Chitooligosaccharide from Pacific White Shrimp Shell Chitosan Ameliorates Inflammation and Oxidative Stress via NF-κB, Erk1/2, Akt and Nrf2/HO-1 Pathways in LPS-Induced RAW264.7 Macrophage Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Material
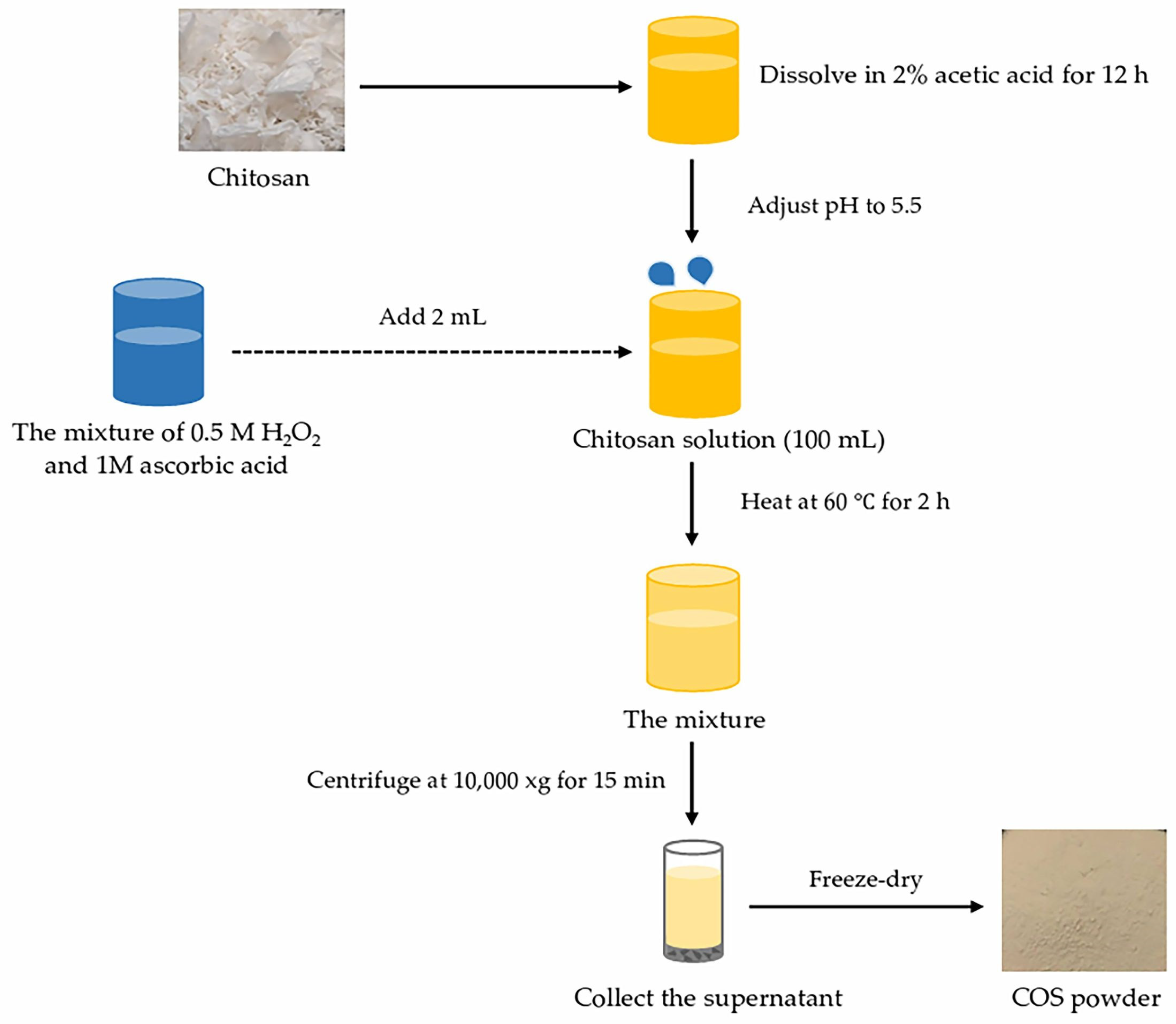
2.2. Chitooligosaccharide (COS) Production
2.3. Cell Viability Test
2.4. Inflammatory Cytokine Assay
2.5. Intracellular ROS Measurement
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
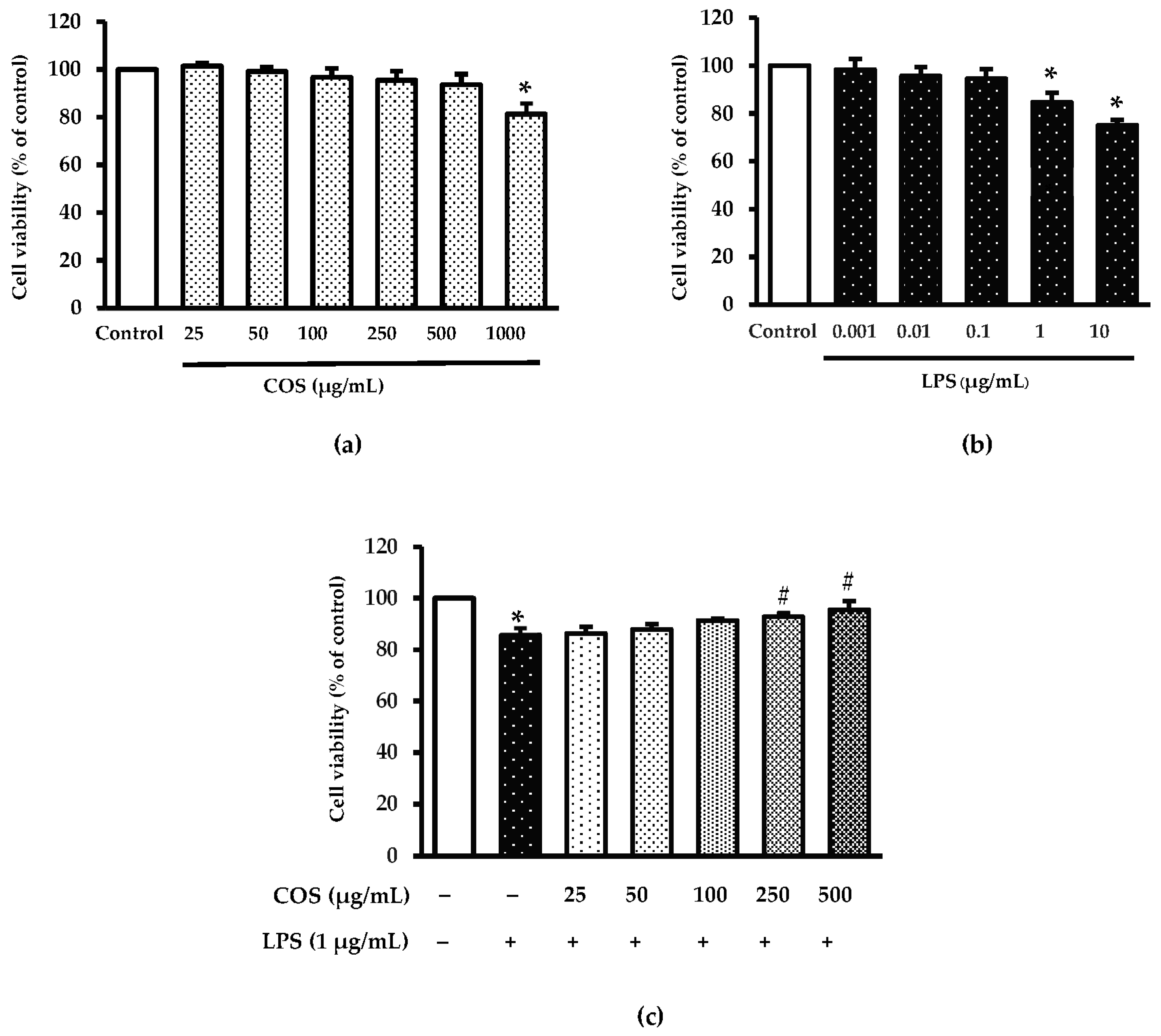
3.1. Impact of COS or LPS at Various Concentrations on Viability of RAW264.7 Macrophage Cell
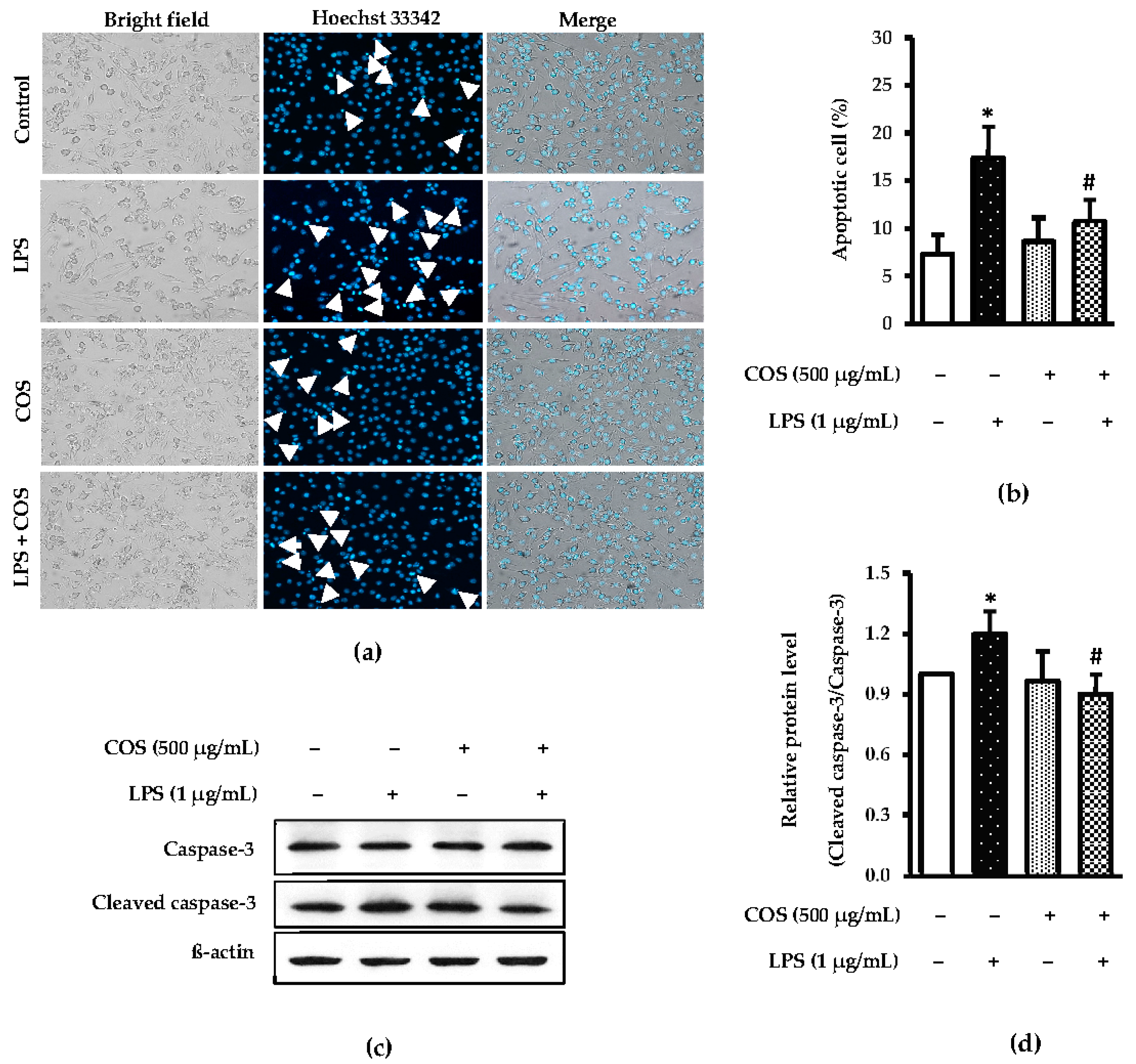
3.2. Effect of COS Combined with LPS at Selected Concentrations on Apoptotic Cells, Caspase 3 and Cleaved-Caspase 3 Expressions in LPS-Treated Cells
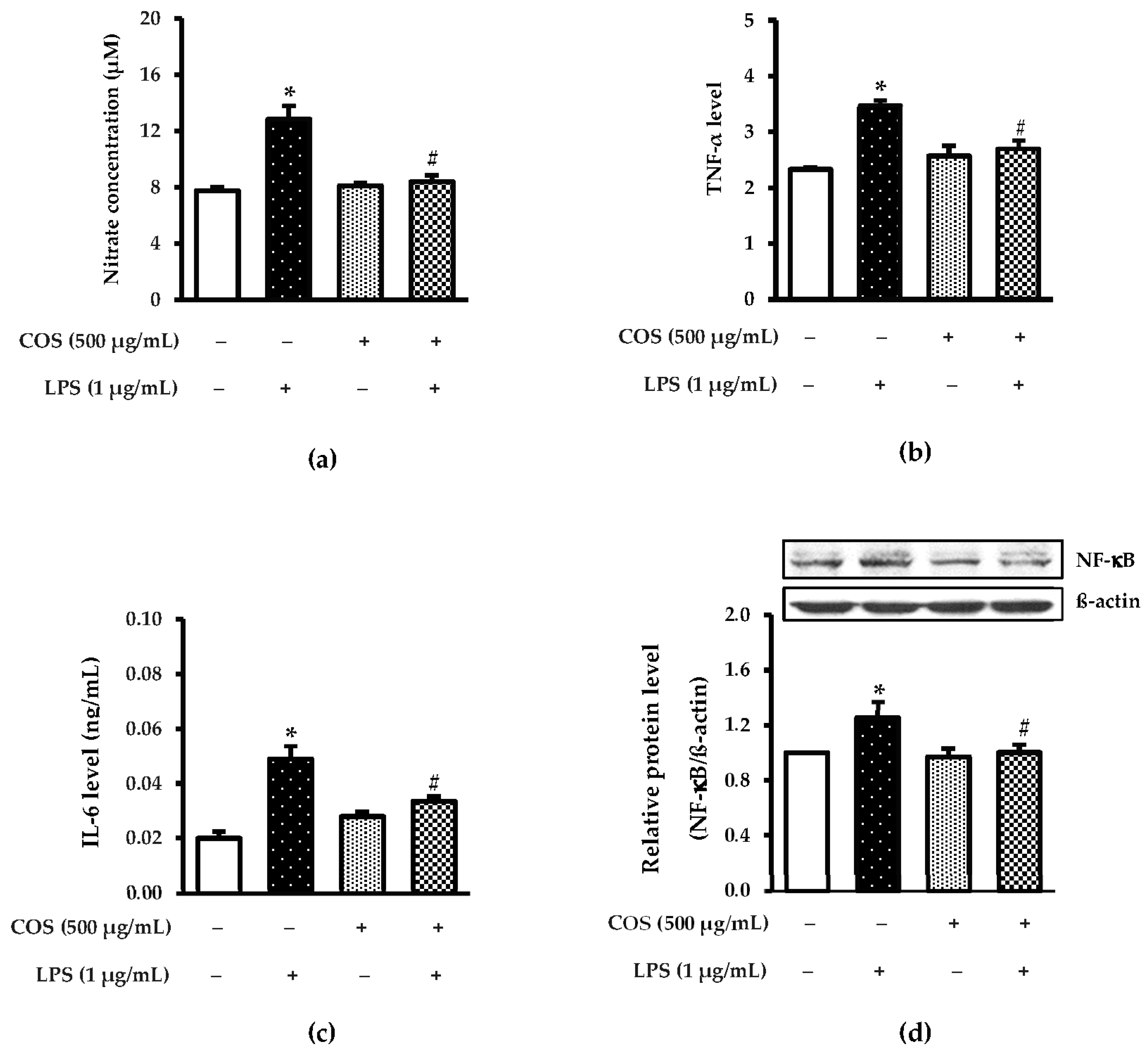
3.3. Protective Effect of COS against Inflammation
3.4. Effect of COS at Selected Concentration on ROS Prohibition of LPS-Activated RAW264.7 Cells
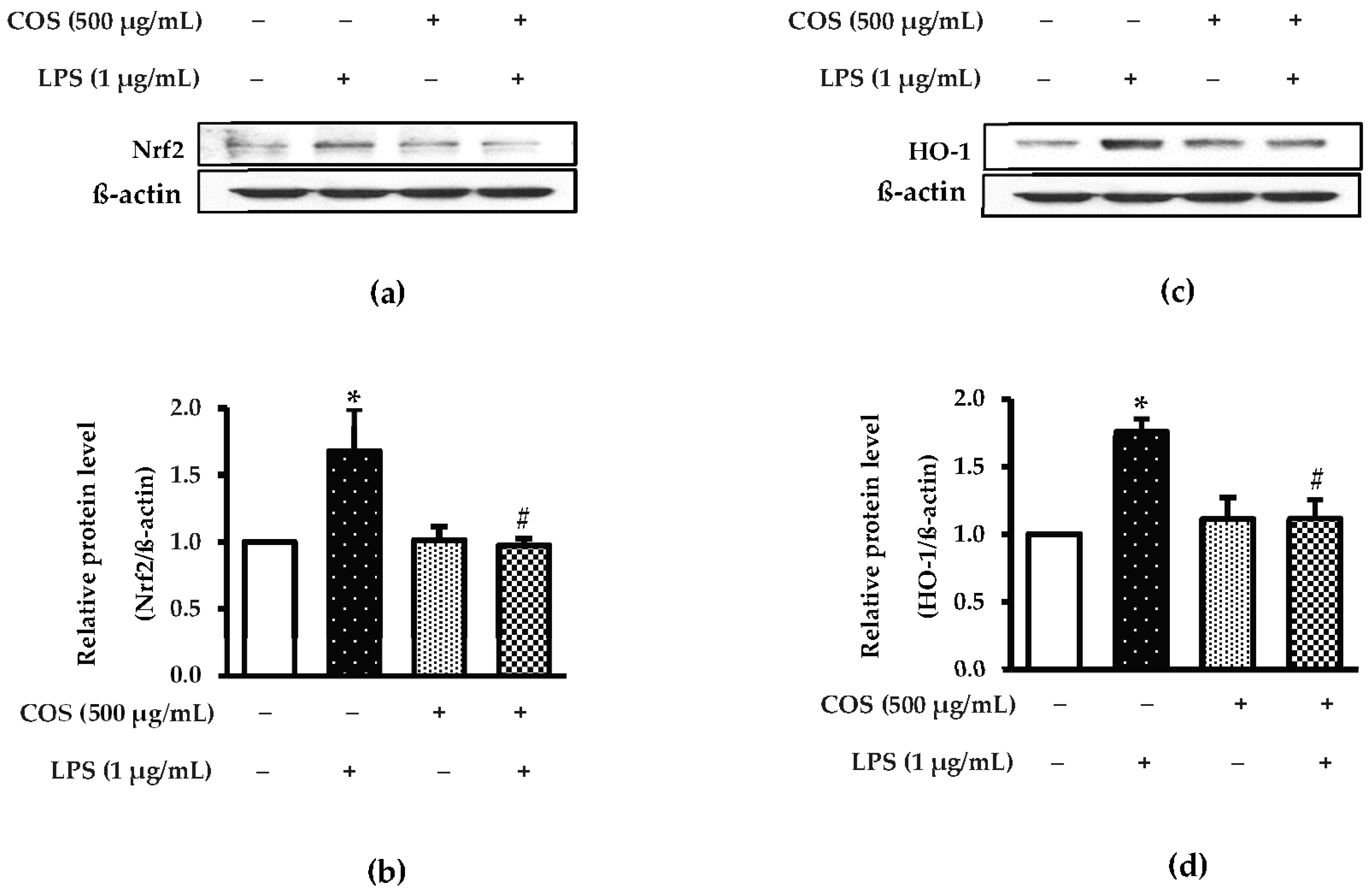
3.5. Effect of COS on Inhibition of Nrf-2 and HO-1 in LPS-Activated RAW264.7 Cells
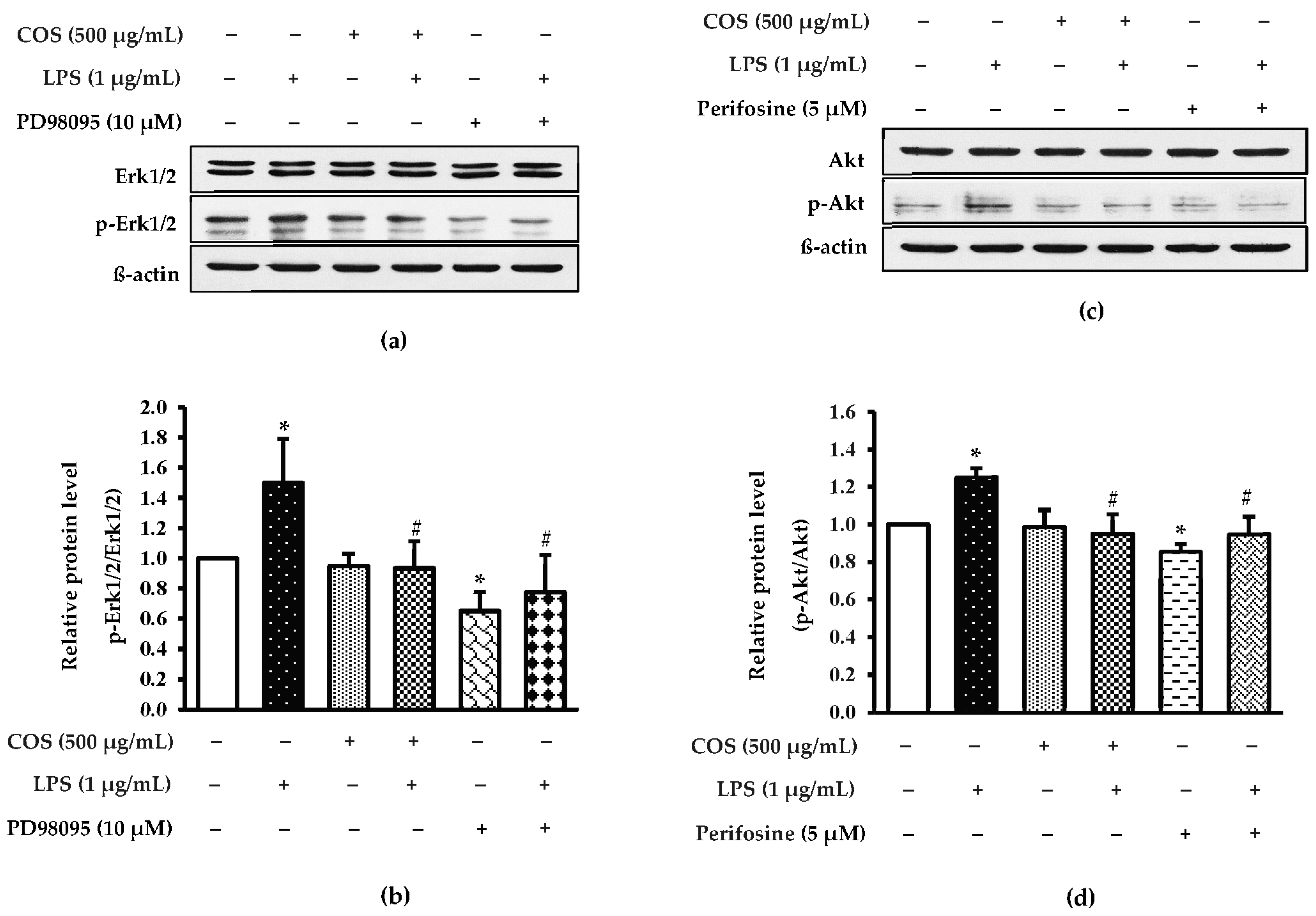
3.6. Effect of COS on Inhibition of Erk1/2 and Akt Expressions in LPS-Activated RAW264.7 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Seemann, S.; Zohles, F.; Lupp, A. Comprehensive comparison of three different animal models for systemic inflammation. J. Biomed. Sci. 2017, 24, 60. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, J.; Ren, G.; Zhang, Y.; Tan, X.; Yang, L. Punicalagin Prevents Inflammation in LPS-Induced RAW264.7 Macrophages by Inhibiting FoxO3a/Autophagy Signaling Pathway. Nutrients 2019, 11, 2794. [Google Scholar] [CrossRef]
- Li, H.; Yoon, J.H.; Won, H.J.; Ji, H.S.; Yuk, H.J.; Park, K.H.; Park, H.Y.; Jeong, T.S. Isotrifoliol inhibits pro-inflammatory mediators by suppression of TLR/NF-κB and TLR/MAPK signaling in LPS-induced RAW264.7 cells. Int. Immunopharmacol. 2017, 45, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Gao, J.; Ji, H.; Li, W.; Xing, X.; Liu, D.; Guo, Q.; Zhou, L.; Jing, F. Benzoylaconine Modulates LPS-Induced Responses Through Inhibition of Toll-Like Receptor-Mediated NF-κB and MAPK Signaling in RAW264.7 Cells. Inflammation 2021, 44, 2018–2032. [Google Scholar] [CrossRef]
