Development of a Magnetic Molecularly Imprinted Microsphere-Based Signal Amplified Semi-Homogeneous Method for Multidetection of Five Progestins in Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Fe3O4@MIM
2.3. Synthesis of Enzyme Labeled Conjugate
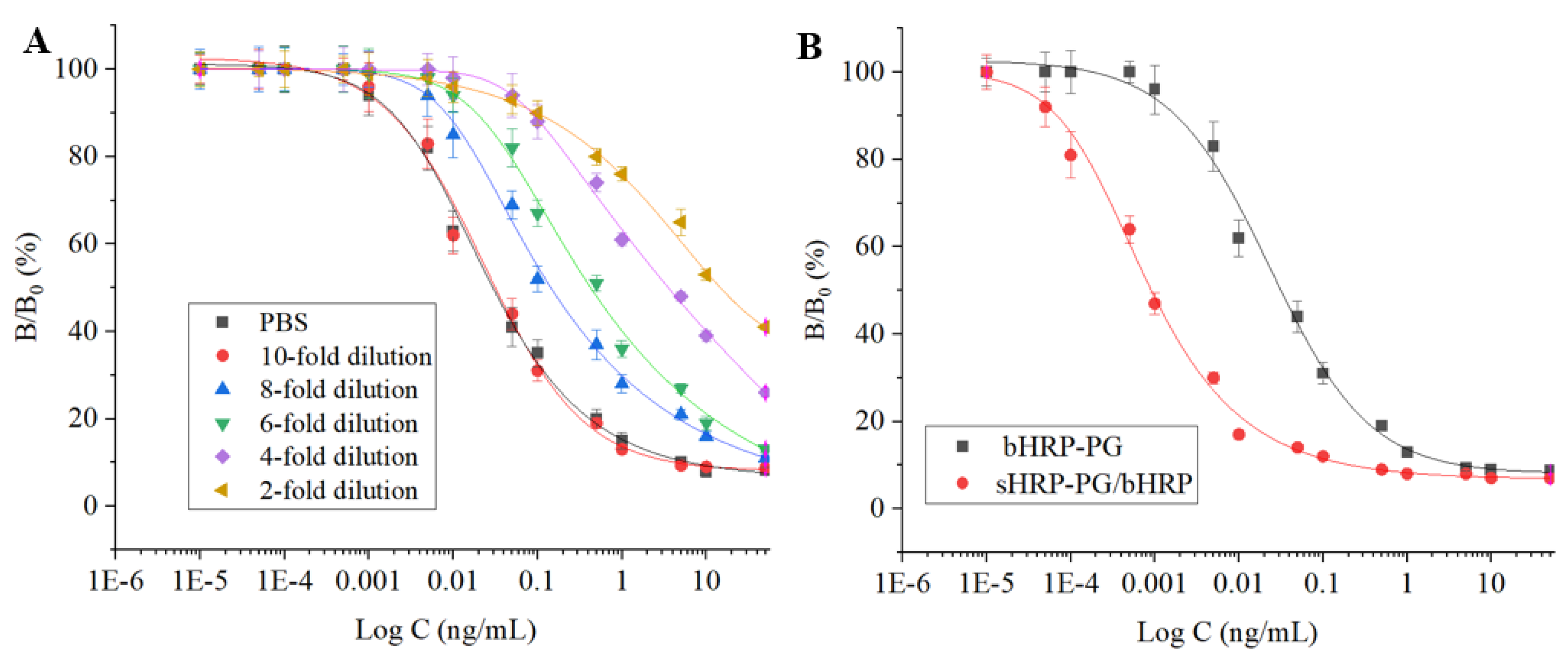
2.4. Development of Semi-Homogeneous Method
2.5. Sample Preparation and Method Application
3. Results and Discussions
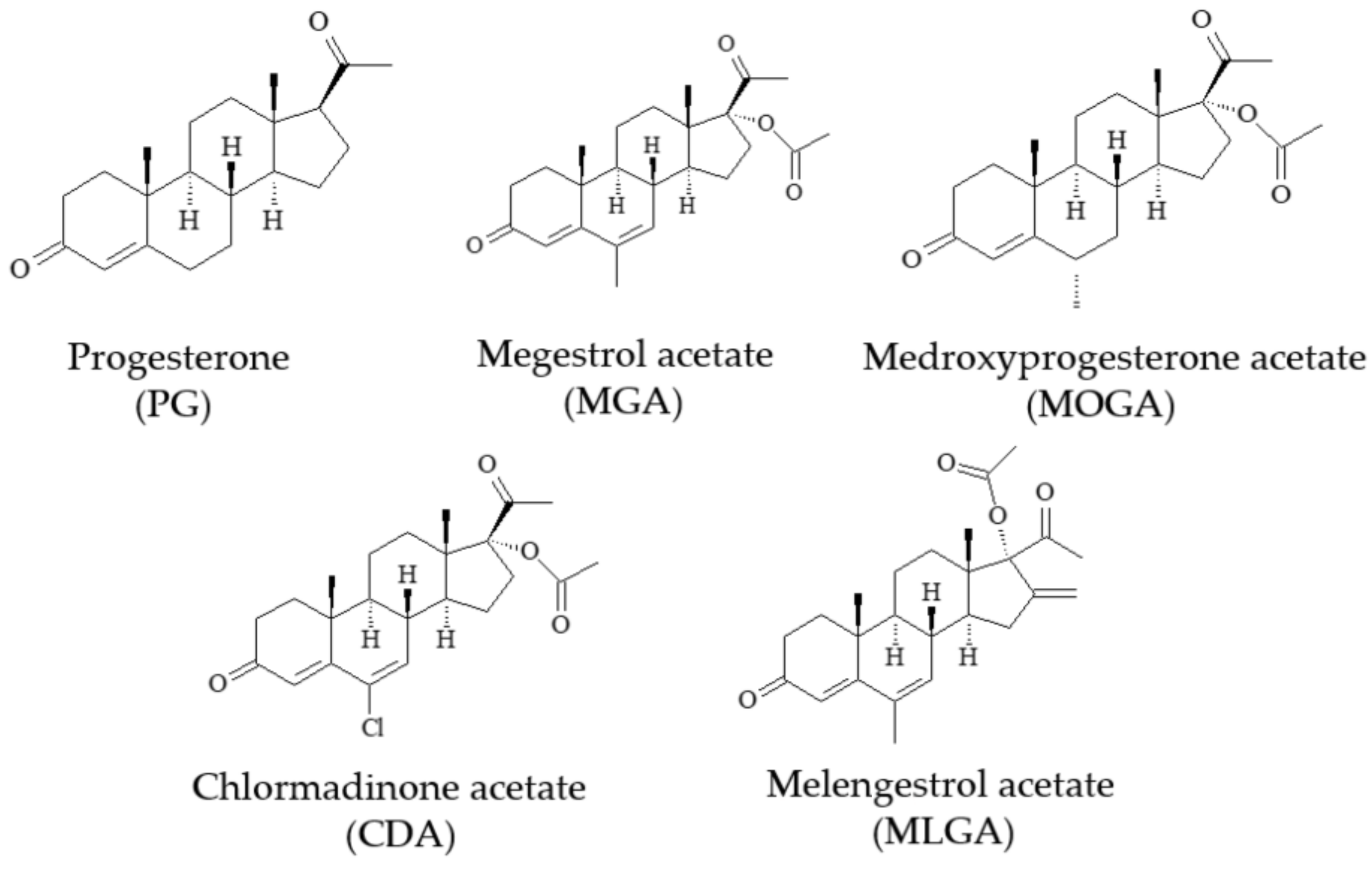
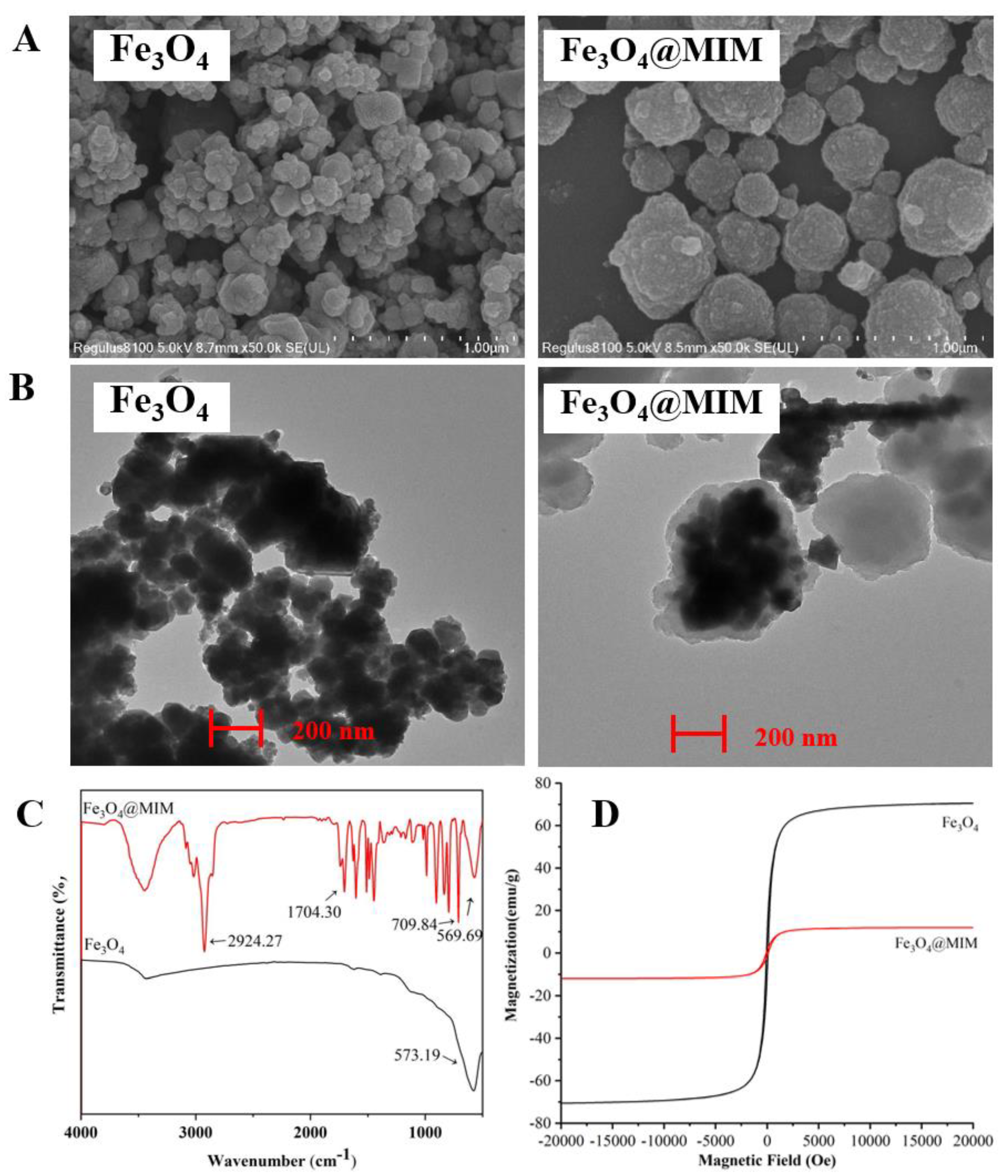
3.1. Fe3O4@MIM
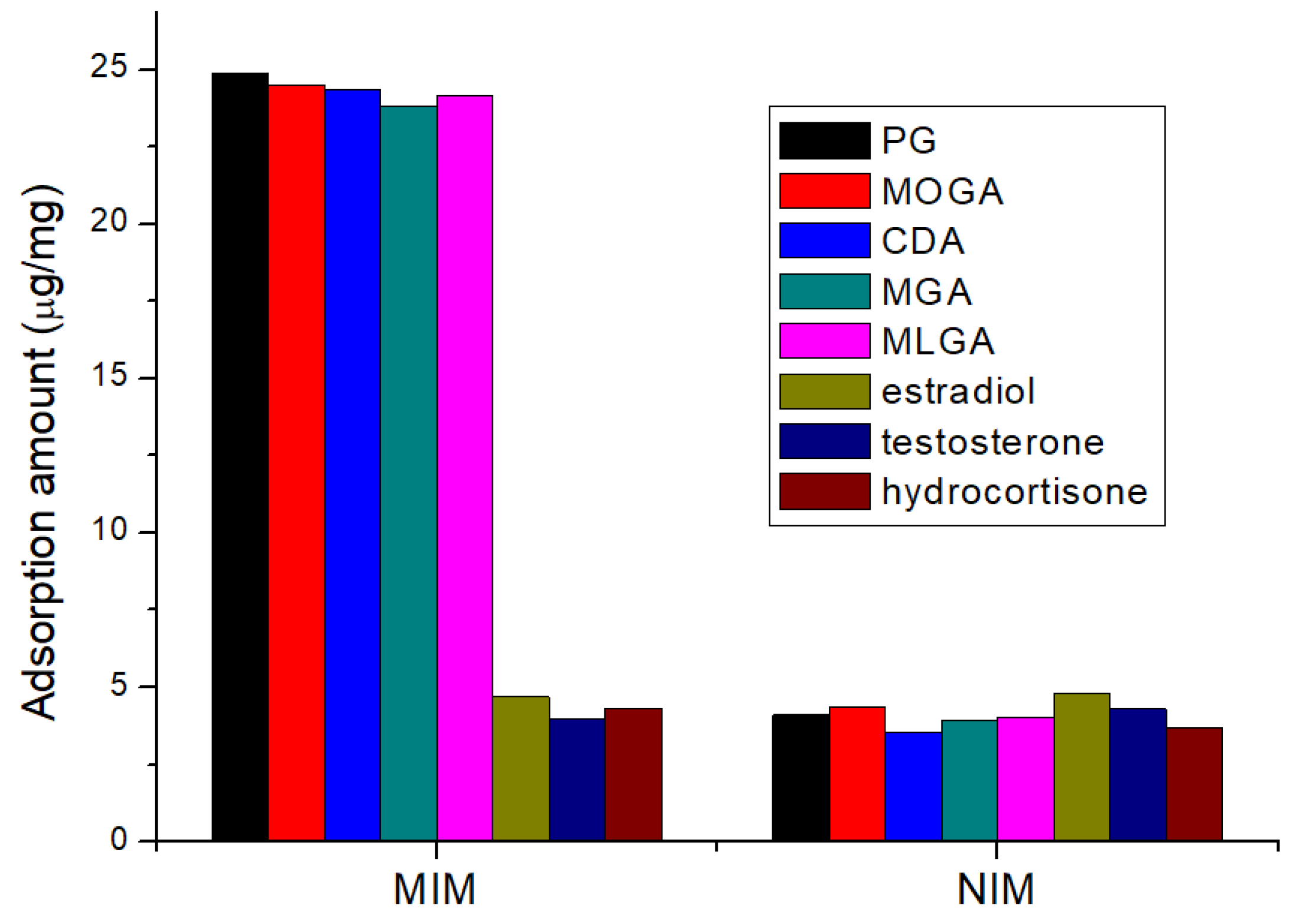
3.2. Selective Absorption Ability of Fe3O4@MIM
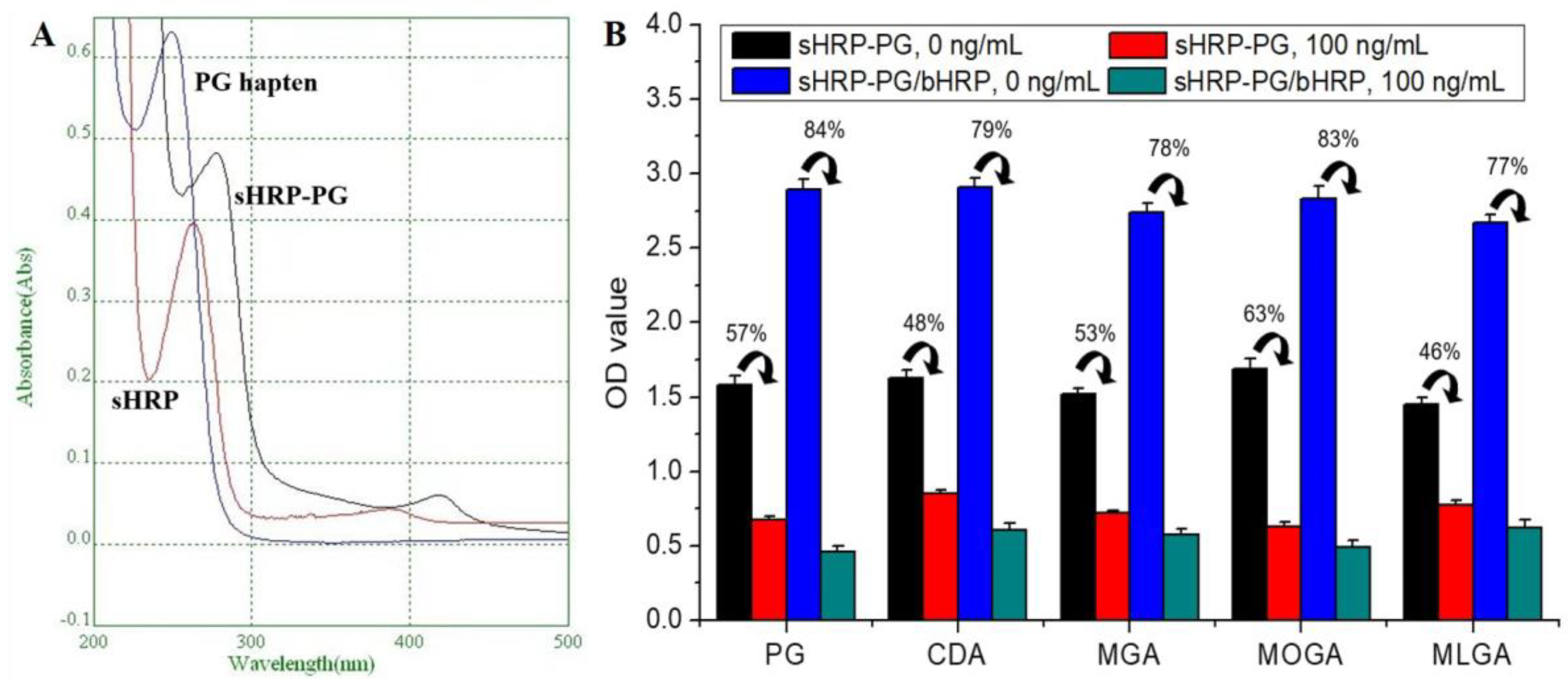
3.3. Characterization of Enzyme Labeled Conjugate
3.4. Evaluation of the Two Signal Systems
3.5. Optimization of Operation Parameters
3.6. Method Performances
3.7. Method Application
3.8. Comparison with Immunoassays and MIP-Based Methods for Progestins
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adebamowo, C.A.; Spiegelman, D.; Danby, F.W.; Frazier, A.L.; Willett, W.C.; Holmes, M.D. High school dietary dairy intake and teenage acne. J. Am. Acad. Dermatol. 2005, 52, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Venkatram, R.; Singhal, R.S. Recent advances in the application of molecularly imprinted polymers (MIPs) in food analysis. Food Control 2022, 139, 109074. [Google Scholar] [CrossRef]
