Microbial Community Succession and Its Correlation with Quality Characteristics during Gray Sufu Fermentation
Abstract
1. Introduction
2. Materials and Methods
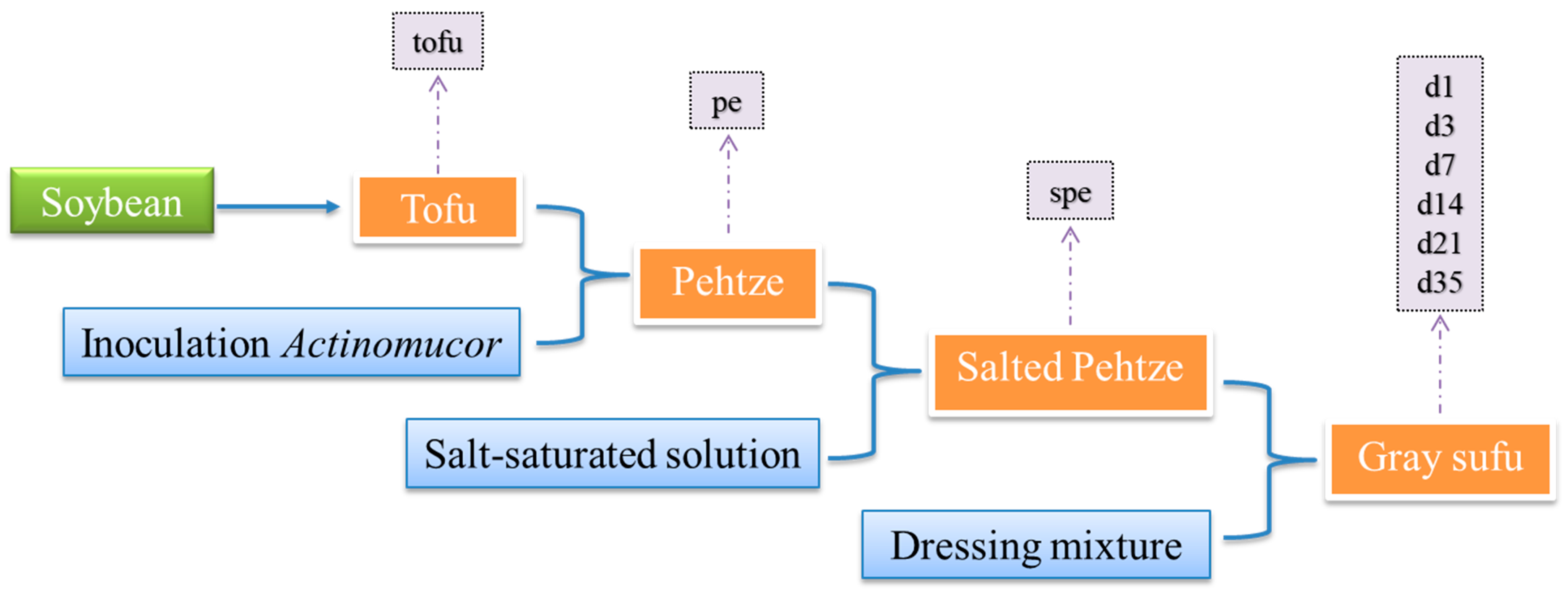
2.1. Sample Collection
2.2. Extraction of Nucleic Acids
2.3. PCR Amplification and Sequencing
2.4. Physicochemical Analysis
2.5. Biogenic Amines and Amino Acids Analysis
2.6. Statistical Analysis
3. Results
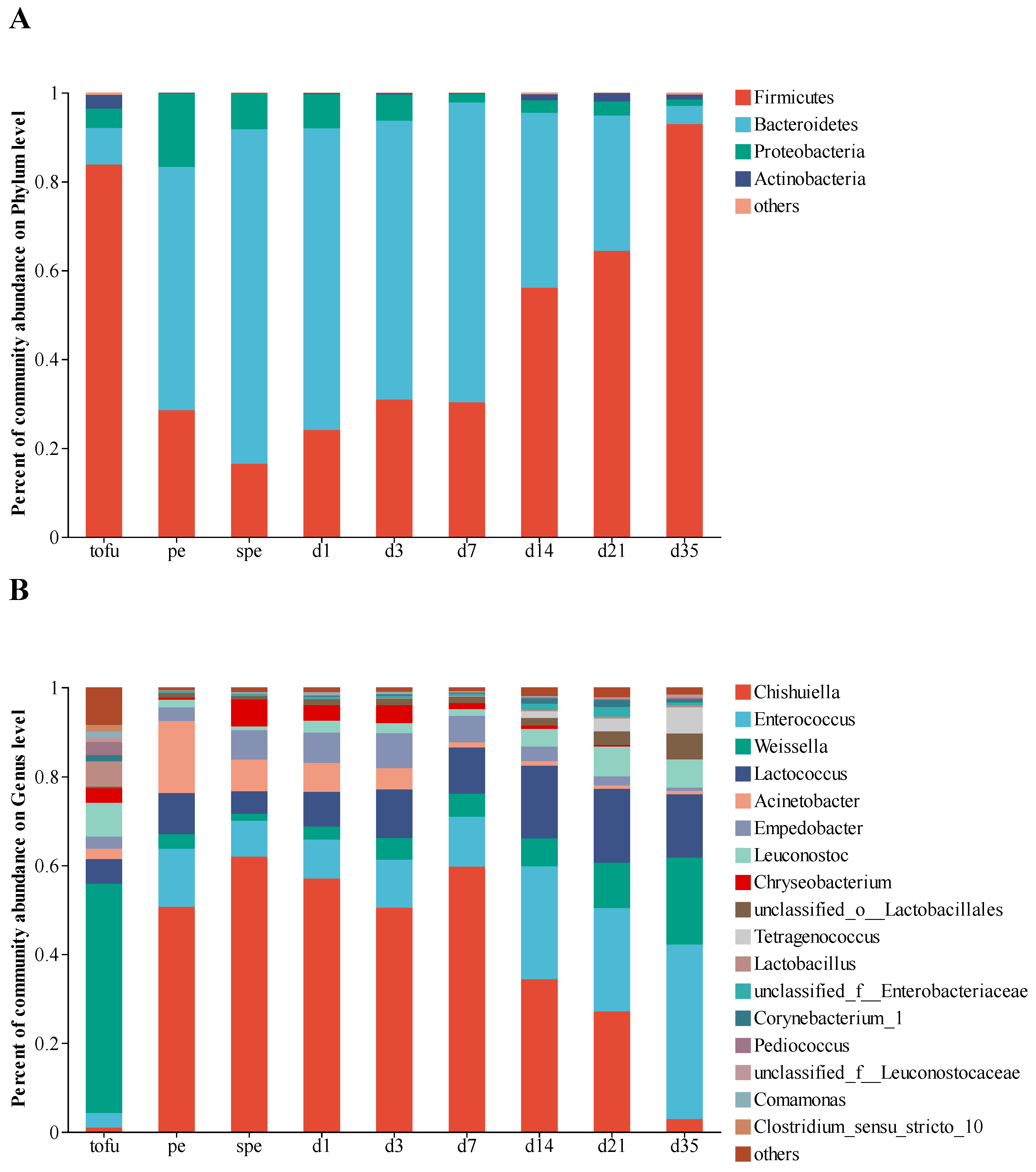
3.1. Dynamics of Bacterial Community
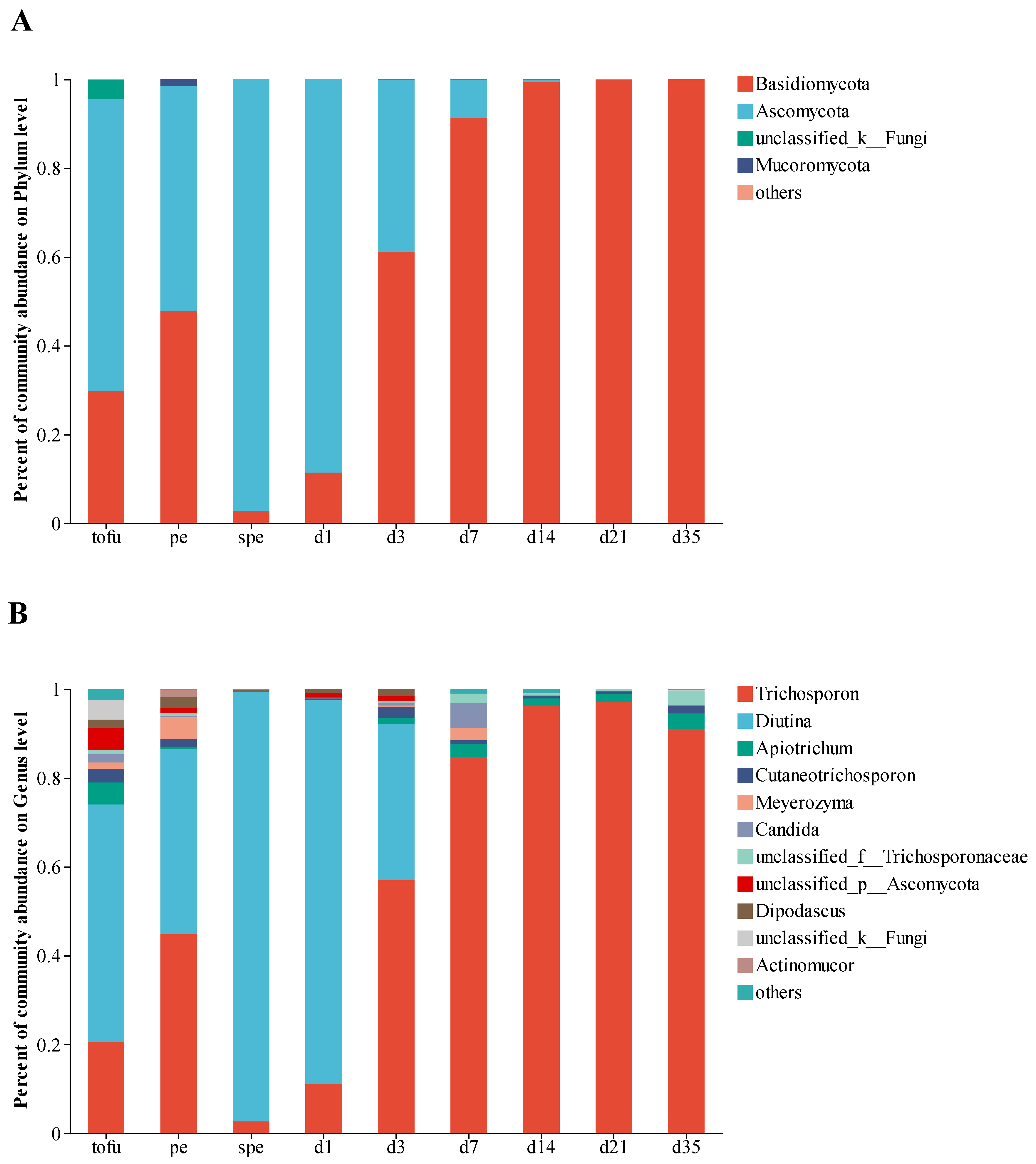
3.2. Dynamics of Fungal Community
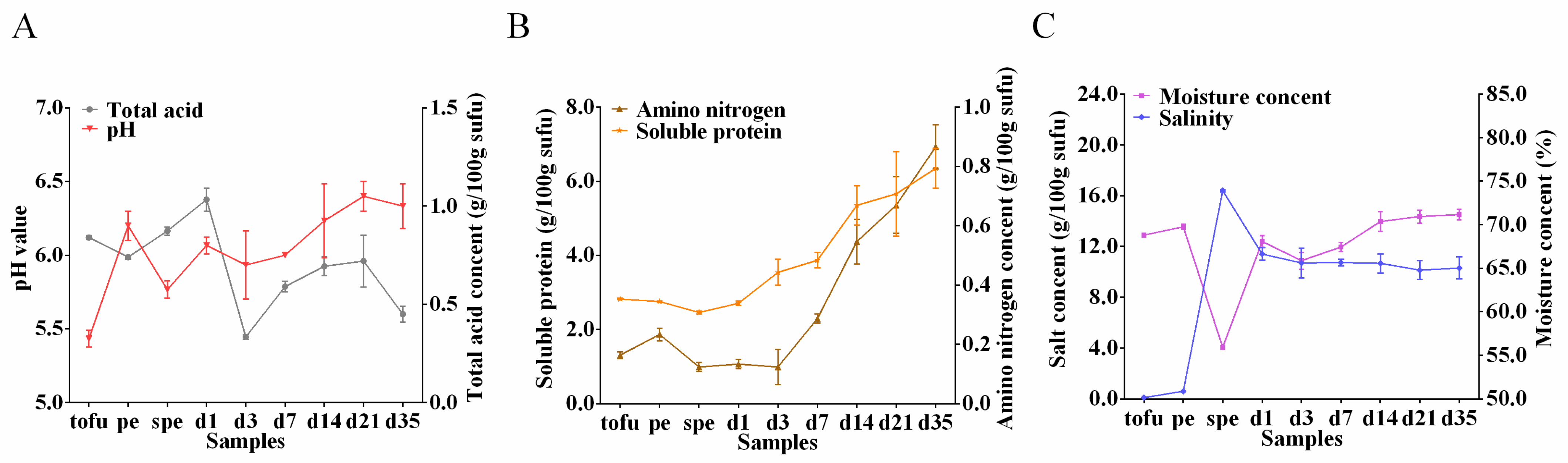
3.3. Physicochemical Properties during Sufu Fermentation
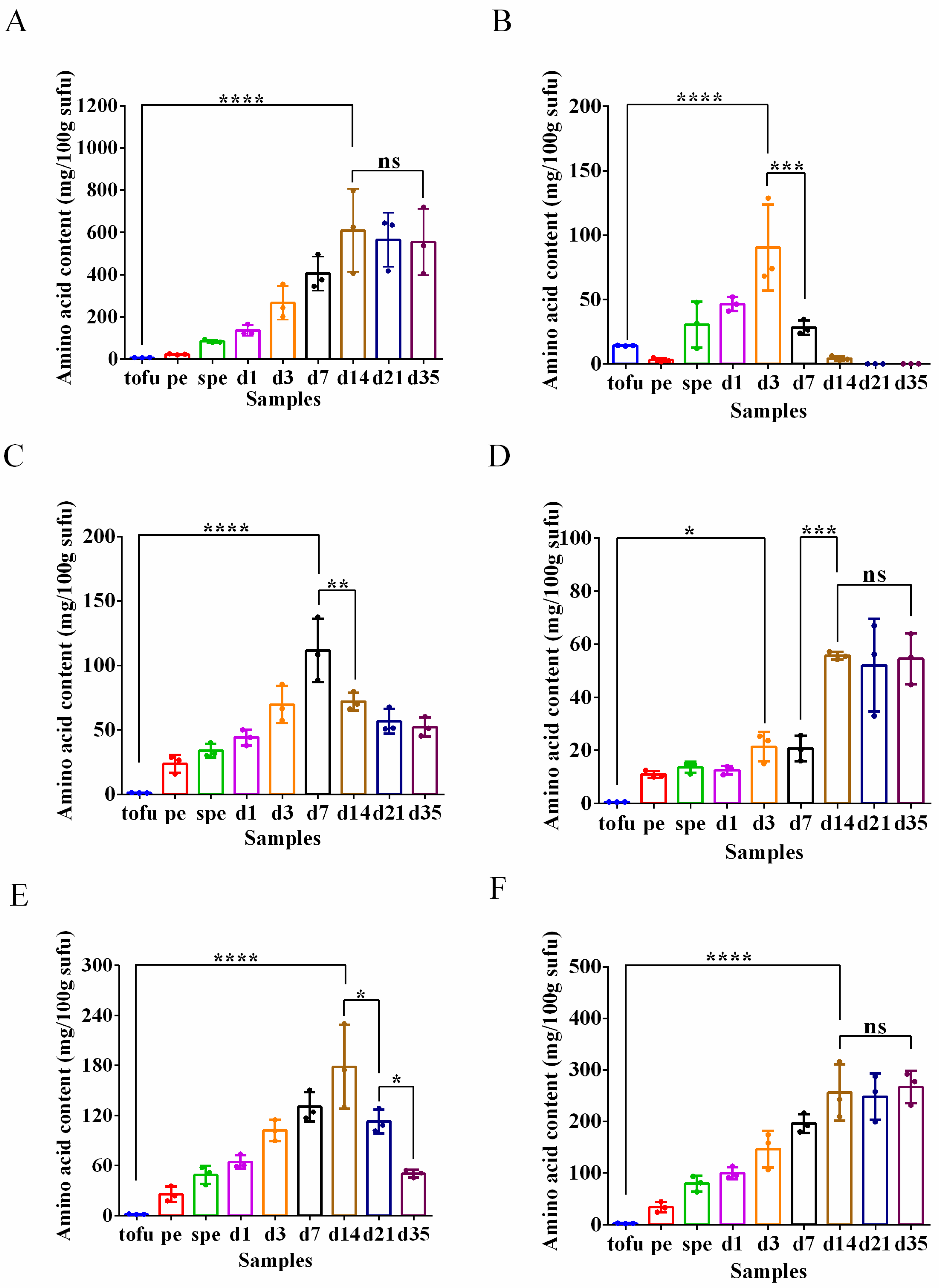
3.4. Biogenic Amine Production and Amino Acid Content during Sufu Fermentation
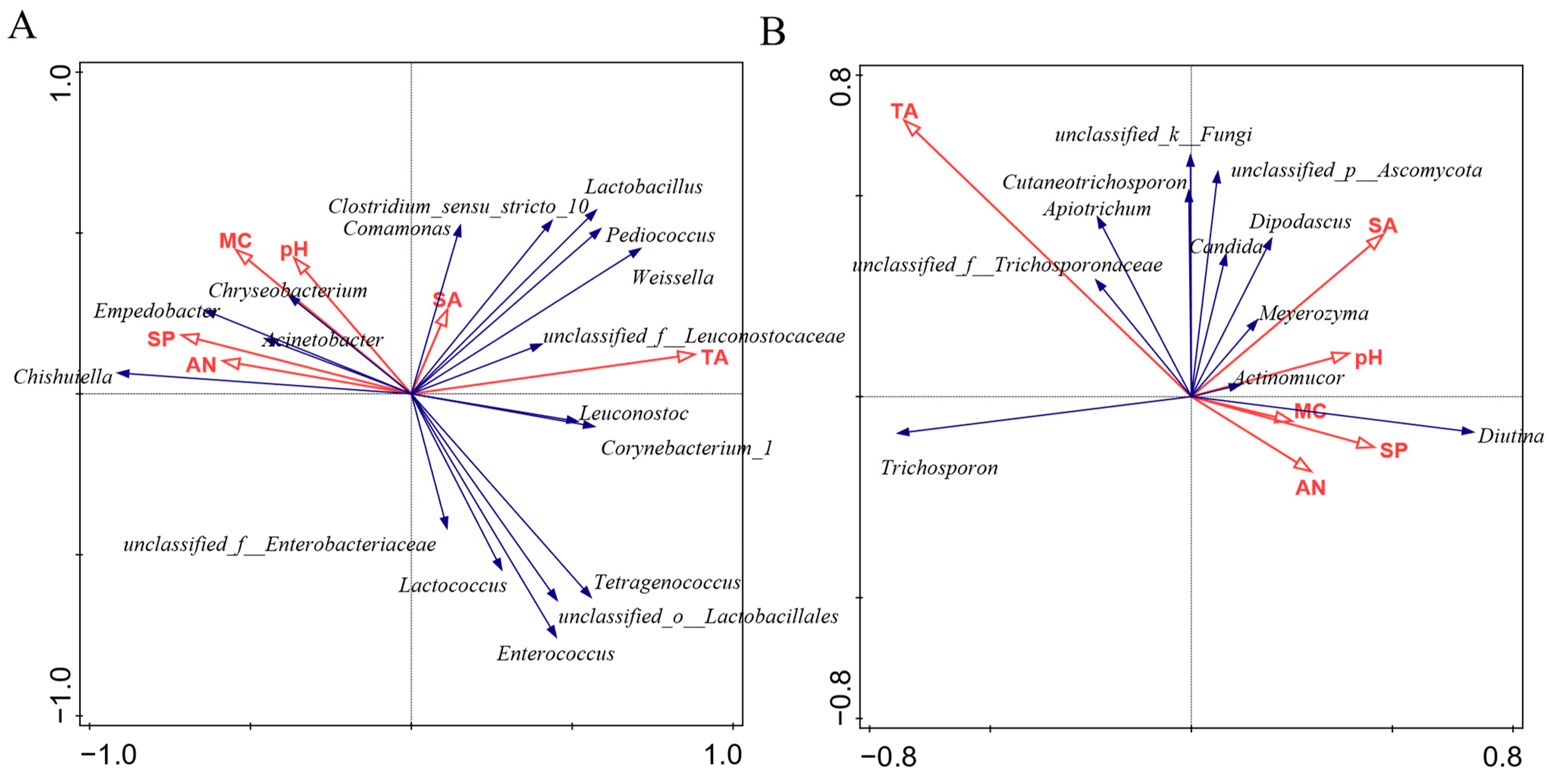
3.5. Correlations between Microbiota and Physicochemical Properties during Sufu Fermentation
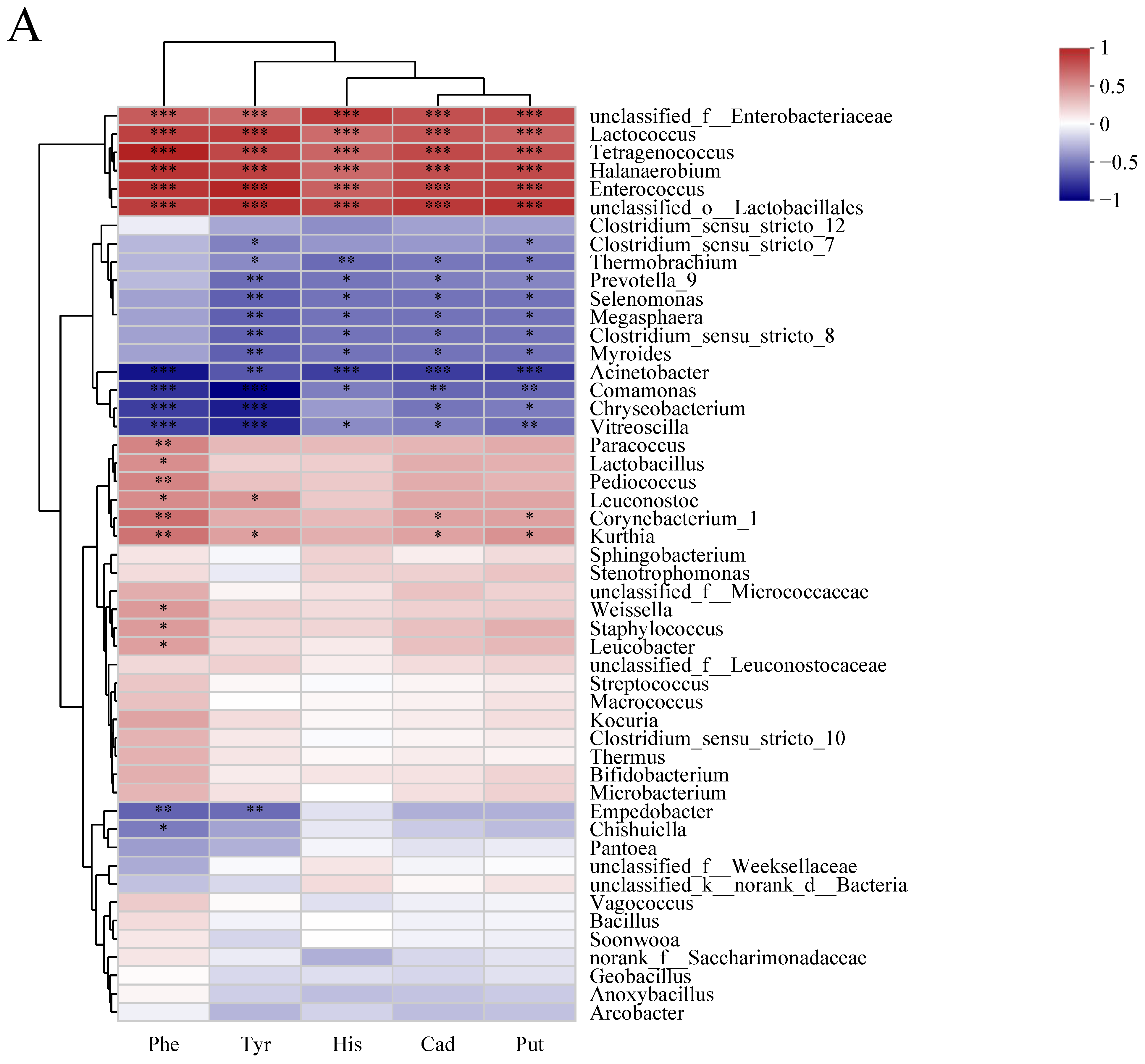
3.6. Correlations between Microbiota and Biogenic Amine during Sufu Fermentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azam, M.; Mohsin, M.; Ijaz, H.; Tulain, U.R.; Ashraf, M.A.; Fayyaz, A.; Ul Abadeen, Z.; Kamran, Q. Lactic acid bacteria in traditional fermented Asian foods. Pak. J. Pharm. Sci. 2017, 30, 1803–1814. [Google Scholar] [PubMed]
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A.; et al. Food fermentations: Microorganisms with technological beneficial use. Int. J. Food Microbiol. 2012, 154, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Han, B.Z.; Beumer, R.R.; Rombouts, M.F.; Nout, M.J.R. Microbiological safety and quality of commercial sufu—A Chinese fermented soybean food. Food Control 2001, 12, 541–547. [Google Scholar] [CrossRef]
