Changes in Lipids and Proteins of Common Carp (Cyprinus carpio) Fillets under Frozen Storage and Establishment of a Radial Basis Function Neural Network (RBFNN)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Thiobarbituric Acid Reactive Substances (TBARS)
2.2.2. Free Fatty Acids (FFA)
2.2.3. Salt-Soluble Protein (SSP)
2.2.4. Ca2+-ATPase Activity
2.2.5. Total Sulfhydryl (SH) Content
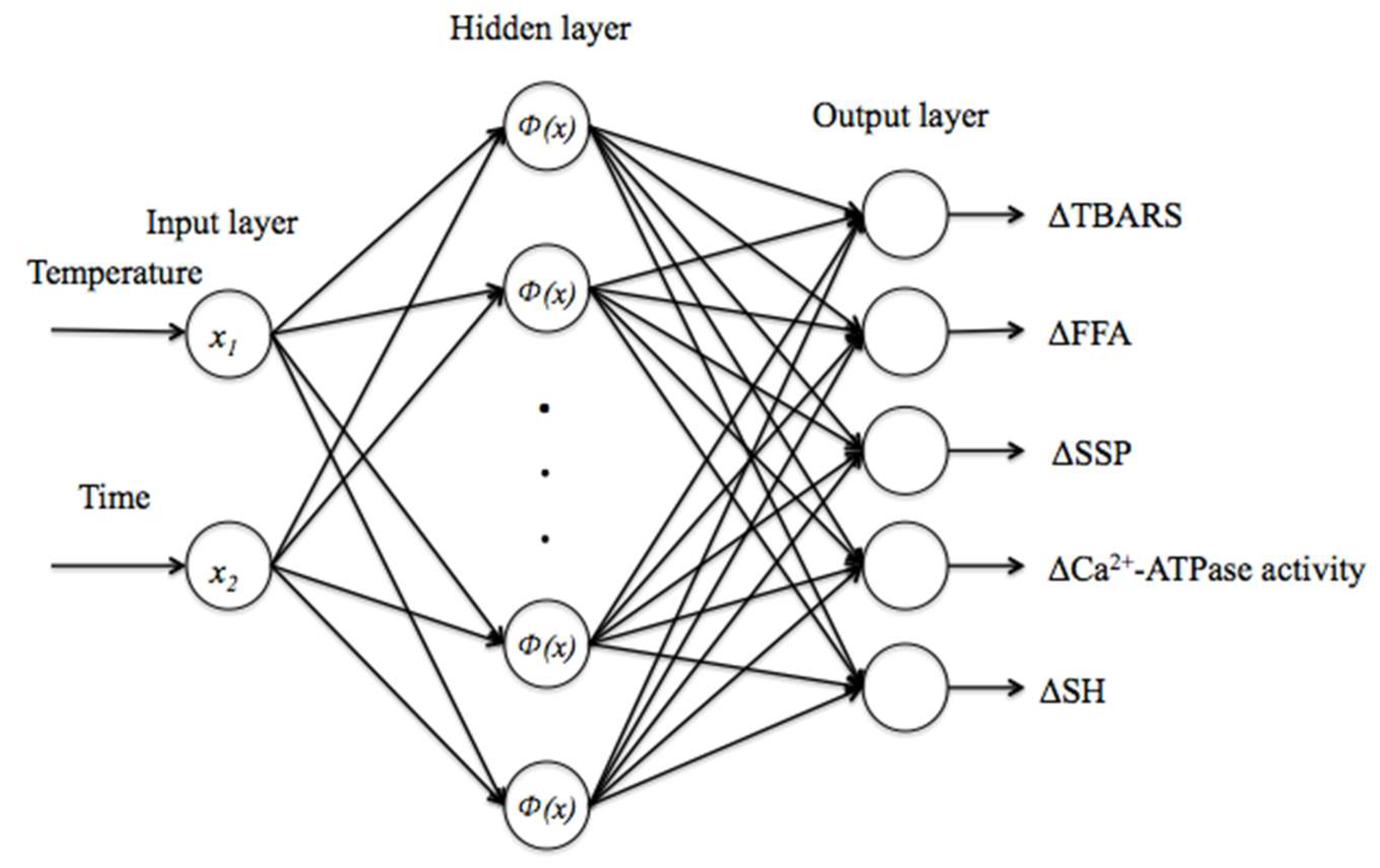
2.2.6. Radial Basis Function Neural Networks (RBFNNs)
2.3. Statistical Analysis
3. Results and Discussion
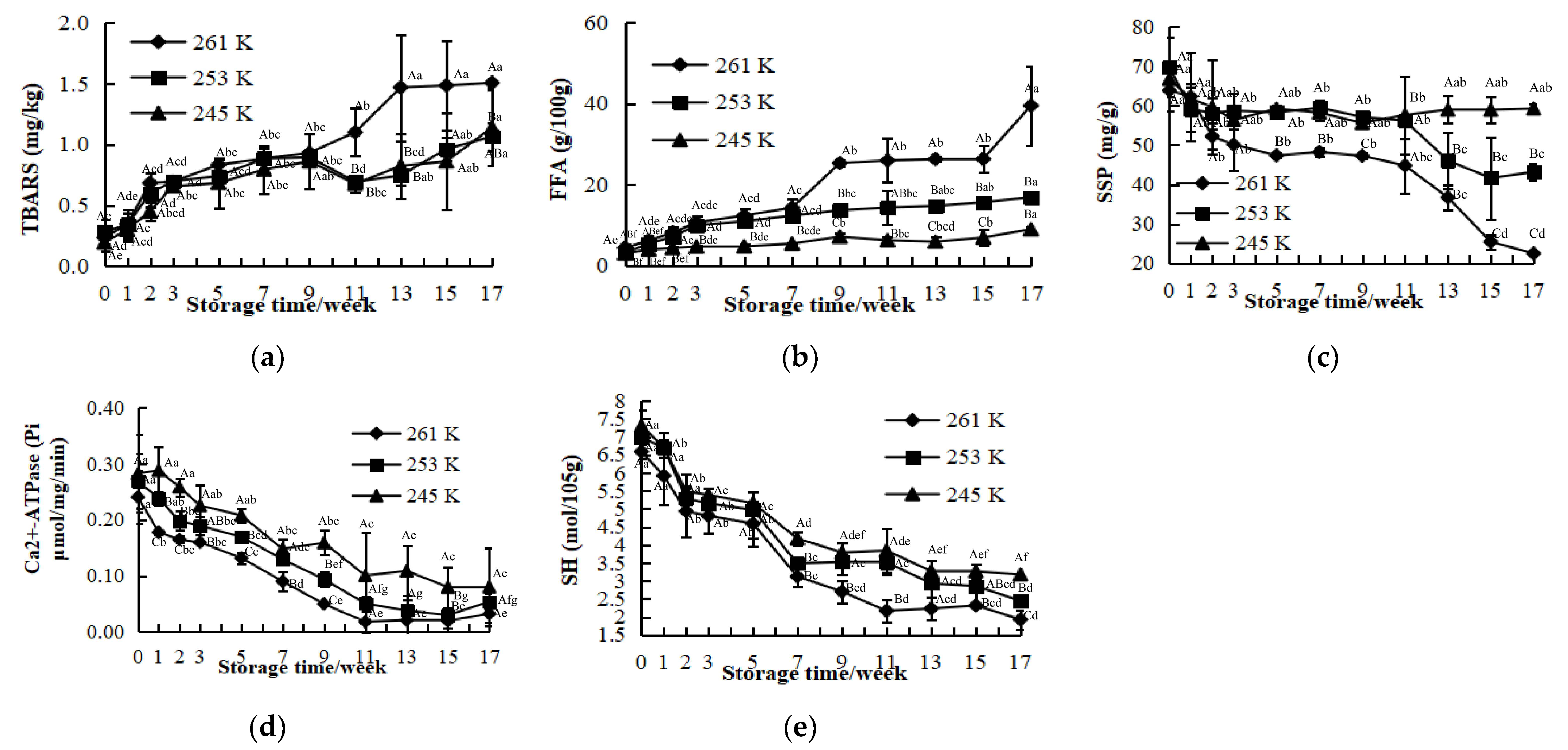
3.1. Thiobarbituric Acid Reactive Substances (TBARS)
3.2. Free Fatty Acids (FFA)
3.3. Salt-Soluble Protein (SSP)
3.4. Ca2+-ATPase Activity
3.5. Total Sulfhydryl (SH) Content
3.6. Relationship between TBARS, FFA, SSP, Ca2+-ATPase, and SH Content
3.7. Establishment and Validation of RBFNN
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hao, R.; Pan, J.; Tilami, S.K.; Shah, B.R.; Mraz, J. Post-mortem quality changes of common carp (Cyprinus carpio) during chilled storage from two culture systems. J. Sci. Food Agric. 2021, 101, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Setiadi, E.; Taufik, I.; Widyastuti, Y.R.; Ardi, I.; Saputra, A. Different substrate of trickling filter on growth, survival rate, and water quality of common carp (Cyprinus carpio) cultivation by using an intensive recirculation system. IOP Conf. Ser. Earth Environ. Sci. 2019, 236, 012027. [Google Scholar] [CrossRef]
- FAO. Understanding-Antimicrobial-Resistance-in-Aquaculture; Asian Fisheries Society: Rome, Italy, 2020. [Google Scholar]
- FAO. Fisheries Situation Report, January to December 2019; Philippine Statistics Authority: Quezon City, Philippines, 2020. [Google Scholar]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attina, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef] [PubMed]
- Walayat, N.; Rincón, M.Á.; Niaz, S.; Nawaz, A.; Niaz, N.; Zahid Farooq, M.; Ahmad, I.; Wang, P.; Zhang, Z. Egg white proteins and β-cyclodextrin: Effective cryoprotectant mixture against oxidative changes in the myofibrillar proteins of Culter alburnus. Int. J. Food Sci. Technol. 2021, 56, 4009–4016. [Google Scholar] [CrossRef]
- Trigo, M.; Rodríguez, A.; Dovale, G.; Pastén, A.; Vega-Gálvez, A.; Aubourg, S.P. The effect of glazing based on saponin-free quinoa (Chenopodium quinoa) extract on the lipid quality of frozen fatty fish. LWT 2018, 98, 231–236. [Google Scholar] [CrossRef]
