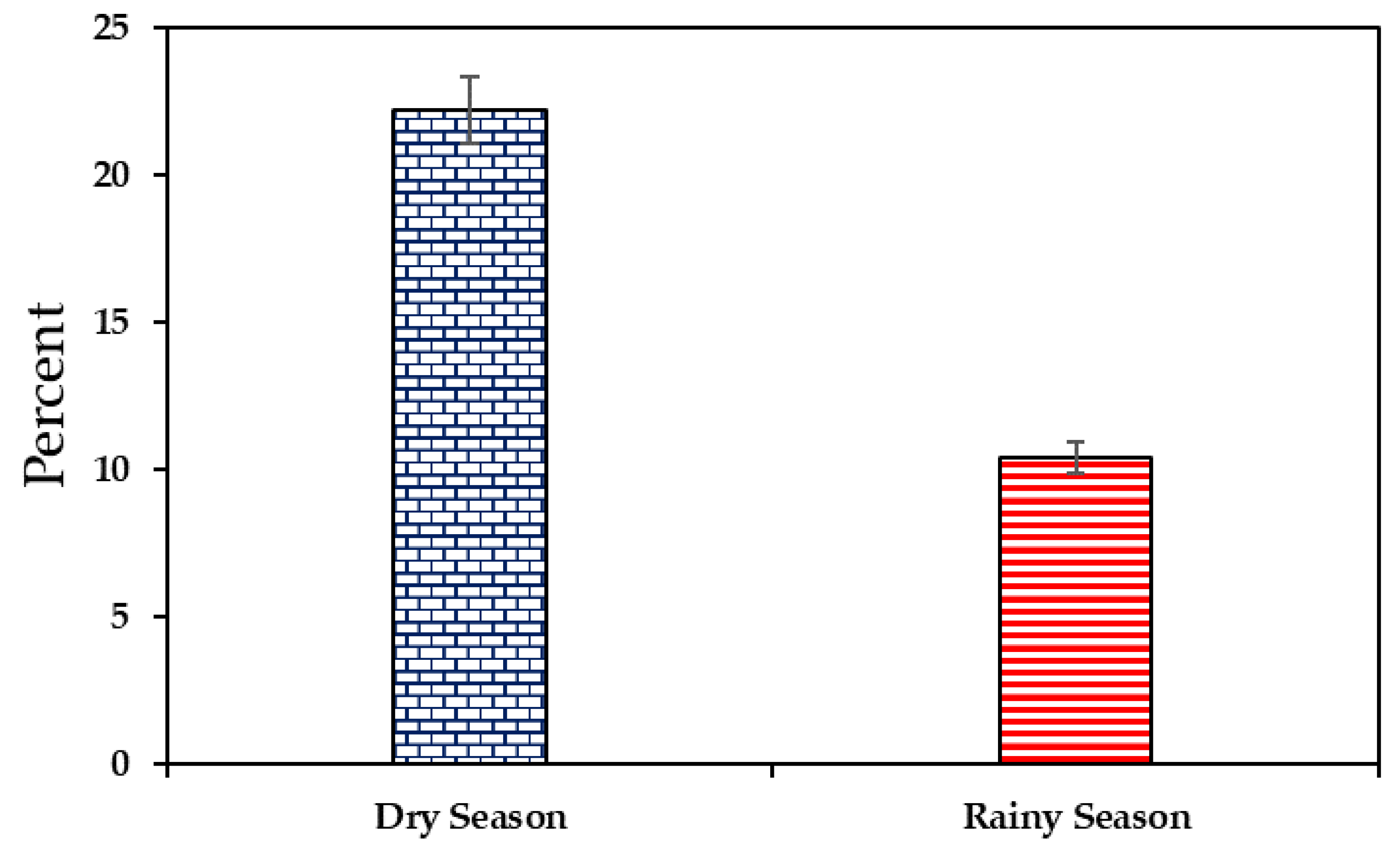
3.2. Prevalence of Listeria monocytogenes in RTE Meat Products
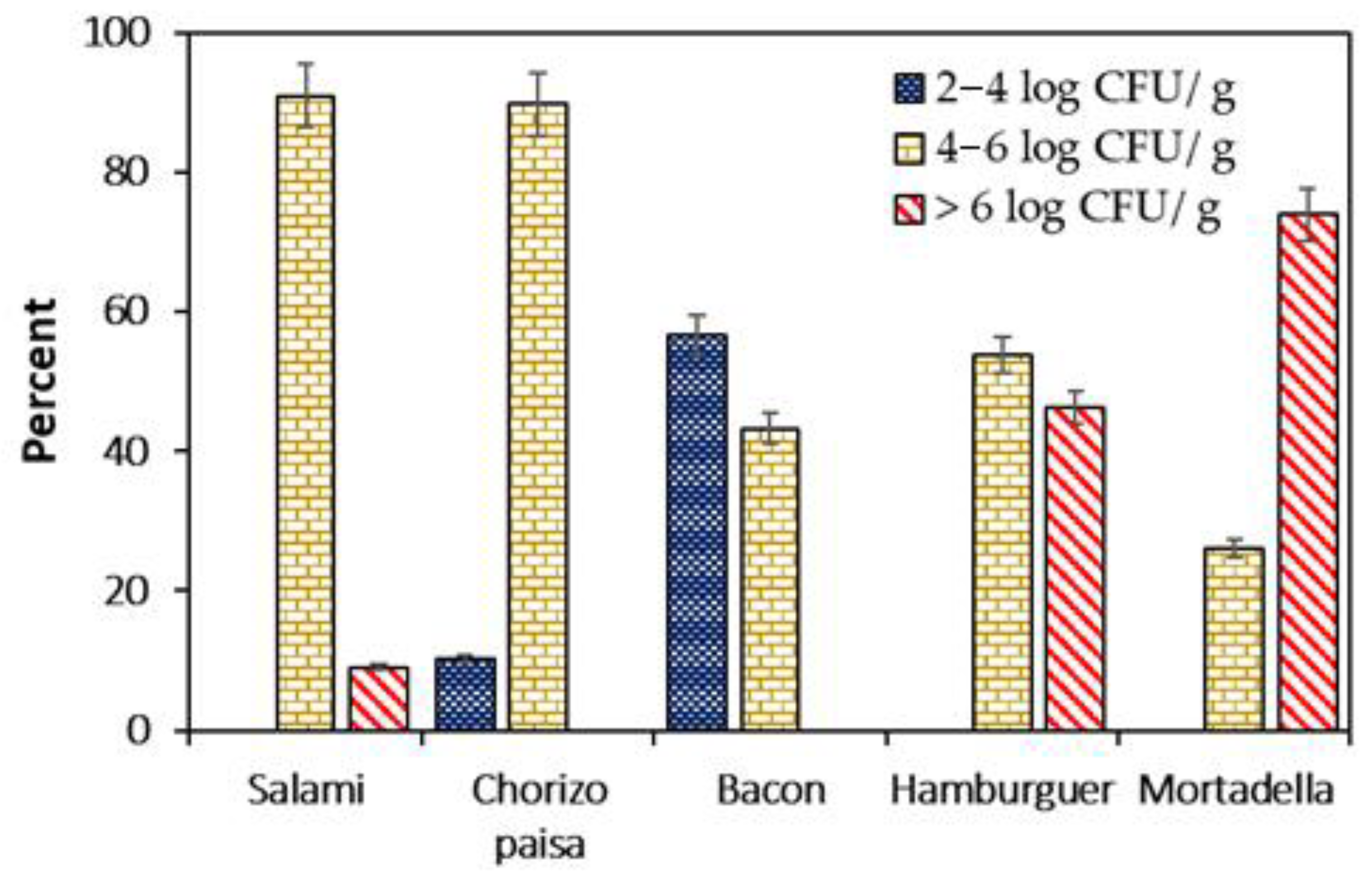
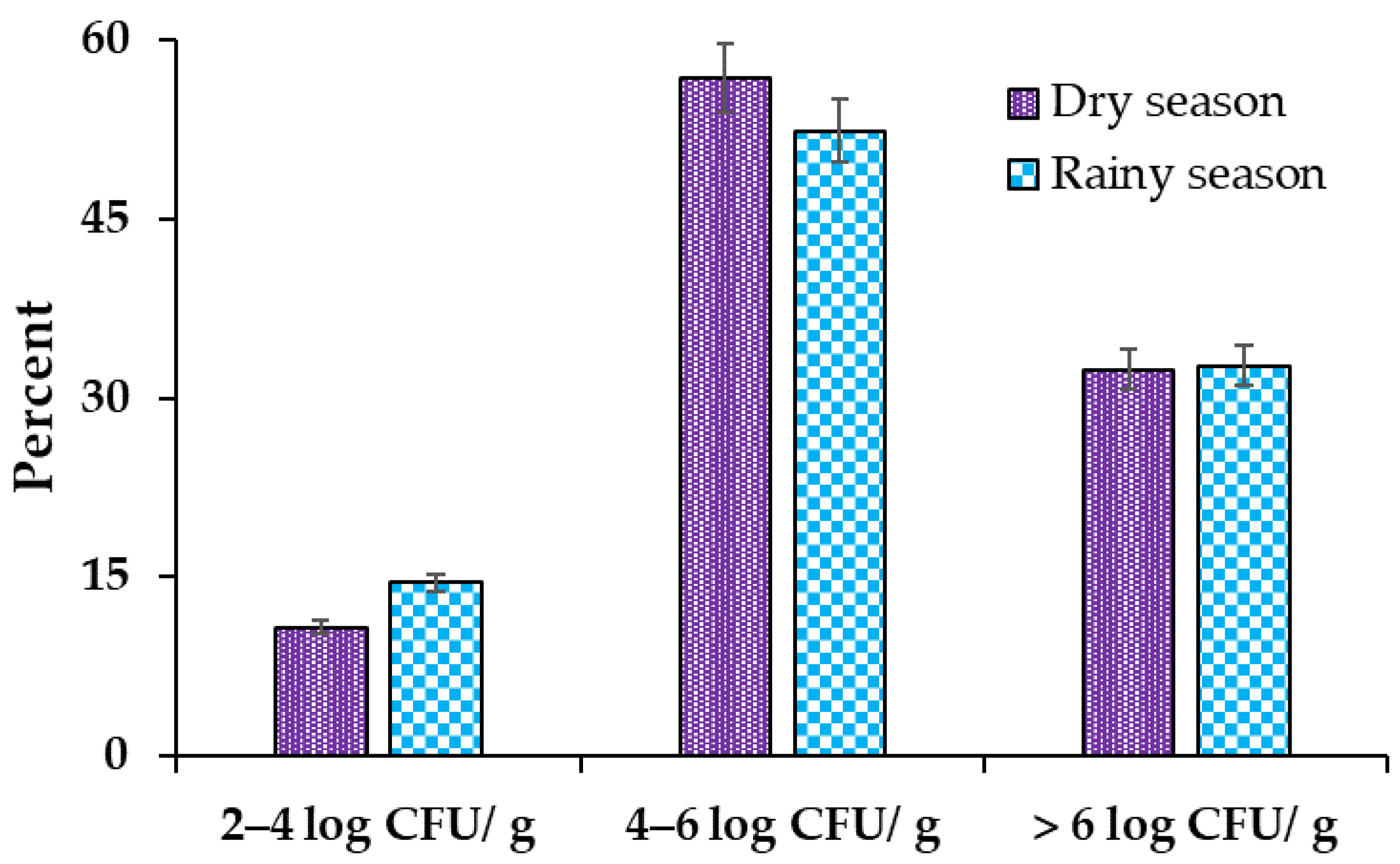
3.3. Listeria monocytogenes Counts in Meat Products
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smigic, N.; Ozilgen, S.; Gómez-López, V.M.; Osés, S.M.; Miloradovic, Z.; Aleksic, B.; Miocinovic, J.; Smole Možina, S.; Kunčič, A.; Guiné, R.; et al. Consumer attitudes and perceptions towards chilled ready-to-eat foods: a multinational study. J. Consum. Prot. Food Saf. 2023, 18, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Grace, D. Food Safety in Low and Middle Income Countries. Int. J. Environ. Res. Public Health 2015, 12, 10490–10507. [Google Scholar] [CrossRef]
- Rodrigues, C.S.; Sá, C.V.G.C.D.; Melo, C.B.D. An overview of Listeria monocytogenes contamination in Ready to Eat meat, dairy and fishery foods. Ciênc. Rural. 2017, 47, e20160721. [Google Scholar] [CrossRef]
- Henri, C.; Félix, B.; Guillier, L.; Leekitcharoenphon, P.; Michelon, D.; Mariet, J.F.; Aarestrup, F.M.; Mistou, M.Y.; Hendriksen, R.S.; Roussel, S. Population Genetic Structure of Listeria monocytogenes Strains as Determined by Pulsed-Field Gel Electrophoresis and Multilocus Sequence Typing. Appl. Environ. Microbiol. 2016, 82, 5720–5728. [Google Scholar] [CrossRef]
- Kaptchouang Tchatchouang, C.-D.; Fri, J.; De Santi, M.; Brandi, G.; Schiavano, G.F.; Amagliani, G.; Ateba, C.N. Listeriosis outbreak in South Africa: A comparative analysis with previously reported cases worldwide. Microorganisms 2020, 8, 135. [Google Scholar] [CrossRef]
- Kramarenko, T.; Roasto, M.; Meremäe, K.; Kuningas, M.; Põltsama, P.; Elias, T. Listeria monocytogenes prevalence and serotype diversity in various foods. Food Control 2012, 30, 24–29. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C.A. Review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Little, C.L.; Amar, C.F.L.; Awofisayo, A.; Grant, K.A. Hospital-acquired listeriosis associated with sandwiches in the UK: A cause for concern. J. Hosp. Infect. 2012, 82, 13–18. [Google Scholar] [CrossRef]