- Cai, Y.; He, X.; Cui, P.L.; Liu, J.; Li, Z.B.; Jia, B.J.; Zhang, T.; Wang, J.P.; Yuan, W.Z. Preparation of a chemiluminescence sensor for multi-detection of benzimidazoles in meat based on molecularly imprinted polymer. Food Chem. 2019, 280, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; He, X.; Cui, P.L.; Yuan, W.Z.; Wang, J.P.; Liu, J. Molecularly imprinted microspheres based multiplexed fluorescence method for simultaneous detection of benzimidazoles and pyrethroids in meat samples. Food Chem. 2020, 319, 126539. [Google Scholar] [CrossRef]
- Chen, C.; Luo, J.; Li, C.; Ma, M.; Yu, W.; Shen, J.; Wang, Z. Molecularly imprinted polymer as an antibody substitutionin pseudo-immunoassays for chemical contaminants infood and environmental samples. J. Agric. Food Chem. 2018, 66, 2561–2571. [Google Scholar] [CrossRef]
- Comin, A.; Renaville, B.; Marchini, E.; Maiero, S.; Cairoli, F.; Prandi, A. Technical note: Direct enzyme immunoassay of progesterone in bovine milk whey. J. Dairy Sci. 2005, 88, 4239–4242. [Google Scholar] [CrossRef]
- Daems, D.; Lu, J.; Delport, F.; Marien, N.; Orbie, L.; Aernouts, B.; Adriaens, I.; Huybrechts, T.; Saeys, W.; Spasic, D.; et al. Competitive inhibition assay for the detection of progesterone in dairy milk using a fiber optic SPR biosensor. Anal. Chim. Acta 2017, 950, 1–6. [Google Scholar] [CrossRef]
- Ganmaa, D.; Sato, A. The possible role of female sex hormones in milk from pregnant cows in the development of breast, ovarian and corpus uteri cancers. Med. Hypotheses 2005, 65, 1028–1037. [Google Scholar] [CrossRef]