- Han, B.Z.; Cao, C.F.; Rombouts, M.F.; Nout, M.J.R. Microbial changes during the production of sufu—A Chinese fermented soybean food. Food Control 2004, 15, 265–270. [Google Scholar] [CrossRef]
- Ding, S.Q.; Tian, M.; Yang, L.; Pan, Y.; Suo, L.L.; Zhu, X.M.; Ren, D.Y.; Yu, H.S. Diversity and dynamics of microbial population during fermentation of gray sufu and their correlation with quality characteristics. LWT-Food Sci. Technol. 2023, 180, 114711. [Google Scholar] [CrossRef]
- Jou, H.J.; Tsai, P.J.; Tu, J.H.; Wu, W.H. Stinky tofu as a rich source of bioavailable S-equol in Asian diets. J. Funct. Foods 2013, 5, 651–659. [Google Scholar] [CrossRef]
- Liu, S.N.; Han, Y.; Zhou, Z.J. Lactic acid bacteria in traditional fermented Chinese foods. Food Res. Int. 2011, 44, 643–651. [Google Scholar] [CrossRef]
- He, W.; Chen, Z.; Chung, H.Y. Dynamic correlations between major enzymatic activities, physicochemical properties and targeted volatile compounds in naturally fermented plain sufu during production. Food Chem. 2022, 378, 131988. [Google Scholar] [CrossRef]
- Chao, S.H.; Tomii, Y.; Watanabe, K.; Tsai, Y.C. Diversity of lactic acid bacteria in fermented brines used to make stinky tofu. Int. J. Food Microbiol. 2008, 123, 134–141. [Google Scholar] [CrossRef]
- Song, Z.; Hu, Y.; Chen, X.; Li, G.; Zhong, Q.; He, X.; Xu, W. Correlation between bacterial community succession and propionic acid during gray sufu fermentation. Food Chem. 2021, 353, 129447. [Google Scholar] [CrossRef]
- Chen, Y.H.; Liu, X.W.; Huang, J.L.; Baloch, S.; Xu, X.; Pei, X.F. Microbial diversity and chemical analysis of Shuidouchi, traditional Chinese fermented soybean. Food Res. Int. 2019, 116, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Schirone, M.; Tofalo, R.; Fasoli, G.; Perpetuini, G.; Corsetti, A.; Manetta, A.C.; Ciarrocchi, A.; Suzzi, G. High content of biogenic amines in Pecorino cheeses. Food Microbiol. 2013, 34, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.A.; Moreno-Arribas, M.V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014, 39, 146–155. [Google Scholar] [CrossRef]