- Hilliard, A.; Leong, D.; O’Callaghan, A.; Culligan, E.P.; Morgan, C.A.; DeLappe, N.; Hill, C.; Jordan, K.; Cormican, M.; Gahan, C.G.M. Genomic Characterization of Listeria monocytogenes Isolates Associated with Clinical Listeriosis and the Food Production Environment in Ireland. Genes 2018, 9, 171. [Google Scholar] [CrossRef]
- Rotela, A.; Valentina, M. Listeriosis diseminada: A propósito de un caso. Rev. Urug. Med. Int. 2023, 8, 38–45. Available online: http://www.scielo.edu.uy/scielo.php?script=sci_arttext&pid=S2393-67972023000100038&lng=es&nrm=iso (accessed on 31 May 2023).
- Pérez-Rodríguez, F.; Carrasco, E.; Bover-Cid, S.; Jofré, A.; Valero, A. Closing gaps for performing a risk assessment on Listeria monocytogenes in Ready-to-Eat (RTE) foods: Activity 2, a quantitative risk characterization on L. monocytogenes in RTE foods; Starting from the Retail Stage. EFSA Support. Publ. 2017, 14, 1252E. [Google Scholar] [CrossRef]
- Lomonaco, S.; Nucera, D.; Filipello, V. The evolution and epidemiology of Listeria monocytogenes in Europe and the United States. Infect. Genet. Evol. 2015, 35, 172–183. [Google Scholar] [CrossRef]
- Sampedro, F.; Pérez-Rodríguez, F.; Servadio, J.L.; Gummalla, S.; Hedberg, C.W. Quantitative Risk assessment model to investigate the public health impact of varying Listeria monocytogenes allowable levels in different food commodities: A Retrospective Analysis. Int. J. Food Microbiol. 2022, 383, 109932. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Tau, N.P.; Smouse, S.L.; Allam, M.; Ismail, A.; Ramalwa, N.R.; Disenyeng, B.; Ngomane, M.; Thom-as, J. Outbreak of Listeria monocytogenes in South Africa 2017–2018: Laboratory activities and experiences associ-ated with whole-genome sequencing analysis of isolates. Foodborne Pathog. Dis. 2019, 16, 524–530. [Google Scholar] [CrossRef]
