Microbial Inhibition by UV Radiation Combined with Nisin and Shelf-Life Extension of Tangerine Juice during Refrigerated Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tangerine Juice Preparation
2.2. Nisin, Chemicals, and Microbial Media
2.3. Microbial Cultures and Inoculation in Tangerine Juice
2.4. UV Sterilization, Nisin Treatment, and Pasteurization of Tangerine Juice
2.5. UV-Irradiated Tangerine Juice for Analysis of Microbial Growth Inhibition Kinetics
2.6. Physicochemical Properties of Nisin- and UV-Treated and Pasteurized Tangerine Juice
2.7. Analysis of Microbial and Physicochemical Characterization during Storage at 4 °C
2.8. Statistical Analysis
3. Results and Discussion
3.1. Impact of UV Radiation on the Microbial Growth and Inhibition Kinetics in Tangerine Juice
3.2. Efficacy of Nisin at Different Concentrations on the Spoilage and Pathogenic Microbial Load of Tangerine Juice
3.3. Combined Effects of UV Irradiation and Nisin on the Spoilage and Pathogenic Microbial Load of Tangerine Juice
3.4. Combined Effects of UV Irradiation and Nisin Concentrations on the Physicochemical Properties of Tangerine Juice
3.5. Combined Effects of UV Radiation and Nisin on the Spoilage Microbial Load of Tangerine Juice during Storage at 4 °C
3.6. Combined Effects of UV Radiation and Nisin on the Physicochemical Quality Changes in Tangerine Juice during Storage at 4 °C
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sovacool, B.K.; Bazilian, M.; Griffiths, S.; Kim, J.; Foley, A.; Rooney, D. Decarbonizing the food and beverages industry: A critical and systematic review of developments, sociotechnical systems and policy options. Renew. Sustain. Energy Rev. 2021, 143, 110856. [Google Scholar] [CrossRef]
- Sirichan, T.; Kijpatanasilp, I.; Asadatorn, N.; Assatarakul, K. Optimization of ultrasound extraction of functional compound from makiang seed by response surface methodology and antimicrobial activity of optimized extract with its application in orange juice. Ultrason. Sonochemistry 2022, 83, 105916. [Google Scholar] [CrossRef] [PubMed]