- Carrera, M.; Fidalgo, L.G.; Vazquez, M.; Saraiva, J.A.; Aubourg, S.P. Comparative effect of a previous 150-MPa treatment on the quality loss of frozen hake stored at different temperatures. J. Sci. Food Agr. 2020, 100, 4245–4251. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, S. Pullulan suppresses the denaturation of myofibrillar protein of grass carp (Ctenopharyngodon idella) during frozen storage. Int. J. Biol. Macromol. 2018, 112, 1171–1174. [Google Scholar] [CrossRef]
- Tatiyaborworntham, N.; Oz, F.; Richards, M.P.; Wu, H. Paradoxical effects of lipolysis on the lipid oxidation in meat and meat products. Food Chem. X 2022, 14, 100317. [Google Scholar] [CrossRef]
- Masniyom, P. Deterioration and shelf-life extension of fish and fishery products by modified atmosphere packaging. Songklanakarin J. Sci. Technol. 2011, 33, 181–192. [Google Scholar]
- Aubourg, S.P. Review: Loss of Quality during the Manufacture of Canned Fish Products. Food Sci. Technol. Int. 2016, 7, 199–215. [Google Scholar] [CrossRef]
- Hematyar, N.; Masilko, J.; Mraz, J.; Sampels, S. Nutritional quality, oxidation, and sensory parameters in fillets of common carp (Cyprinus carpio L.) influenced by frozen storage (−20 °C). J. Food Process. Preserv. 2018, 42, e13589. [Google Scholar] [CrossRef]
- Van Hecke, T.; Goethals, S.; Vossen, E.; De Smet, S. Long-Chain n-3 PUFA Content and n-6/n-3 PUFA Ratio in Mammal, Poultry, and Fish Muscles Largely Explain Differential Protein and Lipid Oxidation Profiles Following In Vitro Gastrointestinal Digestion. Mol. Nutr. Food Res. 2019, 63, e1900404. [Google Scholar] [CrossRef]
- Hematyar, N.; Rustad, T.; Sampels, S.; Kastrup Dalsgaard, T. Relationship between lipid and protein oxidation in fish. Aquac. Res. 2019, 50, 1393–1403. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Diao, X.; Kong, B.; Xia, X. Moisture migration, microstructure damage and protein structure changes in porcine longissimus muscle as influenced by multiple freeze-thaw cycles. Meat Sci. 2017, 133, 10–18. [Google Scholar] [CrossRef]
- Wang, Y.; Miyazaki, R.; Saitou, S.; Hirasaka, K.; Takeshita, S.; Tachibana, K.; Taniyama, S. The effect of ice crystals formations on the flesh quality of frozen horse mackerel (Trachurus japonicus). J. Texture Stud. 2018, 49, 485–491. [Google Scholar] [CrossRef]
- Tian, J.; Walayat, N.; Ding, Y.; Liu, J. The role of trifunctional cryoprotectants in the frozen storage of aquatic foods: Recent developments and future recommendations. Compr. Rev. Food Sci. Food Saf. 2022, 21, 321–339. [Google Scholar] [CrossRef]
- Ke, P.J.; Cervantes, E.; Robles-Martinez, C. Determination of Thiobarbituric Acid Reactive Substances (TBARS) in Fish Tissue by an Improved Distillation-Spectrophotometric Method. J. Sci. Food Agric. 1984, 35, 1248–1254. [Google Scholar] [CrossRef]