- Gillis, E.H.; Gosling, J.P.; Sreenan, J.M.; Kane, M. Development and validation of a biosensor-based immunoassay for progesterone in bovine milk. J. Immunol. Methods 2002, 267, 131–138. [Google Scholar] [CrossRef]
- Hageleit, M.; Daxenberger, A.; Meyer, H.H.D. A sensitive enzyme immunoassay (EIA) for the determination of melengestrol acetate (MGA) in adipose and muscle tissues. Food Addit. Contam. A 2001, 18, 285–291. [Google Scholar] [CrossRef]
- Ivanova, T.; Jevargova, T.G.; Dimova, N. Magnetic nanoparticle-based fluorescent immunoassay for determination of progesterone in milk. Int. J. Dairy Technol. 2018, 71, 309–320. [Google Scholar] [CrossRef]
- Kadhem, A.J.; Gentile, G.J.; Fidalgo de Cortalezzi, M.M. Molecularly imprinted polymers (MIPs) in sensors for environmental and biomedical applications: A review. Molecules 2021, 26, 6233. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; O’Hare, D.; Guo, H.Z.; Yang, C.H.; Lin, H.Y. Electrochemical sensing of urinary progesterone with molecularly imprinted poly(aniline-co-metanilic acid)s. J. Mater. Chem. B 2016, 4, 3782–3787. [Google Scholar] [CrossRef]
- GB 31650-2019; National Food Safety Standard—Maximum Residue Limits for Veterinary Drugs in Foods. Ministry of Agriculture of China: Beijing, China, 6 September 2019.
- Ministry of Agriculture of China. Regulation No. 250 of Ministry of Agriculture, Peoples Republic of China; Ministry of Agriculture of China: Beijing, China, 2020.
