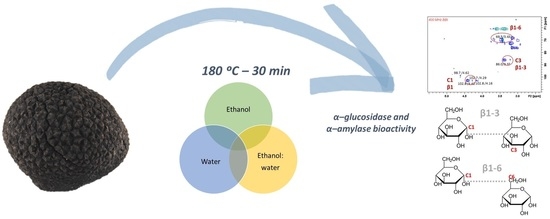
Pressurized Liquid (PLE) Truffle Extracts Have Inhibitory Activity on Key Enzymes Related to Type 2 Diabetes (α-Glucosidase and α-Amylase)
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Reagents
2.3. Pressurized Liquid Extractions (PLE)
2.4. Determination of Carbohydrates in PLE Extracts
2.5. Determination of Lipid Compounds in PLE Extracts
2.6. Determination of Protein and Phenolic Compounds
2.7. Determination of the α-Amylase and α-Glucosidase Inhibitory Activities
2.8. Statistical Analysis
3. Results
3.1. PLE Extractions from Several Truffle Species
3.2. Carbohydrates Composition of PLE Fractions
3.3. Lipid Composition of PLE Fractions
3.4. Total Protein and Phenol Composition of PLE Fractions
3.5. PLE Extract Inhibitory Activity on the Key Enzymes Linked to Diabetes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yaribeygi, H.; Atkin, S.L.; Pirro, M.; Sahebkar, A. A Review of the Anti-inflammatory Properties of Antidiabetic Agents Providing Protective Effects against Vascular Complications in Diabetes. J. Cell Physiol. 2019, 234, 8286–8294. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Antoine, J.-M.; Benton, D.; Björck, I.; Bozzetto, L.; Brouns, F.; Diamant, M.; Dye, L.; Hulshof, T.; Holst, J.J.; et al. Impact of Postprandial Glycaemia on Health and Prevention of Disease. Obes. Rev. 2012, 13, 923–984. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Huang, Y.; Liu, Y.; Huang, M.; Song, G.; Ming, Q.; Ma, X.; Yang, J.; Deng, S.; Wen, Y.; et al. Antidiabetic Activity of Ergosterol from Pleurotus Ostreatus in KK-A y Mice with Spontaneous Type 2 Diabetes Mellitus. Mol. Nutr. Food Res. 2018, 62, 1700444. [Google Scholar] [CrossRef]
- Stojkovic, D.; Smiljkovic, M.; Ciric, A.; Glamoclija, J.; Van Griensven, L.; Ferreira, I.C.F.R.; Sokovic, M. An Insight into Antidiabetic Properties of Six Medicinal and Edible Mushrooms: Inhibition of α-Amylase and α-Glucosidase Linked to Type-2 Diabetes. S. Afr. J. Bot. 2019, 120, 100–103. [Google Scholar] [CrossRef]
- Oliach, D.; Vidale, E.; Brenko, A.; Marois, O.; Andrighetto, N.; Stara, K.; Mart, J.; Arag, D.; Colinas, C.; Bonet, A. Truffle Market Evolution: An Application of the Delphi Method. Forests 2021, 12, 1174. [Google Scholar] [CrossRef]
- Tejedor-Calvo, E.; Morales, D.; Marco, P.; Sánchez, S.; Garcia-Barreda, S.; Smiderle, F.R.; Iacomini, M.; Villalva, M.; Santoyo, S.; Soler-Rivas, C. Screening of Bioactive Compounds in Truffles and Evaluation of Pressurized Liquid Extractions (PLE) to Obtain Fractions with Biological Activities. Food Res. Int. 2020, 132, 109054. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A.; Khan, H.; Khalid, S.; Mubarak, M.S. Potential Health Benefits of Natural Products Derived from Truffles: A Review. Trends Food Sci. Technol. 2017, 70, 1–8. [Google Scholar] [CrossRef]
- Morales, D.; Smiderle, F.R.; Villalva, M.; Abreu, H.; Rico, C.; Santoyo, S.; Iacomini, M.; Soler-Rivas, C. Testing the Effect of Combining Innovative Extraction Technologies on the Biological Activities of Obtained β-Glucan-Enriched Fractions from Lentinula edodes. J. Funct. Foods 2019, 60, 103446. [Google Scholar] [CrossRef]
