Effects of Freeze–Thaw Cycles on the Structures and Functional Properties of Clitocybe squamulosa Protein Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. CSPI Preparation
2.3. F–T Treatment
2.4. Determination of Free Sulfhydryl Content
2.5. Determination of Carbonyl Content
2.6. Determination of Surface Hydrophobicity
2.7. FTIR
2.8. SEM
2.9. Functional Properties
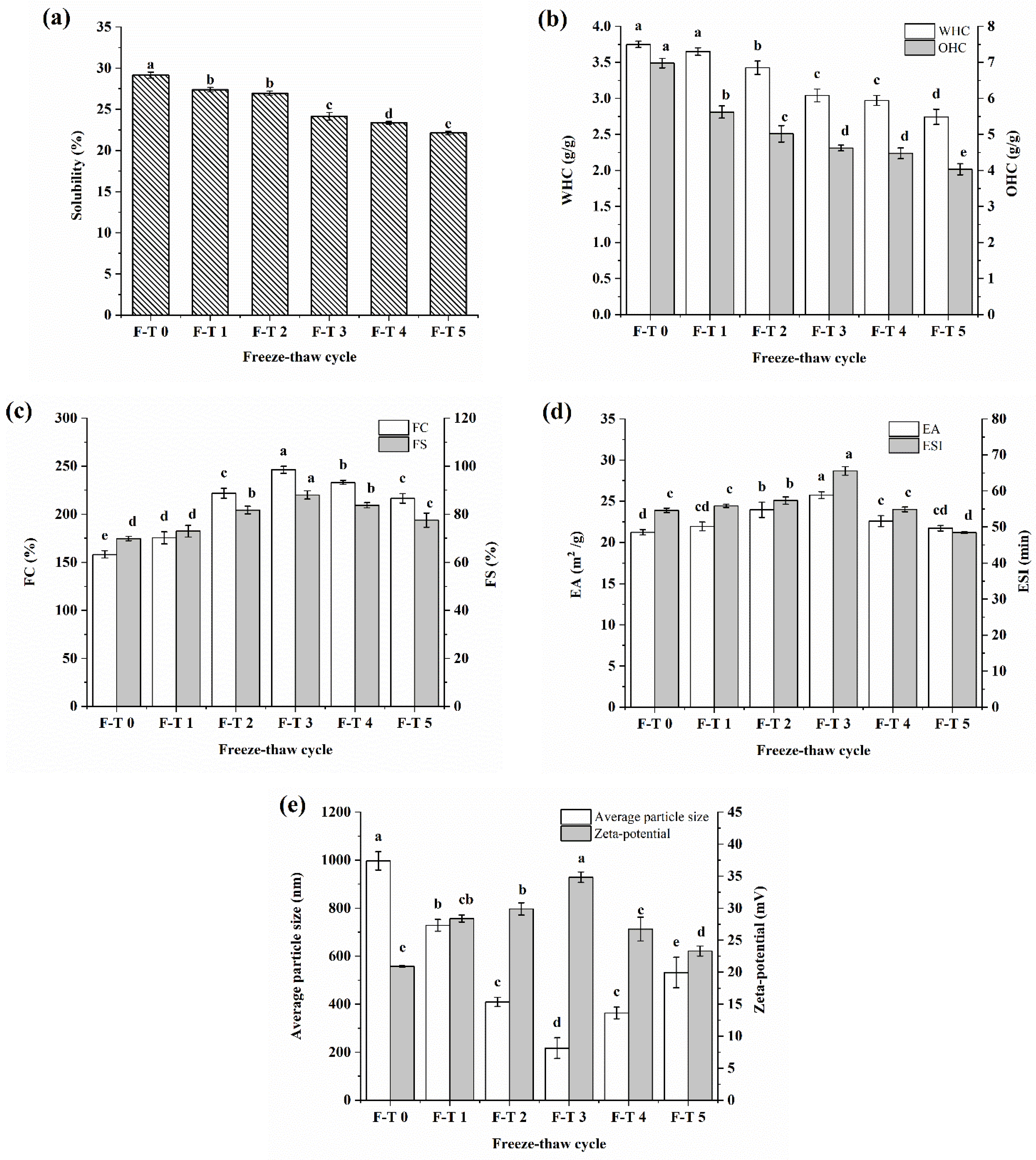
2.9.1. Solubility
2.9.2. WHC and OHC
2.9.3. Foaming Capacity and Foaming Stability
2.9.4. Emulsifying Activity Index and Emulsifying Stability Index
2.9.5. Average Particle Size and Zeta Potential of Emulsions
2.10. IVGD
2.11. The Polypeptide Content
2.12. In Vitro Antioxidant Activities
2.13. Statistical Analysis
3. Results and Discussion
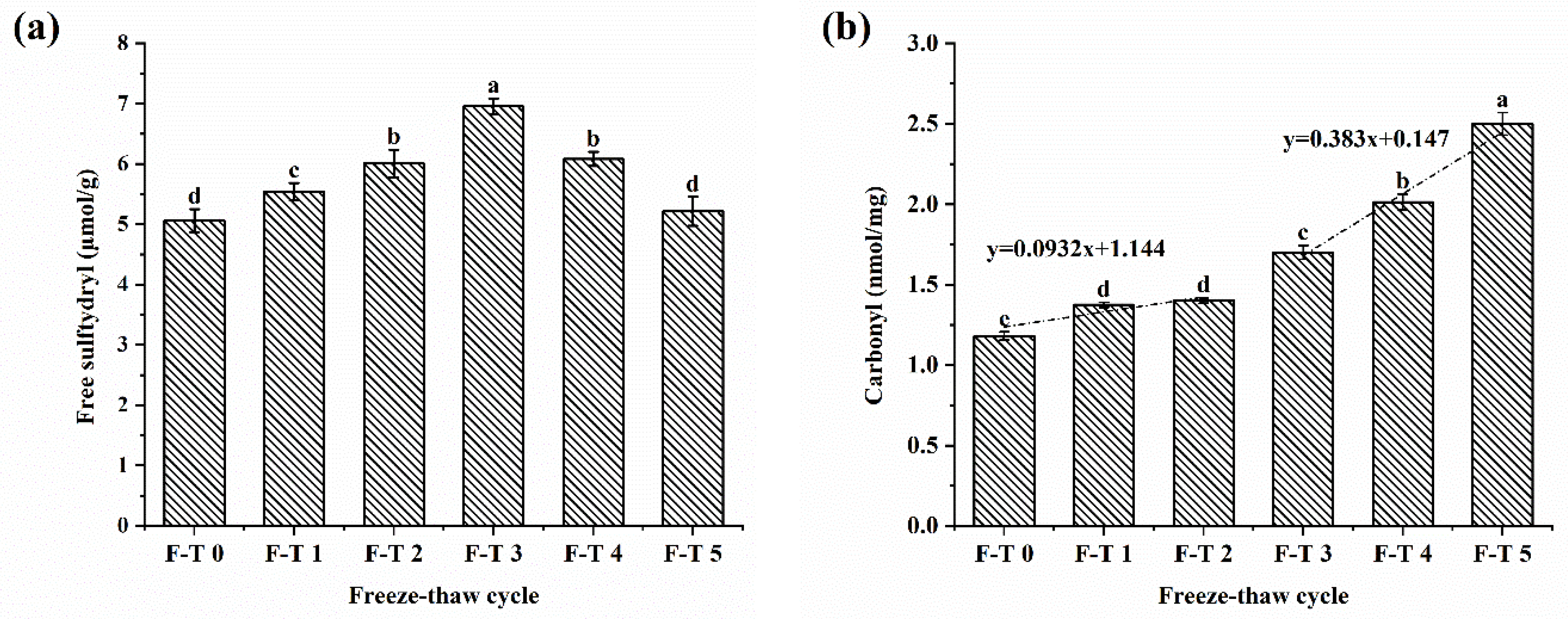
3.1. Free Sulfhydryl Content
3.2. Carbonyl Content
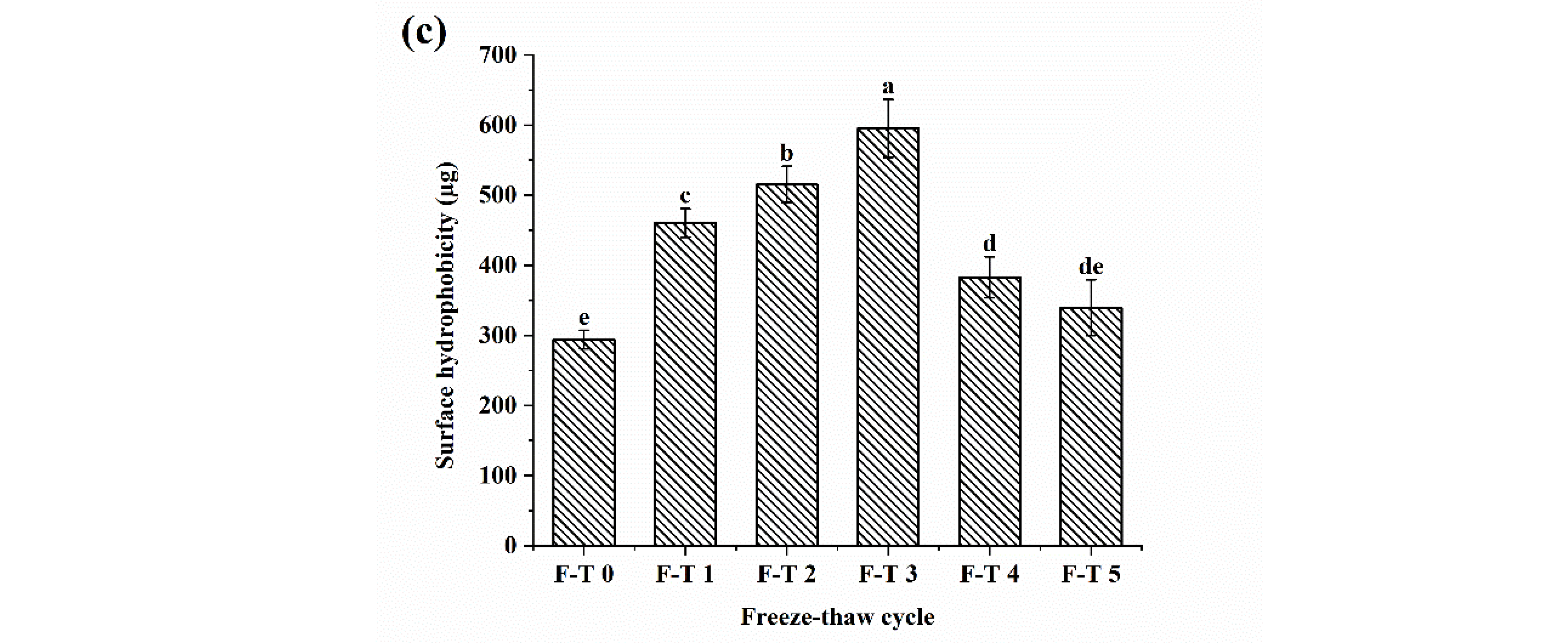
3.3. Surface Hydrophobicity
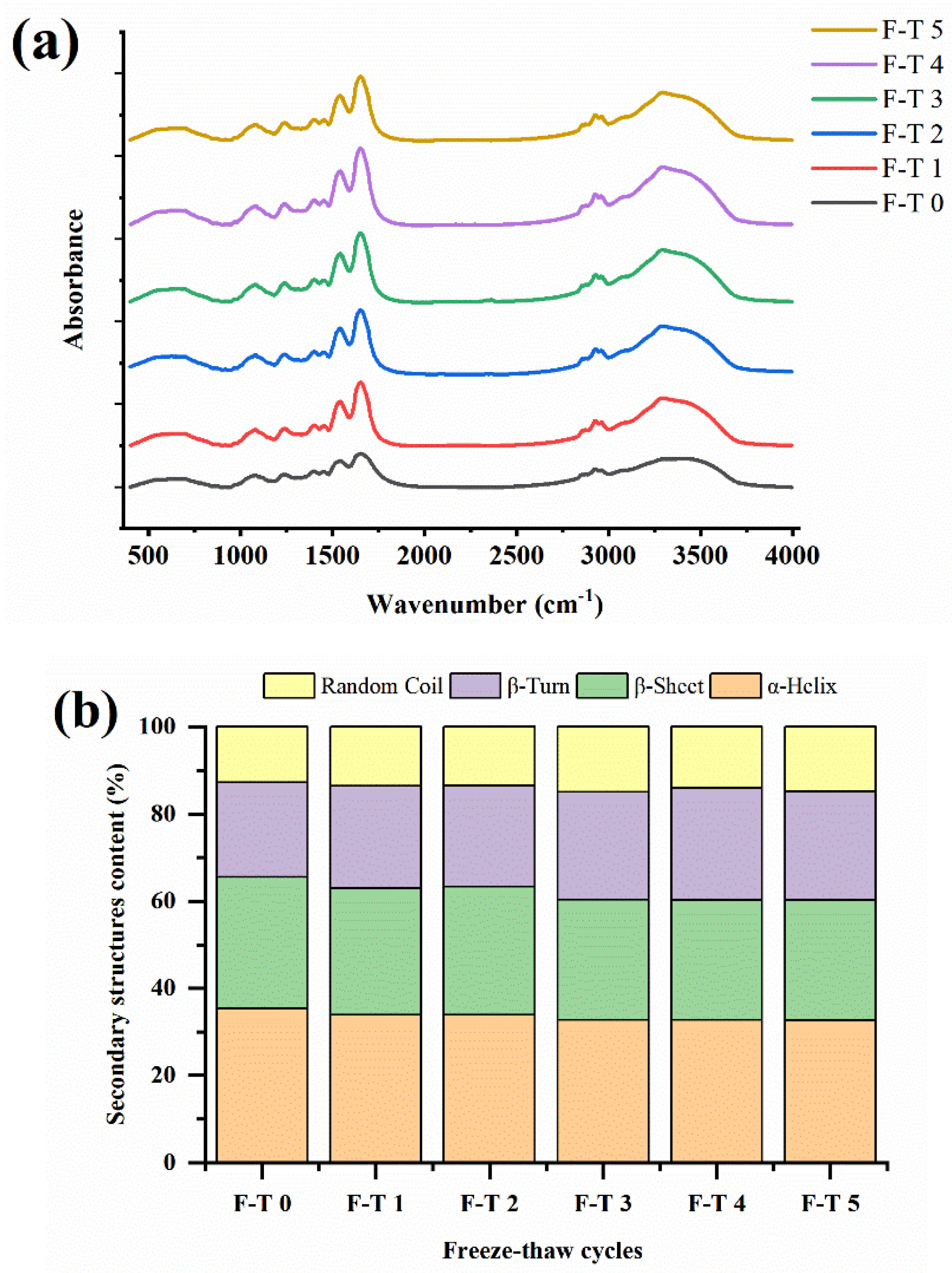
