Comprehensive Analysis of Physiological, Biochemical and Flavor Characteristics Changes in Crucian Carp (Carassius auratus) under Different Concentrations of Eugenol
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Maintenance
2.2. Anesthetic Agents and Preparation
2.3. Sample Collection
2.4. Blood Biochemistry
2.5. Serum Biochemistry
2.6. Glycogen Content Determination
2.7. E-Nose Analysis
2.8. Histopathology
2.9. Statistical Analysis
3. Results
3.1. Blood and Serum Indexes in Crucian Carp
3.1.1. Blood Components
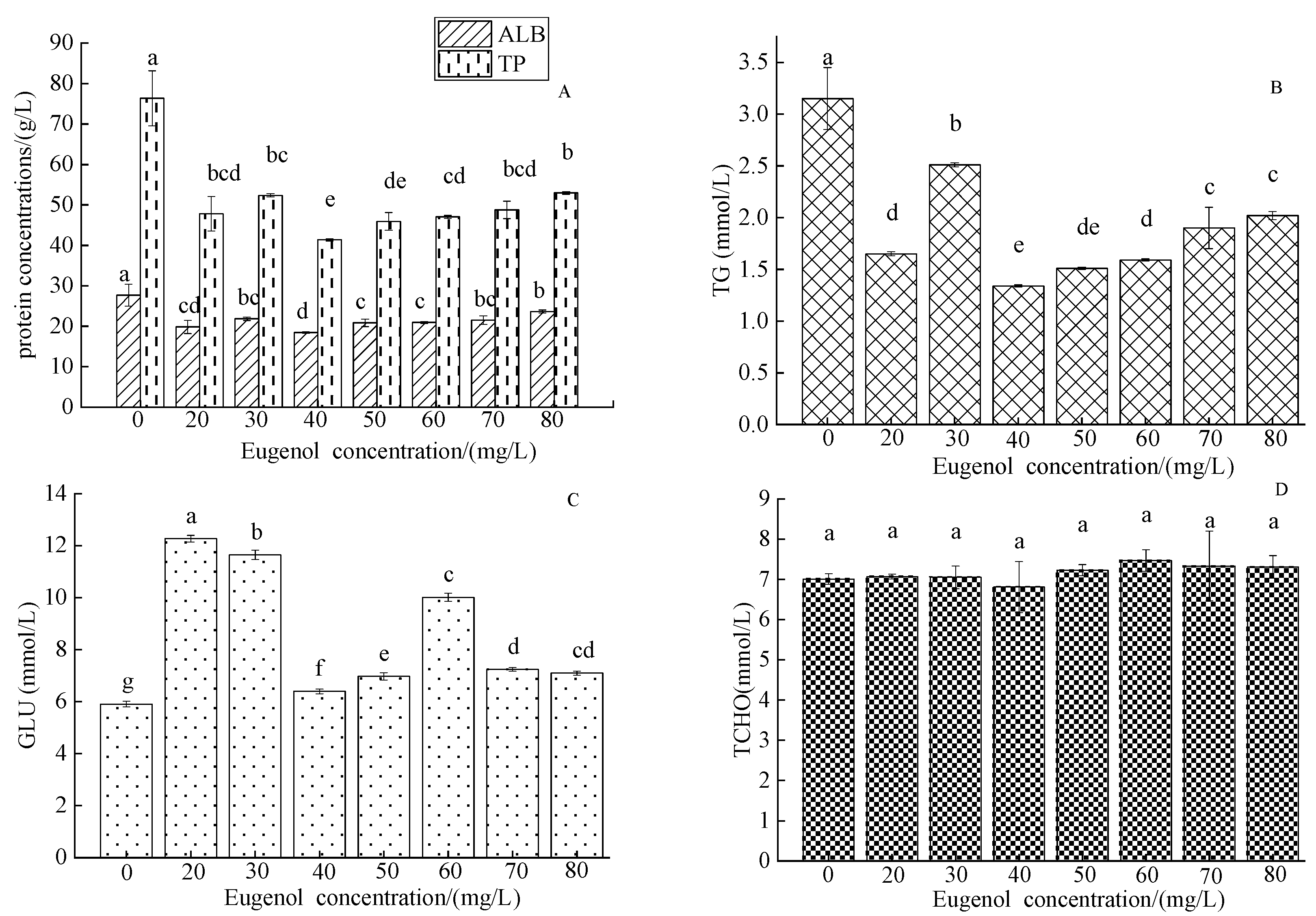
3.1.2. The Changes in Serum Enzymes
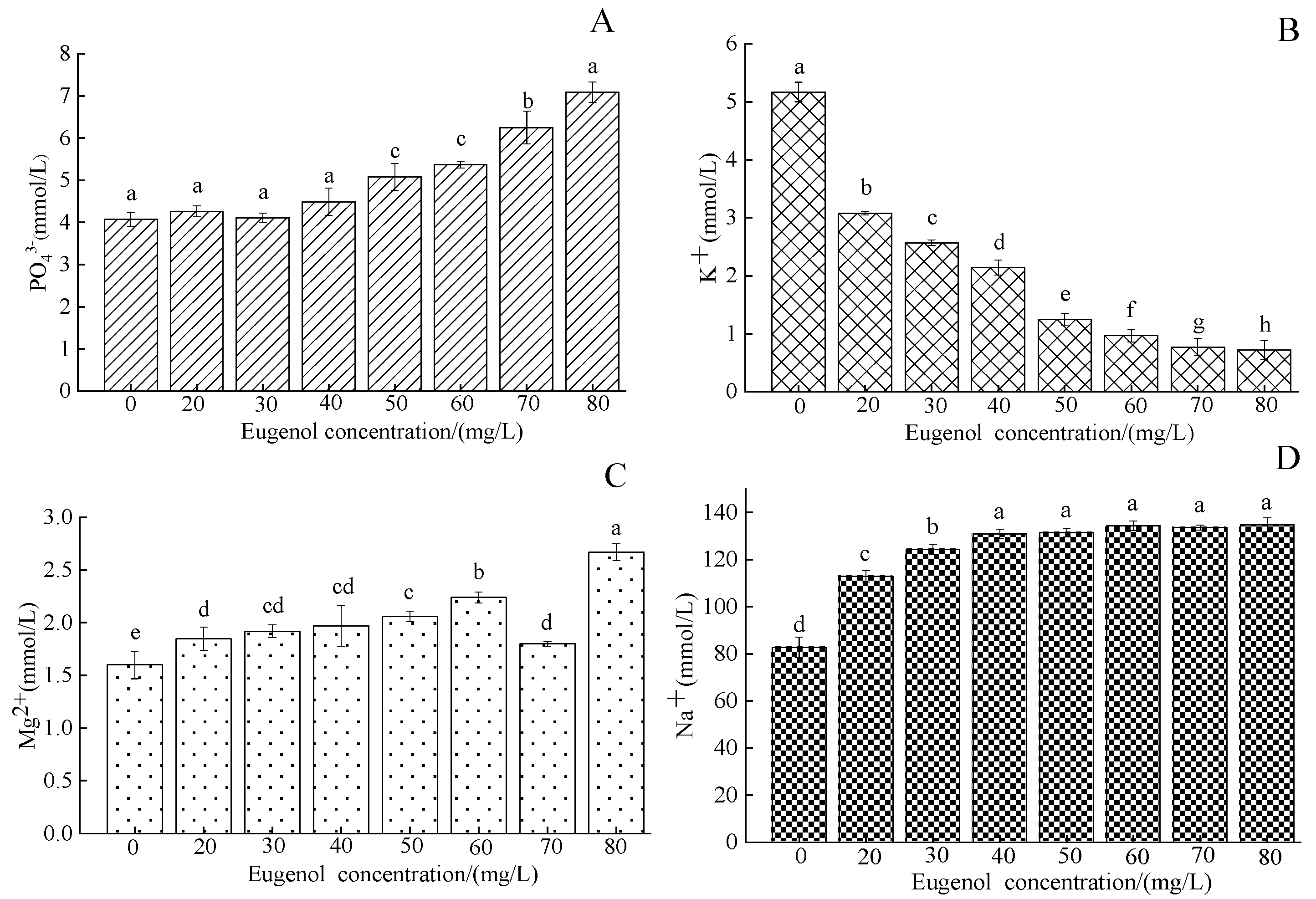
3.1.3. Serum Ion Content
3.1.4. The Serum Concentrations of Organic Components
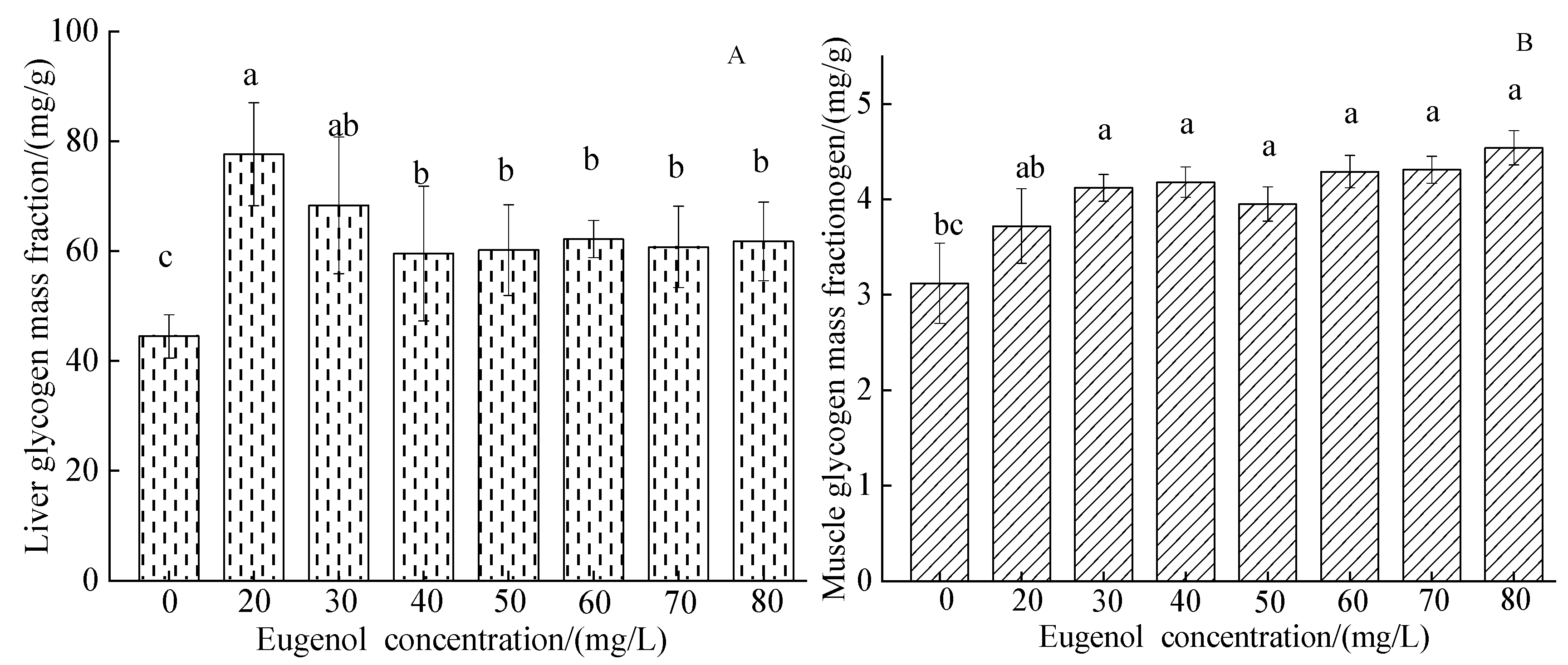
3.1.5. The Glycogen Index
3.2. Pathology
3.2.1. Gills
3.2.2. Liver
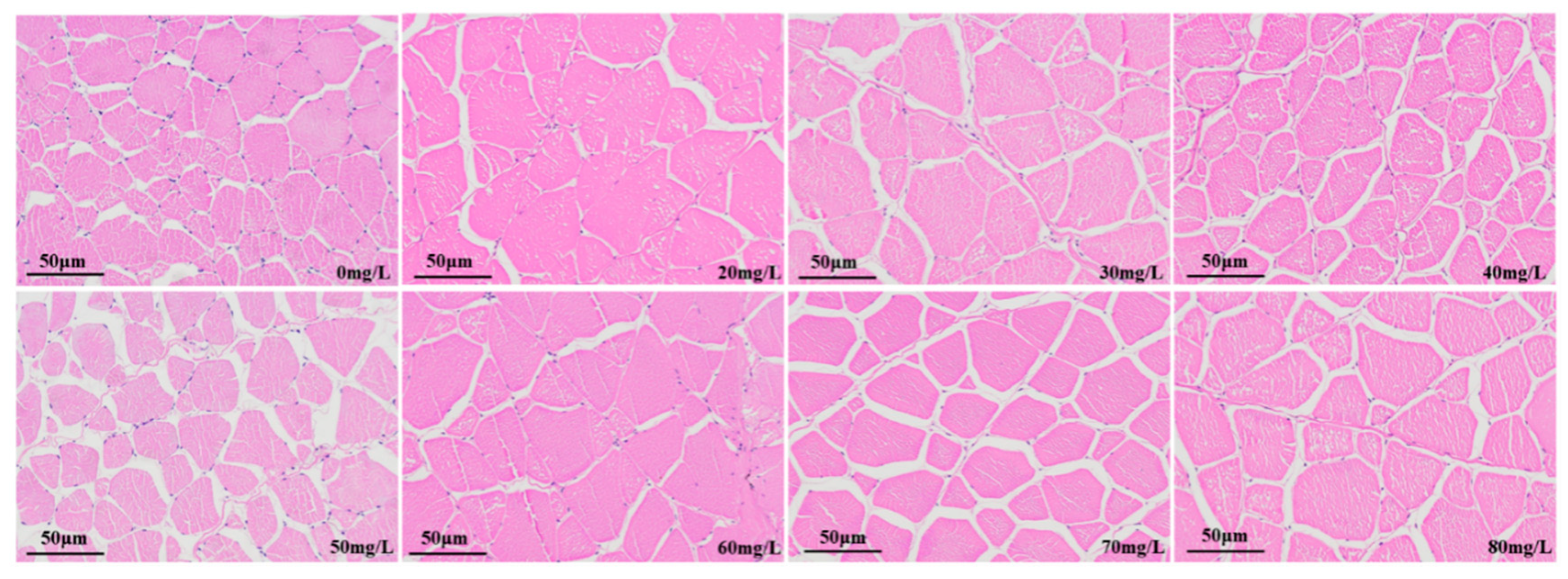
3.2.3. Muscle
3.3. Response of an Electronic Nose to Volatile Flavor Compounds in Crucian Carp Anesthetized with Different Concentrations of Eugenol
3.3.1. Radar Fingerprinting of the Effects of Different Concentrations of Eugenol on Muscle Flavor in Crucian Carp
3.3.2. LDA Analysis Based on the Electronic Nose Method
4. Discussion and Conclusions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Li, M.; Zhu, R.; Yu, Z.; Wang, J.; Duan, J.; Wang, T.; Wu, L. Effects of β-conglycinin on growth performance, antioxidant capacity and intestinal health in juvenile golden crucian carp, Carassius Auratus. Aquac. Res. 2019, 50, 3231–3241. [Google Scholar] [CrossRef]
- Palic, D.; Herolt, D.M.; Andreasen, C.B.; Menzel, B.W.; Roth, J.A. Anesthetic efficacy of tricaine methanesulfonate, metomidate and eugenol: Effects on plasma cortisol concentration and neutrophil function in fathead minnows (Pimephales promelas Rafinesque, 1820). Aquaculture 2006, 254, 675–685. [Google Scholar] [CrossRef]
- Thomas, P.; Robertson, L. Plasma cortisol and glucose stress responses of red drum (Sciaenops ocellatus) to handling and shallow water stressors and anesthesia with MS-222, quinaldine sulfate and metomidate. Aquaculture 1991, 96, 69–86. [Google Scholar] [CrossRef]
- Schreck, C.B.; Tort, L. The concept of stress in fish. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2016; Volume 35, pp. 1–34. [Google Scholar]
- Pan, H.M.; Jiang, L.X.; Zhang, C.F.; Huang, B.S. Research progress and safety evaluation of fishery anesthetics. Preserv. Process. 2021, 21, 136–143. [Google Scholar]