- Guan, R.F.; Liu, Z.F.; Zhang, J.J.; Wei, Y.X.; Wahab, S.; Liu, D.H.; Ye, X.Q. Investigation of biogenic amines in sufu (furu): A Chinese traditional fermented soybean food product. Food Control 2013, 31, 345–352. [Google Scholar] [CrossRef]
- Spanjer, M.C.; Roode, B.A.S.W. Towards a regulatory limit for biogenic amines in fish, cheese and sauerkraut. Ware (N)-Chem. 1991, 21, 139–167. [Google Scholar]
- Zare, D.; Muhammad, K.; Bejo, M.H.; Ghazali, H.M. Determination of urocanic acid, a compound implicated in histamine toxicity, and assessment of biogenic amines relative to urocanic acid content in selected fish and fish products. J. Food Compos. Anal. 2015, 37, 95–103. [Google Scholar] [CrossRef]
- Liu, Z.F.; Wei, Y.X.; Zhang, J.J.; Liu, D.H.; Hu, Y.Q.; Ye, X.Q. Changes in biogenic amines during the conventional production of stinky tofu. Int. J. Food Sci. Technol. 2011, 46, 687–694. [Google Scholar] [CrossRef]
- Allen, G.C.; Flores-Vergara, M.A.; Krasynanski, S.; Kumar, S.; Thompson, W.F. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat. Protoc. 2006, 1, 2320–2325. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.L.; Nie, Q.; Dong, S.; Shao, Y.; Yang, X.N.; Wu, Y.L.; Yang, Y.; Zhong, W.Z. Neoadjuvant crizotinib in resectable locally advanced non–small cell lung cancer with ALK rearrangement. J. Thorac. Oncol. 2019, 14, 726–731. [Google Scholar] [CrossRef]
- Emam, A.; Wu, X.; Xu, S.; Wang, L.; Liu, S.; Wang, B. Stalled replication fork protection limits cGAS–STING and P-body-dependent innate immune signalling. Nat. Cell Biol. 2022, 24, 1154–1164. [Google Scholar] [CrossRef]