- Kijpatanasilp, I.; Narumonlittikrai, P.; Sheikh, K.A.; Jafari, S.; Worobo, R.W.; Assatarakul, K. Microbial inhibition and shelf-life extension of longan (Dimocarpus longan) juice by UV radiation. Food Control 2023, 149, 109694. [Google Scholar] [CrossRef]
- Ibarz, A.; Garvín, A.; Falguera, V. Ultraviolet in food preservation and processing. In Conventional and Advanced Food Processing Technologies; Wiley Online Library: Hoboken, NJ, USA, 2014; pp. 411–436. [Google Scholar]
- Liao, H.; Jiang, L.; Cheng, Y.; Liao, X.; Zhang, R. Application of nisin-assisted thermosonication processing for preservation and quality retention of fresh apple juice. Ultrason. Sonochemistry 2018, 42, 244–249. [Google Scholar] [CrossRef]
- Verma, D.K.; Thakur, M.; Singh, S.; Tripathy, S.; Gupta, A.K.; Baranwal, D.; Patel, A.R.; Shah, N.; Utama, G.L.; Niamah, A.K.; et al. Bacteriocins as antimicrobial and preservative agents in food: Biosynthesis, separation and application. Food Biosci. 2022, 46, 101594. [Google Scholar] [CrossRef]
- Lanclos, C.E. A Study of UV Radiation and Lactic Acid Bacteria Derived Bacteriocins on the Reduction of Bacterial Loads on Shrimp. Master’s Thesis, Louisiana State University, Baton Rouge, LA, USA, 2021. [Google Scholar]
- Ferreira, T.V.; Mizuta, A.G.; de Menezes, J.L.; Dutra, T.V.; Bonin, E.; Castro, J.C.; dos Anjos Szczerepa, M.M.; Pilau, E.J.; Nakamura, C.V.; Mikcha, J.M.G.; et al. Effect of ultraviolet treatment (UV–C) combined with nisin on industrialized orange juice in Alicyclobacillus acidoterrestris spores. LWT 2020, 133, 109911. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Quan, W.; Jia, X.; He, Z.; Wang, Z.; Adhikari, B.; Chen, J.; Zeng, M. Quantitation of furosine, furfurals, and advanced glycation end products in milk treated with pasteurization and sterilization methods applicable in China. Food Res. Int. 2021, 140, 110088. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis of AOAC International, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Jafari, S.; Karami, Z.; Shiekh, K.A.; Kijpatanasilp, I.; Worobo, R.W.; Assatarakul, K. Ultrasound-Assisted Extraction of Bioactive Compounds from Cocoa Shell and Their Encapsulation in Gum Arabic and Maltodextrin: A Technology to Produce Functional Food Ingredients. Foods 2023, 12, 412. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Rungroj, N.; Worobo, R.W.; Assatarakul, K. Kinetic study of selected microorganisms and quality attributes during cold storage of mango and passion fruit smoothie subjected to dimethyl dicarbonate. Int. J. Food Microbiol. 2021, 358, 109404. [Google Scholar] [CrossRef]
- Shiekh, K.A.; Luanglaor, T.; Hanprerakriengkrai, N.; Jafari, S.; Kijpatanasilp, I.; Asadatorn, N.; Assatarakul, K. Antioxidants and Quality Changes of Thermally Processed Purple Corn (Zea mays L.) Milk Fortified with Low Sucrose Content during Cold Storage. Foods 2023, 12, 277. [Google Scholar] [CrossRef]
- Gayán, E.; Condón, S.; Álvarez, I. Biological Aspects in Food Preservation by Ultraviolet Light: A Review. Food Bioprocess Technol. 2014, 7, 1–20. [Google Scholar] [CrossRef]
- Singh, H.; Bhardwaj, S.K.; Khatri, M.; Kim, K.-H.; Bhardwaj, N. UVC radiation for food safety: An emerging technology for the microbial disinfection of food products. Chem. Eng. J. 2021, 417, 128084. [Google Scholar] [CrossRef]
- Aaliya, B.; Sunooj, K.V.; Navaf, M.; Akhila, P.P.; Sudheesh, C.; Mir, S.A.; Sabu, S.; Sasidharan, A.; Hlaing, M.T.; George, J. Recent trends in bacterial decontamination of food products by hurdle technology: A synergistic approach using thermal and non-thermal processing techniques. Food Res. Int. 2021, 147, 110514. [Google Scholar] [CrossRef] [PubMed]
- Colás-Medà, P.; Nicolau-Lapeña, I.; Viñas, I.; Neggazi, I.; Alegre, I. Bacterial Spore Inactivation in Orange Juice and Orange Peel by Ultraviolet-C Light. Foods 2021, 10, 855. [Google Scholar] [CrossRef]
- Liu, G.; Nie, R.; Liu, Y.; Mehmood, A. Combined antimicrobial effect of bacteriocins with other hurdles of physicochemic and microbiome to prolong shelf life of food: A review. Sci. Total Environ. 2022, 825, 154058. [Google Scholar] [CrossRef]