- Sowa-Kucma, M.; Styczen, K.; Siwek, M.; Misztak, P.; Nowak, R.J.; Dudek, D.; Rybakowski, J.K.; Nowak, G.; Maes, M. Lipid Peroxidation and Immune Biomarkers Are Associated with Major Depression and Its Phenotypes, Including Treatment-Resistant Depression and Melancholia. Neurotox. Res. 2018, 33, 448–460. [Google Scholar] [CrossRef]
- Zeng, N.; Huang, R.; Li, N.; Jiang, H.; Li, R.; Wang, F.; Chen, W.; Xia, M.; Wang, Q. MiR-451a attenuates free fatty acids-mediated hepatocyte steatosis by targeting the thyroid hormone responsive spot 14 gene. Mol. Cell Endocrinol. 2018, 474, 260–271. [Google Scholar] [CrossRef]
- Pavillard, L.E.; Marin-Aguilar, F.; Bullon, P.; Cordero, M.D. Cardiovascular diseases, NLRP3 inflammasome, and western dietary patterns. Pharmacol. Res. 2018, 131, 44–50. [Google Scholar] [CrossRef]
- Lee, K.R.; Midgette, Y.; Shah, R. Fish Oil Derived Omega 3 Fatty Acids Suppress Adipose NLRP3 Inflammasome Signaling in Human Obesity. J. Endocr. Soc. 2019, 3, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Andevari, G.T.; Rezaei, M. Effect of gelatin coating incorporated with cinnamon oil on the quality of fresh rainbow trout in cold storage. Int. J. Food Sci. Technol. 2011, 46, 2305–2311. [Google Scholar] [CrossRef]
- Yin, C.; Wang, J.; Qian, J.; Xiong, K.; Zhang, M. Quality changes of rainbow trout stored under different packaging conditions and mathematical modeling for predicting the shelf life. Food Packag. Shelf Life 2022, 32, 100824. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Y.; Shi, W.; Wang, X. Comparison of Arrhenius model and artificial neuronal network for predicting quality changes of frozen tilapia (Oreochromis niloticus). Food Chem. 2022, 372, 131268. [Google Scholar] [CrossRef]
- Chen, S.; Tao, F.; Pan, C.; Hu, X.; Ma, H.; Li, C.; Zhao, Y.; Wang, Y. Modeling quality changes in Pacific white shrimp (Litopenaeus vannamei) during storage: Comparison of the Arrhenius model and Random Forest model. J. Food Process. Preserv. 2020, 45, e14999. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, X.; Wang, H.; Hong, H.; Luo, Y. Comparison between the Arrhenius model and the radial basis function neural network (RBFNN) model for predicting quality changes of frozen shrimp (Solenocera melantho). Int. J. Food Prop. 2017, 20, 2711–2723. [Google Scholar] [CrossRef]
- Li, Q.; Xiong, Q.; Ji, S.; Yu, Y.; Wu, C.; Yi, H. A method for mixed data classification base on RBF-ELM network. Neurocomputing 2021, 431, 7–22. [Google Scholar] [CrossRef]
- Yu, J.; Zhan, J.; Huang, W. Identification of Wine According to Grape Variety Using Near-Infrared Spectroscopy Based on Radial Basis Function Neural Networks and Least-Squares Support Vector Machines. Food Anal. Methods 2017, 10, 3306–3311. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Chen, J.; Lv, Y.; Luo, Y. Quality Attributes and Shelf Life Modeling of Pacific White Shrimp (Litopenaeus vannamei) Stored at Different Temperatures. J. Aquat. Food Prod. Technol. 2018, 27, 998–1008. [Google Scholar] [CrossRef]