- Zhang, H.; Que, F.; Xu, B.; Sun, L.; Zhu, Y.; Chen, W.; Ye, Y.; Dong, Q.; Liu, H.; Zhang, X. Identification of Listeria monocytogenes contamination in a Ready-to-Eat meat processing plant in China. Front. Microbiol. 2021, 12, 628204. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Regan, P.; Laksanalamai, P.; Healey, S.; Hu, Z. Prevalence and methodologies for detection, characterization and subtyping of Listeria monocytogenes and L. ivanovii in foods and environmental sources. Food Sci. Hum. Wellness 2017, 6, 97–120. [Google Scholar] [CrossRef]
- Burnett, J.; Wu, S.T.; den Bakker, H.C.; Cook, P.W.; Veenhuizen, D.R.; Hammons, S.R.; Singh, M.; Oliver, H.F. Listeria monocytogenes is prevalent in retail produce environments but Salmonella enterica is rare. Food Control 2020, 113, 107173. [Google Scholar] [CrossRef]
- Ebakota, D.O.; Abiodun, O.A.; Nosa, O.O. Prevalence of Antibiotics Resistant Listeria monocytogenes Strains in Nigerian Ready-to-eat Foods. Food Saf. 2018, 6, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, Y.; Ida, M.; Nakama, A.; Nishino, Y.; Fukui, R.; Kuroda, S.; Hirai, A.; Kai, A.; Sadamasu, K. Prevalence and contamination levels of Listeria monocytogenes in ready-to-eat foods in Tokyo, Japan. J. Vet. Med. Sci. 2016, 78, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Mackiw, E.; Stasiak, M.; Kowalska, J.; Kucharek, K.; Korsak, D.; Postupolski, J. Occurrence and Characteristics of Listeria monocytogenes in Ready-to-Eat Meat Products in Poland. J. Food Prot. 2020, 83, 1002–1009. [Google Scholar] [CrossRef]
- Parra-Flores, J.; Holý, O.; Bustamante, F.; Lepuschitz, S.; Pietzka, A.; Contreras-Fernández, A.; Castillo, C.; Ovalle, C.; Alarcón-Lavín, M.P.; Cruz-Córdova, A.; et al. Virulence and Antibiotic Resistance Genes in Listeria monocytogenes Strains Isolated From Ready-to-Eat Foods in Chile. Front. Microbiol. 2022, 21, 796040. [Google Scholar] [CrossRef] [PubMed]
- Mata, E.E.; Mejía, L.; Villacís, J.E.; Alban, V.; Zapata, S. Detection and genotyping of Listeria monocytogenes in artisanal soft cheeses from Ecuador. Rev. Argent. Microbiol. 2022, 54, 101–110. [Google Scholar] [CrossRef]
- UNE-EN ISO. UNE-EN ISO 11290-1:2018 Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method (ISO 11290-1:2017). Available online: https://www.une.org/en-cuentra-tu-norma/busca-tu-norma/norma/?Tipo=N&c=N0059546 (accessed on 2 May 2023).
