Camellia japonica Flowers as a Source of Nutritional and Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Evaluation of Camellia as a Promising Source of Nutrients
2.2.1. Lipid Content
Fatty Acid Characterization
2.2.2. Protein Content
2.2.3. Carbohydrate Content (Simple Sugars by Difference)
2.2.4. Dietary Fiber Content
2.2.5. Macro- and Micromineral Content
2.2.6. Moisture and Ash Content
2.2.7. Basic Elemental Composition (C, O, N, H, S)
2.2.8. Thermal Analysis
2.3. Determination of Bioactive Compounds from C. japonica L. Flowers
2.3.1. Extraction Yield
2.3.2. Total Phenolic Content (TPC)
2.3.3. Total Carotenoid Content (TCC)
2.3.4. Total Flavonoid Content (TFC)
2.3.5. Total Anthocyanin Content (TAC)
2.4. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Composition of C. japonica Flowers
3.1.1. Lipid Content and Fatty Acid Profile
3.1.2. Protein Content
3.1.3. Mineral Content
3.2. Thermochemical Characterization of Camellias
3.3. Evaluation of Camellia as a Promising Source of Bioactive Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vilar-Compte, M.; Burrola-Méndez, S.; Lozano-Marrufo, A.; Ferré-Eguiluz, I.; Flores, D.; Gaitán-Rossi, P.; Teruel, G.; Pérez-Escamilla, R. Urban poverty and nutrition challenges associated with accessibility to a healthy diet: A global systematic literature review. Int. J. Equity Health 2021, 20, 40. [Google Scholar] [CrossRef]
- Elder, E.I.R.; Ransom, E. Nutrition of Women and Adolescent Girls: Why It Matters. Popul. Ref. Bur. 2003, 1–16. [Google Scholar]
- Popović-Djordjević, J.B.; Katanić Stanković, J.S.; Mihailović, V.; Pereira, A.G.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Algae as a Source of Bioactive Compounds to Prevent the Development of Type 2 Diabetes Mellitus. Curr. Med. Chem. 2021, 28, 4592–4615. [Google Scholar] [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Pereira, A.G.; Fraga-Corral, M.; Garciá-Oliveira, P.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Carpena, M.; Otero, P.; Gullón, P.; Prieto, M.A.; Simal-Gandara, J. Culinary and nutritional value of edible wild plants from northern Spain rich in phenolic compounds with potential health benefits. Food Funct. 2020, 11, 8493–8515. [Google Scholar] [CrossRef] [PubMed]
- Baldermann, S.; Blagojević, L.; Frede, K.; Klopsch, R.; Neugart, S.; Neumann, A.; Ngwene, B.; Norkeweit, J.; Schröter, D.; Schröter, A.; et al. Are Neglected Plants the Food for the Future? CRC Crit. Rev. Plant Sci. 2016, 35, 106–119. [Google Scholar] [CrossRef]
- Li, X.; Yadav, R.; Siddique, K.H.M. Neglected and Underutilized Crop Species: The Key to Improving Dietary Diversity and Fighting Hunger and Malnutrition in Asia and the Pacific. Front. Nutr. 2020, 7, 254. [Google Scholar] [CrossRef]
- Li, X.; Siddique, K.H.M. Future Smart Food: Harnessing the potential of neglected and underutilized species for Zero Hunger. Matern. Child Nutr. 2020, 16, e13008. [Google Scholar] [CrossRef]
- Rivas-García, L.; Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Forbes-Hernández, T.Y.; Varela-López, A.; Llopis, J.; Sánchez-González, C.; Quiles, J.L. Edible flowers as a health promoter: An evidence-based review. Trends Food Sci. Technol. 2021, 117, 46–59. [Google Scholar] [CrossRef]
- Yang, C.; Liu, X.; Chen, Z.; Lin, Y.; Wang, S. Comparison of Oil Content and Fatty Acid Profile of Ten New Camellia oleifera Cultivars. J. Lipids 2016, 2016, 3982486. [Google Scholar] [CrossRef]
- Pereira, A.G.; Garcia-Perez, P.; Cassani, L.; Chamorro, F.; Cao, H.; Barba, F.J.; Simal-Gandara, J.; Prieto, M.A. Camellia japonica: A phytochemical perspective and current applications facing its industrial exploitation. Food Chem. X 2022, 13, 100258. [Google Scholar] [CrossRef]
- Luan, F.; Zeng, J.; Yang, Y.; He, X.; Wang, B.; Gao, Y.; Zeng, N. Recent advances in Camellia oleifera Abel: A review of nutritional constituents, biofunctional properties, and potential industrial applications. J. Funct. Foods 2020, 75, 104242. [Google Scholar] [CrossRef]
- Teixeira, A.M.; Sousa, C. A review on the biological activity of Camellia species. Molecules 2021, 26, 2178. [Google Scholar] [CrossRef] [PubMed]
- Xunta de Galicia. Ruta de la Camelia. Available online: https://www.turismo.gal/que-facer/ruta-da-camelia?langId=es_ES (accessed on 12 November 2020).
- Salinero, C.; Feás, X.; Pedro Mansilla, J.; Seijas, J.A.; Pilar Vázquez-Tato, M.; Vela, P.; Sainz, M.J. 1H-nuclear magnetic resonance analysis of the triacylglyceride composition of cold-pressed oil from Camellia japonica. Molecules 2012, 17, 6716–6727. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ding, Y.; Chen, G.; Sun, Y.; Zeng, X.; Ye, H. Components identification and nutritional value exploration of tea (Camellia sinensis L.) flower extract: Evidence for functional food. Food Res. Int. 2020, 132, 109100. [Google Scholar] [CrossRef]
- Shi, L.; Gu, Y.; Wu, D.; Wu, X.; Grierson, D.; Tu, Y.; Wu, Y. Hot air drying of tea flowers: Effect of experimental temperatures on drying kinetics, bioactive compounds and quality attributes. Int. J. Food Sci. Technol. 2019, 54, 526–535. [Google Scholar] [CrossRef]
- Fernandes, L.; Ramalhosa, E.; Pereira, J.A.; Saraiva, J.A.; Casal, S. Borage, camellia, centaurea and pansies: Nutritional, fatty acids, free sugars, vitamin E, carotenoids and organic acids characterization. Food Res. Int. 2020, 132, 109070. [Google Scholar] [CrossRef]
- Yoon, I.S.; Park, D.H.; Kim, J.E.; Yoo, J.C.; Bae, M.S.; Oh, D.S.; Shim, J.H.; Choi, C.Y.; An, K.W.; Kim, E.I.; et al. Identification of the biologically active constituents of Camellia japonica leaf and anti-hyperuricemic effect in vitro and in vivo. Int. J. Mol. Med. 2017, 39, 1613–1620. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; AOAC: Rockville, MD, USA, 1995. [Google Scholar]
- Cavonius, L.R.; Carlsson, N.G.; Undeland, I. Quantification of total fatty acids in microalgae: Comparison of extraction and transesterification methods. Anal. Bioanal. Chem. 2014, 406, 7313. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.C.; Zhang, Y. Protein Analysis; Springer: Cham, Switzerland, 2017; Volume 18. [Google Scholar]
- AOAC International. Official Methods of Analysis, 20th ed.; AOAC: Rockville, MD, USA, 2016. [Google Scholar]
- McCleary, B.V.; McLoughlin, C.; Charmier, L.M.J.; McGeough, P. Measurement of available carbohydrates, digestible, and resistant starch in food ingredients and products. Cereal Chem. 2020, 97, 114–137. [Google Scholar] [CrossRef]
- Megazyme. Total Dietary Fiber Assay Procedure. Available online: www.megazyme.com/documents/Assay_Protocol/K-TDFR-200A_DATA.pdf (accessed on 31 May 2022).