- Geng, Z.; Liu, F.; Shang, D.; Han, Y.; Shang, Y.; Chu, C. Early warning and control of food safety risk using an improved AHC-RBF neural network integrating AHP-EW. J. Food Eng. 2021, 292, 110239. [Google Scholar] [CrossRef]
- Noori, S.M.A.; Khanzadi, S.; Fazlara, A.; Najafzadehvarzi, H.; Azizzadeh, M. Effect of lactic acid and ajwain (Carum copticum) on the biogenic amines and quality of refrigerated common carp (Cyprinus carpio). Lwt 2018, 97, 434–439. [Google Scholar] [CrossRef]
- Kong, C.; Wang, H.; Li, D.; Zhang, Y.; Pan, J.; Zhu, B.; Luo, Y. Quality changes and predictive models of radial basis function neural networks for brined common carp (Cyprinus carpio) fillets during frozen storage. Food Chem. 2016, 201, 327–333. [Google Scholar] [CrossRef]
- Shi, J.; Lei, Y.; Shen, H.; Hong, H.; Yu, X.; Zhu, B.; Luo, Y. Effect of glazing and rosemary (Rosmarinus officinalis) extract on preservation of mud shrimp (Solenocera melantho) during frozen storage. Food Chem. 2019, 272, 604–612. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Gao, S.; Bao, Y.; Tan, Y.; Luo, Y.; Li, X.; Hong, H. Effect of protein oxidation in meat and exudates on the water holding capacity in bighead carp (Hypophthalmichthys nobilis) subjected to frozen storage. Food Chem. 2022, 370, 131079. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, J.; Liu, Y.; Zheng, Y.; Pi, R.; Mubango, E.; Tan, Y.; Luo, Y.; Hong, H. Inhibitive effect of cryoprotectants on the oxidative and structural changes in myofibrillar proteins of unwashed mince from silver carp during frozen storage. Food Res. Int. 2022, 161, 111880. [Google Scholar] [CrossRef]
- Lin, J.; Hong, H.; Zhang, L.; Zhang, C.; Luo, Y. Antioxidant and cryoprotective effects of hydrolysate from gill protein of bighead carp (Hypophthalmichthys nobilis) in preventing denaturation of frozen surimi. Food Chem. 2019, 298, 124868. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, F.; Zhang, L.; Wang, H.; Wang, X.-c. Effect of different extent of protein oxidation on the frozen storage stability of muscle protein in obscure pufferfish (Takifugu obscurus). Lwt 2021, 137, 110416. [Google Scholar] [CrossRef]
- Han, H.G.; Chen, Q.L.; Qiao, J.F. An efficient self-organizing RBF neural network for water quality prediction. Neural Netw. 2011, 24, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Javed, M.; Cheng, M.; Xiong, S.; Liu, Y. Gelatin hydrolysates from sliver carp (Hypophthalmichthys molitrix) improve the antioxidant and cryoprotective properties of unwashed frozen fish mince. Int. J. Food Sci. Technol. 2021, 57, 2619–2627. [Google Scholar] [CrossRef]