- Dong, J.; Li, J.; Cui, L.; Wang, Y.; Lin, J.; Qu, Y.; Wang, H. Cortisol modulates inflammatory responses in LPS-stimulated RAW264.7 cells via the NF-κB and MAPK pathways. BMC Vet. Res. 2018, 14, 30. [Google Scholar] [CrossRef]
- Liao, W.; He, X.; Yi, Z.; Xiang, W.; Ding, Y. Chelidonine suppresses LPS-Induced production of inflammatory mediators through the inhibitory of the TLR4/NF-κB signaling pathway in RAW264.7 macrophages. Biomed. Pharmacother. 2018, 107, 1151–1159. [Google Scholar] [CrossRef]
- Xie, C.; Li, X.; Zhu, J.; Wu, J.; Geng, S.; Zhong, C. Magnesium isoglycyrrhizinate suppresses LPS-induced inflammation and oxidative stress through inhibiting NF-κB and MAPK pathways in RAW264.7 cells. Bioorg. Med. Chem. 2019, 27, 516–524. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Lin, F.; Han, K.; Wang, X. Omentin-1 attenuates lipopolysaccharide (LPS)-induced U937 macrophages activation by inhibiting the TLR4/MyD88/NF-κB signaling. Arch. Biochem. Biophys. 2020, 679, 108187. [Google Scholar] [CrossRef]
- Acaroz, U.; Ince, S.; Arslan-Acaroz, D.; Gurler, Z.; Demirel, H.H.; Kucukkurt, I.; Eryavuz, A.; Kara, R.; Varol, N.; Zhu, K. Bisphenol-A induced oxidative stress, inflammatory gene expression, and metabolic and histopathological changes in male Wistar albino rats: Protective role of boron. Toxicol. Res. 2019, 8, 262–269. [Google Scholar] [CrossRef]
- Jayakumar, T.; Huang, C.J.; Yen, T.L.; Hsia, C.W.; Sheu, J.R.; Bhavan, P.S.; Huang, W.C.; Hsieh, C.Y.; Hsia, C.H. Activation of Nrf2 by Esculetin Mitigates Inflammatory Responses through Suppression of NF-κB Signaling Cascade in RAW 264.7 Cells. Molecules 2022, 27, 5143. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Cha, H.J.; Lee, H.; Kim, G.Y.; Choi, Y.H. The regulation of the TLR4/NF-κB and Nrf-2/HO-1 signaling pathways is involved in the inhibition of lipopolysaccharide-induced inflammation and oxidative reactions by morroniside in RAW 264.7 macrophages. Arch. Biochem. Biophys. 2021, 706, 108926. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, L.; Wang, Y.; Zhai, J.; Chen, C.; Hu, K. Gambogic acid induces heme oxygenase-1 through Nrf2 signaling pathway and inhibits NF-κB and MAPK activation to reduce inflammation in LPS-activated RAW264.7 cells. Biomed. Pharmacother. 2019, 109, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Li, S.T.; Dai, Q.; Zhang, S.X.; Liu, Y.J.; Yu, Q.Q.; Tan, F.; Lu, S.H.; Wang, Q.; Chen, J.W.; Huang, H.Q.; et al. Ulinastatin attenuates LPS-induced inflammation in mouse macrophage RAW264.7 cells by inhibiting the JNK/NF-κB signaling pathway and activating the PI3K/Akt/Nrf2 pathway. Acta Pharmacol. Sin. 2018, 39, 1294–1304. [Google Scholar] [CrossRef]
- Ramazani, N.; Mahd Gharebagh, F.; Soleimanzadeh, A.; Arslan, H.O.; Keles, E.; Gradinarska-Yanakieva, D.G.; Arslan-Acaröz, D.; Zhandi, M.; Baran, A.; Ayen, E.; et al. The influence of L-proline and fulvic acid on oxidative stress and semen quality of buffalo bull semen following cryopreservation. Vet. Med. Sci. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chotphruethipong, L.; Sukketsiri, W.; Battino, M.; Benjakul, S. Conjugate between hydrolyzed collagen from defatted seabass skin and epigallocatechin gallate (EGCG): Characteristics, antioxidant activity and in vitro cellular bioactivity. RSC Adv. 2021, 11, 2175–2184. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Binlateh, T.; Hutamekalin, P.; Sukketsiri, W.; Aluko, R.E.; Benjakul, S. Hydrolyzed collagen from defatted sea bass skin and its conjugate with epigallocatechin gallate: In vitro antioxidant, anti-inflammatory, wound-healing and anti-obesity activities. Food Biosci. 2021, 43, 101303. [Google Scholar] [CrossRef]