- Garcia-Amezquita, L.E.; Tejada-Ortigoza, V.; Heredia-Olea, E.; Serna-Saldívar, S.O.; Welti-Chanes, J. Differences in the dietary fiber content of fruits and their by-products quantified by conventional and integrated AOAC official methodologies. J. Food Compos. Anal. 2018, 67, 77–85. [Google Scholar] [CrossRef]
- AOAC International. AOAC 2017.16 as a New Method of Analysis for Total Dietary Fibre; AOAC: Rockville, MD, USA, 2021; p. 34. [Google Scholar]
- Cassani, L.; Lourenço-Lopes, C.; Barral-Martinez, M.; Chamorro, F.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Thermochemical Characterization of Eight Seaweed Species and Evaluation of Their Potential Use as an Alternative for Biofuel Production and Source of Bioactive Compounds. Int. J. Mol. Sci. 2022, 23, 2355. [Google Scholar] [CrossRef]
- Millos, J.; Costas-Rodríguez, M.; Lavilla, I.; Bendicho, C. Multiple small volume microwave-assisted digestions using conventional equipment for multielemental analysis of human breast biopsies by inductively coupled plasma optical emission spectrometry. Talanta 2009, 77, 1490–1496. [Google Scholar] [CrossRef]
- Millos, J.; Costas-Rodríguez, M.; Lavilla, I.; Bendicho, C. Multielemental determination in breast cancerous and non-cancerous biopsies by inductively coupled plasma-mass spectrometry following small volume microwave-assisted digestion. Anal. Chim. Acta 2008, 622, 77–84. [Google Scholar] [CrossRef] [PubMed]
- UNE-EN 14774-1; Biocombustibles Sólidos. Determinación del Contenido de Humedad. Método de Secado en Estufa. UNE: Madrid, Spain, 2010; p. 34.
- UNE-EN 14775; Biocombustibles Sólidos. Método para la Determinación del Contenido en Cenizas. UNE: Madrid, Spain, 2010.
- Fadeeva, V.P.; Tikhova, V.D.; Nikulicheva, O.N. Elemental analysis of organic compounds with the use of automated CHNS analyzers. J. Anal. Chem. 2008, 63, 1094–1106. [Google Scholar] [CrossRef]
- Attia, A.K.; Abdel-Moety, M.M. Thermoanalytical Investigation of Terazosin Hydrochloride. Adv. Pharm. Bull. 2013, 3, 147. [Google Scholar]
- Pereira, A.G.; Silva, A.; Barral-Martine, M.; Echave, J.; Chamorro, F.; Mansour, S.S.; Cassani, L.; Otero, P.; Xiao, J.; Barroso, F.; et al. Antimicrobial activity screening of Camellia japonica flowers (var. Conde de la Torre). Med. Sci. Forum 2022, 1, 15. [Google Scholar]
- Silva, A.; Rodrigues, C.; Garcia-Oliveira, P.; Lourenço-Lopes, C.; Silva, S.A.; Garcia-Perez, P.; Carvalho, A.P.; Domingues, V.F.; Barroso, M.F.; Delerue-Matos, C.; et al. Screening of bioactive properties in brown algae from the northwest Iberian Peninsula. Foods 2021, 10, 1915. [Google Scholar] [CrossRef]
- Cassani, L.; Tomadoni, B.; Ponce, A.; Agüero, M.V.; Moreira, M.R. Combined Use of Ultrasound and Vanillin to Improve Quality Parameters and Safety of Strawberry Juice Enriched with Prebiotic Fibers. Food Bioprocess Technol. 2017, 10, 1454–1465. [Google Scholar] [CrossRef]
- Scott, K.J. Detection and Measurement of Carotenoids by UV/VIS Spectrophotometry. Curr. Protoc. Food Anal. Chem. 2001, 1, F2-2. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Kim, B.-S.; Choi, O.-J.; Shim, K.-H. Properties of Chemical Components of Camellia japonica L. loaves According to Picking Time. J. Korean Soc. Food Sci. Nutr. 2005, 34, 681–686. [Google Scholar]
- Barreiro, R.; Rodríguez-Solana, R.; Alonso, L.; Salinero, C.; Sánchez, J.I.L.; Pérez-Santín, E. Fast1h-nmr species differentiation method for Camellia seed oils applied to spanish ornamentals plants. Comparison with traditional gas chromatography. Plants 2021, 10, 1984. [Google Scholar] [CrossRef] [PubMed]
- de Haro Bailón, A.; Obregón, S.; del Río Celestino, M.; Mansilla, P.; Salinero, M.C. Variability in seed storage components (protein, oil and fatty acids) in a Camellia germplasm collection. In Proceedings of the International Camellia Congress, Pontevedra, Spain, 11–15 March 2014; pp. 302–309. [Google Scholar]