- Klein, R.D.; Rosa, C.E.; Colares, E.P.; Robaldo, R.B.; Martinez, P.E.; Bianchini, A. Antioxidant defense system and oxidative status in Antarctic fishes: The sluggish rockcod Notothenia coriiceps versus the active marbled notothen Notothenia rossii. J. Therm. Biol. 2017, 68 (Pt A), 119–127. [Google Scholar] [CrossRef]
- Yu, Y.J.; Yang, S.P.; Lin, T.; Qian, Y.F.; Xie, J.; Hu, C. Effect of Cold Chain Logistic Interruptions on Lipid Oxidation and Volatile Organic Compounds of Salmon (Salmo salar) and Their Correlations With Water Dynamics. Front. Nutr. 2020, 7, 155. [Google Scholar] [CrossRef]
- Aguiar Saldanha Pinheiro, A.C.; Tappi, S.; Patrignani, F.; Lanciotti, R.; Romani, S.; Rocculi, P. Effects of novel modified atmosphere packaging on lipid quality and stability of sardine (Sardina pilchardus) fillets. Int. J. Food Sci. Technol. 2020, 56, 945–953. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, Q.; Sun, D.-W. Measuring and controlling ice crystallization in frozen foods: A review of recent developments. Trends Food Sci. Technol. 2019, 90, 13–25. [Google Scholar] [CrossRef]
- Jia, H.; Roy, K.; Pan, J.; Mraz, J. Icy affairs: Understanding recent advancements in the freezing and frozen storage of fish. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1383–1408. [Google Scholar] [CrossRef]
- Li, T.; Kuang, S.; Xiao, T.; Hu, L.; Nie, P.; Ramaswamy, H.S.; Yu, Y. The Effect of Pressure-Shift Freezing versus Air Freezing and Liquid Immersion on the Quality of Frozen Fish during Storage. Foods 2022, 11, 1842. [Google Scholar] [CrossRef]
- Tanaka, R.; Nakazawa, N.; Fukushima, H.; Watanabe, M.; Maekawa, K.; Okano, T.; Hiraishi, K.; Okazaki, E. Effects of Initial Freshness Level, Frozen Storage Temperature, and Storage Period on Lipid Deterioration and K-value in Meat Blocks from Chub Mackerel Scomber japonicus. J. Aquat. Food Prod. Technol. 2021, 31, 47–59. [Google Scholar] [CrossRef]
- Bejaoui, S.; Ghribi, F.; Chetoui, I.; Aouini, F.; Bouaziz, M.; Houas-Gharsallah, I.; Soudani, N.; El Cafsi, M. Effect of storage temperature and time on the fatty acids and nutritional quality of the commercial mussel (Mytilus galloprovincialis). J. Food Sci. Technol. 2021, 58, 3493–3503. [Google Scholar] [CrossRef]
- Gomez-Estaca, J.; Gomez-Guillen, M.C.; Marin-Penalver, D.; Montero, M.P. Functional aptitude of hake minces with added TMAO-demethylase inhibitors during frozen storage. Food Chem. 2020, 309, 125683. [Google Scholar] [CrossRef]