- Zheng, H.Z.; Cui, C.L.; Jeong, W.S.; Chung, S.K. Anti-inflammatory effect of unripe apple polyphenols-chitooligosaccharides microcapsule against LPS-induced RAW 264.7 cells. Appl. Biol. Chem. 2020, 63, 51. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.S.; Hwang, J.W.; Kim, S.K.; Jeon, Y.J.; Je, J.Y.; Ahn, C.B.; Moon, S.H.; Jeon, B.T.; Park, P.J. Chitooligosaccharide and its derivatives: Preparation and biological applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef]
- Mittal, A.; Singh, A.; Hong, H.; Benjakul, S. Chitooligosaccharides from shrimp shell chitosan prepared using H2O2 or ascorbic acid/H2O2 redox pair hydrolysis: Characteristics, antioxidant and antimicrobial activities. Int. J. Food Sci. Technol. 2022, 58, 2645–2660. [Google Scholar] [CrossRef]
- Sánchez, Á.; Mengíbar, M.; Fernández, M.; Alemany, S.; Heras, A.; Acosta, N. Influence of preparation methods of chitooligosaccharides on their physicochemical properties and their anti-inflammatory effects in mice and in RAW264. 7 macrophages. Mar. Drugs 2018, 16, 430. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Lin, Y.Y.; Wu, G.J.; Huang, C.H.; Tsai, G.J. Effect of chitosan molecular weight on anti-inflammatory activity in the RAW 264.7 macrophage model. Int. J. Biol. Macromol. 2019, 131, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Spindola, H.; De Sousa, V.; Santos-Silva, A.; Pintado, M.E.; Malcata, F.X.; Carvalho, J.E. Anti-inflammatory activity of chitooligosaccharides in vivo. Mar. Drugs 2010, 8, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Jitprasertwong, P.; Khamphio, M.; Petsrichuang, P.; Eijsink, V.G.; Poolsri, W.; Muanprasat, C.; Rangnoi, K.; Yamabhai, M. Anti-inflammatory activity of soluble chito-oligosaccharides (CHOS) on VitD3-induced human THP-1 monocytes. PLoS ONE 2021, 16, e024638. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A. Production, properties, and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Binlateh, T.; Hutamekalin, P.; Aluko, R.E.; Tepaamorndech, S.; Zhang, B.; Benjakul, S. Impact of hydrolyzed collagen from defatted sea bass skin on proliferation and differentiation of preosteoblast MC3T3-E1 cells. Foods 2021, 10, 1476. [Google Scholar] [CrossRef]