- Jensen, C.; Li, H.; Vestergaard, M.; Dalsgaard, A.; Frees, D.; Leisner, J.J. Nisin Damages the Septal Membrane and Triggers DNA Condensation in Methicillin-Resistant Staphylococcus aureus. Front. Microbiol. 2020, 11, 1007. [Google Scholar] [CrossRef]
- Mok, J.H.; Pyatkovskyy, T.; Yousef, A.; Sastry, S.K. Effects of combination shear stress, moderate electric field (MEF), and nisin on kinetics and mechanisms of inactivation of Escherichia coli K12 and Listeria innocua in fresh apple-kale blend juice. J. Food Eng. 2021, 292, 110262. [Google Scholar] [CrossRef]
- Jeon, M.J.; Ha, J.W. Inactivating foodborne pathogens in apple juice by combined treatment with fumaric acid and ultraviolet-A light, and mechanisms of their synergistic bactericidal action. Food Microbiol. 2020, 87, 103387. [Google Scholar] [CrossRef]
- Ling, J.K.U.; Sam, J.H.; Jeevanandam, J.; Chan, Y.S.; Nandong, J. Thermal Degradation of Antioxidant Compounds: Effects of Parameters, Thermal Degradation Kinetics, and Formulation Strategies. Food Bioprocess Technol. 2022, 15, 1919–1935. [Google Scholar] [CrossRef]
- de Oliveira Junior, A.A.; Couto, H.G.S.D.A.; Barbosa, A.A.T.; Carnelossi, M.A.G.; de Moura, T.R. Stability, antimicrobial activity, and effect of nisin on the physico-chemical properties of fruit juices. Int. J. Food Microbiol. 2015, 211, 38–43. [Google Scholar] [CrossRef]
- Pravallika, K.; Chakraborty, S. Effect of nonthermal technologies on the shelf life of fruits and their products: A review on the recent trends. Appl. Food Res. 2022, 2, 100229. [Google Scholar] [CrossRef]
- Visuthiwan, S.; Assatarakul, K. Kinetic modeling of microbial degradation and antioxidant reduction in lychee juice subjected to UV radiation and shelf life during cold storage. Food Control 2021, 123, 107770. [Google Scholar] [CrossRef]