- Botrel, B.M.C.; Abreu, D.C.P.; Bazana, M.J.F.; e Rosa, P.V.; Saczk, A.A. Development, optimization, and validation of the HS-SPME/GC-MS method for the residual determination of menthol in fish. Food Anal. Methods 2019, 12, 1390–1398. [Google Scholar] [CrossRef]
- Gabriel, N.N.; Erasmus, V.N.; Namwoonde, A. Effects of different fish sizes, temperatures and concentration levels of sodium bicarbonate on anaesthesia in mozambique tilapia (Oreochromis mossambicus). Aquaculture 2020, 529, 735716. [Google Scholar] [CrossRef]
- Tang, Z.L.; Zhang, J.J.; Zhou, G.Q.; Chen, S.Q.; Xu, G.C.; Xu, P.; Qiang, J.; Wang, P.P. Effects of eugenol on transport water quality and physiological indexes of blood and muscle of juvenile ‘Youyu 3’. Fish. Sci. Technol. Inf. 2023, 50, 44–52. [Google Scholar]
- Qian, W.; Sun, Z.; Wang, T.; Yang, M.; Liu, M.; Zhang, J.; Li, Y. Antimicrobial activity of eugenol against carbapenem-resistant Klebsiella pneumoniae and its effect on biofilms. Microb. Pathog. 2020, 139, 103924. [Google Scholar] [CrossRef]
- Komala, V.V.; Ratnavathi, C.V.; Kumar, B.S.V.; Das, I.K. Inhibition of aflatoxin B1 production by an antifungal component, eugenol in stored sorghum grains. Food Control 2012, 26, 139–146. [Google Scholar] [CrossRef]
- He, R.; Lei, B.; Su, Y. Effectiveness of eugenol as an anesthetic for adult spotted sea bass (Lateolabrax maculatus). Aquaculture 2020, 523, 735180. [Google Scholar] [CrossRef]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological properties and health benefits of eugenol: A comprehensive review. Oxidative Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef]
- Ito, M.; Murakami, K.; Yoshino, M. Antioxidant action of eugenol compounds: Role of metal ion in the inhibition of lipid peroxidation. Food Chem. Toxicol. 2005, 43, 461–466. [Google Scholar] [CrossRef]
- Zahran, E.; Risha, E.; Rizk, A. Comparison propofol and eugenol anesthetics efficacy and effects on general health in Nile tilapia. Aquaculture 2021, 534, 736251. [Google Scholar] [CrossRef]
- Anderson, W.G.; Mckinley, R.S.; Colavecchia, M. The use of clove oil as an anesthetic for rainbow trout and its effects on swimming performance. N. Am. J. Fish. Manag. 1997, 17, 301–307. [Google Scholar] [CrossRef]
- Joint, F. WHO Technical Report Series 934: Evaluation of Certain Food Additives; World Health Organization: Geneva, Switzerland, 2006; pp. 49–54.