- Nawaz, T.; Ahmad, M.; Yu, J.Y.; Wang, S.Q.; Wei, T. Biomimetic detection of progesterone by novel bifunctional group monomer based molecularly imprinted polymer prepared in UV light. New J. Chem. 2020, 44, 6992–7000. [Google Scholar] [CrossRef]
- Peng, D.; Li, Z.; Wang, Y.; Liu, Z.; Sheng, F.; Yuan, Z. Enzyme-linked immunoassay based on imprinted microspheres for the detection of sulfamethazine residue. J. Chromatogr. A 2017, 1506, 9–17. [Google Scholar] [CrossRef]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Development of a competitive lateral flow immunoassay for progesterone: Influence of coating conjugates and buffer components. Anal. Bioanal. Chem. 2008, 392, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.; Hsu, S.Y.; Rossi, E.; Salahshoor, Z.; Lin, C.H.; Parada, L.P.; Fidalgo, M. Detection of progesterone in aqueous samples by molecularly imprinted photonic polymers. Microchim. Acta 2022, 189, 174. [Google Scholar] [CrossRef]
- Ren, S.; Wang, X.; Lin, Z.; Li, Z.; Ying, X.; Chen, G.; Lin, J.M. Development of a high-throughput, indirect antibody immobilization format chemiluminescence enzyme immunoassay (CLEIA) for the determination of progesterone in human serum. Luminescence 2008, 23, 175–181. [Google Scholar] [CrossRef]
- Samsonova, J.V.; Safronova, V.A.; Osipov, A.P. Rapid flow-through enzyme immunoassay of progesterone in whole cows’ milk. Anal. Biochem. 2018, 545, 43–48. [Google Scholar] [CrossRef]