- Fundo, J.F.; Miller, F.A.; Mandro, G.F.; Tremarin, A.; Brandão, T.R.S.; Silva, C.L.M. UV-C light processing of Cantaloupe melon juice: Evaluation of the impact on microbiological, and some quality characteristics, during refrigerated storage. LWT 2019, 103, 247–252. [Google Scholar] [CrossRef]
- Polydera, A.C.; Stoforos, N.G.; Taoukis, P.S. Comparative Shelf Life Study and Vitamin C Loss Kinetics in Pasteurised and High Pressure Processed Reconstituted Orange Juice. J. Food Eng. 2003, 60, 21–29. [Google Scholar] [CrossRef]
- Chia, S.L.; Rosnah, S.; Noranizan, M.A.; Wan Ramli, W.D. The effect of storage on the quality attributes of ultraviolet-irradiated and thermally pasteurised pineapple juices. Int. Food Res. J. 2012, 19, 1001–1010. [Google Scholar]
- Mandha, J.; Shumoy, H.; Matemu, A.O.; Raes, K. Characterization of fruit juices and effect of pasteurization and storage conditions on their microbial, physicochemical, and nutritional quality. Food Biosci. 2023, 51, 102335. [Google Scholar] [CrossRef]
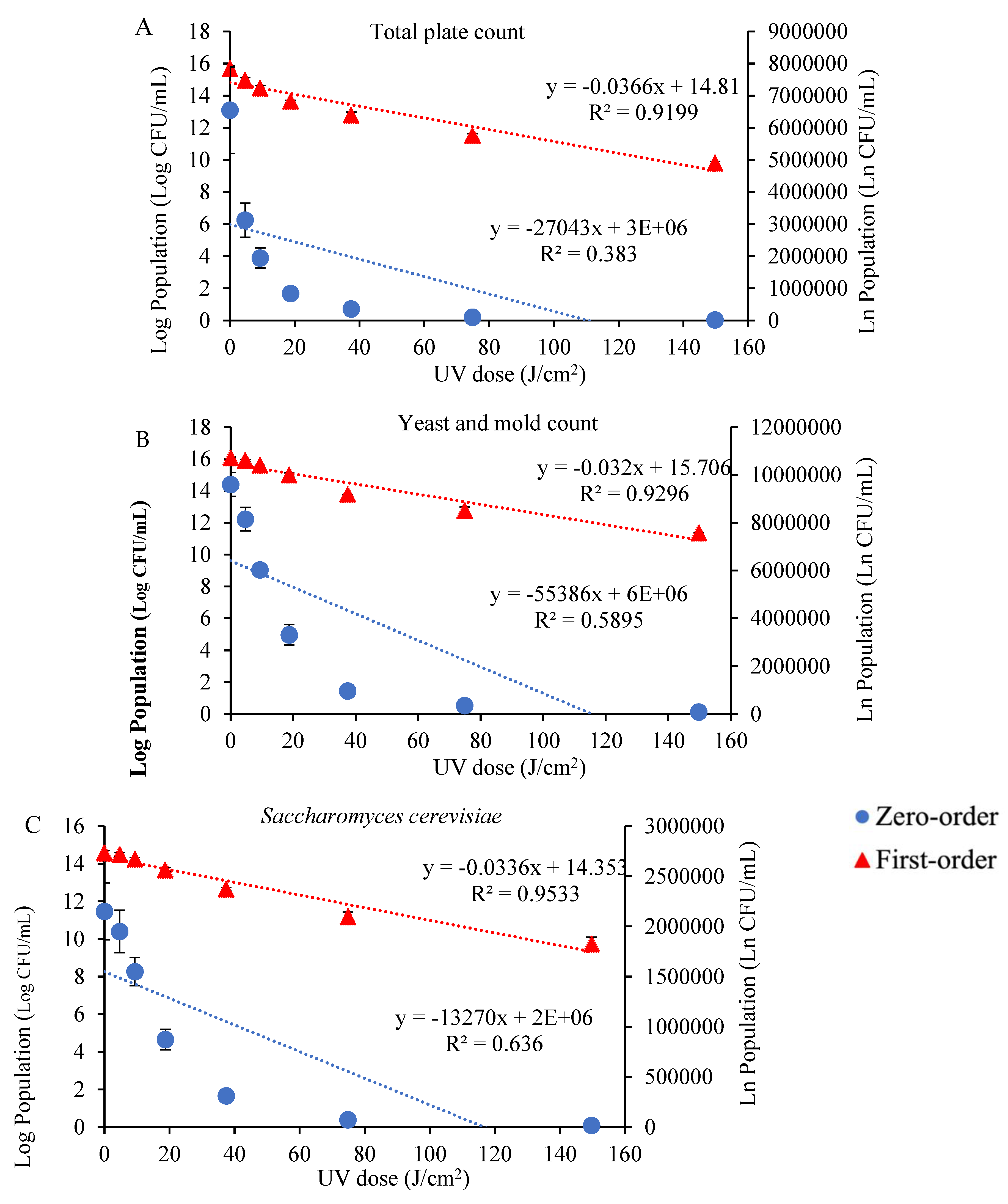
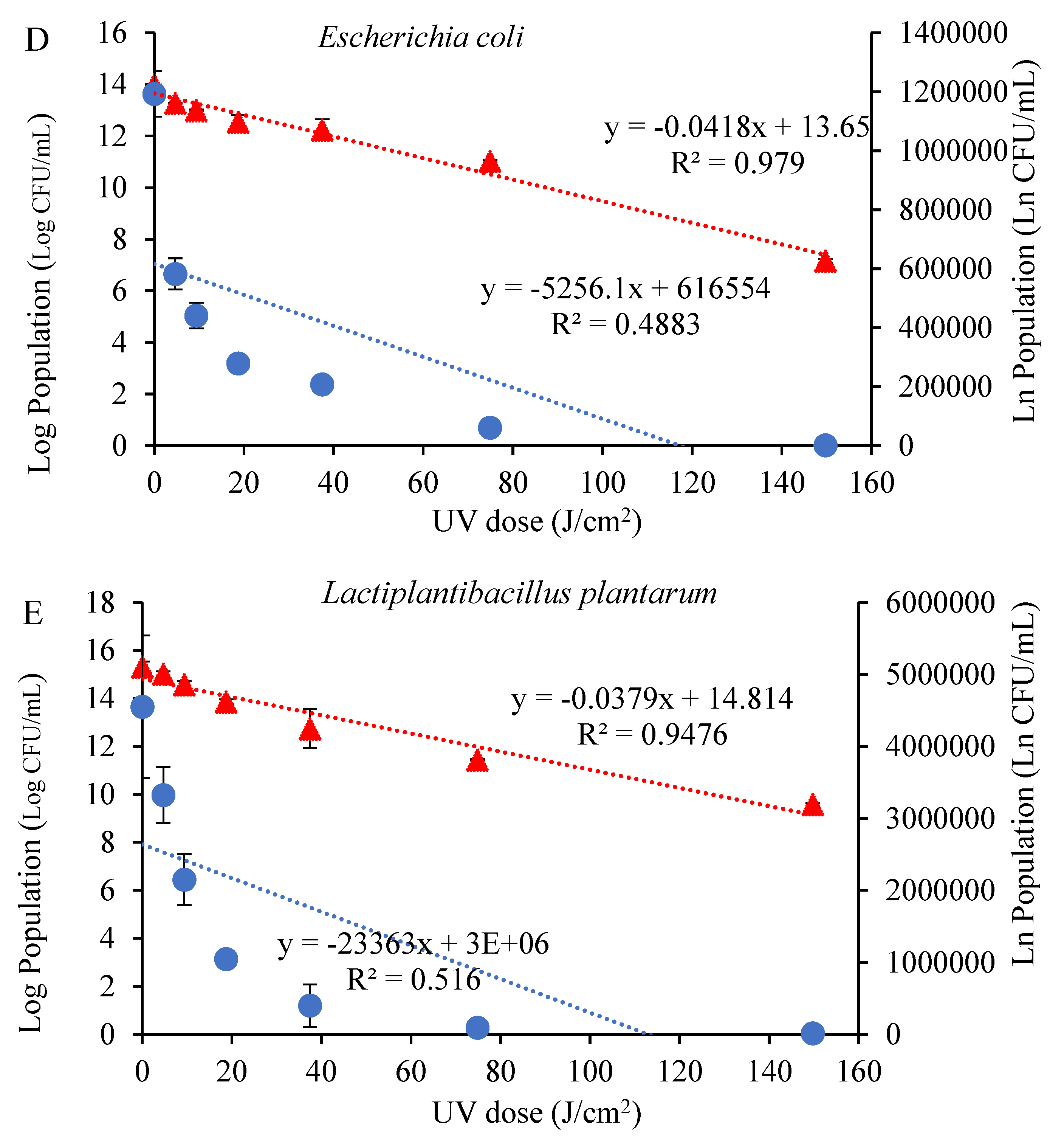
| Microorganisms | Zero-Order | First-Order | ||
|---|---|---|---|---|
| Rate Constant (k) | Coefficient of Determination (R2) | Rate Constant (k) | Coefficient of Determination (R2) | |
| Total plate count | 27,043 | 0.3830 | 0.0366 | 0.9199 |
| Yeast and mold count | 55,386 | 0.5895 | 0.0320 | 0.9296 |
| E. coli | 52,561 | 0.4883 | 0.0418 | 0.9790 |
| L. plantarum | 23,363 | 0.516 | 0.0379 | 0.9476 |
| S. cerevisiae | 13,270 | 0.6360 | 0.0336 | 0.9533 |
| Sample Treatments | Total Plate Count (log CFU/mL) | Yeast and Mold Count (log CFU/mL) | E. coli (log CFU/mL) | L. plantarum (log CFU/mL) | S. cerevisiae (log CFU/mL) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Population | Log Reduction | Population | Log Reduction | Population | Log Reduction | Population | Log Reduction | Population | Log Reduction | |
| Control | 6.75 ± 0.27 a | 0.00 ± 0.00 | 6.86 ± 0.09 a | 0.00 ± 0.00 | 7.59 ± 0.02 a | 0.00 ± 0.00 | 7.85 ± 0.06 a | 0.00 ± 0.0 | 7.02 ± 0.03 a | 0.00 ± 0.00 |
| UV | 4.21 ± 0.05 b | 2.54 ± 0.04 | 4.84 ± 0.001 b | 2.02 ± 0.02 | 4.58 ± 0.02 b | 3.01 ± 0.07 | 5.36 ± 0.03 b | 2.49 ± 0.06 | 4.94 ± 0.16 c | 2.08 ± 0.05 |