- Wang, W.; Dong, H.; Sun, Y.; Sun, C.; Duan, Y.; Gu, Q.; Li, Y.; Xie, M.; Zhang, J. Immune and physiological responses of juvenile Chinese Sea Bass (Lateolabrax maculatus) to eugenol and tricaine methanesulfonate (MS-222) in gills. Aquac. Rep. 2020, 18, 100554. [Google Scholar] [CrossRef]
- Su, M.M.; Sun, X.Q.; Yang, C.G.; Peng, X.T.; Liu, H.Y.; Cao, J.J. Research progress on application and detection methods of fishery anesthetic MS-222 and eugenol in the transportation of fresh aquatic products. J. Food Saf. Qual. Insp. 2015, 6, 25–29. [Google Scholar]
- Hu, W.J.; Wang, C.H.; Feng, G.P.; Zhuang, P.; Zheng, Y.P.; Ji, Q. Anesthetic effects of eugenol and MS-222 on Trachidermus fasciatus. Mar. Fish. 2023, 45, 10. [Google Scholar]
- Huang, X.L.; Dai, C.; Yu, W.; Yang, J.; Yang, Y.K.; Li, T.; Li, T.; Lin, H.Z.; Huang, Z.; Sun, X.Y.; et al. Anesthetic effect of eugenol on juvenile Trachinotus ovatus. J. Guangdong Ocean. Univ. 2020, 40, 124–131. [Google Scholar]
- Wang, J.L.; Wang, W.L.; Zhang, B.B.; Tang, D.M. Comparison of anesthetic effects of MS-222 and eugenol on Yadong salmon. J. Gansu Agric. Univ. 2021, 56, 26–32+40. [Google Scholar]
- Tarkhani, R.; Imani, A.; Jamali, H. Anaesthetic Efficacy of eugenol on various size classes of angelfish (Pterophyllum scalare schultze, 1823). Aquac. Res. 2017, 48, 5263–5270. [Google Scholar] [CrossRef]
- Oliveira, C.P.B.; Lemos, C.H.P.; Vidal, L.V.O. Anaesthesia with eugenol in hybrid amazon catfish (Pseudoplatystoma reticulatum × Leiarius marmoratus) handling: Biochemical and haematological responses. Aquaculture 2019, 501, 255–259. [Google Scholar] [CrossRef]
- Favero, G.C.; Silva, W.S.; Boaventura, T.P.; Leme, F.O.P.; Luz, R.K. Eugenol or salt to mitigate stress during the transport of juvenile Lophiosilurus alexandri, a neotropical carnivorous freshwater catfish. Aquaculture 2019, 512, 734321. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, P.; Wang, B.; Lu, Y.; Li, L.; Li, Y.; Liu, S. Evaluation of the effects of astragalus polysaccharides as immunostimulants on the immune response of crucian carp and against SVCV in vitro and in vivo. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 253, 109249. [Google Scholar] [CrossRef]