- Vlaicu, P.A.; Untea, A.E.; Turcu, R.P.; Saracila, M.; Panaite, T.D.; Cornescu, G.M. Nutritional Composition and Bioactive Compounds of Basil, Thyme and Sage Plant Additives and Their Functionality on Broiler Thigh Meat Quality. Foods 2022, 11, 1105. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Vasilakoglou, I.B.; Petrotos, K.; Barros, L.; Ferreira, I.C.F.R. Nutritional value, chemical composition and cytotoxic properties of common purslane (Portulaca oleracea L.) in relation to harvesting stage and plant part. Antioxidants 2019, 8, 293. [Google Scholar] [CrossRef]
- Uddin, M.K.; Juraimi, A.S.; Ali, M.E.; Ismail, M.R. Evaluation of antioxidant properties and mineral composition of purslane (Portulaca oleracea L.) at different growth stages. Int. J. Mol. Sci. 2012, 13, 10257–10267. [Google Scholar] [CrossRef]
- Lebaka, V.R.; Wee, Y.J.; Ye, W.; Korivi, M. Nutritional composition and bioactive compounds in three different parts of mango fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef] [PubMed]
- Leite Neta, M.T.S.; De Santana, K.L.; Dos Santos, R.A.R.; Galvão, M.S.; Fontes, A.S.; Narain, N. Fatty acids profile of pulp and peel of mango (Mangifera indica Linn.) cultivar ‘Espada’ from Brazilian northeast region. Acta Hortic. 2018, 1198, 197–198. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef]
- Casaril, K.B.P.B.; Kasuya, M.C.M.; Vanetti, M.C.D. Antimicrobial activity and mineral composition of shiitake mushrooms cultivated on agricultural waste. Brazilian Arch. Biol. Technol. 2011, 54, 991–1002. [Google Scholar] [CrossRef]
- Sundriyal, M.; Sundriyal, R.C.; Url, S. Wild Edible Plants of the Sikkim Himalaya: Nutritive Values of Selected Species. Economic 2001, 55, 377–390. [Google Scholar] [CrossRef]
- Giwa, S.; Ogunbona, C. Sweet almond (Prunus amygdalus “dulcis”) seeds as a potential feedstock for Nigerian Biodiesel Automotive Project. Rev. Ambient. Agua 2014, 9, 37–45. [Google Scholar] [CrossRef]
- Koba, K.; Yanagita, T. Health benefits of conjugated linoleic acid (CLA). Obes. Res. Clin. Pract. 2014, 8, e525–e532. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.E.G. Ácido linoleico conjugado; Elsevier: Amsterdam, The Netherlands, 2009; pp. 42–49. [Google Scholar]
- Simopoulos, A.P.; DiNicolantonio, J.J. The importance of a balanced ω-6 to ω-3 ratio in the prevention and management of obesity. Open Heart 2016, 3, 385. [Google Scholar] [CrossRef]
- Noh, S.; Yoon, S.H. Stereospecific Positional Distribution of Fatty Acids of Camellia (Camellia japonica L.) Seed Oil. J. Food Sci. 2012, 77, C1055–C1057. [Google Scholar] [CrossRef]
- Zeng, W.; Endo, Y. Lipid Characteristics of Camellia Seed Oil. J. Oleo Sci 2019, 68, 649–658. [Google Scholar] [CrossRef]
- Chung, K.H. Transesterification of Camellia japonica and Vernicia fordii seed oils on alkali catalysts for biodiesel production. J. Ind. Eng. Chem. 2010, 16, 506–509. [Google Scholar] [CrossRef]
- Garcia-Jares, C.; Sanchez-Nande, M.; Lamas, J.P.; Lores, M. Profiling the fatty acids content of ornamental camellia seeds cultivated in galicia by an optimized matrix solid-phase dispersion extraction. Bioengineering 2017, 4, 87. [Google Scholar] [CrossRef]
- Hu, J.B.; Yang, G.L. Physiochemical characteristics, fatty acid profile and tocopherol composition of the oil from Camellia oleifera abel cultivated in henan, China. Grasas Aceites 2018, 69, e255. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, J.; Cao, J.; Zeng, Y.; Li, X.; Ma, J.; Huang, Z.; Jiang, M.; Sun, L. The Beneficial Effects of Aluminum on the Plant Growth in Camellia japonica. J. Soil Sci. Plant Nutr. 2020, 20, 1799–1809. [Google Scholar] [CrossRef]
- Nakamura, N.; Mori, M.; Suzuki, H. Chemical characterization of the callose plug isolated from Camellia japonica pollen tube. Plant Cell Physiol. 1984, 25, 233–238. [Google Scholar]
- Joshi, R.; Poonam; Gulati, A. Biochemical attributes of tea flowers (Camellia sinensis) at different developmental stages in the Kangra region of India. Sci. Hortic. 2011, 130, 266–274. [Google Scholar] [CrossRef]