- Samsonova, J.V.; Safronova, V.A.; Osipov, A.P. Pretreatment-free lateral flow enzyme immunoassay for progesterone detection in whole cows’ milk. Talanta 2015, 132, 685–689. [Google Scholar] [CrossRef]
- Scarth, J.P.; Kay, J.; Teale, P.; Akre, C.; Le Bizec, B.; De Brabander, H.F.; Vanhaecke, L.; Van Ginkelf, L.; Points, J. A review of analytical strategies for the detection of ‘endogenous’ steroid abuse in food production. Drug Test. Anal. 2012, 4, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Schenk, J.A.; Gajovic-Eichelmann, N.; Sellrie, F.; Bier, F.F. A new one-step antigen heterologous homogeneous fluorescence immunoassay for progesterone detection in serum. Talanta 2015, 134, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Trapiella-Alfonso, L.; Costa-Fernández, J.M.; Pereiro, R.; Sanz-Medel, A. Development of a quantum dot-based fluorescent immunoassay for progesterone determination in bovine milk. Biosens. Bioelectron. 2011, 26, 4753–4759. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Wang, J.P.; Wu, N.P. Determination of 35 sulfonamides in pork by magnetic molecularly imprinted polymer based dispersive solid phase extraction and ultra performance liquid chromatography photodiode array method. J. Sci. Food Agric. 2023, 103, 1954–1963. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhang, Z.; Wu, C.; Han, L.; Zhang, H. Molecularly imprinted polymer grafted paper-based method for the detection of 2017, 17β-estradiol. Food Chem. 2017, 221, 82–86. [Google Scholar] [CrossRef]