- Gao, X.; Dai, J.; Li, G.; Dai, X. Gambogic acid protects LPS-induced apoptosis and inflammation in a cell model of neonatal pneumonia through the regulation of TrkA/Akt signaling pathway. BMC Pharmacol. Toxicol. 2021, 22, 28. [Google Scholar] [CrossRef]
- George, L.; Ramasamy, T.; Sirajudeen, K.; Manickam, V. LPS-induced apoptosis is partially mediated by hydrogen sulphide in RAW 264.7 murine macrophages. Immunol. Investig. 2019, 48, 451–465. [Google Scholar] [CrossRef]
- Notarte, K.I.R.; Quimque, M.T.J.; Macaranas, I.T.; Khan, A.; Pastrana, A.M.; Villaflores, O.B.; Arturo, H.C.P.; Pilapil IV, D.Y.H.; Tan, S.M.M.; Wei, D.Q.; et al. Attenuation of lipopolysaccharide-induced inflammatory responses through inhibition of the NF-κB pathway and the increased Nrf2 level by a flavonol-enriched n-butanol fraction from Uvaria alba. ACS Omega 2023, 8, 5377–5392. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, L.; He, J. Plantanone C attenuates LPS-stimulated inflammation by inhibiting NF-κB/iNOS/COX-2/MAPKs/Akt pathways in RAW264.7 macrophages. Biomed. Pharmacother. 2021, 143, 112104. [Google Scholar] [CrossRef]
- Raza, H.; John, A.; Shafarin, J. Potentiation of LPS-induced apoptotic cell death in human hepatoma HepG2 cells by aspirin via ROS and mitochondrial dysfunction: Protection by NAcetyl cysteine. PLoS ONE 2016, 11, e0159750. [Google Scholar] [CrossRef]
- Lim, D.W.; Choi, H.J.; Park, S.D.; Kim, H.; Yu, G.R.; Kim, J.E.; Park, W.H. Activation of the Nrf2/HO-1 pathway by Amomum villosum extract suppresses LPS-induced oxidative stress In Vitro and Ex Vivo. Evid. Based Complement. Altern. Med. 2020, 2020, 2837853. [Google Scholar] [CrossRef]
- Kang, X.; Li, P.; Zhang, C.; Zhao, Y.; Hu, H.; Wen, G. The TLR4/ERK/PD-L1 axis may contribute to NSCLC initiation. Int. J. Oncol. 2020, 57, 456–465. [Google Scholar] [CrossRef]