- Shi, L.; Yang, T.; Xiong, G.; Li, X.; Wang, X.; Ding, A.; Qiao, Y.; Wu, W.; Liao, L.; Wang, L. Influence of frozen storage temperature on the microstructures and physicochemical properties of pre-frozen perch (Micropterus salmoides). LWT 2018, 92, 471–476. [Google Scholar] [CrossRef]
- Gullian-Klanian, M.; Terrats-Preciat, M.; Pech-Jiménez, E.C.; Cutz De Ocampo, J. Effect of Frozen Storage on Protein Denaturation and Fatty Acids Profile of the Red Octopus (Octopus maya). J. Food Process. Preserv. 2017, 41, e13072. [Google Scholar] [CrossRef]
- Odoli, C.O.; Oduor-Odote, P.; Arason, S. The influence of lipid content and pretreatment methods on protein conformation in fish (capelin, Mallotus villosus) during smoking and drying. Food Sci. Nutr. 2019, 7, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Sriket, P.; La-ongnual, T. Quality Changes and Discoloration of Basa (Pangasius bocourti) Fillet during Frozen Storage. J. Chem. 2018, 2018, 5159080. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Z.; Luo, Y.; Hong, H.; Shen, H. Quality Changes and Establishment of Predictive Models for Bighead Carp (Aristichthys nobilis) Fillets During Frozen Storage. Food Bioprocess. Technol. 2014, 7, 3381–3389. [Google Scholar] [CrossRef]
- Konno, K. Myosin Denaturation Study for the Quality Evaluation of Fish Muscle-based Products. Food Sci. Technol. Res. 2017, 23, 9–21. [Google Scholar] [CrossRef]
- Welle, M.; Pedersen, J.T.; Ravnsborg, T.; Hayashi, M.; Maass, S.; Becher, D.; Jensen, O.N.; Stohr, C.; Palmgren, M. A conserved, buried cysteine near the P-site is accessible to cysteine modifications and increases ROS stability in the P-type plasma membrane H+-ATPase. Biochem. J. 2021, 478, 619–632. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Zhao, L.; Chen, L.; He, Y.; Yang, H. Vacuum impregnation of fish gelatin combined with grape seed extract inhibits protein oxidation and degradation of chilled tilapia fillets. Food Chem. 2019, 294, 316–325. [Google Scholar] [CrossRef]
- Cai, L.; Nian, L.; Cao, A.; Zhang, Y.; Li, X. Effect of Carboxymethyl Chitosan Magnetic Nanoparticles Plus Herring Antifreeze Protein on Conformation and Oxidation of Myofibrillar Protein From Red Sea Bream (Pagrosomus major) After Freeze-Thaw Treatment. Food Bioprocess. Technol. 2019, 13, 355–366. [Google Scholar] [CrossRef]
- Gao, W.; Huang, Y.; Zeng, X.A.; Brennan, M.A. Effect of soluble soybean polysaccharides on freeze-denaturation and structure of myofibrillar protein of bighead carp surimi with liquid nitrogen freezing. Int. J. Biol. Macromol. 2019, 135, 839–844. [Google Scholar] [CrossRef]
- Qian, P.; Zhang, Y.; Shen, Q.; Ren, L.; Jin, R.; Xue, J.; Yao, H.; Dai, Z. Effect of cryogenic immersion freezing on quality changes of vacuum-packed bighead carp (Aristichthys nobilis) during frozen storage. J. Food Process. Preserv. 2018, 42, e13640. [Google Scholar] [CrossRef]