- Chen, D.; Chen, G.; Sun, Y.; Zeng, X.; Ye, H. Physiological genetics, chemical composition, health benefits and toxicology of tea (Camellia sinensis L.) flower: A review. Food Res. Int. 2020, 137, 109584. [Google Scholar] [CrossRef]
- Xiong, Z.; Qi, X.; Wei, X.; Chen, Z.; Tang, H.; Chai, S. Nutrient composition in leaves of cultivated and wild Camellia nitidissima. Pakistan J. Bot. 2012, 44, 635–638. [Google Scholar]
- Xu, R.; Ye, H.; Sun, Y.; Tu, Y.; Zeng, X. Preparation, preliminary characterization, antioxidant, hepatoprotective and antitumor activities of polysaccharides from the flower of tea plant (Camellia sinensis). Food Chem. Toxicol. 2012, 50, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, L.; Montesano, D.; Senatore, A. The distribution of minerals and flavonoids in the tea plant (Camellia sinensis). Farmaco 2001, 56, 397–401. [Google Scholar] [CrossRef]
- Marles, R.J. Mineral nutrient composition of vegetables, fruits and grains: The context of reports of apparent historical declines. J. Food Compos. Anal. 2017, 56, 93–103. [Google Scholar] [CrossRef]
- Ben Amira, A.; Blecker, C.; Richel, A.; Arias, A.A.; Fickers, P.; Francis, F.; Besbes, S.; Attia, H. Influence of the ripening stage and the lyophilization of wild cardoon flowers on their chemical composition, enzymatic activities of extracts and technological properties of cheese curds. Food Chem. 2018, 245, 919–925. [Google Scholar] [CrossRef]
- Mikołajczak, N.; Sobiechowska, D.A.; Tańska, M. Edible flowers as a new source of natural antioxidants for oxidative protection of cold-pressed oils rich in omega-3 fatty acids. Food Res. Int. 2020, 134, 109216. [Google Scholar] [CrossRef]
- Platače, R.; Adamovičs, A. The evaluation of ash content in grass biomass used for energy production. WIT Trans. Ecol. Environ. 2014, 190, 1057–1065. [Google Scholar]
- Munawar, M.A.; Khoja, A.H.; Naqvi, S.R.; Mehran, M.T.; Hassan, M.; Liaquat, R.; Dawood, U.F. Challenges and opportunities in biomass ash management and its utilization in novel applications. Renew. Sustain. Energy Rev. 2021, 150, 111451. [Google Scholar] [CrossRef]
- López-Cervantes, J.; Sánchez-Machado, D.I.; Cruz-Flores, P.; Mariscal-Domínguez, M.F.; Servín de la Mora-López, G.; Campas-Baypoli, O.N. Antioxidant capacity, proximate composition, and lipid constituents of Aloe vera flowers. J. Appl. Res. Med. Aromat. Plants 2018, 10, 93–98. [Google Scholar] [CrossRef]
- Uche Christina, O.; Baxter-Grillo, D.; Helen, N.; Okwuonu Uche Christina, C.; Tracy, I.P. Phytochemical, proximate and elemental constituents of Aspilia africana (Wild sunflower) flowers. J. Med. Plants Stud. 2017, 5, 22–27. [Google Scholar]
- Fernandes, E.R.K.; Marangoni, C.; Souza, O.; Sellin, N. Thermochemical characterization of banana leaves as a potential energy source. Energy Convers. Manag. 2013, 75, 603–608. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Yguatyara de Luna, M.; Marcelo Rodrigues, P.; Antônia Mabrysa Torres, G.; Antônio Eufrázio, D.C.J.; Jackson Queiroz, M.; Selma Elaine, M.; Maria Alexsandra de Sousa, R. A thermogravimetric analysis of biomass wastes from the northeast region of Brazil as fuels for energy recovery. Energy Sources Part A Recover. Util. Environ. Eff. 2019, 41, 1557–1572. [Google Scholar] [CrossRef]
- Frankowski, J.; Zaborowicz, M.; Dach, J.; Czekała, W.; Przybył, J. Biological Waste Management in the Case of a Pandemic Emergency and Other Natural Disasters. Determination of Bioenergy Production from Floricultural Waste and Modeling of Methane Production Using Deep Neural Modeling Methods. Energies 2020, 13, 3014. [Google Scholar] [CrossRef]