- Kin, D.Y.; Jun, J.H.; Lee, H.L.; Woo, K.M.; Ryoo, H.M.; Kim, G.S.; Baek, J.H.; Han, S.B. N-acetylcysteine prevents LPS-induced pro-inflammatory cytokines and MMP2 production in gingival fibroblasts. Arch. Pharmacal Res. 2007, 30, 1283–1292. [Google Scholar] [CrossRef]
- Ei, Z.Z.; Hutamekalin, P.; Prommeenate, P.; Singh, A.; Benjakul, S.; Visuttijai, K.; Chanvorachote, P. Chitooligosaccharide prevents vascular endothelial cell apoptosis by attenuation of endoplasmic reticulum stress via suppression of oxidative stress through Nrf2-SOD1 up-regulation. Pharm. Biol. 2022, 60, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Anil, S. Potential medical applications of Chitooligosaccharides. Polymers 2022, 14, 3558. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xing, R.; Liu, S.; Li, R.; Qin, Y.; Meng, X.; Li, P. Separation of chito-oligomers with several degrees of polymerization and study of their antioxidant activity. Carbohydr. Polym. 2012, 88, 896–903. [Google Scholar] [CrossRef]
- Mengibar, M.; Mateos-Aparicio, I.; Miralles, B.; Heras, A. Influence of the physico-chemical characteristics of chito-oligosaccharides (COS) on antioxidant activity. Carbohydr. Polym. 2013, 97, 776–782. [Google Scholar] [CrossRef]
- Ponder, K.G.; Boise, L.H. The prodomain of caspase-3 regulates its own removal and caspase activation. Cell Death Discov. 2019, 5, 56. [Google Scholar] [CrossRef]
- Yoon, H.J.; Moon, M.E.; Park, H.S.; Im, S.Y.; Kim, Y.H. Chitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW264.7 macrophage cells. Biochem. Biophys. Res. Commun. 2007, 358, 954–959. [Google Scholar] [CrossRef]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.R.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Yu, K.; Chen, X.; Chen, H.; Hong, J.; Cheng, S.; Peng, L. Piperine inhibits LPS induced expression of inflammatory mediators in RAW 264.7 cells. Cell. Immunol. 