- Chen, D.J.; Zhang, M.G.; Nie, X.B.; Jiang, P.H.; Zhang, Y.H.; Zhang, C.F.; Ren, F. Detection of turbot quality treated by electrostatic field based on gas phase ion mobility spectrometry. Food Sci. 2019, 40, 313–319. [Google Scholar]
- Jia, Z.; Chen, X.T.; Pan, N.; Cai, S.L.; Zhang, Y.; Liu, Z.Y. Changes of volatile flavor substances in Takifugu bimaculatus during cold storage. Food Sci. 2021, 42, 188–196. [Google Scholar]
- Li, H.D.; Xu, Q.S.; Liang, Y.Z. LibPLS: An integrated library for partial least squares regression and linear discriminant analysis. Chemom. Intell. Lab. Syst. 2018, 176, 34–43. [Google Scholar] [CrossRef]
- Qi, Q.; Liu, X.Y.; Liu, H.B.; Zhang, Y.; Zhang, Y.W.; Zhang, H.Y.; Peng, X.; Sun, D.J. Effects of compound Chinese herbal medicine on serum biochemical indexes of Siberian sturgeon broodstock. J. Northeast. Agric. Univ. 2012, 43, 134–138. [Google Scholar]
- Safuan, S.N.M.; Tomari, M.R.M.; Zakaria, W.N.W. White blood cell (WBC) counting analysis in blood smear images using various color segmentation methods. Measurement 2018, 116, 543–555. [Google Scholar] [CrossRef]
- Kumar, M.; Matoba, O.; Quan, X.; Rajput, S.K.; Morita, M.; Awatsuji, Y. Quantitative dynamic evolution of physiological parameters of RBC by highly stable digital holographic microscopy. Opt. Lasers Eng. 2022, 151, 106887. [Google Scholar] [CrossRef]
- Wang, K.; Bian, X.; Zheng, M.; Liu, P.; Lin, L.; Tan, X. Rapid determination of hemoglobin concentration by a novel ensemble extreme learning machine method combined with near-infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120138. [Google Scholar] [CrossRef]
- Liang, Z.Y.; An, L.N.; Dong, Z.J.; Miao, L.H.; Xu, P.; Xie, Z. Anesthetic effect of clove oil on Tilapia and its effects on blood indexes and hormone levels. J. Shanghai Ocean. Univ. 2009, 18, 629–635. [Google Scholar]
- Amenyogbe, E.; Yang, E.; Xie, R.; Huang, J.; Chen, G. Influences of indigenous isolates Pantoea agglomerans RCS2 on growth, proximate analysis, haematological parameters, digestive enzyme activities, serum biochemical parameters, antioxidants activities, intestinal morphology, disease resistance, and molecular immune response in juvenile’s cobia fish (Rachycentron canadum). Aquaculture 2022, 551, 737942. [Google Scholar]
- Yong, W.; Huang, Y.; Li, X.; Wu, X. Changes of AST, ALP, CK and LDH-1 level in biological injury models caused by 18.4 mm rubber bullet. Int. J. Lab. Med. 2012, 33, 3. [Google Scholar]