| NS | 3.68 ± 0.16 c | 2.07 ± 0.05 | 6.66 ± 0.04 c | 0.20 ± 0.08 | 6.08 ± 0.30 c | 1.51 ± 0.09 | 4.81 ± 0.20 c | 3.04 ± 0.02 | 6.84 ± 0.07 b | 0.18 ± 0.01 |
| UV + NS | 1.89 ± 0.02 d | 4.86 ± 0.02 | 4.49 ± 0.01 d | 2.37 ± 0.04 | 2.79 ± 0.07 d | 4.80 ± 0.10 | 1.81 ± 0.01 b | 6.04 ± 0.15 | 4.73 ± 0.03 d | 2.29 ± 0.02 |
| Pasteurization | 0.74 ± 0.00 e | 6.07 ± 0.09 | 0.73 ± 0.01 e | 6.25 ± 0.02 | - | 6.60 ± 0.10 | - | 6.08 ± 0.03 | - | 6.02 ± 0.06 |
| Properties | Control | UV | NS | UV + NS | Pasteurization |
|---|---|---|---|---|---|
| pH | 3.87 ± 0.01 a | 3.87 ± 0.01 a | 3.86 ± 0.01 a | 3.85 ± 0.07 a | 3.87 ± 0.07 a |
| TSS | 9.0 ± 0.04 a | 8.9 ± 0.14 a | 8.9 ± 0.04 a | 8.9 ± 0.04 a | 9.0 ± 0.01 a |
| TA | 0.44 ± 0.01 a | 0.42 ± 0.01 a | 0.44 ± 0.01 a | 0.44 ± 0.01 a | 0.42 ± 0.01 a |
| L* | 71.90 ± 0.07 ab | 71.81 ± 0.06 ab | 71.94 ± 0.02 a | 71.76 ± 0.035 b | 70.95 ± 0.06 c |
| a* | 11.62 ± 0.04 c | 12.11 ± 0.12 b | 11.82 ± 0.10 bc | 11.91 ± 0.018 bc | 12.53 ± 0.10 a |
| b* | 74.73 ± 0.11 a | 73.70 ± 0.28 c | 74.26 ± 0.03 bc | 73.92 ± 0.046 b | 72.95 ± 0.12 d |
| TPC | 203.75 ± 6.38a | 148.33 ± 2.70 b | 197.35 ± 4.62 a | 139.00 ± 1.57 b | 101.61 ± 13.01 c |
| TFC | 185.90 ± 0.78 a | 169.23 ± 3.77 b | 181.88 ± 4.93 a | 163.79 ± 3.85 b | 156.45 ± 2.95 c |
| Vitamin C | 41.61 ± 0.71 a | 36.00 ± 0.60 b | 41.11 ± 0.41 a | 35.83 ± 0.71 b | 19.80 ± 0.94 c |
| Carotenoid | 17.36 ± 0.38 a | 12.00 ± 0.30 b | 15.95 ± 0.11 a | 11.32 ± 1.94c | 4.76 ± 0.11 d |
| DPPH | 344.09 ± 8.61 a | 328.36 ± 2.31 ab | 339.00 ± 9.26 ab | 322.00 ± 5.40 b | 292.00 ± 8.74 c |
| FRAP | 348.19 ± 3.85 a | 322.00 ± 3.60 b | 343.11 ± 1.36 a | 317.40 ± 3.47 b | 285.12 ± 6.95 c |
| Storage Time (Days) | Total Plate Count (log CFU/mL) | Yeast and Mold Count (log CFU/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | UV | NS | UV + NS | Pasteurization | Control | UV | NS | UV + NS | Pasteurization | |
| 0 | 3.08 ± 0.02 | 0.29 ± 0.16 | 0.99 ± 0.06 | - | - | 3.46 ± 0.04 | 0.99 ± 0.06 | 3.21 ± 0.05 | 0.72 ± 0.03 | - |