2013, 285, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Muniandy, K.; Gothai, S.; Badran, K.M.H.; Kumar, S.S.; Esa, N.M.; Arulselvan, P. Suppression of Proinflammatory Cytokines and Mediators in LPS-Induced RAW 264.7 Macrophages by Stem Extract of Alternanthera sessilis via the Inhibition of the NF-κB Pathway. J. Immunol. Res. 2018, 2018, 3430684. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Y.; Ling, T.; Zhao, C.; Li, Y.; Geng, M.; Gai, S.; Qi, W.; Luo, X.; Chen, L.; et al. Chitosan Oligosaccharides Alleviate Colitis by Regulating Intestinal Microbiota and PPARγ/SIRT1-Mediated NF-κB Pathway. Mar. Drugs 2022, 20, 96. [Google Scholar] [CrossRef]
- Hu, H.; Xia, H.; Zou, X.; Li, X.; Zhang, Z.; Yao, X.; Yin, M.; Tian, D.; Liu, H. N-acetyl-chitooligosaccharide attenuates inflammatory responses by suppression of NF-κB signaling, MAPK and NLRP3 inflammasome in macrophages. J. Funct. Foods 2021, 78, 104364. [Google Scholar] [CrossRef]
- Cho, B.O.; Ryu, H.W.; So, Y.; Lee, C.W.; Jin, C.H.; Yook, H.S.; Jeong, Y.W.; Park, J.C.; Jeong, I.Y. Anti-Inflammatory Effect of Mangostenone F in Lipopolysaccharide-Stimulated RAW264.7 Macrophages by Suppressing NF-κB and MAPK Activation. Biomol. Ther. 2014, 22, 288–294. [Google Scholar] [CrossRef]
- Kang, S.G.; Lee, G.B.; Vinayagam, R.; Do, G.S.; Oh, S.Y.; Yang, S.J.; Kwon, J.B.; Singh, M. Anti-Inflammatory, Antioxidative, and Nitric Oxide-Scavenging Activities of a Quercetin Nanosuspension with Polyethylene Glycol in LPS-Induced RAW 264.7 Macrophages. Molecules 2022, 27, 7432. [Google Scholar] [CrossRef]
- Yang, C.; Yang, R.; Gu, M.; Hao, J.; Wang, S.; Li, C. Chitooligosaccharides derivatives protect ARPE-19 cells against acrolein-induced oxidative injury. Mar. Drugs 2023, 21, 137. [Google Scholar] [CrossRef]
- Han, S.; Gao, H.; Chen, S.; Wang, Q.; Li, X.; Du, L.J.; Li, J.; Luo, Y.Y.; Li, J.X.; Zhao, L.C.; et al. Procyanidin A1 Alleviates Inflammatory Response induced by LPS through NF-κB, MAPK, and Nrf2/HO-1 Pathways in RAW264.7 cells. Sci. Rep. 2019, 9, 15087. [Google Scholar] [CrossRef]
