Genomic and Antimicrobial Surveillance of Campylobacter Population in Italian Poultry
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Strains Selection
2.2. DNA Extraction and PCR Identification
2.3. Antimicrobial Susceptibility Tests
2.4. Whole Genome Sequencing and Genomic Characterization
2.5. Resistome and Virulome Characterization
3. Results
3.1. MLST Analysis of C. jejuni and C. coli Isolates from Italian Poultry
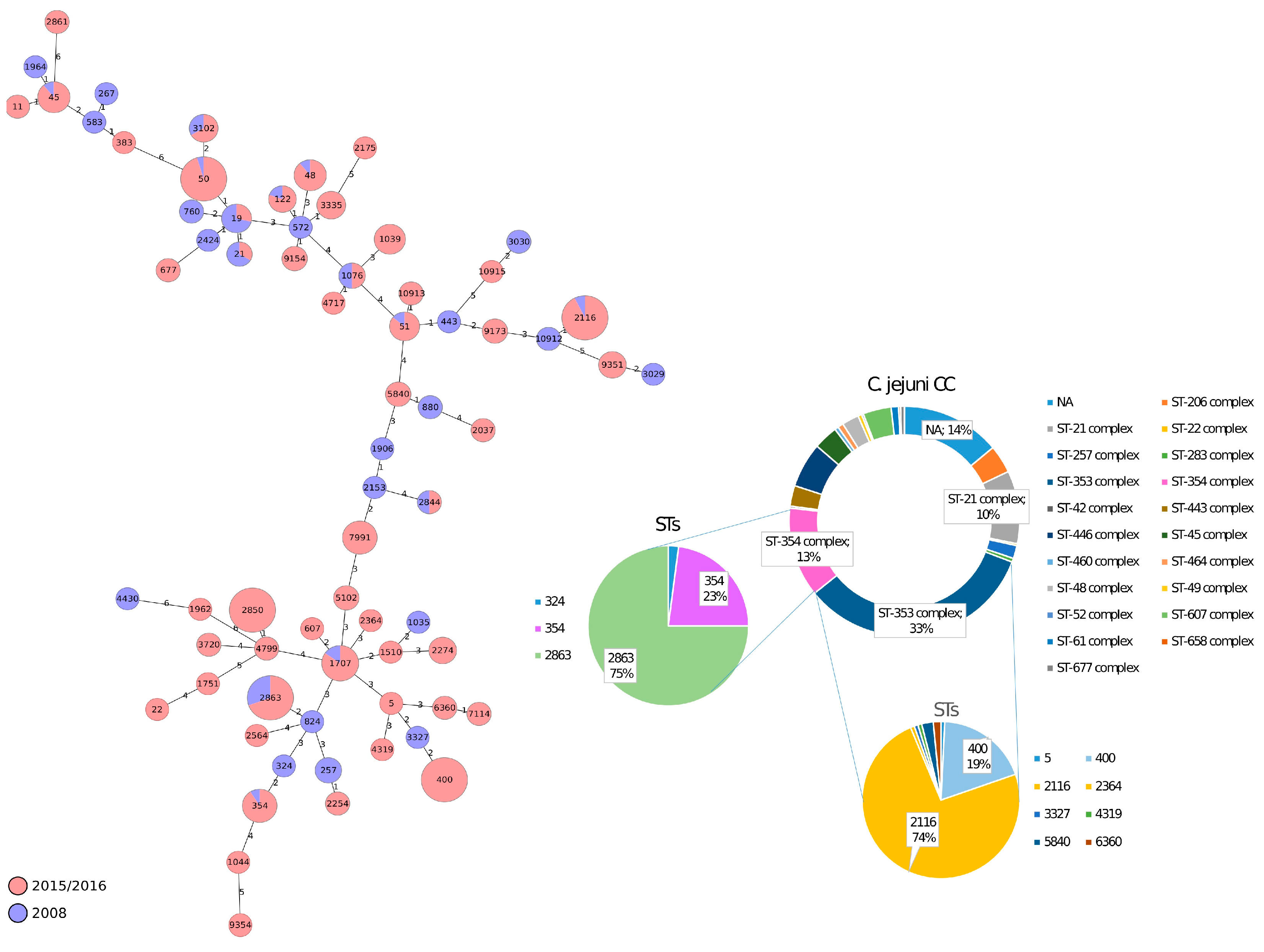
3.2. cgMLST Analysis of C. jejuni and C. coli Isolates from Italian Poultry
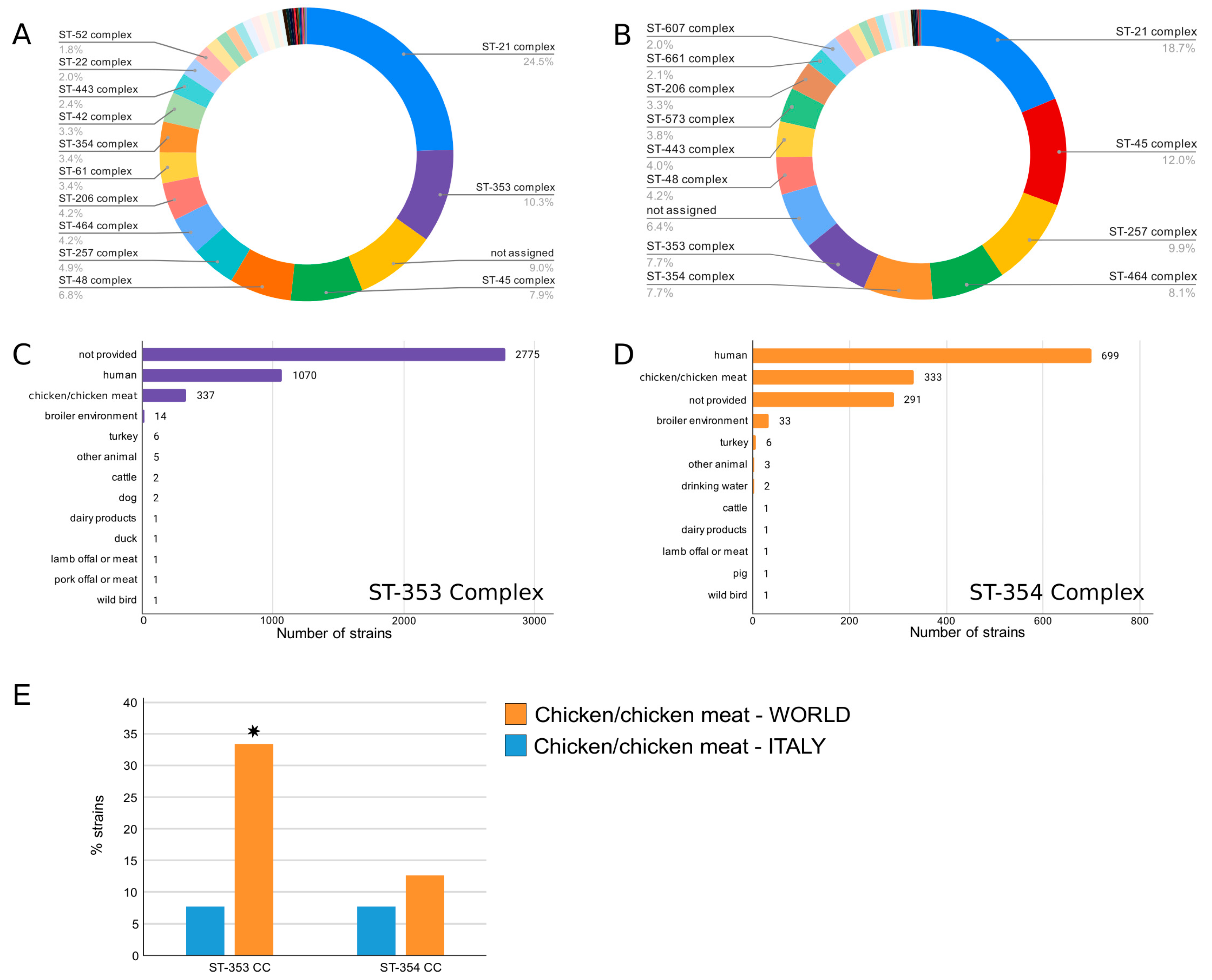
3.3. Comparison of Italian and Global Strains of C. jejuni CC353 and CC354 Isolated from Poultry