- Ottaviani, F.; Ottaviani, M.; and Agosti, M. Differential agar medium for Listeria monocytogenes. Ind. Aliment. 1997, 36, 3. [Google Scholar]
- Martínez, P.M.; Isquierdo, D.Z.; Ulloa, V.A. Identificación de Listeria monocytogenes por amplificación del gen iap a partir de cultivos de Listeria sp. procedente de lugares de expendio de carne de res, pescado y verduras en Trujillo (Perú). REBIOL 2014, 34, 21–28. [Google Scholar]
- Farber, J.M.; Daley, E.M.; Mackie, M.T.; Limerick, B. A small outbreak of listeriosis potentially linked to the consumption of imitation crab meat. Lett. Appl. Microbiol. 2000, 31, 100–104. [Google Scholar] [CrossRef]
- Gandhi, M.; Chikindas, M.L. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef]
- Sofos, J.N.; Geornaras, I. Overview of current meat hygiene and safety risks and summary of recent studies on biofilms, and control of Escherichia coli O157:H7 in nonintact, and Listeria monocytogenes in Ready-to-Eat, meat products. Meat Sci. 2010, 86, 2–14. [Google Scholar] [CrossRef]
- Swaminathan, B.; Gerner-Smidt, P. The epidemiology of human listeriosis. Microbes Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef]
- Meloni, D. Presence of Listeria monocytogenes in Mediterranean-Style Dry Fermented Sausages. Foods 2015, 4, 34–50. [Google Scholar] [CrossRef]
- Augustin, J.C.; Carlier, V. Modelling the growth rate of Listeria monocytogenes with a multiplicative type model including interactions between environmental factors. Int. J. Food Microbiol. 2000, 56, 53–70. [Google Scholar] [CrossRef]
- Iacumin, L.; Cappellari, G.; Colautti, A.; Comi, G. Listeria monocytogenes Survey in Cubed Cooked Ham Packaged in Modified Atmosphere and Bioprotective Effect of Selected Lactic Acid Bacteria. Microorganisms 2020, 8, 898. [Google Scholar] [CrossRef]
- Figuera, B.; Cabello, A.; Villalobos, L.; Márquez, Y.; Vallenilla, O. Crecimiento de Listeria monocytogenes en atún ahumado empacado al vacío. Zootec. Trop. 2005, 23, 171–181. [Google Scholar]
- Authority, E.F.S.; European Centre for Disease Prevention and Control. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2015. EFSA J. 2016, 14, e04634. [Google Scholar] [CrossRef]