3.4. Variation of Secondary Structures
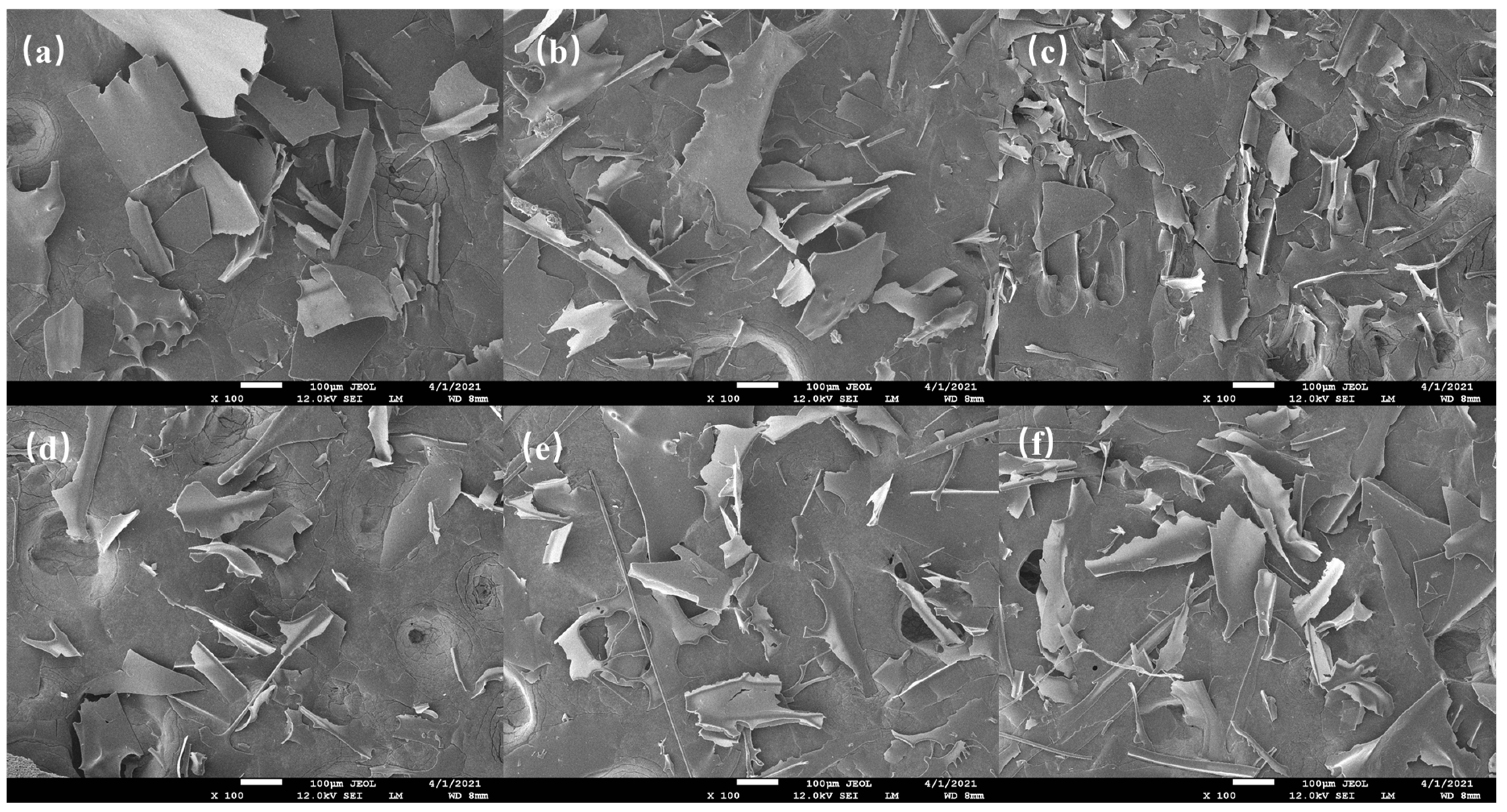
3.5. SEM
3.6. Functional Properties
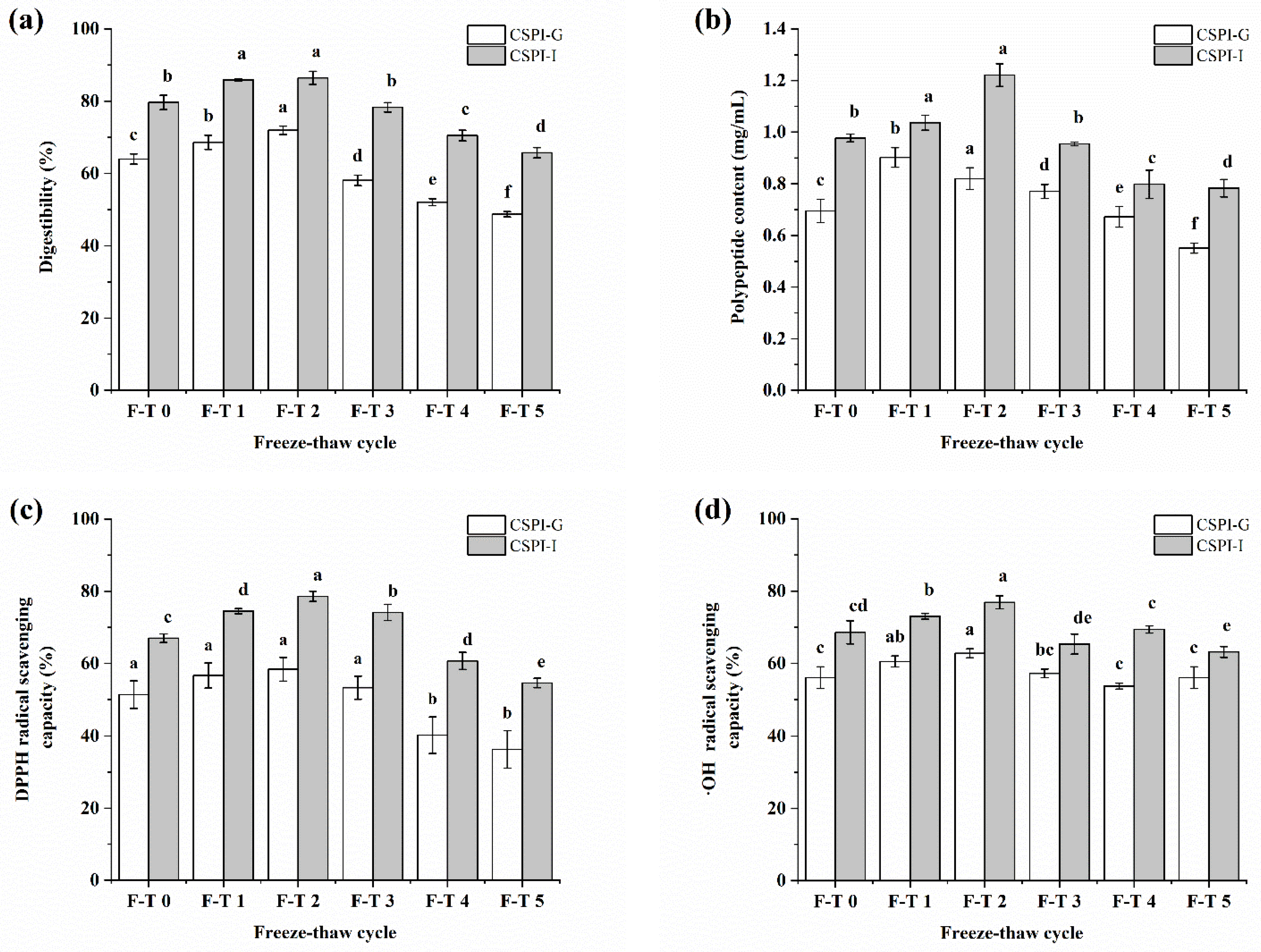
3.7. Effect of F–T Treatment on IVGD of CSPI
3.8. Polypeptide Content
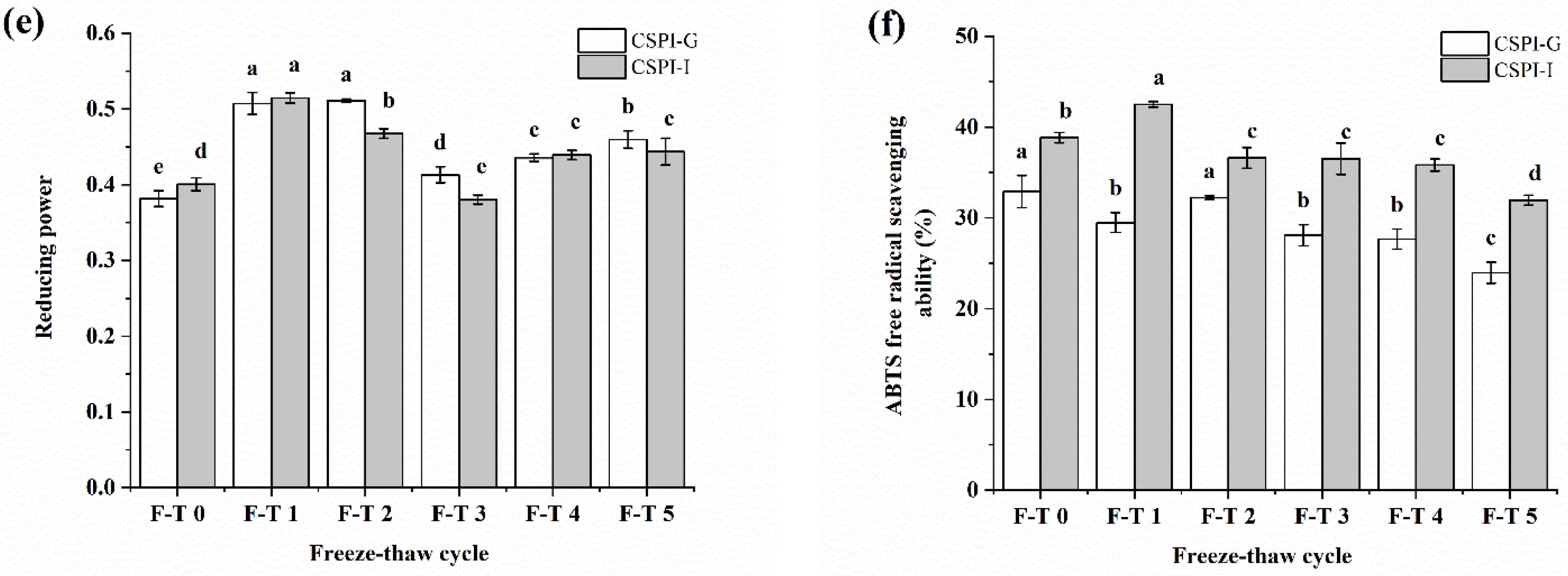
3.9. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lappi, J.; Silventoinen-Veijalainen, P.; Vanhatalo, S.; Rosa-Sibakov, N.; Sozer, N. The Nutritional Quality of Animal-Alternative Processed Foods Based on Plant or Microbial Proteins and the Role of the Food Matrix. Trends Food Sci. Technol. 2022, 129, 144–154. [Google Scholar] [CrossRef]
- González, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodríguez, R.; Contreras, J.; Belmares, R. Edible Mushrooms as a Novel Protein Source for Functional Foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef]
- Guo, D.; Lei, J.; He, C.; Peng, Z.; Liu, R.; Pan, X.; Meng, J.; Feng, C.; Xu, L.; Cheng, Y.; et al. In Vitro Digestion and Fermentation by Human Fecal Microbiota of Polysaccharides from Clitocybe Squamulose. Int. J. Biol. Macromol. 