| 3 | 4.36 ± 0.05 | 1.09 ± 0.13 | 2.21 ± 0.10 | - | - | 4.65 ± 0.27 | 1.54 ± 0.09 | 4.52 ± 0.14 | 1.20 ± 0.05 | - |
| 6 | 6.07 ± 0.09 | 1.93 ± 0.07 | 3.68 ± 0.08 | - | 0.74 ± 0.01 | 6.24 ± 0.64 | 3.68 ± 0.14 | 6.87 ± 0.14 | 3.03 ± 0.01 | - |
| 9 | 7.26 ± 0.05 | 3.83 ± 0.18 | 4.78 ± 0.10 | 0.55 ± 0.03 | 0.93 ± 0.03 | 7.85 ± 0.07 | 4.86 ± 0.17 | 7.96 ± 0.05 | 4.43 ± 0.02 | - |
| 12 | 7.60 ± 0.27 | 5.71 ± 0.12 | 6.68 ± 0.17 | 0.91 ± 0.01 | 1.54 ± 0.01 | 8.73 ± 0.08 | 6.03 ± 0.13 | 8.49 ± 0.02 | 5.71 ± 0.01 | - |
| 15 | 7.95 ± 0.04 | 6.52 ± 0.46 | 6.98 ± 0.04 | 1.44 ± 0.02 | 1.91 ± 0.03 | 9.21 ± 0.07 | 7.27 ± 0.041 | 9.04 ± 0.03 | 7.09 ± 0.05 | - |
| 18 | 8.28 ± 0.03 | 6.77 ± 0.23 | 7.66 ± 0.01 | 1.95 ± 0.04 | 2.64 ± 0.01 | 9.32 ± 0.13 | 7.96 ± 0.002 | 9.14 ± 0.11 | 7.73 ± 0.06 | - |
| 21 | 9.49 ± 0.05 | 7.24 ± 0.19 | 7.88 ± 0.08 | 2.28 ± 0.02 | 3.11 ± 0.03 | 8.99 ± 0.03 | 9.20 ± 0.038 | 9.25 ± 0.11 | 8.98 ± 0.02 | - |
| Storage Time (Days) | pH | Total Soluble Solid (Brix) | Titratable Acidity (TA % Malic Acid) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | UV | NS | UV + NS | Pasteurization | Control | UV | NS | UV + NS | Pasteurization | Control | UV | NS | UV + NS | Pasteurization | |
| 0 | 3.88 ± 0.025 | 3.88 ± 0.018 | 3.88 ± 0.021 | 3.88 ± 0.001 | 3.88 ± 0.005 | 8.51 ± 0.05 | 9.30 ± 0.14 | 8.75 ± 0.01 | 9.30 ± 0.07 | 9.38 ± 0.04 | 0.42 ± 0.029 | 0.42 ± 0.029 | 0.42 ± 0.029 | 0.42 ± 0.032 | 0.42 ± 0.029 |
| 3 | 3.82 ± 0.021 | 3.86 ± 0.021 | 3.85 ± 0.025 | 3.86 ± 0.005 | 3.87 ± 0.005 | 8.34 ± 0.05 | 9.00 ± 0.01 | 8.60 ± 0.01 | 9.15 ± 0.01 | 9.30 ± 0.01 | 0.44 ± 0.011 | 0.43 ± 0.016 | 0.44 ± 0.034 | 0.41 ± 0.005 | 0.40 ± 0.001 |
| 6 | 3.77 ± 0.004 | 3.86 ± 0.032 | 3.83 ± 0.046 | 3.85 ± 0.010 | 3.87 ± 0.001 | 8.23 ± 0.04 | 8.70 ± 0.21 | 8.48 ± 0.04 | 8.85 ± 0.01 | 9.20 ± 0.01 | 0.49 ± 0.027 | 0.44 ± 0.018 | 0.46 ± 0.02 | 0.42 ± 0.005 | 0.40 ± 0.001 |