- Morales, D.; Tejedor-Calvo, E.; Jurado-Chivato, N.; Polo, G.; Tabernero, M.; Ruiz-Rodríguez, A.; Largo, C.; Soler-Rivas, C. In Vitro and In Vivo Testing of the Hypocholesterolemic Activity of Ergosterol- and β-Glucan-Enriched Extracts Obtained from Shiitake Mushrooms (Lentinula edodes). Food Funct. 2019, 10, 7325–7332. [Google Scholar] [CrossRef]
- Palanisamy, M.; Aldars-García, L.; Gil-Ramírez, A.; Ruiz-Rodríguez, A.; Marín, F.R.; Reglero, G.; Soler-Rivas, C. Pressurized Water Extraction of β-Glucan Enriched Fractions with Bile Acids-Binding Capacities Obtained from Edible Mushrooms. Biotechnol. Prog. 2014, 30, 391–400. [Google Scholar] [CrossRef]
- Tejedor-calvo, E.; García-Barreda, S.; Sánchez, S. Application of Pressurized Liquid Extractions to Obtain Bioactive Compounds from Tuber aestivum and Terfezia claveryi. Foods 2022, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ramírez, A.; Caz, V.; Martin-Hernandez, R.; Marín, F.R.; Largo, C.; Rodríguez-Casado, A.; Tabernero, M.; Ruiz-Rodríguez, A.; Reglero, G.; Soler-Rivas, C. Modulation of Cholesterol-Related Gene Expression by Ergosterol and Ergosterol-Enriched Extracts Obtained from Agaricus bisporus. Eur. J. Nutr. 2016, 55, 1041–1057. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cheng, J.; Wang, L.; Yan, S.; Wang, R.; Zhang, H.; Shao, H.; Yang, X. Valorization of Spent Shiitake Substrate for Recovery of Antitumor Fungal Sterols by Ultrasound-Assisted Extraction. J. Food Biochem. 2018, 42, e12602. [Google Scholar] [CrossRef]
- Kobori, M.; Yoshida, M.; Ohnishi-Kameyama, M.; Shinmoto, H. Ergosterol Peroxide from an Edible Mushroom Suppresses Inflammatory Responses in RAW264.7 Macrophages and Growth of HT29 Colon Adenocarcinoma Cells. Br. J. Pharmacol. 2007, 150, 209–219. [Google Scholar] [CrossRef]
- Sommer, K.; Vetter, W. Gas Chromatography with Mass Spectrometry Detection and Characterization of 27 Sterols in Two Truffle (Tuber) Species. J. Food Compos. Anal. 2020, 94, 103650. [Google Scholar] [CrossRef]
- Beara, I.; Majkić, T.; Torović, L. Bioguided Design of New Black Truffle (Tuber aestivum Vittad.) Product Enriched with Herbs and Spices. LWT 2021, 138, 110637. [Google Scholar] [CrossRef]
- Bhotmange, D.U.; Wallenius, J.H.; Singhal, R.S.; Shamekh, S.S. Enzymatic Extraction and Characterization of Polysaccharide from Tuber aestivum. Bioact. Carbohydr. Diet. Fibre 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Deveci, E.; Çayan, F.; Tel-Çayan, G.; Duru, M.E. Inhibitory Activities of Medicinal Mushrooms on α-Amylase and α-Glucosidase-Enzymes Related to Type 2 Diabetes. S. Afr. J. Bot. 2021, 137, 19–23. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T.; Radović-Jakovljević, M.; Ćirić, A.; Grujičić, D.; Milošević-Djordjević, O. Craterellus cornucopioides Edible Mushroom as Source of Biologically Active Compounds. Nat. Prod. Commun. 2019, 14, 1934578X1984361. [Google Scholar] [CrossRef]
- Bach, F.; Zielinski, A.A.F.; Helm, C.V.; Maciel, G.M.; Pedro, A.C.; Stafussa, A.P.; Ávila, S.; Haminiuk, C.W.I. Bio Compounds of Edible Mushrooms: In Vitro Antioxidant and Antimicrobial Activities. LWT 2019, 107, 214–220. [Google Scholar] [CrossRef]