2022, 208, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Du, L.; Sun, Y.; Zhou, C.; Pan, D. Recent Developments in Phosphorylation Modification on Food Proteins: Structure Characterization, Site Identification and Function. Food Hydrocoll. 2023, 137, 108390. [Google Scholar] [CrossRef]
- Kumar, P.K.; Sivabalan, S.; Parhi, A.; Sablani, S.S. Modification of Pea Protein Isolate Functionality by Freeze—Thaw Cycling. J. Food Meas. Charact. 2022, 16, 162–170. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Diao, X.; Kong, B.; Xia, X. Moisture Migration, Microstructure Damage and Protein Structure Changes in Porcine Longissimus Muscle as Influenced by Multiple Freeze-Thaw Cycles. Meat Sci. 2017, 133, 10–18. [Google Scholar] [CrossRef]
- Duan, X.; Li, J.; Zhang, Q.; Zhao, T.; Li, M.; Xu, X.; Liu, X. Effect of a Multiple Freeze-Thaw Process on Structural and Foaming Properties of Individual Egg White Proteins. Food Chem. 2017, 228, 243–248. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, F.; Li, Y.; Kong, B.; Liu, Q. Effect of Freeze-Thaw Cycles on the Emulsion Activity and Structural Characteristics of Soy Protein Isolate. Process Biochem. 2015, 50, 1607–1613. [Google Scholar] [CrossRef]
- Fernández-Martín, F.; Pérez-Mateos, M.; Dadashi, S.; Gómez-Guillén, C.M.; Sanz, P.D. Impact of Magnetic Assisted Freezing in the Physicochemical and Functional Properties of Egg Components. Part 1: Egg White. Innov. Food Sci. Emerg. 2017, 44, 131–138. [Google Scholar] [CrossRef]
- Feng, H.; Jin, H.; Gao, Y.; Yan, S.; Zhang, Y.; Zhao, Q.; Xu, J. Effects of Freeze-Thaw Cycles on the Structure and Emulsifying Properties of Peanut Protein Isolates. Food Chem. 2020, 330, 127215. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, H.; Guo, H.; Zhang, L.; Zhang, X.; Chen, Y. High Intensity Ultrasound-Assisted Quality Enhancing of the Marinated Egg: Gel Properties and in Vitro Digestion Analysis. Ultrason. Sonochem. 