- Jemmi, T.; Stephan, R. Listeria monocytogenes: Food-Borne pathogen and hygiene indicator. Rev. Sci. Tech. Int. Off. Epizoot. 2006, 25, 571–580. [Google Scholar] [CrossRef]
- Junttila, J.; Hirn, J.; Hill, P.; Nurmi, E. Effect of different levels of nitrite and nitrate on the survival of Listeria monocytogenes during the manufacture of fermented sausage. J. Food Prot. 1989, 52, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.A.; Germano, P.M.L. Listeria monocytogenes in Ready-to-Eat, sliced, cooked ham and salami products, marketed in the city of São Paulo, Brazil: Occurrence, quantification, and serotyping. Food Control 2011, 2, 297–302. [Google Scholar] [CrossRef]
- Teixeira, A.; Domínguez, R.; Ferreira, I.; Pereira, E.; Estevinho, L.; Rodrigues, S.; Lorenzo, J.M. Effect of NaCl replacement by other salts on the quality of bísaro pork sausages (PGI Chouriça de Vinhais). Foods 2021, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Uyttendaele, M.; De Troy, P.; Debevere, J. Incidence of Listeria monocytogenes in different types of meat products on the Belgian retail market, Int. J. Food Microbiol. 1999, 53, 75–80. [Google Scholar] [CrossRef]
- Elson, R.; Burgess, F.; Little, C.L.; Mitchell, R.T.; The Local Authorities Coordinators of Regulatory Services and the Health Protection Agency. Microbiological examination of Ready-to-Eat cold sliced meats and pâté from catering and retail premises in the UK. J. Appl. Microbiol. 2004, 96, 499–509. [Google Scholar] [CrossRef]
- Wong, T.L.; Carey-Smith, G.V.; Hollis, L.; Hudson, J.A. Microbiological survey of prepackaged pâté and ham in New Zealand. Lett. Appl. Microbiol. 2005, 41, 106–111. [Google Scholar] [CrossRef]
- Uyttendaele, M.; Busschaert, P.; Valero, A.; Geeraerd, A.H.; Vermeulen, A.; Jacxsens, L.; Goh, K.K.; De Loy, A.; Van Impe, J.F.; Devlieghere, F. Prevalence and challenge tests of Listeria monocytogenes in Belgian produced and retailed mayonnaise-Based Deli-Salads, cooked meat products and smoked fish between 2005 and 2007. Int. J. Food Microbiol. 2009, 133, 94–104. [Google Scholar] [CrossRef]