- Jóvér, J.; Antal, K.; Zsembeli, J.; Blaskó, L.; Tamás, J. Assessment of gross calorific value of crop and bio-energy residues. Res. Agric. Eng. 2018, 64, 121–127. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Kim, C.-H.; Lee, J.-Y.; Lee, M.-S.; Goo, H.-K.; Park, J.-H.; Park, S.-H.; Ryu, J.-H. Exploration of Alternative Woody Biomass for Manufacturing Biopellets. J. Korea TAPPI 2020, 52, 253–3200. [Google Scholar] [CrossRef]
- Adamovics, A.; Platace, R.; Gulbe, I.; Ivanovs, S. The content of carbon and hydrogen in grass biomass and its influence on heating value. Eng. Rural Dev. 2018, 17, 1277–1281. [Google Scholar]
- Andrade, L.A.; Barrozo, M.A.S.; Vieira, L.G.M. Thermo-chemical behavior and product formation during pyrolysis of mango seed shell. Ind. Crops Prod. 2016, 85, 174–180. [Google Scholar] [CrossRef]
- Arora, K.; Kumar, P.; Bose, D.; Li, X.; Kulshrestha, S. Potential applications of algae in biochemical and bioenergy sector. 3 Biotech 2021, 11, 296. [Google Scholar] [CrossRef]
- Kanth, B.K.; Lee, K.Y.; Lee, G.J. Antioxidant and radical-scavenging activities of petal extracts of Camellia japonica ecotypes. Hortic. Environ. Biotechnol. 2014, 55, 335–341. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Xiao, Y.; Fu, N.L. Antioxidant capacities and total phenolic contents of 30 flowers. Ind. Crop. Prod. 2018, 111, 430–445. [Google Scholar] [CrossRef]
- Trinh, L.T.P.; Choi, Y.S.; Bae, H.J. Production of phenolic compounds and biosugars from flower resources via several extraction processes. Ind. Crop. Prod. 2018, 125, 261–268. [Google Scholar] [CrossRef]
- Li, A.N.; Li, S.; Li, H.B.; Xu, D.P.; Xu, X.R.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Foods 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Páscoa, R.N.M.J.; Teixeira, A.M.; Sousa, C. Antioxidant capacity of Camellia japonica cultivars assessed by near- and mid-infrared spectroscopy. Planta 2019, 249, 1053–1062. [Google Scholar] [CrossRef]
- Jang, E.B.; Ho, T.T.; Park, S.Y. Effect of light quality and tissue origin on phenolic compound accumulation and antioxidant activity in Camellia japonica calli. Vitr. Cell. Dev. Biol. Plant 2020, 56, 567–577. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Hwang, E.-J.; Kim, G.-H.; Choi, Y.-B.; Lim, C.-Y.; Kim, S.-M. Antifungal and Antioxidant Activities of Extracts from Leaves and Flowers of Camellia japonica L. Korean J. Med. Crop Sci. 2005, 13, 93–100. [Google Scholar]
- Roslan, A.S.; Ando, Y.; Azlan, A.; Ismail, A. Effect of glucose and ascorbic acid on total phenolic content estimation of green tea and commercial fruit juices by using Folin Ciocalteu and fast blue BB assays. Pertanika J. Trop. Agric. Sci. 2019, 42, 545–556. [Google Scholar]
- Wei, X.; Hou, Y.J.; Ye, Y.L.; Zhang, Q.Y.; Zheng, H.W.; Liang, G.L.; Tong, H.R. Determination of Carotenoid Contents in 31 Elite Chinese Tea [Camellia sinensis (L.) O. Kuntze] Cultivars. Acad. J. Agric. Res. 2016, 4, 525–533. [Google Scholar]
- Moon, S.H.; Kim, M.Y. Phytochemical profile, antioxidant, antimicrobial and antipancreatic lipase activities of fermented Camellia japonica L. leaf extracts. Trop. J. Pharm. Res. 2018, 17, 905–912. [Google Scholar] [CrossRef]
- Dorkbuakaew, N.; Ruengnet, P.; Pradmeeteekul, P.; Nimkamnerd, J.; Nantitanon, W.; Thitipramote, N. Bioactive compounds and antioxidant activities of Camellia sinensis var. assamica in different leave maturity from Northern Thailand. Int. Food Res. J. 2016, 23, 2291–2295. [Google Scholar]
- Phuoc Minh, N. Freeze-Drying (Lyophilization) of Yellow Camellia (Camellia chrysantha) Flower Bud. Biosci. Res. 2021, 18, 1604–1607. [Google Scholar]
- Fan, M.; Zhang, Y.; Yang, M.; Wu, S.; Yin, H.; Li, J.; Li, X. Transcriptomic and Chemical Analyses Reveal the Hub Regulators of Flower Color Variation from Camellia japonica Bud Sport. Horticulturae 2022, 8, 129. [Google Scholar] [CrossRef]
- Xiaowei, H.; Xiaobo, Z.; Jiewen, Z.; Jiyong, S.; Xiaolei, Z.; Holmes, M. Measurement of total anthocyanins content in flowering tea using near infrared spectroscopy combined with ant colony optimization models. Food Chem. 2014, 164, 536–543. [Google Scholar] [CrossRef]