- Zeng, Q.; An, S. Identifying the biogeographic patterns of pare and abundant bacterial communities using different primer sets on the loess plateau. Microorganisms 2021, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, W.; Zhang, F.; Zhu, X.; Kong, W.; Niu, S.; Gao, K.; Yang, H. Community composition and trophic mode diversity of fungi associated with fruiting body of medicinal Sanghuangporus vaninii. BMC Microbiol. 2022, 22, 251. [Google Scholar] [CrossRef]
- Xing, H.; Liu, H.; Pan, J. High-throughput sequencing of oral microbiota in Candida carriage sjögren’s syndrome patients: A pilot cross-sectional study. J. Clin. Med. 2023, 12, 1559. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, Y.; Huang, G.; Zheng, N.; Zhao, S.; Wang, J. Ruminal bacterial community is associated with the variations of total milk solid content in Holstein lactating cows. Anim. Nutr. 2022, 9, 175–183. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta 2022, 1, 12. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.P.; Warnow, T.; Pop, M.; White, B. A perspective on 16S rRNA operational taxonomic unit clustering using sequence similarity. NPJ Biofilms Microbiomes 2016, 2, 16004. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Eldred, L.E.; Thorn, R.G.; Smith, D.R. Simple matching using QIIME 2 and RDP reveals misidentified sequences and an underrepresentation of fungi in reference datasets. Front. Genet. 2021, 12, 768473. [Google Scholar] [CrossRef]
- Rogers, M.B.; Firek, B.; Shi, M.; Yeh, A.; Brower-Sinning, R.; Aveson, V.; Kohl, B.L.; Fabio, A.; Carcillo, J.A.; Morowitz, M.J. Disruption of the microbiota across multiple body sites in critically ill children. Microbiome 2016, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.; van der Heijden, M.G.A.; Wittwer, R.A.; Banerjee, S.; Walser, J.C.; Schlaeppi, K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 2018, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, J.; Zhang, Y.; Gong, H.; Hou, R.; Sun, Z.; Ouyang, Z. Soil microbes from saline-alkali farmland can form carbonate precipitates. Agronomy 2023, 13, 372. [Google Scholar] [CrossRef]