3.4. Virulence Determinants in C. jejuni Isolates
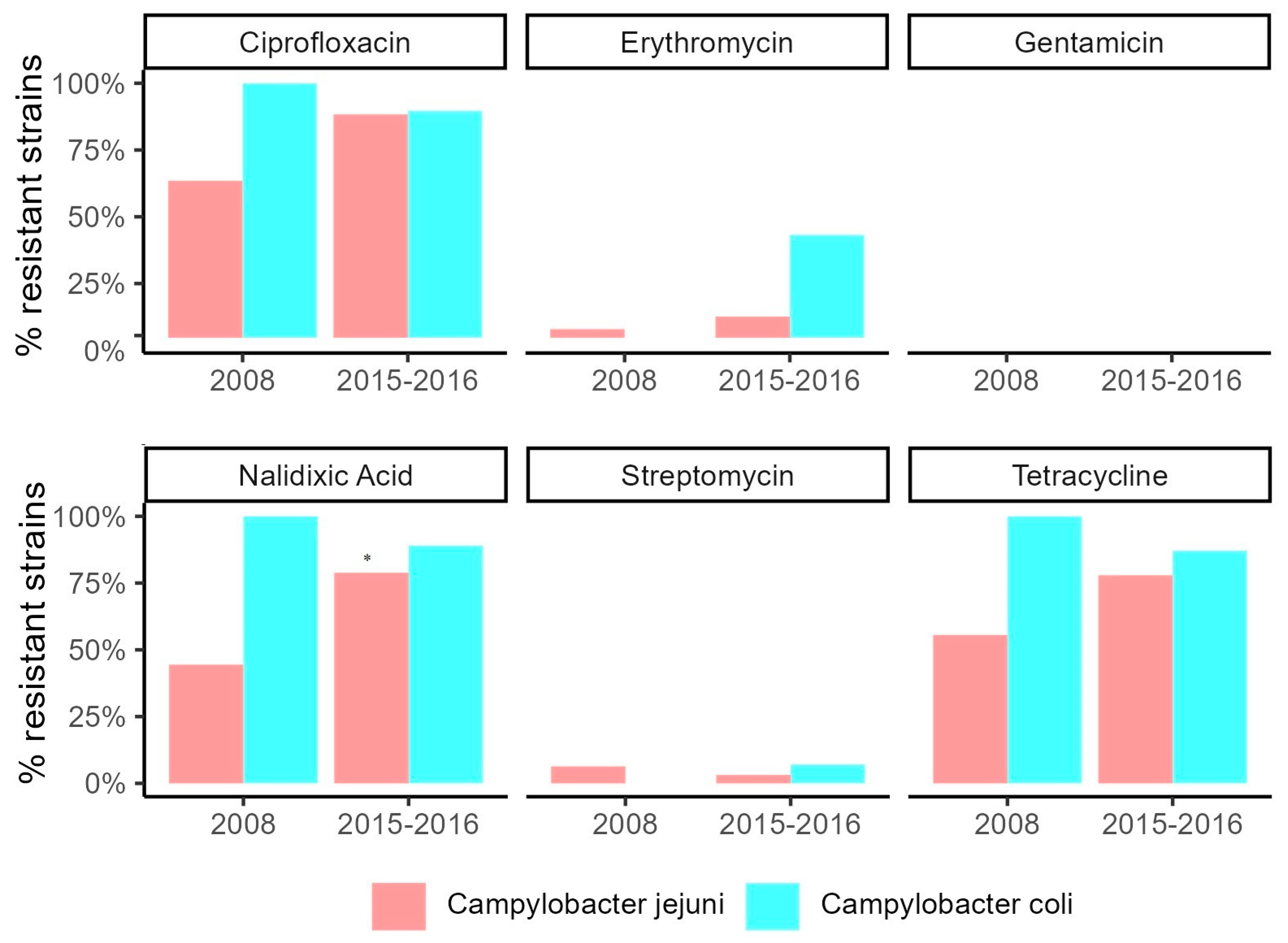
3.5. Antimicrobial Susceptibility
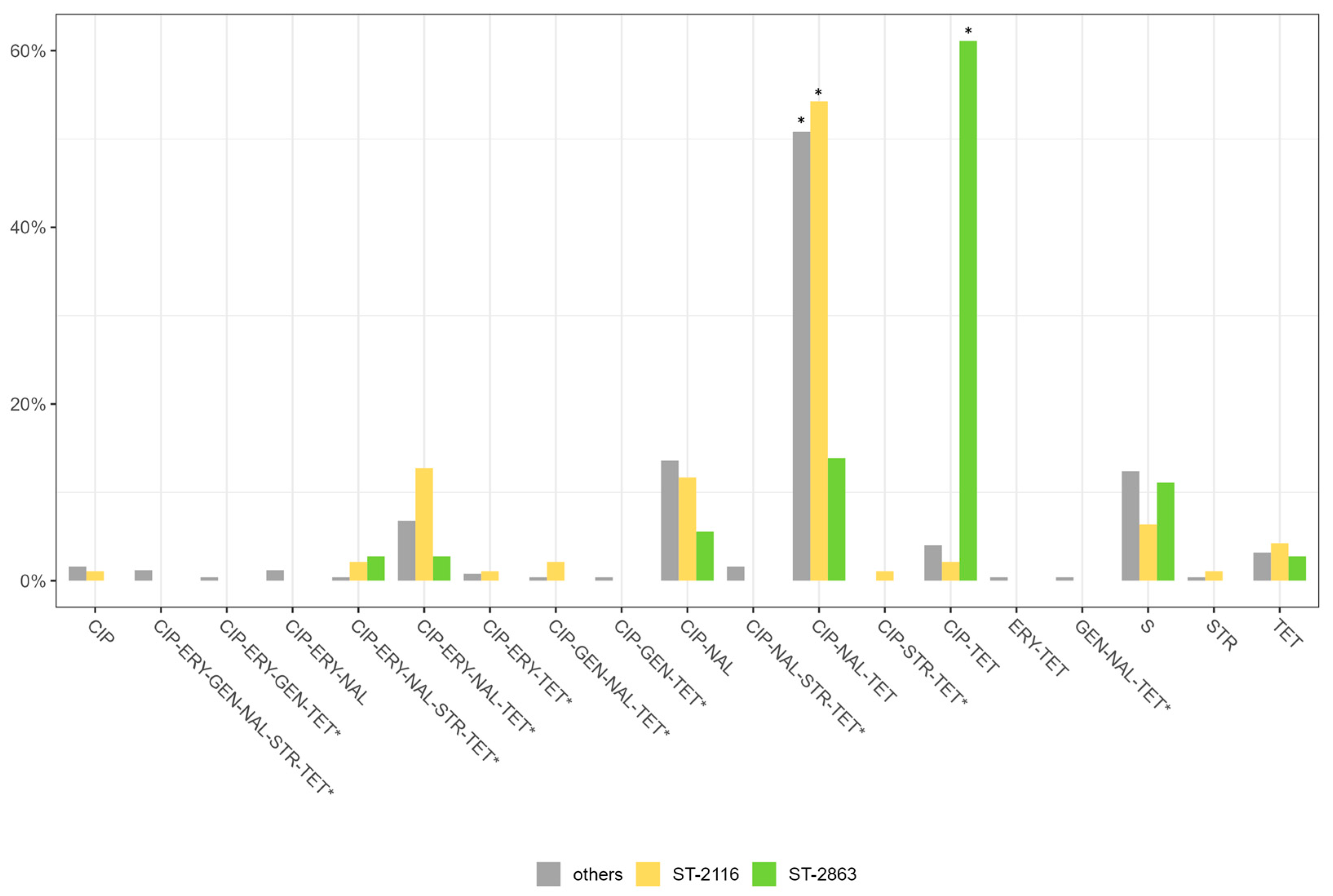
3.6. Antimicrobial Resistance Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.L.; Bulach, D.; McLure, A.; Varrone, L.; Jennison, A.V.; Valcanis, M.; Smith, J.J.; Polkinghorne, B.G.; Glass, K.; Kirk, M.D. Antimicrobial Resistance of Campylobacter spp. Causing Human Infection in Australia: An International Comparison. Microb. Drug Resist. 2021, 27, 518–528. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, 7666. [Google Scholar] [CrossRef]