- Fu, M.; Yang, X.; Zheng, J.; Wang, L.; Yang, X.; Tu, Y.; Ye, J.; Zhang, W.; Liao, Y.; Cheng, S.; et al. Unraveling the Regulatory Mechanism of Color Diversity in Camellia japonica Petals by Integrative Transcriptome and Metabolome Analysis. Front. Plant Sci. 2021, 12, 1119. [Google Scholar] [CrossRef]
- Žilić, S.; Serpen, A.; Akillioǧlu, G.; Gökmen, V.; Vančetović, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant activity and healthy benefits of natural pigments in fruits: A review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef]
- Bader Ul Ain, H.; Tufail, T.; Javed, M.; Tufail, T.; Arshad, M.U.; Hussain, M.; Gull Khan, S.; Bashir, S.; Al Jbawi, E.; Abdulaali Saewan, S. Phytochemical profile and pro-healthy properties of berries. Int. J. Food Prop. 2022, 25, 1714–1735. [Google Scholar] [CrossRef]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.C. Correlation study of antioxidant activity with phenolic and flavonoid compounds in 12 indonesian indigenous herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Frond, A.D.; Iuhas, C.I.; Stirbu, I.; Leopold, L.; Socaci, S.; Andreea, S.; Ayvaz, H.; Andreea, S.; Mihai, S.; Diaconeasa, Z.; et al. Phytochemical Characterization of Five Edible Purple-Reddish Vegetables: Anthocyanins, Flavonoids, and Phenolic Acid Derivatives. Molecules 2019, 24, 1536. [Google Scholar] [CrossRef] [PubMed]
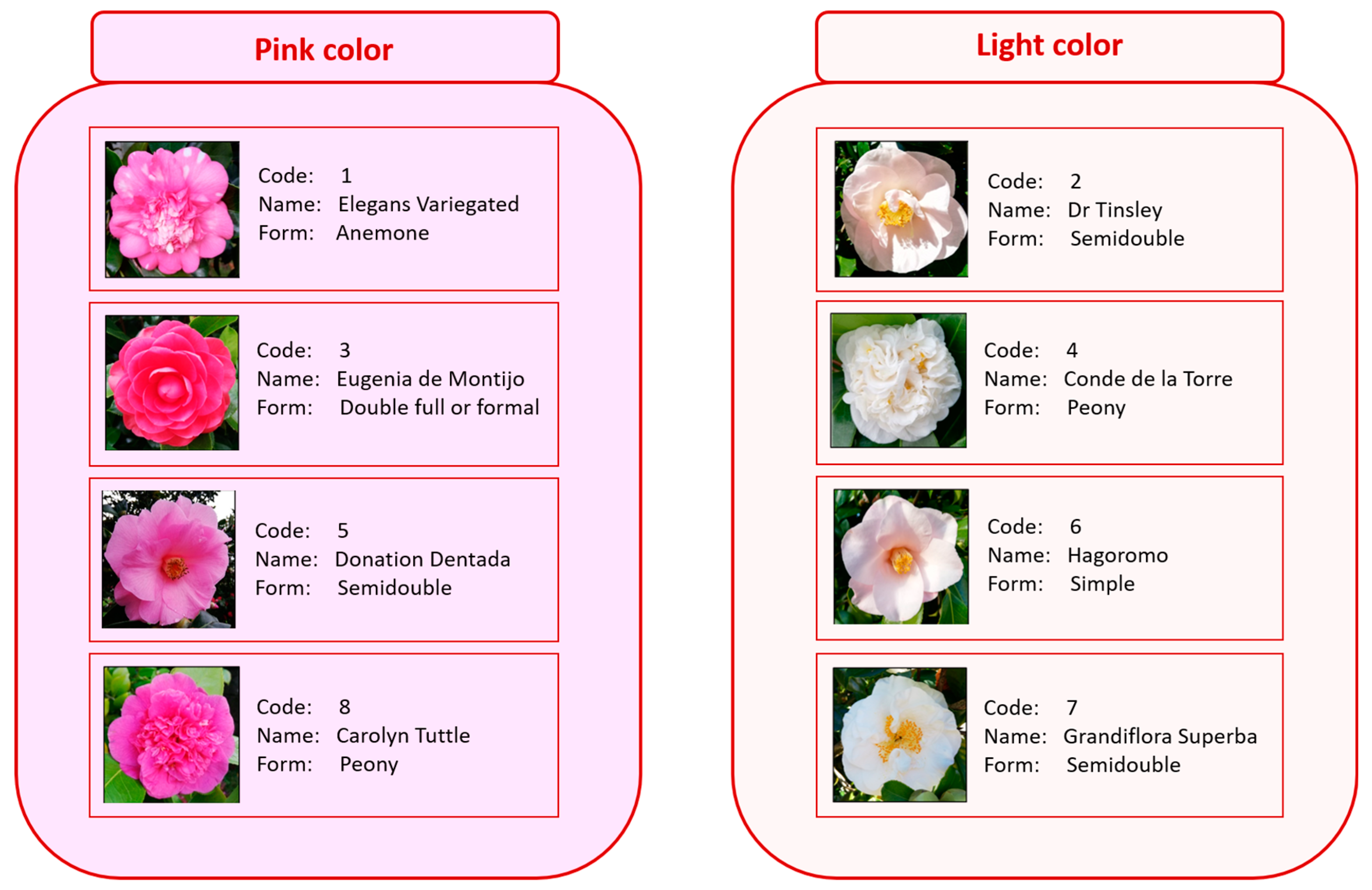
| Camellia | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| EV | DT | EM | CT | DD | HA | GS | TU | p-Value | |
| Nutritional composition (g/100 g dry weight) | |||||||||
| Protein | 6.3 ± 0.3 a | 4.4 ± 0.1 c | 4.7 ± 0.1 bc | 6 ± 1 abc | 5.7 ± 0.1 abc | 5.7 ± 0.5 abc | 6.1 ± 0.4 ab | 5.4 ± 0.4 abc | 9.93 × 10−3 |
| Lipids | 1.2 ± 0.01 c | 1.5 ± 0.1 ab | 1.2 ± 0.01 c | 1.3 ± 0.1 bc | 1.6 ± 0.1 a | 1.4 ± 0.1 abc | 0.9 ± 0.1 d | 1.2 ± 0.1 c | 9.02 × 10−7 |
| Fatty acid composition (% total lipids) | |||||||||
| Saturated FAs | 29.28 ± 1.1 | 34.38 ± 0.3 | 33.48 ± 13.8 | 25.62 ± 0.6 | 32.78 ± 0.6 | 26.08 ± 0.5 | 33.90 ± 2.3 | 24.39 ± 0.5 | 0.33 |
| C14:0 Myristic acid | 1.3 ± 0.2 ab | 1.2 ± 0.2 ab | 1.1 ± 0.3 ab | 0.79 ± 0.04 b | 1.33 ± 0.04 ab | 1.1 ± 0.1 ab | 1.7 ± 0.3 a | 0.72 ± 0.01 b | 0.01 |
| C16:0 Palmitic acid | 21.8 ± 0.1 | 22 ± 1 | 25 ± 10 | 19 ± 1 | 22 ± 1 | 16 ± 1 | 20.2 ± 0.4 | 19 ± 1 | 0.39 |
| C18:0 Stearic acid | 2.0 ± 0.4 | 2.4 ± 0.2 | 2 ± 1 | 1.8 ± 0.3 | 3.1 ± 0.3 | 2.2 ± 0.2 | 2.8 ± 0.4 | 1.7 ± 0.1 | 0.07 |
| C20:0 Arachidic acid | 1.4 ± 0.1 | 1.7 ± 0.1 | 2 ± 1 | 1.52 ± 0.01 | 2.0 ± 0.4 | 2.1 ± 0.3 | 2.5 ± 0.5 | 1.83 ± 0.03 | 0.62 |
| C22:0 Behenic acid | 2.8 ± 0.4 bc | 7.30 ± 0.02 a | 3 ± 1 bc | 2.4 ± 0.3 bc | 4 ± 1 bc | 5 ± 1 ab | 7 ± 1 a | 1.94± 0.04 c | 2.66 × 10−4 |
| Monounsaturated FAs | 4.3 ± 0.3 ab | 4.2 ± 0.3 ab | 4.1 ± 0.3 ab | 3.8 ± 0.2 b | 4.7 ± 0.1 a | 4.2 ± 0.2 ab | 3.9 ± 0.1 a | 4.6 ± 0.1 ab | 0.03 |
| C20:1 cis-11-Eicosenoic acid | 1.6 ± 0.1 | 1.4 ± 0.1 | 1.7 ± 0.4 | 1.4 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.1 | nd | 1.89 a ± 0.03 | 0.24 |
| C18:1 cis-Oleic acid | 2.6 ± 0.4 | 2.8 ± 0.3 | 2 ± 1 | 2.4 ± 0.2 | 3.0 ± 0.2 | 2.4 ± 0.3 | 4 ± 1 | 2.7 ± 0.2 | 0.16 |
| Polyunsaturated FAs | 66.5 ± 1.4 | 61.0 ± 0.1 | 62.4 ± 14.2 | 70.6 ± 0.4 | 62.5 ± 0.7 | 69.8 ± 0.7 | 61.5 ± 2.2 | 71.1 ± 0.6 | 0.33 |
| C18:2 cis-Linoleic acid (ω-6) | 45 ± 1 | 44.3 ± 0.3 | 48 ± 9 | 51.8 ± 0.2 | 45.95 ± 0.04 | 46 ± 1 | 46 ± 2 | 52.93 a ± 0.04 | 0.19 |
| C18:3 Linolenic acid (ω-3) | 19.60 ± 0.03 ab | 16.7 ± 0.4 ab | 13 ± 5 b | 18.0 ± 0.5 ab | 17 ± 1 ab | 22.7 ± 0.1 a | 15.9 ± 0.5 ab | 16.82 ± 0.56 ab | 0.02 |
| C20:2 cis-11,14-Eicosadienoic acid (ω-6) | 1.62 ± 0.02 a | nd | 1.15 ± 0.10 ab | 0.9 ± 0.1 ab | nd | 1.13± 0.04 ab | nd | 1.31 ± 0.01 a | 4.64 × 10−4 |
| PUFAs ω6 | 46.9 ± 1.3 | 44.8 ± 0.3 | 49.5 ± 9.1 | 52.6 ± 0.1 | 46.0 ± 0.1 | 47.0 ± 0.9 | 45.6 ± 1.7 | 54.2 ± 0.1 | 0.15 |