- Ochi, N. Simultaneous determination of eight underivatized biogenic amines in salted mackerel fillet by ion-pair solid-phase extraction and volatile ion-pair reversed-phase liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2019, 1601, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wu, Z.Y.; Zhang, W.X. Bioinformatics analysis of amino acid decarboxylases related to four major biogenic amines in pickles. Food Chem. 2022, 393, 133339. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, Y.; Liang, J.; Finkelman, F.D. Molecular regulation of histamine synthesis. Front. Immunol. 2018, 9, 1392. [Google Scholar] [CrossRef]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef]
- Hui, H.; Bai, Y.; Fan, T.P.; Zheng, X.; Cai, Y. Biosynthesis of putrescine from L-arginine using engineered Escherichia coli whole cells. Catalysts 2020, 10, 947. [Google Scholar] [CrossRef]
- Marcobal, A.; De Las Rivas, B.; Landete, J.M.; Tabera, L.; Muñoz, R. Tyramine and phenylethylamine biosynthesis by food bacteria. Crit. Rev. Food Sci. Nutr. 2012, 52, 448–467. [Google Scholar] [CrossRef]
- Zhou, N.; Zhang, A.; Wei, G.; Yang, S.; Xu, S.; Chen, K.; Ouyang, P. Cadaverine production from L-lysine with chitin-binding protein-mediated lysine decarboxylase immobilization. Front. Bioeng. Biotechnol. 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Wang, Y.; Cheng, Y.; Liu, Y.; Yadav, M.P.; Yin, L. Reduction of biogenic amines in sufu by ethanol addition during ripening stage. Food Chem. 2018, 239, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, D.; Shi, R.; Wang, J.; Guo, S.; Ma, Y.; Xiong, K. Effects of microbial community succession on volatile profiles and biogenic amine during sufu fermentation. LWT-Food Sci. Technol. 2019, 114, 108379. [Google Scholar] [CrossRef]