- Xu, C.; Yang, W.; Xia, C.; Wu, L.; Zhang, H. Development of a competitive lateral flow immunoassay for progesterone in dairy cows’ milk. Med. Weter. 2016, 72, 494–497. [Google Scholar] [CrossRef][Green Version]
- Yadav, S.K.; Chandra, P.; Goyal, R.N.; Shim, Y. A review on determination of steroids in biological samples exploiting nanobio-electroanalytical methods. Anal. Chim. Acta 2013, 762, 14–24. [Google Scholar] [CrossRef]
- Ye, F.; Liu, J.; Huang, Y.; Li, S.; Zhao, S. Competitive immunoassay of progesterone by microchip electrophoresis with chemiluminescence detection. J. Chromatogr. B 2013, 936, 74–79. [Google Scholar] [CrossRef]
- Yu, J.Y.; Jiao, S.Q.; Nawaz, T.; Wang, S.Q.; Wei, T.X. Surface plasmone resonance sensor for biomimetic detection of progesterone with macroporous molecularly imprinted polymers prepared by visible light. IOP Conf. Ser. Mater. Sci. Eng. 2019, 688, 033032. [Google Scholar] [CrossRef]


| Analyte | sHRP-PG | sHRP-PG/bHPR | IC10 Improvement Fold | ||
|---|---|---|---|---|---|
| IC50 (pg/mL) | IC10 (pg/mL) | IC50 (pg/mL) | IC10 (pg/mL) | ||
| PG | 24 | 3 | 0.9 | 0.05 | 60 |
| CDA | 35 | 4 | 1.1 | 0.09 | 44 |
| MGA | 22 | 3 | 0.8 | 0.04 | 75 |
| MOGA | 47 | 5 | 1.5 | 0.08 | 63 |
| MLGA | 52 | 6 | 1.8 | 0.1 | 60 |
| Analyte | Added (ng/mL) | Intra-Day | Inter-Day | ||
|---|---|---|---|---|---|
| Recovery (%) | CV (%) | Recovery (%) | CV (%) | ||
| PG | 0.1 | 65.4 | 7.3 | 69.4 | 9.3 |