- Zhang, T.; Jayachandran, M.; Ganesan, K.; Xu, B. Black Truffle Aqueous Extract Attenuates Oxidative Stress and Inflammation in STZ-Induced Hyperglycemic Rats via Nrf2 and NF-ΚB Pathways. Front. Pharmacol. 2018, 9, 1257. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Morales, D.; Gil-Ramírez, A.; de Jesus, L.I.; Gilbert-López, B.; Iacomini, M.; Soler-Rivas, C. Evaluation of Microwave-Assisted and Pressurized Liquid Extractions to Obtain β-D-Glucans from Mushrooms. Carbohydr. Polym. 2017, 156, 165–174. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Aldars-García, L.; Palanisamy, M.; Jiverdeanu, R.M.; Ruiz-Rodríguez, A.; Marín, F.R.; Reglero, G.; Soler-Rivas, C. Sterol Enriched Fractions Obtained from Agaricus bisporus Fruiting Bodies and By-Products by Compressed Fluid Technologies (PLE and SFE). Innov. Food Sci. Emerg. Technol. 2013, 18, 101–107. [Google Scholar] [CrossRef]
- Rivera, C.S.; Blanco, D.; Marco, P.; Oria, R.; Venturini, M.E. Effects of Electron-Beam Irradiation on the Shelf Life, Microbial Populations and Sensory Characteristics of Summer Truffles (Tuber aestivum) Packaged under Modified Atmospheres. Food Microbiol. 2011, 28, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.; Smiderle, F.R.; Piris, A.J.; Soler-Rivas, C.; Prodanov, M. Production of a β-d-Glucan-Rich Extract from Shiitake Mushrooms (Lentinula edodes) by an Extraction/Microfiltration/Reverse Osmosis (Nanofiltration) Process. Innov. Food Sci. Emerg. Technol. 2018, 51, 80–90. [Google Scholar] [CrossRef]
- Pettolino, F.A.; Walsh, C.; Fincher, G.B.; Bacic, A. Determining the Polysaccharide Composition of Plant Cell Walls. Nat. Protoc. 2012, 7, 1590–1607. [Google Scholar] [CrossRef]
- Tejedor-Calvo, E.; Morales, D.; Marco, P.; Venturini, M.E.; Blanco, D.; Soler-Rivas, C. Effects of Combining Electron-Beam or Gamma Irradiation Treatments with Further Storage under Modified Atmospheres on the Bioactive Compounds of Tuber Melanosporum Truffles. Postharvest Biol. Technol. 2019, 155, 149–155. [Google Scholar] [CrossRef]
- Malone Steverson, E.; Korus, R.A.; Admassu, W.; Heimsch, R.C. Kinetics of the Amylase System of Saccharomycopsis fibuliger. Enzym. Microb. Technol. 1984, 6, 549–554. [Google Scholar] [CrossRef]
- Khan, A.A.; Gani, A.; Khanday, F.A.; Masoodi, F.A. Biological and Pharmaceutical Activities of Mushroom β-Glucan Discussed as a Potential Functional Food Ingredient. Bioact. Carbohydr. Diet. Fibre 2018, 16, 1–13. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Therapeutic Potential of Mushrooms in Diabetes Mellitus: Role of Polysaccharides. Int. J. Biol. Macromol. 2020, 164, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Wunjuntuk, K.; Ahmad, M.; Techakriengkrai, T.; Chunhom, R.; Jaraspermsuk, E.; Chaisri, A.; Kiwwongngam, R.; Wuttimongkolkul, S.; Charoenkiatkul, S. Proximate Composition, Dietary Fibre, Beta-Glucan Content, and Inhibition of Key Enzymes Linked to Diabetes and Obesity in Cultivated and Wild Mushrooms. J. Food Compos. Anal. 2022, 105, 104226. [Google Scholar] [CrossRef]