- Igwaran, A.; Okoh, A.I. Human campylobacteriosis: A public health concern of global importance. Heliyon 2019, 5, e02814. [Google Scholar] [CrossRef]
- Yahara, K.; Méric, G.; Taylor, A.J.; de Vries, S.P.; Murray, S.; Pascoe, B.; Mageiros, L.; Torralbo, A.; Vidal, A.; Ridley, A.; et al. Genome—Wide association of functional traits linked with C ampylobacter jejuni survival from farm to fork. Environ. Microbiol. 2017, 19, 361–380. [Google Scholar] [CrossRef]
- Chen, S.; Fegan, N.; Kocharunchitt, C.; Bowman, J.; Duffy, L. Changes of the bacterial community diversity on chicken carcasses through an Australian poultry processing line. Food Microbiol. 2020, 86, 103350. [Google Scholar] [CrossRef]
- Sheppard, S.K.; Dallas, J.F.; Strachan, N.J.C.; MacRae, M.; McCarthy, N.D.; Wilson, D.J.; Gormley, F.J.; Falush, D.; Ogden, I.D.; Maiden, M.C.J.; et al. Campylobacter Genotyping to Determine the Source of Human Infection. Clin. Infect. Dis. 2009, 48, 1072–1078. [Google Scholar] [CrossRef]
- Wieczorek, K.; Bocian, Ł.; Osek, J. Prevalence and antimicrobial resistance of Campylobacter isolated from carcasses of chickens slaughtered in Poland—A retrospective study. Food Control 2020, 112, 107159. [Google Scholar] [CrossRef]
- Colles, F.M.; Cain, R.J.; Nickson, T.; Smith, A.L.; Roberts, S.J.; Maiden, M.C.; Lunn, D.; Dawkins, M.S. Monitoring chicken flock behaviour provides early warning of infection by human pathogen Campylobacter. Proc. R. Soc. B Boil. Sci. 2016, 283, 20152323. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Update and review of control options for Campylobacter in broilers at primary production. EFSA J. 2020, 18, e06090. [Google Scholar]
- FAO (2018) FAOSTAT: Production—Livestock Primary, Chicken Meat and Canned Chicken Meat. 2017. Available online: http://faostat.fao.org (accessed on 13 March 2020).
- AVEC. Association of Poultry Processors and Poultry Trade in the EU Countries—Annual Report 2022. 2022. Available online: https://avec-poultry.eu/wp-content/uploads/2022/09/AVEC-annual-report-2022_FINAL-WEB.pdf (accessed on 1 February 2023).