- Nieto-Lozano, J.C.; Reguera-Useros, J.I.; Peláez-Martínez, M.D.C.; Sacristán-Pérez-Minayo, G.; Gutiérrez-Fernández, Á.J.; de la Torre, A.H. The effect of the Pediocin PA-1 produced by Pediococcus acidilactici against Listeria monocytogenes and Clostridium perfringens in Spanish dry-fermented sausages and frankfurters. Food Control 2010, 21, 679–685. [Google Scholar] [CrossRef]
- Rivera, P.F.H.; Wesley, I.; Hurd, S.; Simoes, D.; Sosa, A.; Rivera, S. Determinación microbiológica y molecular de Listeria sp. y Listeria monocytogenes en Cerdas a nivel de una planta beneficiadora en EUA. Rev. Científica 2006, 16, 297–307. [Google Scholar]
- Nørrung, B. Microbiological criteria for Listeria monocytogenes in foods under special consideration of risk assessment ap-proaches. Int. J. Food Microbiol. 2000, 62, 217–221. [Google Scholar] [CrossRef]
- Petruzzelli, A.; Blasi, G.; Masini, L.; Calza, L.; Duranti, A.; Santarelli, S.; Fisichella, S.; Pezzotti, G.; Aquilanti, L.; Osimani, A.; et al. Occurrence of Listeria monocytogenes in salami manufactured in the Marche Region (Central Italy). J. Vet. Med. Sci. 2010, 72, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Mataragas, M.; Bellio, A.; Rovetto, F.; Astegiano, S.; Greci, C.; Hertel, C.; Decastelli, L.; Cocolin, L. Quantification of persistence of the foodborne pathogens Listeria monocytogenes and Salmonella enterica during manufacture of Italian fermented sausages. Food Control 2015, 47, 552–559. [Google Scholar] [CrossRef]
- Simpson, C.A.; Geornaras, I.; Yoon, Y.; Scanga, J.A.; Kendall, P.A.; Sofos, J.N. Effect of inoculum preparation procedure and storage time and temperature on the fate of Listeria monocytogenes on inoculated salami. J. Food Prot. 2008, 71, 494–501. [Google Scholar] [CrossRef]
| Meat Product Type | Product Description | Storage Temperature | Geographical Zone (Number of Samples) | |||
|---|---|---|---|---|---|---|
| Dry Season | Rainy Season | South (%) | Central (%) | North (%) | ||
| A: Salami | Ripened product (200 g slices) | 2–14 °C | 4–14 °C | 33 | 33 | 34 |