- Tang, Y.; Li, H.-M.; Tang, Y.-J. Comparison of Sterol Composition between Tuber Fermentation Mycelia and Natural Fruiting Bodies. Food Chem. 2012, 132, 1207–1213. [Google Scholar] [CrossRef]
- Yeh, C.W.; Kan, S.C.; Lin, C.C.; Shieh, C.J.; Liu, Y.C. Polyhydroxylated Steroids and Triterpenoids from an Entophytic Fungus, Hypocreales sp. NCHU01 Isolated from Tuber Magnatum. J. Taiwan. Inst. Chem. Eng. 2016, 64, 22–30. [Google Scholar] [CrossRef]
- Mello, A.; Murat, C.; Bonfante, P. Truffles: Much More than a Prized and Local Fungal Delicacy. FEMS Microbiol. Lett. 2006, 260, 1–8. [Google Scholar] [CrossRef]
- Tejedor-Calvo, E.; Marco, P.; Spègel, P.; Soler-Rivas, C. Extraction and Trapping of Truffle Flavoring Compounds into Food Matrices Using Supercritical CO2. Food Res. Int. 2023, 164, 112422. [Google Scholar] [CrossRef] [PubMed]
- Tejedor-Calvo, E.; Morales, D.; García-Barreda, S.; Sánchez, S.; Venturini, M.E.; Blanco, D.; Soler-Rivas, C.; Marco, P. Effects of Gamma Irradiation on the Shelf-Life and Bioactive Compounds of Tuber aestivum Truffles Packaged in Passive Modified Atmosphere. Int. J. Food Microbiol. 2020, 332, 108774. [Google Scholar] [CrossRef] [PubMed]
- Tejedor-Calvo, E.; Amara, K.; Reis, F.S.; Barros, L.; Martins, A.; Calhelha, R.C.; Eugenia Venturini, M.; Blanco, D.; Redondo, D.; Marco, P.; et al. Chemical Composition and Evaluation of Antioxidant, Antimicrobial and Antiproliferative Activities of Tuber and Terfezia Truffles. Food Res. Int. 2020, 140, 110071. [Google Scholar] [CrossRef]
- Shah, N.N.; Hokkanen, S.; Pastinen, O.; Eljamil, A.; Shamekh, S. A Study on the Fatty Acid Composition of Lipids in Truffles Selected from Europe and Africa. 3 Biotech 2020, 10, 415. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Y.; Sang, X.; Fan, L. Nutritional Value, Chemical Composition and Antioxidant Activity of Three Tuber Species from China. AMB Express 2017, 7, 136. [Google Scholar] [CrossRef]
- Yadav, D.; Negi, P.S. Bioactive Components of Mushrooms: Processing Effects and Health Benefits. Food Res. Int. 2021, 148, 110599. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Yanni, A.E.; Koutrotsios, G.; Aloupi, M. Bioactive Microconstituents and Antioxidant Properties of Wild Edible Mushrooms from the Island of Lesvos, Greece. Food Chem. Toxicol. 2013, 55, 378–385. [Google Scholar] [CrossRef]
- Muszyńska, B.; Grzywacz-Kisielewska, A.; Kała, K.; Gdula-Argasińska, J. Anti-Inflammatory Properties of Edible Mushrooms: A Review. Food Chem. 2018, 243, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant Properties of Phenolic Compounds Occurring in Edible Mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar] [CrossRef]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, Vegetables, and Mushrooms for the Preparation of Extracts with α-Amylase and α-Glucosidase Inhibition Properties: A Review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Y.; Zhang, J.Y.; Chen, L.J.; Liu, X.C.; Liu, Y.; Wang, W.X.; Zhang, Y.M. Comparative Evaluation of Polysaccharides Isolated from Astragalus, Oyster Mushroom, and Yacon as Inhibitors of α-Glucosidase. Chin. J. Nat. Med. 2014, 12, 290–293. [Google Scholar] [CrossRef]