- Bharti, S.; Rasool, F. Analysis of the biochemical and histopathological impact of a mild dose of commercial malathion on Channa punctatus (Bloch) fish. Toxicol. Rep. 2021, 8, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.H.; Zhu, X.J.; Ji, Y.P.; Zhou, B.; Wang, Y.J.; Xu, S.L.; Wang, D.L. Effects of stocking density on growth, metabolic enzyme activity and related gene expression of juvenile silver pomfret. J. Trop. Oceanogr. 2020, 39, 54–64. [Google Scholar]
- Mirghaed, A.T.; Yasari, M.; Mirzargar, S.S.; Hoseini, S.M. Rainbow trout (Oncorhynchus mykiss) anesthesia with myrcene: Efficacy and physiological responses in comparison with eugenol. Fish Physiol. Biochem. 2018, 44, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Bardell, D. Determination of reference intervals for equine arterial blood-gas, acid-base and electrolyte analysis. Vet. Anaesth. Analg. 2019, 46, 765–771. [Google Scholar] [CrossRef]
- Bijvelds, M.J.; Van, D.V.; Kolar, Z.I. Magnesium transport in freshwater teleosts. J. Exp. Biol. 1998, 201, 1981–1990. [Google Scholar] [CrossRef]
- Feng, G.P.; Zhuang, P.; Zhang, L.Z.; Yan, S.W.; Qian, S. Effects of anesthetic eugenol on oxygen consumption rate and blood biochemical indexes of juvenile Siberian sturgeon. J. Dalian Fish. Coll. 2010, 25, 113–118. [Google Scholar]
- Hu, X.; Ding, C.Y.; Ye, Q.; Wu, R.H.; Zhang, Q.; Li, H.; Li, Y. Effects of carbon dioxide anesthesia on physiological and biochemical indexes and expression of neural regulation related genes in silver carp. Freshw. Fish. 2020, 50, 9–14. [Google Scholar]
- Sun, P.; Tang, B.J.; Jiang, Y.Z. Changes of serum biochemical indexes and liver antioxidant activity of large yellow croaker under stress state. Mar. Fish. 2020, 45, 634–640. [Google Scholar]
- Gao, X.Q.; Wang, X.; Wang, X.Y.; Li, H.X.; Xu, L.; Huang, B.; Meng, X.S.; Zhang, T.; Chen, H.-B.; Xing, R.; et al. Effects of different feeding frequencies on the growth, plasma biochemical parameters, stress status, and gastric evacuation of juvenile tiger puffer fish (Takifugu rubripes). Aquaculture 2022, 548, 737718. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.; Wei, Z.; Liu, Q.; Mai, H.; Liu, Y.; Liu, B.; Zhuang, Y.; Zou, D.; Zhang, W.; et al. Effects of dietary dandelion extract on the growth performance, serum biochemical parameters, liver histology, and immune and apoptosis-related genes expression of hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀) at different feeding period. Fish Shellfish. Immunol. 2022, 120, 280–286. [Google Scholar]