- Liaw, J.; Hong, G.; Davies, C.; Elmi, A.; Sima, F.; Stratakos, A.; Stef, L.; Pet, I.; Hachani, A.; Corcionivoschi, N.; et al. The Campylobacter jejuni Type VI Secretion System Enhances the Oxidative Stress Response and Host Colonization. Front. Microbiol. 2019, 10, 2864. [Google Scholar] [CrossRef] [PubMed]
- Truccollo, B.; Whyte, P.; Burgess, C.M.; Bolton, D.J. Genomic Characterisation of Campylobacter jejuni Isolates Recovered During Commercial Broiler Production. Front. Microbiol. 2021, 12, 2678. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Leite, D.; Fernandes, M.; Mena, C.; Gibbs, P.A.; Teixeira, P. Campylobacter spp. as a foodborne pathogen: A review. Front. Microbiol. 2011, 2, 200. [Google Scholar] [CrossRef]
- Mourkas, E.; Florez-Cuadrado, D.; Pascoe, B.; Calland, J.K.; Bayliss, S.C.; Mageiros, L.; Méric, G.; Hitchings, M.D.; Quesada, A.; Porrero, C.; et al. Gene pool transmission of multidrug resistance among Campylobacter from livestock, sewage and human disease. Environ. Microbiol. 2019, 21, 4597–4613. [Google Scholar] [CrossRef]
- Davies, R.; Wales, A. Antimicrobial Resistance on Farms: A Review Including Biosecurity and the Potential Role of Disinfectants in Resistance Selection. Compr. Rev. Food Sci. Food Saf. 2019, 18, 753–774. [Google Scholar] [CrossRef]
- Stetsenko, V.V.; Efimochkina, N.R.; Pichugina, T.V. Growth and Persistence of Campylobacter jejuni in Foodstuffs. Bull. Exp. Biol. Med. 2019, 166, 759–765. [Google Scholar] [CrossRef]
- Wysok, B.; Wojtacka, J.; Hänninen, M.-L.; Kivistö, R. Antimicrobial Resistance and Virulence-Associated Markers in Campylobacter Strains From Diarrheic and Non-diarrheic Humans in Poland. Front. Microbiol. 2020, 11, 1799. [Google Scholar] [CrossRef]
- Quino, W.; Caro-Castro, J.; Hurtado, V.; Flores-León, D.; Gonzalez-Escalona, N.; Gavilan, R.G. Genomic Analysis and Antimicrobial Resistance of Campylobacter jejuni and Campylobacter coli in Peru. Front. Microbiol. 2022, 12, 4181. [Google Scholar] [CrossRef]
- Uelze, L.; Grützke, J.; Borowiak, M.; Hammerl, J.A.; Juraschek, K.; Deneke, C.; Tausch, S.H.; Malorny, B. Typing methods based on whole genome sequencing data. One Health Outlook 2020, 2, 3. [Google Scholar] [CrossRef]
- Zhao, S.; Tyson, G.H.; Chen, Y.; Li, C.; Mukherjee, S.; Young, S.; Lam, C.; Folster, J.P.; Whichard, J.M.; McDermott, P.F. Whole-Genome Sequencing Analysis Accurately Predicts Antimicrobial Resistance Phenotypes in Campylobacter spp. Appl. Environ. Microbiol. 2016, 82, 459–466. [Google Scholar] [CrossRef]
- Marotta, F.; Garofolo, G.; Di Marcantonio, L.; Di Serafino, G.; Neri, D.; Romantini, R.; Sacchini, L.; Alessiani, A.; Di Donato, G.; Nuvoloni, R. Antimicrobial resistance genotypes and phenotypes of Campylobacter jejuni isolated in Italy from humans, birds from wild and urban habitats, and poultry. PLoS ONE 2019, 14, e0223804. [Google Scholar] [CrossRef] [PubMed]
- Di Giannatale, E.; Prencipe, V.; Colangeli, P.; Alessiani, A.; Barco, L.; Staffolani, M.; Tagliabue, S.; Grattarola, C.; Cerrone, A.; Costa, A.; et al. Prevalence of thermotolerant Campylobacter in broiler flocks and broiler carcasses in Italy. Veter-Ital. 2010, 46, 405–423. [Google Scholar]