- Gu, J.; Liu, T.; Hou, J.; Pan, L.; Sadiq, F.A.; Yuan, L.; Yang, H.; He, G. Analysis of bacterial diversity and biogenic amines content during the fermentation processing of stinky tofu. Food Res. Int. 2018, 111, 689–698. [Google Scholar] [CrossRef]
- Shaffer, B.T.; Lighthart, B. Survey of culturable airborne bacteria at four diverse locations in Oregon: Urban, rural, forest, and coastal. Microb. Ecol. 1997, 34, 167–177. [Google Scholar] [CrossRef]
- Kim, M.J.; Kwak, H.S.; Jung, H.Y.; Kim, S.S. Microbial communities related to sensory attributes in Korean fermented soy bean paste (doenjang). Food Res. Int. 2016, 89, 724–732. [Google Scholar] [CrossRef]
- Li, Z.; Rui, J.; Li, X.; Li, J.; Dong, L.; Huang, Q.; Huang, C.; Wang, Z.; Li, L.; Xuan, P.; et al. Bacterial community succession and metabolite changes during doubanjiang-meju fermentation, a Chinese traditional fermented broad bean (Vicia faba L.) paste. Food Chem. 2017, 218, 534–542. [Google Scholar] [CrossRef]
- Liang, H.; Chen, H.; Zhang, W.; Yu, C.; Ji, C.; Lin, X. Investigation on microbial diversity of industrial Zhacai paocai during fermentation using high-throughput sequencing and their functional characterization. LWT-Food Sci. Technol. 2018, 91, 460–466. [Google Scholar] [CrossRef]
- Liang, T.; Xie, X.; Ma, J.; Wu, L.; Xi, Y.; Zhao, H.; Li, L.; Li, H.; Feng, Y.; Xue, L.; et al. Microbial communities and physicochemical characteristics of traditional dajiang and sufu in North China revealed by high-throughput sequencing of 16S rRNA. Front. Microbiol. 2021, 12, 665243. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, Y.; Yin, L.; Wang, J.; Li, L. Effects of processing and NaCl on angiotensin I-converting enzyme inhibitory activity and γ-aminobutyric acid content during sufu manufacturing. Food Bioprocess Technol. 2013, 6, 1782–1789. [Google Scholar] [CrossRef]