- Zhu, T.B.; Li, F.; Wu, X.B.; Zhu, Y.J.; Guo, W.; Yang, D.G. Effects of electrical anesthesia on behavior and blood biochemical indexes of juvenile Coreius guichenoti. Sichuan J. Zool. 2015, 34, 885–888. [Google Scholar]
- Zhu, T.T.; Li, Q.; Zhu, H.Y.; Wang, H.W.; Li, E.C.; Chen, L.Q. Effects of dietary lipid levels on growth, blood biochemical parameters and antioxidant capacity of juvenile Russian sturgeon (Acipenser gueldenstaedti). Mar. Fish. 2017, 39, 58–67. [Google Scholar]
- Cheng, Z.J.; Hardy, R.W. Protein and lipid sources affect cholesterol concentrations of juvenile pacific white shrimp, Litopenaeus vannamei (Boone). J. Anim. Sci. 2004, 82, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.Q.; Jiang, K.Y.; Han, L.J.; Yuan, K.S.; Qiu, C.W.; Wang, B.J.; Liu, M.; Wang, L.; Wen, H.S. Effects of continuous cooling on metabolic function of adult turbot. Mar. Sci. 2014, 38, 46–53. [Google Scholar]
- Li, L.; Cao, Z.D.; Fu, S.J. Changes of lactic acid, glycogen and glucose levels in juvenile catfish after exhaustive exercise. Acta Hydrobiol. Sin. 2007, 6, 880–885. [Google Scholar]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Han, Q.; Xu, Y.; Jiang, H.; Xing, H.; Teng, X. Impaired immune function and structural integrity in the gills of common carp (Cyprinus carpio L.) caused by chlorpyrifos exposure: Through oxidative stress and apoptosis. Fish Shellfish Immunol. 2019, 86, 239–245. [Google Scholar] [CrossRef]
- Cappello, T.; Brandao, F.; Guilherme, S.; Santos, M.A.; Maisano, M.; Mauceri, A.; Canario, J.; Pacheco, M.; Pereira, P. Insights into the mechanisms underlying mercury-induced oxidative stress in gills of wild fish (Liza aurata) combining 1H NMR metabolomics and conventional biochemical assays. Sci. Total Environ. 2016, 548, 13–24. [Google Scholar] [CrossRef]
- De Domenico, E.; Mauceri, A.; Giordano, D.; Maisano, M.; Giannetto, A.; Parrino, V.; Natalotto, A.; D’Agata, A.; Cappello, T.; Fasulo, S. Biological responses of juvenile european sea bass (Dicentrarchus labrax) exposed to contaminated sediments. Ecotoxicol. Environ. Saf. 2013, 97, 114–123. [Google Scholar] [CrossRef]
- Qu, Y.J.; Liu, Q.Q.; Wen, J.F.; Li, J.E.; Li, H. Effects of acute low temperature stress on the structure of liver, muscle and gill of juvenile Eleutheronema tetradactylum. Ecoscience 2018, 37, 53–59. [Google Scholar]