| 1.0 | 76.2 | 8.2 | 83.2 | 8.5 | |
| 10 | 79.3 | 6.4 | 80.4 | 10.6 | |
| CDA | 0.1 | 70.6 | 8.2 | 75.3 | 9.7 |
| 1.0 | 83.4 | 6.7 | 79.5 | 13.4 | |
| 10 | 81.0 | 6.1 | 71.3 | 10.6 | |
| MGA | 0.1 | 67.4 | 8.8 | 74.3 | 11.8 |
| 1.0 | 74.2 | 8.2 | 79.6 | 8.9 | |
| 10 | 79.3 | 8.4 | 85.0 | 7.3 | |
| MOGA | 0.1 | 69.5 | 9.4 | 71.5 | 14.2 |
| 1.0 | 82.5 | 7.3 | 83.2 | 10.3 | |
| 10 | 85.3 | 7.0 | 87.3 | 8.8 | |
| MLGA | 0.1 | 72.5 | 8.2 | 75.3 | 11.2 |
| 1.0 | 81.5 | 8.5 | 77.8 | 12.4 | |
| 10 | 88.2 | 8.4 | 70.6 | 10.7 | |
| Recognition Reagent | Method | Analyte | Assay Time (from Addition of Sample) | LOD (ng/g) | Sample | Reusable? | Ref. |
|---|---|---|---|---|---|---|---|
| pAb | ELISA | PG | Overnight | 0.0015 | milk | No | [7] |
| pAb | ELISA | MLGA | Overnight | 0.1 | beef | No | [8] |
| pAb | chemiluminescence immunoassay | PG | 90 min | 0.06 | human serum | No | [9] |
| mAb | fluorescence immunoassay | PG | 13 min | 5.0 | human serum | No | [10] |
| mAb | fluorescence immunoassay | PG | 12 min | 0.065 | milk | No | [11] |
| mAb | fluorescence immunoassay | PG | -- | 0.1 | milk | No | [12] |
| mAb | surface plasmon resonance sensor | PG | 5 min | 3.56 | milk | Yes | [17] |
| mAb | chemiluminescence sensor | PG | 35 min | 1.2 | human serum | Yes | [18] |
| mAb | surface plasmon resonance sensor | PG | 45 min | 0.5 | milk | Yes | [19] |
| MIP film | electrochemical sensor | PG | 30 min | 0.001 | human urine | Yes | [27] |