2022, 86, 106036. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, Y.; Zhang, C.; Wan, J.; Shah, B.R.; Pei, Y.; Zhou, B.; Li, J.; Li, B. High Intensity Ultrasound Modified Ovalbumin: Structure, Interface and Gelation Properties. Ultrason. Sonochem. 2016, 31, 302–309. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, Y.; Han, J.; Chen, Q.; Kong, B. Structure-Modification by Moderate Oxidation in Hydroxyl Radical-Generating Systems Promote the Emulsifying Properties of Soy Protein Isolate. Food Struct. 2015, 6, 21–28. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, A.; Yang, R.; Jia, R.; Zhang, J.; Xu, D.; Yang, W. Myofibrillar Protein Structure and Gel Properties of Trichiurus haumela Surimi Subjected to High Pressure or High Pressure Synergistic Heat. Food Bioprocess Technol. 2020, 13, 589–598. [Google Scholar] [CrossRef]
- Zhao, Q.; Xie, T.; Hong, X.; Zhou, Y.; Fan, L.; Liu, Y.; Li, J. Modification of Functional Properties of Perilla Protein Isolate by High-Intensity Ultrasonic Treatment and the Stability of o/w Emulsion. Food Chem. 2022, 368, 130848. [Google Scholar] [CrossRef]
- Kim, T.K.; Yong, H.I.; Kim, Y.B.; Jung, S.; Kim, H.W.; Choi, Y.S. Effects of Organic Solvent on Functional Properties of Defatted Proteins Extracted from Protaetia brevitarsis Larvae. Food Chem. 2021, 336, 127679. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J. Changes in the Antioxidant Properties of Rice Bran Protein Isolate upon Simulated Gastrointestinal Digestion. LWT 2020, 126, 109206. [Google Scholar] [CrossRef]
- Shi, J.; Wu, M.; Quan, M. Effects of Protein Oxidation on Gelatinization Characteristics during Rice Storage. J. Cereal Sci. 2017, 75, 228–233. [Google Scholar] [CrossRef]
- Han, T.; Wang, M.; Wang, Y.; Tang, L. Effects of High-Pressure Homogenization and Ultrasonic Treatment on the Structure and Characteristics of Casein. LWT 2020, 130, 109560. [Google Scholar] [CrossRef]