| PUFAs ω3 | 19.6 ± 0.1 ab | 16.7 ± 0.4 ab | 12.9 ± 5.1 b | 17.9 ± 0.5 ab | 16.6 ± 0.7 ab | 22.7 ± 0.2 a | 15.9 ± 0.5 ab | 16.8 ± 0.6 ab | 0.02 |
| Ratio ω6/ω3 | 2.4 ± 0.1 b | 2.7 ± 0.1 b | 3.8 ± 0.9 a | 2.9 ± 0.1 ab | 2.8 ± 0.1 ab | 2.1 ± 0.1 b | 2.9 ± 0.1 ab | 3.2 ± 0.1 ab | 8.52 × 10−3 |
| OB | TV | SO | PO | MI | LE | PA | CJ | |
|---|---|---|---|---|---|---|---|---|
| Nutritional composition (%) | ||||||||
| Proteins | 22.53 | 15.38 | 9.56 | 1.57 | 0.82 | 0.89 | 20.8 | 4.4–6.3 |
| Lipids | 1.51 | 2.09 | 3.15 | 0.157 | 1.36 | 0.35 | 58.9 | 0.9–1.6 |
| Fatty acid composition (% total lipids) | ||||||||
| Saturated FAs | 40.52 | 43.86 | 38.79 | 27.58 | 79.74 | 15.10 | 11.11 | 24.39–34.38 |
| C14:0 Myristic acid | 5.20 | 21.41 | 0.43 | 0.73 | nd | nd | nd | 0.72–1.7 |
| C16:0 Palmitic acid | 22.98 | 17.12 | 21.38 | 9.81 | 64.42 | 10.3 | 9.3 | 16–25 |
| C18:0 Stearic acid | 8.16 | 3.06 | 4.10 | 2.52 | 9.55 | 1.6 | 1.8 | 1.7–2.8 |
| C20:0 Arachidic acid | nd | nd | nd | nd | 0.63 | nd | nd | 1.4–2.1 |
| C22:0 Behenic acid | nd | nd | nd | nd | nd | nd | nd | 1.94–7.30 |
| Monounsaturated FAs | 21.99 | 11.98 | 19.70 | 5.89 | 18.62 | 2.9 | 70.0 | 3.8–4.7 |
| C20:1 cis-11-Eicosenoic acid | nd | nd | nd | nd | nd | nd | nd | nd–1.89 |
| C18:1 cis-Oleic acid | 17.85 | 7.54 | 12.65 | 5.29 | 9.01 | 2.3 | 69.7 | 2.0–4.0 |
| Polyunsaturated FAs | 36.57 | 43.19 | 40.96 | 66.53 | 1.65 | 82.0 | 18.9 | 61.0–71.1 |
| C18:2 cis-Linoleic acid (ω-6) | 17.36 | 12.62 | 11.40 | 11.40 | 1.65 | 81.1 | 18.2 | 44.3–52.93 |
| C18:3 Linolenic acid (ω-3) | 15.95 | 27.96 | 12.61 | 54.92 | 0.1 | 0.7 | 13.0–22.7 | |
| C20:2 cis-11,14-Eicosadienoic acid (ω-6) | nd | 0.17 | 3.21 | nd | nd | nd | nd | nd–1.62 |
| Macroelemental composition of the studied camellias (mg/kg). | ||||||||
| Ca | nd | nd | nd | 43.55 | 11.0 | 6.26 | 0.23 | 1482.0–2544.5 |
| K | nd | nd | nd | 33.05 | 168.0 | 50.82 | nd | 6557.0–10,422.0 |
| Mg | nd | nd | nd | nd | 10.00 | 10.46 | nd | 730.0–1172.0 |
| Na | nd | nd | nd | 14.44 | 1.0 | nd | nd | 221.0–419.5 |
| P | nd | nd | nd | nd | 14.0 | 228.5 | 0.49 | 1091.0–1566.0 |
| Microelemental composition of the studied camellias (mg/kg). | ||||||||
| Fe | 624.51 | 690.05 | 732.72 | 4.44 | 0.16 | nd | 3.5 | 13.59–33.49 |
| Mn | 78.46 | 96.11 | 68.92 | nd | nd | nd | nd | 34.67–214.21 |
| Zn | 54.63 | 31.73 | 38.87 | 2.08 | 0.09 | nd | nd | 4.57–9.58 |
| Cu | 27.69 | 7.41 | 7.89 | nd | 0.04 | nd | nd | 3.54–6.24 |
| Se | nd | nd | nd | nd | 0.6 | nd | nd | 0.11–0.17 |
| Reference | [44] | [44] | [44] | [45,46] | [47,48] | [49,50] | [51,52] | This study |
| Camellia | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| EV | DT | EM | CT | DD | HA | GS | TU | p-Value | |
| Macroelemental composition of the studied camellias (mg/kg). | |||||||||
| Ca | 1693.0 ± 145.7 de | 2295.5 ± 47.4 b | 1482.0 ± 21.2 e | 2067.5 ± 5.0 bc | 1852.5 ± 17.7 c | 2544.5 ± 44.6 a | 1633.5 ± 31.8 de | 1822.5 ± 0.7 d | 1.17 × 10−6 |
| K | 9968.5 ± 27.6 ab | 9056.5 ± 61.5 bc | 7570.5 ± 279.3 de | 10,322.5 ± 726.2 a | 7985.0 ± 297.0 cd | 9646.5 ± 64.4 ab | 10,422.0 ± 116.0 a | 6557.0 ± 76.4 e | 8.56 × 10−6 |
| Mg | 994.5 ± 7.8 bc | 1156.0 ± 14.1 a | 730.0 ± 33.9 d | 1017.5 ± 46.0 b | 904.0 ± 5.7 c | 1020.5 ± 40.3 b | 1172.0 ± 9.9 a | 934.0 ± 33.9 bc | 5.49 × 10−6 |
| Na | 259.5± 5.0 cd | 419.5 ± 23.2 a | 415.0 ± 35.4 ab | 337.0 ± 4.2 abc | 221.0 ± 42.4 d | 312.5 ± 26.2 bcd | 286.5 ± 6.4 cd | 250.5 ± 31.8 cd | 3.80 × 10−4 |
| P | 1566.0 ± 36.8 a | 1219.5 ± 27.6 cd | 1145.0 ± 28.3 d | 1403.0 ± 33.4 b | 1463.5 ± 58.7 ab | 1536.5 ± 20.5 a | 1347.0 ± 26.9 bc | 1091.0 ± 1.4 d | 3.13 × 10−6 |
| Microelemental composition of the studied camellias (mg/kg). | |||||||||
| Fe | 19.87 ± 2.32 ab | 21.76 ± 0.48 ab | 13.59 ± 0.57 b | 18.57 ± 1.49 ab | 19.10 ± 0.93 ab | 26.26 ± 12.32 ab | 33.49 ± 3.25 a | 15.89 ± 0.57 ab | 0.04 |
| Mn | 40.92 ± 1.72 e | 175.73 ± 7.65 b | 43.65 ± 0.08 e | 102.34 ± 1.90 d | 152.655 ± 8.75 c | 34.67 ± 1.74 e | 146.20 ± 0.33 c | 214.21 ± 6.36 a | 1.41 × 10−9 |