- Xu, D.; Wang, P.; Zhang, X.; Zhang, J.; Sun, Y.; Gao, L.; Wang, W. High-throughput sequencing approach to characterize dynamic changes of the fungal and bacterial communities during the production of sufu, a traditional Chinese fermented soybean food. Food Microbiol. 2020, 86, 103340. [Google Scholar] [CrossRef] [PubMed]
- Han, B.Z.; Wang, J.H.; Rombouts, F.M.; Nout, M.R. Effect of NaCl on textural changes and protein and lipid degradation during the ripening stage of sufu, a Chinese fermented soybean food. J. Sci. Food Agric. 2003, 83, 899–904. [Google Scholar] [CrossRef]
- Wójcik, W.; Łukasiewicz, M.; Puppel, K. Biogenic amines: Formation, action and toxicity—A review. J. Sci. Food Agric. 2021, 101, 2634–2640. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef]
- ten Brink, B.; Damink, C.; Joosten, H.M.L.J.; Huis in’t Veld, J.H.J. Occurrence and formation of biologically active amines in foods. Int. J. Food Microbiol. 1990, 11, 73–84. [Google Scholar] [CrossRef]
- Hao, Y.; Sun, B. Analysis of bacterial diversity and biogenic amines content during fermentation of farmhouse sauce from Northeast China. Food Control 2020, 108, 106861. [Google Scholar] [CrossRef]
- Gao, X.; Li, C.; He, R.; Zhang, Y.; Wang, B.; Zhang, Z.H.; Ho, C.T. Research advances on biogenic amines in traditional fermented foods: Emphasis on formation mechanism, detection and control methods. Food Chem. 2023, 405, 134911. [Google Scholar] [CrossRef]
- Freudenberg, R.A.; Wittemeier, L.; Einhaus, A.; Baier, T.; Kruse, O. Advanced pathway engineering for phototrophic putrescine production. Plant Biotechnol. J. 2022, 20, 1968–1982. [Google Scholar] [CrossRef]