| MIP film | surface plasmon resonance sensor | PG | 16 min | 3.1 × 10−6 | water | Yes | [28] |
| MIP film | surface plasmon resonance sensor | PG | 20 min | 0.9 × 10−9 | water | Yes | [29] |
| MIP film | optical sensor | PG | 12 min | 0.5 | water | Yes | [30] |
| Fe3O4@MIM | semi-homogeneous method | 5 drugs | 20 min | 0.0004–0.001 | milk | Yes | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Liu, G.; Hou, H.; Peng, Y.; Wang, J. Development of a Magnetic Molecularly Imprinted Microsphere-Based Signal Amplified Semi-Homogeneous Method for Multidetection of Five Progestins in Milk. Foods 2023, 12, 2818. https://doi.org/10.3390/foods12152818
Su Y, Liu G, Hou H, Peng Y, Wang J. Development of a Magnetic Molecularly Imprinted Microsphere-Based Signal Amplified Semi-Homogeneous Method for Multidetection of Five Progestins in Milk. Foods. 2023; 12(15):2818. https://doi.org/10.3390/foods12152818
Chicago/Turabian StyleSu, Yan, Gelin Liu, Haozhe Hou, Yaojia Peng, and Jianping Wang. 2023. "Development of a Magnetic Molecularly Imprinted Microsphere-Based Signal Amplified Semi-Homogeneous Method for Multidetection of Five Progestins in Milk" Foods 12, no. 15: 2818. https://doi.org/10.3390/foods12152818
APA StyleSu, Y., Liu, G., Hou, H., Peng, Y., & Wang, J. (2023). Development of a Magnetic Molecularly Imprinted Microsphere-Based Signal Amplified Semi-Homogeneous Method for Multidetection of Five Progestins in Milk. Foods, 12(15), 2818. https://doi.org/10.3390/foods12152818