- Dong, H.; Wang, W.; Duan, Y.; Li, H.; Liu, Q.; Sun, Y.; Zhang, J. Transcriptomic analysis of juvenile Chinese sea bass (Lateolabrax maculatus) anesthetized by MS-222 (Tricaine Methanesulfonate) and eugenol. Fish Physiol. Biochem. 2020, 46, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.P.B.; Lemos, C.H.P.; Felix, S.A.; de Souza, S.A.; Albinati, A.C.L.; Lima, A.O.; Copatti, C.E. Use of eugenol for the anaesthesia and transportation of freshwater angelfish (Pterophyllum scalare). Aquaculture 2019, 513, 734409. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Metsoviti, M.N.; Gkalogianni, E.Z.; Psofakis, P.; Asimaki, A.; Katsoulas, N.; Papapolymerou, G.; Zarkadas, I. The effects of replacing fishmeal by Chlorella vulgaris and fish oil by Schizochytrium sp. and Microchloropsis gaditana blend on growth performance, feed efficiency, muscle fatty acid composition and liver histology of gilthead seabream (Sparus aurata). Aquaculture 2022, 561, 738709. [Google Scholar] [CrossRef]
- Velisek, J.; Svobodova, Z.; Piackova, V.; Groch, L.; Nepejchalova, L. Effects of clove oil anaesthesia on common carp (Cyprinus carpio L.). Vet. Med. 2005, 50, 269–275. [Google Scholar] [CrossRef]
- Wilson, A.D. Application of electronic-nose technologies and voc-biomarkers for the noninvasive early diagnosis of gastrointestinal diseases. Sensors 2018, 18, 2613. [Google Scholar] [CrossRef]
- Li, P.; Niu, Z.; Shao, K.; Wu, Z. Quantitative analysis of fish meal freshness using an electronic nose combined with chemometric methods. Measurement 2021, 179, 109484. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Pei, Z.; Wei, P.; Xiang, D.; Cao, X.; Shen, X.; Li, C. Volatile flavour components and the mechanisms underlying their production in golden pompano (Trachinotus blochii) fillets subjected to different drying methods: A comparative study using an electronic nose, an electronic tongue and SDE-GC-MS. Food Res. Int. 2019, 123, 217–225. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhang, J.; Li, X.; Wang, J.; Yi, S.; Zhu, W.; Xu, Y.; Li, J. Prediction of the freshness of horse mackerel (Trachurus japonicus) using e-nose, e-tongue, and colorimeter based on biochemical indexes analyzed during frozen storage of whole fish. Food Chem. 2023, 402, 134325. [Google Scholar] [CrossRef] [PubMed]