- Grossmann, L.; McClements, D.J. Current Insights into Protein Solubility: A Review of Its Importance for Alternative Proteins. Food Hydrocoll. 2023, 137, 108416. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, L.; Zhang, C.; Xie, P.; Cheng, J.; Wang, X.; Li, S. Physicochemical and Functional Properties of Chinese Quince Seed Protein Isolate. Food Chem. 2019, 283, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Miedzianka, J.; Pȩksa, A.; Pokora, M.; Rytel, E.; Tajner-Czopek, A.; Kita, A. Improving the Properties of Fodder Potato Protein Concentrate by Enzymatic Hydrolysis. Food Chem. 2014, 159, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.S.H.; Nickerson, M.T. Food Proteins: A Review on Their Emulsifying Properties Using a Structure-Function Approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Wang, X.; Xu, Y.; Meng, J.; Feng, C.; Geng, X.; Cheng, Y.; Chang, M. Effects of Freeze–Thaw Cycles on the Structures and Functional Properties of Clitocybe squamulosa Protein Isolates. Foods 2023, 12, 2948. https://doi.org/10.3390/foods12152948
Xu L, Wang X, Xu Y, Meng J, Feng C, Geng X, Cheng Y, Chang M. Effects of Freeze–Thaw Cycles on the Structures and Functional Properties of Clitocybe squamulosa Protein Isolates. Foods. 2023; 12(15):2948. https://doi.org/10.3390/foods12152948
Chicago/Turabian StyleXu, Lijing, Xin Wang, Yaping Xu, Junlong Meng, Cuiping Feng, Xueran Geng, Yanfen Cheng, and Mingchang Chang. 2023. "Effects of Freeze–Thaw Cycles on the Structures and Functional Properties of Clitocybe squamulosa Protein Isolates" Foods 12, no. 15: 2948. https://doi.org/10.3390/foods12152948
APA StyleXu, L., Wang, X., Xu, Y., Meng, J., Feng, C., Geng, X., Cheng, Y., & Chang, M. (2023). Effects of Freeze–Thaw Cycles on the Structures and Functional Properties of Clitocybe squamulosa Protein Isolates. Foods, 12(15), 2948. https://doi.org/10.3390/foods12152948