- Jasek-Gajda, E.; Jurkowska, H.; Jasińska, M.; Lis, G.J. Targeting the MAPK/ERK and PI3K/AKT Signaling Pathways Affects NRF2, Trx and GSH Antioxidant Systems in Leukemia Cells. Antioxidants 2020, 9, 633. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.J.; Li, Z.Q.; Mo, Z.Q.; Xu, S.; Mao, H.H.; Shi, D.; Li, Z.W.; Dan, X.M.; Luo, X.C. Immunomodulatory Effects of N-Acetyl Chitooligosaccharides on RAW264.7 Macrophages. Mar. Drugs 2020, 18, 421. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chotphruethipong, L.; Chanvorachote, P.; Reudhabibadh, R.; Singh, A.; Benjakul, S.; Roytrakul, S.; Hutamekalin, P. Chitooligosaccharide from Pacific White Shrimp Shell Chitosan Ameliorates Inflammation and Oxidative Stress via NF-κB, Erk1/2, Akt and Nrf2/HO-1 Pathways in LPS-Induced RAW264.7 Macrophage Cells. Foods 2023, 12, 2740. https://doi.org/10.3390/foods12142740
Chotphruethipong L, Chanvorachote P, Reudhabibadh R, Singh A, Benjakul S, Roytrakul S, Hutamekalin P. Chitooligosaccharide from Pacific White Shrimp Shell Chitosan Ameliorates Inflammation and Oxidative Stress via NF-κB, Erk1/2, Akt and Nrf2/HO-1 Pathways in LPS-Induced RAW264.7 Macrophage Cells. Foods. 2023; 12(14):2740. https://doi.org/10.3390/foods12142740
Chicago/Turabian StyleChotphruethipong, Lalita, Pithi Chanvorachote, Ratchaneekorn Reudhabibadh, Avtar Singh, Soottawat Benjakul, Sittiruk Roytrakul, and Pilaiwanwadee Hutamekalin. 2023. "Chitooligosaccharide from Pacific White Shrimp Shell Chitosan Ameliorates Inflammation and Oxidative Stress via NF-κB, Erk1/2, Akt and Nrf2/HO-1 Pathways in LPS-Induced RAW264.7 Macrophage Cells" Foods 12, no. 14: 2740. https://doi.org/10.3390/foods12142740
APA StyleChotphruethipong, L., Chanvorachote, P., Reudhabibadh, R., Singh, A., Benjakul, S., Roytrakul, S., & Hutamekalin, P. (2023). Chitooligosaccharide from Pacific White Shrimp Shell Chitosan Ameliorates Inflammation and Oxidative Stress via NF-κB, Erk1/2, Akt and Nrf2/HO-1 Pathways in LPS-Induced RAW264.7 Macrophage Cells. Foods, 12(14), 2740. https://doi.org/10.3390/foods12142740