- Lu, H.; Liang, Y.; Zhang, L.; Shi, J. Modeling relationship between protein oxidation and denaturation and texture, moisture loss of bighead carp (Aristichthys Nobilis) during frozen storage. J. Food Sci. 2021, 86, 4430–4443. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Fu, Z.; Zhang, H.; Zhang, L.; Li, B.; Tan, Y.; Hong, H.; Luo, Y. Uncovering quality changes of salted bighead carp fillets during frozen storage: The potential role of time-dependent protein denaturation and oxidation. Food Chem. 2023, 414, 135714. [Google Scholar] [CrossRef]
- Kaymak-Ertekin, F.; Gedik, A. Kinetic modelling of quality deterioration in onions during drying and storage. J. Food Eng. 2005, 68, 443–453. [Google Scholar] [CrossRef]
| Indicators | TBARS | FFA | SSP | Ca2+-ATPase | SH | |
|---|---|---|---|---|---|---|
| TBARS | Person’s correlation | 1 | 0.82 ** | −0.84 ** | −0.85 ** | −0.91 ** |
| FFA | Person’s correlation | 1 | −0.90 ** | −0.77 ** | −0.76 ** | |
| SSP | Person’s correlation | 1 | 0.74 ** | 0.74 ** | ||
| Ca2+-ATPase | Person’s correlation | 1 | 0.96 ** | |||
| SH | Person’s correlation | 1 | ||||
| Neurons | MSE | Neurons | MSE | Spread | MSE |
|---|---|---|---|---|---|
| 0 | 0.26884 | 16 | 0.00638 | 0.05 | 0.00191 |
| 2 | 0.13414 | 18 | 0.00488 | 0.10 | 0.00014 |
| 4 | 0.07946 | 20 | 0.00313 | 0.50 | 0.00006 |
| 6 | 0.04552 | 22 | 0.00204 | 1.00 | 0.00011 |
| 8 | 0.02378 | 24 | 0.00147 | 1.50 | 0.00020 |
| 10 | 0.01771 | 26 | 0.00077 | 2.00 | 0.00059 |
| 12 | 0.01144 | 28 | 0.00006 | 2.50 | 0.00114 |
| 14 | 0.00815 | 3.00 | 0.00157 |
| Parameters | Storage Time (Week) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | 7 | 9 | 11 | 13 | 15 | 17 | ||
| TBARS | Predicted value | 0.34 | 0.59 | 0.70 | 0.74 | 0.88 | 0.89 | 0.69 | 0.74 | 0.96 | 1.06 |
| Experimental value | 0.34 ± 0.09 | 0.60 ± 0.11 | 0.70 ± 0.01 | 0.74 ± 0.02 | 0.88 ± 0.08 | 0.89 ± 0.04 | 0.69 ± 0.05 | 0.75 ± 0.08 | 0.96 ± 0.09 | 1.06 ± 0.11 | |
| Relative errors (%) | −0.66 | 0.77 | −0.52 | −0.35 | 0.19 | 0.23 | 0.19 | 0.33 | −0.00 | 0.19 | |
| FFA | Predicted value | 5.23 | 7.42 | 9.48 | 11.18 | 12.02 | 13.82 | 14.14 | 14.79 | 15.54 | 16.82 |
| Experimental value | 5.34 ± 0.96 | 7.06 ± 0.60 | 9.88 ± 0.33 | 10.90 ± 1.27 | 12.27 ± 1.43 | 13.61 ± 0.68 | 14.30 ± 4.35 | 14.69 ± 0.25 | 15.59 ± 1.29 | 16.81 ± 0.99 | |
| Relative errors (%) | 2.05 | −5.16 | 4.05 | −2.56 | 2.03 | −1.56 | 1.11 | −0.69 | 0.30 | −0.06 | |
| SSP | Predicted value | 58.79 | 58.88 | 57.82 | 58.82 | 58.90 | 57.56 | 55.80 | 46.28 | 41.49 | 43.17 |