- Cui, J.; Gu, X.; Wang, F.; Ouyang, J.; Wang, J. Purification and Structural Characterization of an α-Glucosidase Inhibitory Polysaccharide from Apricot (Armeniaca sibirica L. Lam.) Pulp. Carbohydr. Polym. 2015, 121, 309–314. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Bobak, Ł.; Nowicka, P. Anti-Oxidant and Anti-Enzymatic Activities of Sea Buckthorn (Hippophaë rhamnoides L.) Fruits Modulated by Chemical Components. Antioxidants 2019, 8, 618. [Google Scholar] [CrossRef]
- Su, C.-H.; Hsu, C.-H.; Ng, L.-T. Inhibitory Potential of Fatty Acids on Key Enzymes Related to Type 2 Diabetes. BioFactors 2013, 39, 415–421. [Google Scholar] [CrossRef]
- Kaewnarin, K.; Suwannarach, N.; Kumla, J.; Lumyong, S. Phenolic Profile of Various Wild Edible Mushroom Extracts from Thailand and Their Antioxidant Properties, Anti-Tyrosinase and Hyperglycaemic Inhibitory Activities. J. Funct. Foods 2016, 27, 352–364. [Google Scholar] [CrossRef]



| Species | Yield % | TCH | β-Glucans | Chitin | Soluble Proteins | Total Sterols | Ergosterol | Brassicasterol | Ergosta-7,22-Dienol | Stigmasterol | TPC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| W | |||||||||||
| M. terfezoides | 69.62 ± 5.32 | 46.39 ± 1.29 | 16.12 ± 1.98 | 8.83 ± 0.23 | 7.15 ± 0.85 | - | - | - | - | - | 2.77 ± 0.07 |
| T. aestivum var. uncinatum | 58.72 ± 3.31 | 38.01 ± 2.56 | 16.47 ± 1.03 | 11.21 ± 1.02 | 3.83 ± 0.35 | - | - | - | - | - | 1.54 ± 0.12 |
| T. borchii | 56.75 ± 2.87 | 37.30 ± 1.98 | 15.82 ± 2.01 | 12.16 ± 1.14 | 7.85 ± 0.70 | - | - | - | - | - | 2.07 ± 0.02 |
| T. brumale | 48.51 ± 3.02 | 37.37 ± 2.07 | 26.20 ± 1.51 | 11.36 ± 0.99 | 4.58 ± 0.28 | - | - | - | - | - | 1.52 ± 0.14 |
| T. brumale var. moschatum | 47.30 ± 2.09 | 29.43 ± 3.01 | 14.61 ± 0.57 | 10.93 ± 0.12 | 6.44 ± 0.13 | - | - | - | - | - | 1.36 ± 0.10 |
| T. indicum | 49.62 ± 4.47 | 35.51 ± 0.87 | 18.88 ± 2.12 | 12.54 ± 0.67 | 4.35 ± 0.82 | - | - | - | - | - | 1.93 ± 0.08 |
| T. magnatum | 49.46 ± 4.67 | 38.60 ± 1.64 | 12.74 ± 1.22 | 10.01 ± 0.43 | 4.56 ± 0.86 | - | - | - | - | - | 1.48 ± 0.13 |
| W:E | |||||||||||
| M. terfezoides | 49.41 ± 3.45 | 21.99 ± 0.87 | 5.22 ± 1.09 | 8.62 ± 0.65 | 2.26 ± 0.35 | 2.06 ± 0.15 | 0.61 ± 0.01 | 0.85 ± 0.01 | 0.61 ± 0.01 | - | 1.99 ± 0.12 |
| T. aestivum var. uncinatum | 40.25 ± 1.47 | 12.55 ± 1.13 | 4.10 ± 0.67 | 10.41 ± 1.10 | 1.84 ± 0.41 | 3.90 ± 0.83 | 1.52 ± 0.12 | 1.71 ± 0.43 | - | 0.65 ± 0.08 | 1.26 ± 0.20 |
| T. borchii | 40.43 ± 2.16 | 16.18 ± 2.25 | 3.19 ± 0.34 | 12.91 ± 3.17 | 2.41 ± 0.15 | 2.00 ± 0.14 | 0.76 ± 0.01 | 0.74 ± 0.05 | 0.52 ± 0.01 | - | 1.62 ± 0.07 |
| T. brumale | 34.07 ± 1.68 | 9.24 ± 1.65 | 2.08 ± 0.15 | 6.55 ± 1.06 | 1.68 ± 0.17 | 1.82 ± 0.56 | 0.35 ± 0.10 | 0.99 ± 0.09 | 0.50 ± 0.23 | - | 1.40 ± 0.14 |
| T. brumale var. moschatum | 37.78 ± 1.78 | 9.96 ± 1.89 | 2.30 ± 0.87 | 7.07 ± 0.82 | 3.01 ± 0.20 | 1.48 ± 0.38 | 0.52 ± 0.10 | 0.95 ± 0.11 | - | - | 1.26 ± 0.17 |
| T. indicum | 42.80 ± 2.46 | 13.49 ± 2.09 | 3.04 ± 1.12 | 10.26 ± 1.33 | 2.27 ± 0.09 | 2.17 ± 0.56 | 1.10 ± 0.24 | 1.08 ± 0.22 | - | - | 1.41 ± 0.10 |