| Zn | 4.57 ± 0.30 e | 9.58 ± 0.16 a | 5.15 ± 0.16 de | 5.85 ± 0.45 cd | 8.33 ± 0.49 b | 8.52 ± 0.01 ab | 6.62 ± 0.08 c | 5.67 ± 0.08 cd | 6.37 × 10−7 |
| Cu | 5.91 ± 0.11 a | 3.92 ± 0.03 b | 3.72 ± 0.17 b | 5.39 ± 0.39 a | 4.14 ± 0.18 b | 6.24 ± 0.35 a | 5.53 ± 0.18 a | 3.54 ± 0.11 b | 7.09 × 10−6 |
| Se | 0.13 ± 0.06 | 0.12 ±0.01 | 0.12 ± 0.04 | 0.17 ± 0.04 | 0.12 ± 0.05 | 0.15 ± 0.04 | 0.11 ± 0.05 | 0.14 ± 0.03 | 0.91 |
| Thermal analysis (%) | |||||||||
| Moisture | 3.82 ± 0.51 b | 5.55 ± 0.33 b | 5.96 ± 2.22 b | 4.78 ± 0.40 b | 4.59 ± 0.09 b | 4.12 ± 0.37 b | 9.91 ± 0.23 a | 5.45 ± 0.44 b | 2.08 × 10−3 |
| Ash | 1.75 ± 1.63 | 2.60 ± 0.01 | 3.80 ± 1.56 | 2.90 ± 0.42 | 2.35 ± 0.92 | 2.50 ± 0.28 | 3.30 ± 0.71 | 2.00 ± 0.42 | 0.47 |
| Volatile | 73.53 ± 2.50 a | 70.97 ± 0.44 a | 69.88 ± 0.50 a | 71.11 ± 0.20 a | 70.77 ± 0.51 a | 72.83 ± 0.11 a | 65.03 ± 0.38 b | 72.79 ± 0.03 a | 3.73 × 10−4 |
| Fixed C | 20.91 ± 0.32 bc | 20.65 ± 0.39 bc | 20.54 ± 0.03 bc | 20.27 ± 0.27 c | 22.33 ± 0.28 a | 20.49 ± 0.67 bc | 21.75 ± 0.09 ab | 19.74 ± 0.07 c | 9.39 × 10−4 |
| Elemental composition (%) | |||||||||
| N | 1.02 ± 0.05 a | 0.71 ± 0.02 c | 0.75 ± 0.02 bc | 0.93 ± 0.10 abc | 0.92 ± 0.02 abc | 0.92 ± 0.09 abc | 0.98 ± 0.06 ab | 0.86 ± 0.07 abc | 0.01 |
| C | 44.09 ± 0.29 bc | 44.22 ± 0.20 b | 43.18 ± 0.14 cd | 44.10 ± 0.09 bc | 45.78 ± 0.43a | 44.81 ± 0.02 b | 42.27 ± 0.28 d | 43.98 ± 0.23 bc | 1.83 × 10−5 |
| H | 6.39 ± 0.04 | 6.45 ± 0.17 | 6.34 ± 0.19 | 6.47 ± 0.28 | 6.35 ± 0.64 | 6.49 ± 0.08 | 6.25 ± 0.04 | 6.55 ± 0.10 | 0.95 |
| O | 48.50 ± 0.30 bc | 48.62 ± 0.38 bc | 49.74 ± 0.35 ab | 48.51 ± 0.08 bc | 46.97 ± 1.06c | 47.78 ± 0.01c | 50.52 ± 0.18 a | 48.61 ± 0.40 bc | 1.38 × 10−3 |
| HHV(MJ/kg) | 21.32 | 21.49 | 21.26 | 21.17 | 21.61 | 21.18 | 22.33 | 21.36 | |
| Camellia | YIELD | TPC | TCC | TFC | TAC |
|---|---|---|---|---|---|
| (%) | (mg GAE/g DW) | (µg C/g DW) | (µg Q/g DW) | (µg cyd/g DW) | |
| EV | 61.23 ± 0.80 ab | 95.73 ± 20.41 ab | 33.35 ± 1.89 d | 782.12 ± 174.69 bc | 991.96 ± 25.94 c |
| DT | 58.96 ± 2.01 bc | 104.79 ± 22.41 a | 26.03 ± 3.49 ef | 703.98 ± 132.7 bc | 20.57 ± 6.60 e |
| EM | 61.87 ± 0.96 ab | 96.57 ± 22.22 ab | 73.21 ± 4.75 a | 656.43 ± 151.15 bc | 1820.45 ± 147.96 a |
| CT | 57.00 ± 2.40 cd | 108.64 ± 31.39 a | 49.44 ± 1.13 c | 796.45 ± 296.97 abc | nd |
| DD | 55.14 ± 0.41 d | 95.63 ± 30.12 ab | 30.71 ± 0.62 de | 1081.44 ± 153.03 a | 400.61 ± 10.06 d |
| HA | 62.02 ± 2.28 ab | 103.13 ± 19.38 a | 24.80 ± 1.59 f | 855.00 ± 239.48 abc | 23.00 ± 4.98 e |
| GS | 62.68 ± 2.05 a | 93.35 ± 11.16 ab | 28.85 ± 2.00 def | 566.17 ± 78.82 c | nd |
| TU | 61.61 ± 0.79 ab | 78.45 ± 13.00 b | 63.90 ± 1.87 b | 892.96 ± 257.13 ab | 1230.53 ± 45.44 b |
| p-value | 4.01 × 10−8 | 3.44 × 10−3 | 2.00 × 10−16 | 8.89 × 10−8 | 1.06 × 10−18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.G.; Cassani, L.; Liu, C.; Li, N.; Chamorro, F.; Barreira, J.C.M.; Simal-Gandara, J.; Prieto, M.A. Camellia japonica Flowers as a Source of Nutritional and Bioactive Compounds. Foods 2023, 12, 2825. https://doi.org/10.3390/foods12152825
Pereira AG, Cassani L, Liu C, Li N, Chamorro F, Barreira JCM, Simal-Gandara J, Prieto MA. Camellia japonica Flowers as a Source of Nutritional and Bioactive Compounds. Foods. 2023; 12(15):2825. https://doi.org/10.3390/foods12152825
Chicago/Turabian StylePereira, Antia G., Lucia Cassani, Chao Liu, Ningyang Li, Franklin Chamorro, João C. M. Barreira, Jesus Simal-Gandara, and Miguel A. Prieto. 2023. "Camellia japonica Flowers as a Source of Nutritional and Bioactive Compounds" Foods 12, no. 15: 2825. https://doi.org/10.3390/foods12152825
APA StylePereira, A. G., Cassani, L., Liu, C., Li, N., Chamorro, F., Barreira, J. C. M., Simal-Gandara, J., & Prieto, M. A. (2023). Camellia japonica Flowers as a Source of Nutritional and Bioactive Compounds. Foods, 12(15), 2825. https://doi.org/10.3390/foods12152825