| B: “Chorizo paisa” | Raw cured product, vacuum-packed presentation of 300 g | 33 | 33 | 34 | ||
| C: Bacon | Cured, smoked product, presented in 200 g slices | 33 | 33 | 34 | ||
| D: Hamburger meat | Roasted product, in 110 g package | 33 | 33 | 34 | ||
| E: Mortadella | Cooked product, 100 g presented in vacuum package. | 33 | 33 | 34 | ||
| Types of the Meat Product | Number of Samples Collected in the Two Seasons | Number of Samples (Dry and Rainy Season) Positive for L. monocytogenes | Percentage of Positive Samples |
|---|---|---|---|
| A: Salami | 200 | 21 | 10.5% |
| B: “Chorizo paisa” | 200 | 29 | 14.5% |
| C: Bacon | 200 | 30 | 15.0% |
| D: Hamburger meat | 200 | 38 | 19.0% |
| E: Mortadella | 200 | 45 | 22.5% |
| Type of Meat Product | Season | ||||
|---|---|---|---|---|---|
| Dry (%) | Rainy (%) | South (%) | Central (%) | North (%) | |
| A: Salami | 12 | 9 | 39.3 | 40.5 | 20.2 |
| B: “Chorizo paisa” | 21 | 8 | |||
| C: Bacon | 22 | 8 | |||
| D: Hamburger meat | 30 | 8 | |||
| E: Mortadella | 26 | 19 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meza-Bone, G.A.; Meza Bone, J.S.; Cedeño, Á.; Martín, I.; Martín, A.; Maddela, N.R.; Córdoba, J.J. Prevalence of Listeria monocytogenes in RTE Meat Products of Quevedo (Ecuador). Foods 2023, 12, 2956. https://doi.org/10.3390/foods12152956
Meza-Bone GA, Meza Bone JS, Cedeño Á, Martín I, Martín A, Maddela NR, Córdoba JJ. Prevalence of Listeria monocytogenes in RTE Meat Products of Quevedo (Ecuador). Foods. 2023; 12(15):2956. https://doi.org/10.3390/foods12152956
Chicago/Turabian StyleMeza-Bone, Gary Alex, Jessica Sayonara Meza Bone, Ángel Cedeño, Irene Martín, Alberto Martín, Naga Raju Maddela, and Juan J. Córdoba. 2023. "Prevalence of Listeria monocytogenes in RTE Meat Products of Quevedo (Ecuador)" Foods 12, no. 15: 2956. https://doi.org/10.3390/foods12152956
APA StyleMeza-Bone, G. A., Meza Bone, J. S., Cedeño, Á., Martín, I., Martín, A., Maddela, N. R., & Córdoba, J. J. (2023). Prevalence of Listeria monocytogenes in RTE Meat Products of Quevedo (Ecuador). Foods, 12(15), 2956. https://doi.org/10.3390/foods12152956