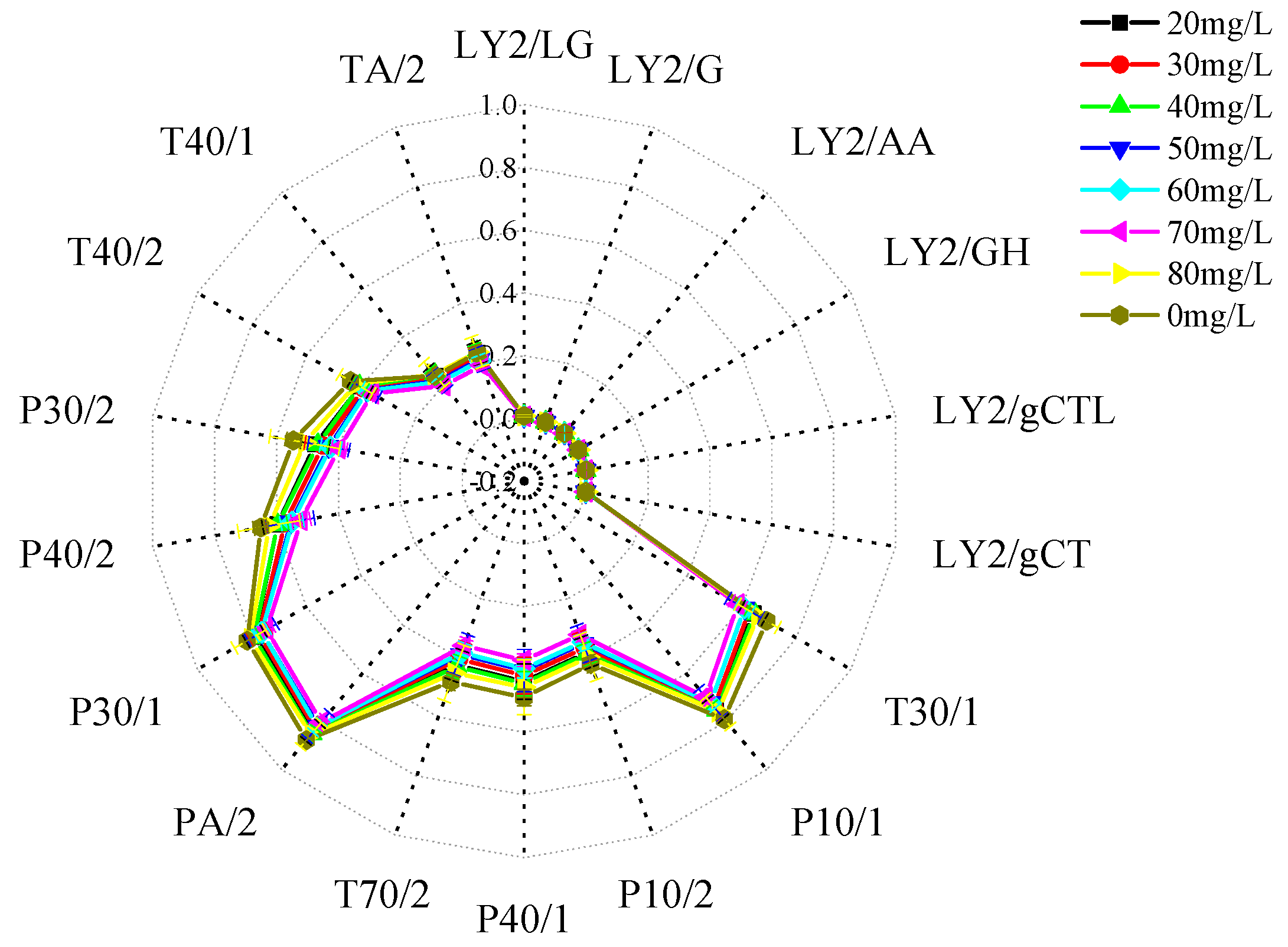
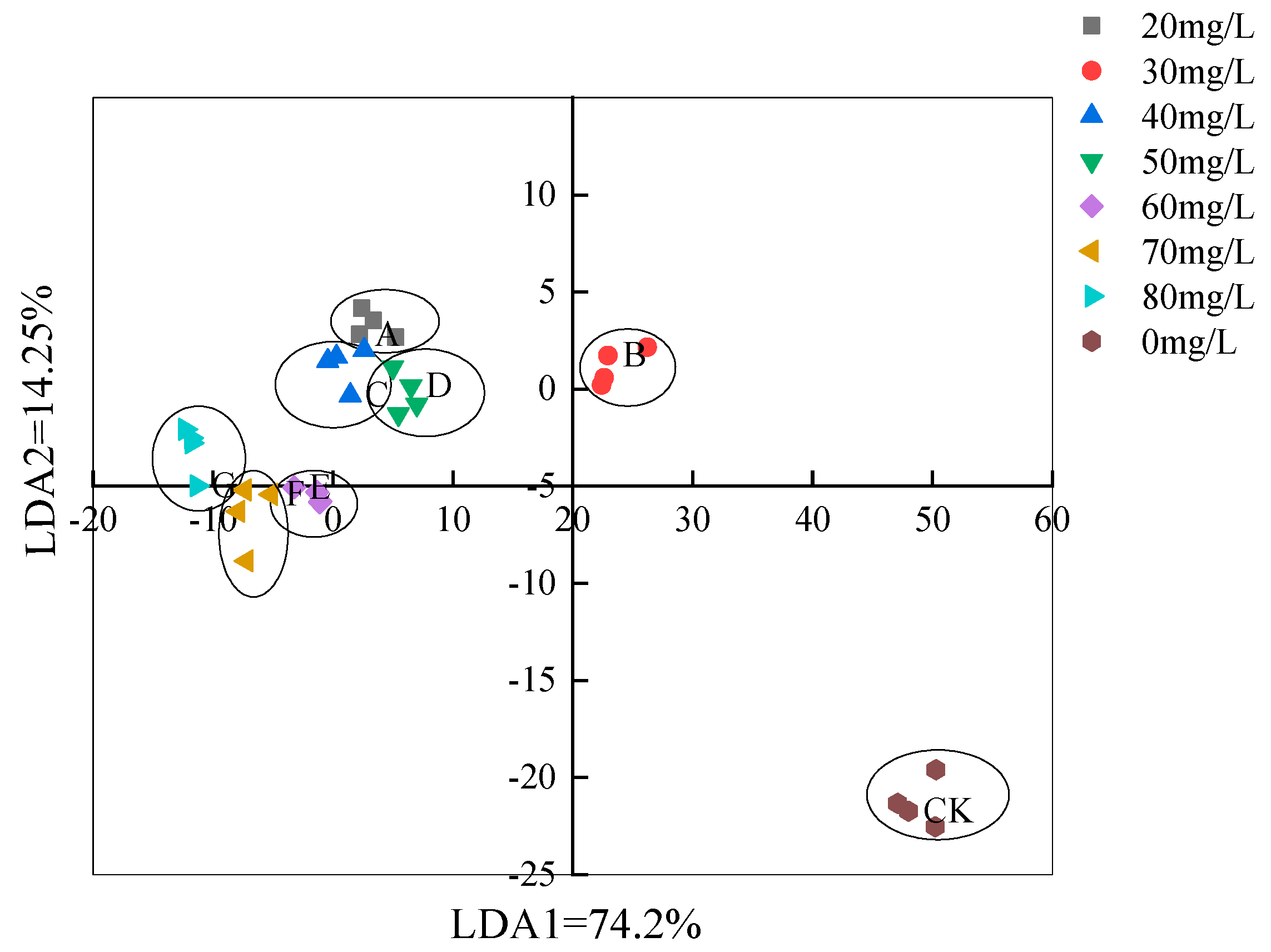
| Serial Number | Sensor Name | Sensor Response Characteristics |
|---|---|---|
| 1 | LY2/LG | Sensitive to gases with high oxidation capacity |
| 2 | LY2/G | Sensitive to toxic gases |
| 3 | LY2/AA | Sensitive to organic compounds |
| 4 | LY2/GH | Sensitive to toxic gases |
| 5 | LY2/gCTL | Sensitive to toxic gases |
| 6 | LY2/gCT | Sensitive to flammable gases |
| 7 | T30/1 | Sensitive to organic compounds |
| 8 | P10/1 | Sensitive to combustible gases |
| 9 | P10/2 | Sensitive to flammable gases |
| 10 | P40/1 | Sensitive to gases with high oxidation capacity |
| 11 | T70/2 | Sensitive to aromatic compounds |
| 12 | PA/2 | Sensitive to organic compounds, toxic gases |
| 13 | P30/1 | Sensitive to combustible gases, organic compounds |
| 14 | P40/2 | Sensitive to gases with high oxidation capacity |
| 15 | P30/2 | Sensitive to organic compounds |
| 16 | T40/2 | Sensitive to gases with high oxidation capacity |
| 17 | T40/1 | Sensitive to gases with high oxidation capacity |
| 18 | TA/2 | Sensitive to organic compounds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Huang, B.; Tang, J.; Jiang, P.; Chen, D.; Zhang, C. Comprehensive Analysis of Physiological, Biochemical and Flavor Characteristics Changes in Crucian Carp (Carassius auratus) under Different Concentrations of Eugenol. Foods 2023, 12, 2820. https://doi.org/10.3390/foods12152820
Jiang L, Huang B, Tang J, Jiang P, Chen D, Zhang C. Comprehensive Analysis of Physiological, Biochemical and Flavor Characteristics Changes in Crucian Carp (Carassius auratus) under Different Concentrations of Eugenol. Foods. 2023; 12(15):2820. https://doi.org/10.3390/foods12152820
Chicago/Turabian StyleJiang, Lexia, Baosheng Huang, Jiaming Tang, Peihong Jiang, Dongjie Chen, and Changfeng Zhang. 2023. "Comprehensive Analysis of Physiological, Biochemical and Flavor Characteristics Changes in Crucian Carp (Carassius auratus) under Different Concentrations of Eugenol" Foods 12, no. 15: 2820. https://doi.org/10.3390/foods12152820
APA StyleJiang, L., Huang, B., Tang, J., Jiang, P., Chen, D., & Zhang, C. (2023). Comprehensive Analysis of Physiological, Biochemical and Flavor Characteristics Changes in Crucian Carp (Carassius auratus) under Different Concentrations of Eugenol. Foods, 12(15), 2820. https://doi.org/10.3390/foods12152820