- Sang, X.; Li, K.; Zhu, Y.; Ma, X.; Hao, H.; Bi, J.; Zhang, G.; Hou, H. The impact of microbial diversity on biogenic amines formation in grasshopper sub shrimp paste during the fermentation. Front. Microbiol. 2020, 11, 782. [Google Scholar] [CrossRef]
- Aymerich, T.; Martín, B.; Garriga, M.; Vidal-Carou, M.C.; Bover-Cid, S.; Hugas, M. Safety properties and molecular strain typing of lactic acid bacteria from slightly fermented sausages. J. Appl. Microbiol. 2006, 100, 40–49. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Liu, Y.; Xu, Q.; Yu, Y.; Zheng, G.; Wang, Y.; Zhang, Q.; Xu, X.; Zhang, N.; Chu, J.; et al. Microbial Community Succession and Its Correlation with Quality Characteristics during Gray Sufu Fermentation. Foods 2023, 12, 2767. https://doi.org/10.3390/foods12142767
Zhao L, Liu Y, Xu Q, Yu Y, Zheng G, Wang Y, Zhang Q, Xu X, Zhang N, Chu J, et al. Microbial Community Succession and Its Correlation with Quality Characteristics during Gray Sufu Fermentation. Foods. 2023; 12(14):2767. https://doi.org/10.3390/foods12142767
Chicago/Turabian StyleZhao, Lei, Yang Liu, Qiong Xu, Yi Yu, Guojian Zheng, Yue Wang, Qingping Zhang, Xiaoqian Xu, Nana Zhang, Jiayue Chu, and et al. 2023. "Microbial Community Succession and Its Correlation with Quality Characteristics during Gray Sufu Fermentation" Foods 12, no. 14: 2767. https://doi.org/10.3390/foods12142767
APA StyleZhao, L., Liu, Y., Xu, Q., Yu, Y., Zheng, G., Wang, Y., Zhang, Q., Xu, X., Zhang, N., Chu, J., Zhang, Y., Sun, Y., Zhao, Q., Zhang, Y., Qu, Q., & Zhong, J. (2023). Microbial Community Succession and Its Correlation with Quality Characteristics during Gray Sufu Fermentation. Foods, 12(14), 2767. https://doi.org/10.3390/foods12142767