| Experimental value | 59.02 ± 5.57 | 58.10 ± 3.97 | 58.69 ± 4.48 | 58.22 ± 0.23 | 59.45 ± 1.92 | 57.08 ± 0.52 | 56.16 ± 1.03 | 46.05 ± 7.10 | 41.60 ± 10.39 | 43.14 ± 2.07 | |
| Relative errors (%) | 0.39 | −1.34 | 1.47 | −1.03 | 0.92 | −0.83 | 0.64 | −0.49 | 0.27 | −0.07 | |
| Ca2+-ATP ase | Predicted value | 0.24 | 0.20 | 0.19 | 0.17 | 0.13 | 0.10 | 0.05 | 0.04 | 0.03 | 0.06 |
| Experimental value | 0.24 ± 0.01 | 0.20 ± 0.02 | 0.19 ± 0.02 | 0.17 ± 0.01 | 0.13 ± 0.00 | 0.09 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.07 | 0.03 ± 0.01 | 0.05 ± 0.01 | |
| Relative errors (%) | −0.38 | −0.03 | 0.04 | 0.81 | 0.03 | −4.92 | 3.35 | −1.30 | 3.81 | −9.18 | |
| SH | Predicted value | 6.71 | 5.28 | 5.16 | 4.96 | 3.50 | 3.52 | 3.52 | 2.92 | 2.86 | 2.45 |
| Experimental value | 6.71 ± 0.27 | 5.29 ± 0.18 | 5.15 ± 0.02 | 4.97 ± 0.78 | 3.49 ± 0.16 | 3.53 ± 0.34 | 3.52 ± 0.33 | 2.93 ± 0.76 | 2.85 ± 0.59 | 2.45 ± 0.09 | |
| Relative errors (%) | −0.05 | 0.24 | −0.26 | 0.18 | −0.24 | 0.34 | −0.21 | 0.27 | −0.15 | 0.07 | |
| Parameters | Temperatures (K) | MSE | R2 | Parameters | Temperatures (K) | MSE | R2 |
|---|---|---|---|---|---|---|---|
| TBARS | 261 | 0.000 | 1.000 | Ca2+-ATPase activity | 261 | 0.000 | 0.999 |
| 253 | 0.000 | 1.000 | 253 | 0.000 | 0.999 | ||
| 245 | 0.000 | 1.000 | 245 | 0.000 | 0.999 | ||
| FFA | 261 | 0.000 | 1.000 | SH | 261 | 0.000 | 1.000 |
| 253 | 0.066 | 0.995 | 253 | 0.000 | 1.000 | ||
| 245 | 0.000 | 1.000 | 245 | 0.000 | 1.000 | ||
| SSP | 261 | 0.001 | 1.000 | ||||
| 253 | 0.308 | 0.994 | |||||
| 245 | 0.001 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, C.; Duan, C.; Zhang, Y.; Shi, C.; Luo, Y. Changes in Lipids and Proteins of Common Carp (Cyprinus carpio) Fillets under Frozen Storage and Establishment of a Radial Basis Function Neural Network (RBFNN). Foods 2023, 12, 2741. https://doi.org/10.3390/foods12142741
Kong C, Duan C, Zhang Y, Shi C, Luo Y. Changes in Lipids and Proteins of Common Carp (Cyprinus carpio) Fillets under Frozen Storage and Establishment of a Radial Basis Function Neural Network (RBFNN). Foods. 2023; 12(14):2741. https://doi.org/10.3390/foods12142741
Chicago/Turabian StyleKong, Chunli, Caiping Duan, Yixuan Zhang, Ce Shi, and Yongkang Luo. 2023. "Changes in Lipids and Proteins of Common Carp (Cyprinus carpio) Fillets under Frozen Storage and Establishment of a Radial Basis Function Neural Network (RBFNN)" Foods 12, no. 14: 2741. https://doi.org/10.3390/foods12142741
APA StyleKong, C., Duan, C., Zhang, Y., Shi, C., & Luo, Y. (2023). Changes in Lipids and Proteins of Common Carp (Cyprinus carpio) Fillets under Frozen Storage and Establishment of a Radial Basis Function Neural Network (RBFNN). Foods, 12(14), 2741. https://doi.org/10.3390/foods12142741