- Wang, G.; Clark, C.G.; Taylor, T.M.; Pucknell, C.; Barton, C.; Price, L.; Woodward, D.L.; Rodgers, F.G. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J. Clin. Microbiol. 2002, 40, 4744–4747. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2016, 45, D566–D573. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Allesøe, R.; Joensen, K.G.; Cavaco, L.M.; Lund, O.; Aarestrup, F.M. PointFinder: A novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J. Antimicrob. Chemother. 2017, 72, 2764–2768. [Google Scholar] [CrossRef]
- Concha-Toloza, M.; Lopez-Cantillo, M.; Molina-Mora, J.A.; Collado, L. Genomic Characterization of Antibiotic-Resistant Campylobacterales Isolated from Chilean Poultry Meat. Antibiotics 2023, 12, 917. [Google Scholar] [CrossRef]
- Mäesaar, M.; Meremäe, K.; Ivanova, M.; Roasto, M. Antimicrobial resistance and multilocus sequence types of Campylobacter jejuni isolated from Baltic broiler chicken meat and Estonian human patients. Poult. Sci. 2018, 97, 3645–3651. [Google Scholar] [CrossRef]
- Griekspoor, P.; Engvall, E.O.; Olsen, B.; Waldenström, J. Multilocus sequence typing of Campylobacter jejuni from broilers. Veter-Microbiol. 2010, 140, 180–185. [Google Scholar] [CrossRef]
- Llarena, A.-K.; Huneau, A.; Hakkinen, M.; Hänninen, M.-L. Predominant Campylobacter jejuni Sequence Types Persist in Finnish Chicken Production. PLoS ONE 2015, 10, e0116585. [Google Scholar] [CrossRef]
- Lai, C.-K.; Chen, Y.-A.; Lin, C.-J.; Lin, H.-J.; Kao, M.-C.; Huang, M.-Z.; Lin, Y.-H.; Chuan, C.-N.; Chen, C.-J.; Lo, U.-G.; et al. Molecular Mechanisms and Potential Clinical Applications of Campylobacter jejuni Cytolethal Distending Toxin. Front. Cell. Infect. Microbiol. 2016, 6, 9. [Google Scholar] [CrossRef]
- Scuron, M.D.; Boesze-Battaglia, K.; Dlakić, M.; Shenker, B.J. The Cytolethal Distending Toxin Contributes to Microbial Virulence and Disease Pathogenesis by Acting As a Tri-Perditious Toxin. Front. Cell. Infect. Microbiol. 2016, 6, 168. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- P´erez-Rodríguez, F.; Mercanoglu Taban, B. A state-of-art review on multi-drug resistant pathogens in foods of animal origin: Risk factors and mitigation strategies. Front. Microbiol. 2019, 10, 2091. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Inglis, G.D.; Gusse, J.F.; House, K.E.; Shelton, T.G.; Taboada, E.N. Tetracycline Resistant Campylobacter jejuni Subtypes Emanating from Beef Cattle Administered Non-Therapeutic Chlortetracycline are Longitudinally Transmitted within the Production Continuum but are Not Detected in Ground Beef. Microorganisms 2019, 8, 23. [Google Scholar] [CrossRef]
- Hachesoo, B.A.; Khoshbakht, R.; Yazdi, H.S.; Tabatabaei, M.; Hosseinzadeh, S.; Asasi, K. Tetracycline Resistance Genes in Campylobacter jejuni and C. coli Isolated From Poultry Carcasses. Jundishapur J. Microbiol. 2014, 7, e12129. [Google Scholar] [CrossRef]
- Portes, A.B.; Panzenhagen, P.; dos Santos, A.M.P.; Junior, C.A.C. Antibiotic Resistance in Campylobacter: A Systematic Review of South American Isolates. Antibiotics 2023, 12, 548. [Google Scholar] [CrossRef]