| T. magnatum | 40.92 ± 3.55 | 15.91 ± 2.16 | 3.64 ± 0.93 | 10.97 ± 1.27 | 3.42 ± 0.39 | 3.64 ± 0.74 | 0.92 ± 0.23 | 0.93 ± 0.23 | 0.90 ± 0.12 | 0.90 ± 0.07 | 1.15 ± 0.09 |
| E | |||||||||||
| M. terfezoides | 19.85 ± 3.21 | - | - | - | - | 3.43 ± 0.35 | 0.82 ± 0.10 | 0.93 ± 0.08 | 0.91 ± 0.01 | 0.76 ± 0.09 | 0.68 ± 0.07 |
| T. aestivum var. uncinatum | 17.08 ± 2.87 | - | - | - | - | 9.31 ± 0.65 | 2.60 ± 0.12 | 2.66 ± 0.16 | 2.58 ± 0.27 | 1.46 ± 0.08 | 0.54 ± 0.01 |
| T. borchii | 20.78 ± 2.69 | - | - | - | - | 4.04 ± 0.41 | 1.13 ± 0.18 | 1.10 ± 0.10 | 0.72 ± 0.01 | 1.06 ± 0.19 | 0.55 ± 0.03 |
| T. brumale | 12.99 ± 1.15 | - | - | - | - | 6.00 ± 0.64 | 2.52 ± 0.02 | 2.60 ± 0.38 | 0.85 ± 0.12 | - | 0.60 ± 0.12 |
| T. brumale var. moschatum | 16.77 ± 1.90 | - | - | - | - | 4.17 ± 0.70 | 2.43 ± 0.24 | 1.75 ± 0.37 | - | - | 0.68 ± 0.09 |
| T. indicum | 18.86 ± 2.01 | - | - | - | - | 2.92 ± 0.45 | 1.53 ± 0.11 | 1.39 ± 0.24 | - | - | 0.70 ± 0.10 |
| T. magnatum | 14.76 ± 3.00 | - | - | - | - | 7.86 ± 0.54 | 2.31 ± 0.02 | 2.34 ± 0.02 | 2.30 ± 0.27 | 0.88 ± 0.12 | 0.77 ± 0.11 |
| Species | Mannose (%) | Glucose (%) | Galactose (%) |
|---|---|---|---|
| M. terfezoides | 15.1 | 76.3 | 8.6 |
| T. aestivum var. uncinatum | 26.0 | 68.6 | 5.4 |
| T. borchii | 20.3 | 78.8 | 0.9 |
| T. brumale | 16.1 | 83.5 | 0.4 |
| T. brumale var. moschatum | 27.8 | 70.2 | 2.0 |
| T. indicum | 31.8 | 59.6 | 8.6 |
| T. magnatum | 33.5 | 65.8 | 0.7 |
| α-Amylase IC50 (mg/mL) | α-Glucosidase IC50 (mg/mL) | |
|---|---|---|
| Water | ||
| M. terfezoides | 29.22 | 119.43 |
| Ethanol | ||
| M. terfezoides | 6.03 | 23.0 |
| T. aestivum var. uncinatum | 19.95 | 7.93 |
| T. brumale | 26.22 | 9.19 |
| T. brumale var. moschatum | 9.39 | 28.33 |
| Arcabosa (1 mg/mL) | 0.67 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejedor-Calvo, E.; Morales, D.; Morillo, L.; Vega, L.; Caro, M.; Smiderle, F.R.; Iacomini, M.; Marco, P.; Soler-Rivas, C. Pressurized Liquid (PLE) Truffle Extracts Have Inhibitory Activity on Key Enzymes Related to Type 2 Diabetes (α-Glucosidase and α-Amylase). Foods 2023, 12, 2724. https://doi.org/10.3390/foods12142724
Tejedor-Calvo E, Morales D, Morillo L, Vega L, Caro M, Smiderle FR, Iacomini M, Marco P, Soler-Rivas C. Pressurized Liquid (PLE) Truffle Extracts Have Inhibitory Activity on Key Enzymes Related to Type 2 Diabetes (α-Glucosidase and α-Amylase). Foods. 2023; 12(14):2724. https://doi.org/10.3390/foods12142724
Chicago/Turabian StyleTejedor-Calvo, Eva, Diego Morales, Laura Morillo, Laura Vega, Mercedes Caro, Fhernanda Ribeiro Smiderle, Marcello Iacomini, Pedro Marco, and Cristina Soler-Rivas. 2023. "Pressurized Liquid (PLE) Truffle Extracts Have Inhibitory Activity on Key Enzymes Related to Type 2 Diabetes (α-Glucosidase and α-Amylase)" Foods 12, no. 14: 2724. https://doi.org/10.3390/foods12142724
APA StyleTejedor-Calvo, E., Morales, D., Morillo, L., Vega, L., Caro, M., Smiderle, F. R., Iacomini, M., Marco, P., & Soler-Rivas, C. (2023). Pressurized Liquid (PLE) Truffle Extracts Have Inhibitory Activity on Key Enzymes Related to Type 2 Diabetes (α-Glucosidase and α-Amylase). Foods, 12(14), 2724. https://doi.org/10.3390/foods12142724