- Alaboudi, A.R.; Malkawi, I.M.; Osaili, T.M.; Abu-Basha, E.A.; Guitian, J. Prevalence, antibiotic resistance and genotypes of Campylobacter jejuni and Campylobacter coli isolated from chickens in Irbid governorate, Jordan. Int. J. Food Microbiol. 2020, 327, 108656. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.; Kowalcyk, B.; Buck, P.; Blaser, M.J.; Frenkel, J.K.; Lorber, B.; Smith, J.; Tarr, P.I. The Long-Term Health Outcomes of Selected Foodborne Pathogens; The Center for Foodborne Illness Research & Prevention: Parsippany, NJ, USA, 2009. [Google Scholar]
- Bolinger, H.; Kathariou, S. The Current State of Macrolide Resistance in Campylobacter spp.: Trends and Impacts of Resistance Mechanisms. Appl. Environ. Microbiol. 2017, 83, e00416–e00417. [Google Scholar] [CrossRef]
- Bravo, V.; Katz, A.; Porte, L.; Weitzel, T.; Varela, C.; Gonzalez-Escalona, N.; Blondel, C.J. Genomic analysis of the diversity, antimicrobial resistance and virulence potential of clinical Campylobacter jejuni and Campylobacter coli strains from Chile. PLOS Neglected Trop. Dis. 2021, 15, e0009207. [Google Scholar] [CrossRef]
- Linn, K.Z.; Furuta, M.; Nakayama, M.; Masuda, Y.; Honjoh, K.-I.; Miyamoto, T. Characterization and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from chicken and pork. Int. J. Food Microbiol. 2021, 360, 109440. [Google Scholar] [CrossRef] [PubMed]



| Adherence | Toxin CDT | Flagellar Glycosylation | Capsule Biosynthesis and Transport | Immune Evasion | Motility | |||
|---|---|---|---|---|---|---|---|---|
| STs | porA | cdtA | cdtB | cdtC | maf4 | no. 25 a | no. 9 b | no. 9 c |
| 2116 (n = 94) | 100% | 100% | 100% | 100% | 20% | 20% | 12% | 81% |
| 2863 (n = 36) | 100% | 97% | 100% | 100% | 39% | 91% | 27% | 95% |
| Others (n = 250) | 78% | 99% | 99% | 100% | 11% | 34% | 30% | 65% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marotta, F.; Janowicz, A.; Romantini, R.; Di Marcantonio, L.; Di Timoteo, F.; Romualdi, T.; Zilli, K.; Barco, L.; D’Incau, M.; Mangone, I.; et al. Genomic and Antimicrobial Surveillance of Campylobacter Population in Italian Poultry. Foods 2023, 12, 2919. https://doi.org/10.3390/foods12152919
Marotta F, Janowicz A, Romantini R, Di Marcantonio L, Di Timoteo F, Romualdi T, Zilli K, Barco L, D’Incau M, Mangone I, et al. Genomic and Antimicrobial Surveillance of Campylobacter Population in Italian Poultry. Foods. 2023; 12(15):2919. https://doi.org/10.3390/foods12152919
Chicago/Turabian StyleMarotta, Francesca, Anna Janowicz, Romina Romantini, Lisa Di Marcantonio, Federica Di Timoteo, Teresa Romualdi, Katiuscia Zilli, Lisa Barco, Mario D’Incau, Iolanda Mangone, and et al. 2023. "Genomic and Antimicrobial Surveillance of Campylobacter Population in Italian Poultry" Foods 12, no. 15: 2919. https://doi.org/10.3390/foods12152919
APA StyleMarotta, F., Janowicz, A., Romantini, R., Di Marcantonio, L., Di Timoteo, F., Romualdi, T., Zilli, K., Barco, L., D’Incau, M., Mangone, I., Cito, F., Di Domenico, M., Pomilio, F., Ricci, L., & Garofolo, G. (2023). Genomic and Antimicrobial Surveillance of Campylobacter Population in Italian Poultry. Foods, 12(15), 2919. https://doi.org/10.3390/foods12152919