| 9 | 3.68 ± 0.007 | 3.85 ± 0.032 | 3.78 ± 0.007 | 3.85 ± 0.001 | 3.87 ± 0.005 | 8.05 ± 0.01 | 8.50 ± 0.21 | 8.35 ± 0.14 | 8.75 ± 0.01 | 9.05 ± 0.01 | 0.52 ± 0.032 | 0.47 ± 0.014 | 0.5 ± 0.029 | 0.44 ± 0.016 | 0.40 ± 0.001 |
| 12 | 3.59 ± 0.032 | 3.82 ± 0.039 | 3.76 ± 0.014 | 3.84 ± 0.005 | 3.87 ± 0.001 | 7.70 ± 0.21 | 8.40 ± 0.35 | 8.23 ± 0.25 | 8.65 ± 0.01 | 8.95 ± 0.01 | 0.58 ± 0.027 | 0.5 ± 0.007 | 0.53 ± 0.018 | 0.45 ± 0.009 | 0.40 ± 0.001 |
| 15 | 3.54 ± 0.011 | 3.78 ± 0.060 | 3.66 ± 0.011 | 3.81 ± 0.005 | 3.87 ± 0.005 | 7.45 ± 0.28 | 8.25 ± 0.35 | 7.95 ± 0.28 | 8.50 ± 0.01 | 8.85 ± 0.01 | 0.61 ± 0.036 | 0.52 ± 0.011 | 0.55 ± 0.009 | 0.46 ± 0.011 | 0.40 ± 0.001 |
| 18 | 3.52 ± 0.011 | 3.70 ± 0.021 | 3.62 ± 0.018 | 3.78 ± 0.005 | 3.86 ± 0.005 | 7.18 ± 0.18 | 8.03 ± 0.25 | 7.68 ± 0.18 | 8.30 ± 0.01 | 8.85 ± 0.01 | 0.63 ± 0.016 | 0.55 ± 0.009 | 0.58 ± 0.014 | 0.47 ± 0.009 | 0.4010.001 |
| 21 | 3.50 ± 0.011 | 3.66 ± 0.025 | 3.56 ± 0.007 | 3.72 ± 0.005 | 3.86 ± 0.001 | 7.11 ± 0.05 | 9.30 ± 0.14 | 8.75 ± 0.01 | 9.30 ± 0.07 | 9.38 ± 0.04 | 0.66 ± 0.007 | 0.57 ± 0.014 | 0.61 ± 0.018 | 0.49 ± 0.009 | 0.41 ± 0.001 |
| Storage Time (Days) | L* Values | a* Values | b* Values | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | UV | NS | UV + NS | Pasteurization | Control | UV | NS | UV + NS | Pasteurization | Control | UV | NS | UV + NS | Pasteurization | |
| 0 | 71.3 ± 0.04 | 71.0 ± 0.05 | 71.2 ± 0.00 | 71.0 ± 0.01 | 70.28 ± 0.09 | 11.6 ± 0.12 | 12.0 ± 0.01 | 11.5 ± 0.06 | 12.0 ± 0.01 | 12.5 ± 0.05 | 74.2 ± 0.04 | 73.9 ± 0.1 | 74.0 ± 0.02 | 74.0 ± 0.03 | 73.0 ± 0.03 |
| 3 | 71.4 ± 0.26 | 71.0 ± 0.01 | 71.2 ± 0.01 | 71.1 ± 0.03 | 70.12 ± 0.14 | 11.5 ± 0.01 | 12.0 ± 0.01 | 11.5 ± 0.01 | 12.1 ± 0.04 | 12.5 ± 0.07 | 74.2 ± 0.03 | 73.9 ± 0.2 | 74.1 ± 0.05 | 74.0 ± 0.00 | 73.0 ± 0.11 |
| 6 | 71.4 ± 0.04 | 71.1 ± 0.00 | 71.2 ± 0.18 | 71.1 ± 0.01 | 70.22 ± 0.04 | 11.5 ± 0.02 | 12.1 ± 0.01 | 11.6 ± 0.05 | 12.1 ± 0.01 | 12.5 ± 0.06 | 74.3 ± 0.00 | 74.1 ± 0.1 | 74.2 ± 0.05 | 74.1 ± 0.01 | 73.0 ± 0.01 |
| 9 | 71.5 ± 0.07 | 71.1 ± 0.05 | 71.4 ± 0.10 | 71.1 ± 0.01 | 70.30 ± 0.08 | 11.6 ± 0.15 | 12.1 ± 0.01 | 11.6 ± 0.07 | 12.1 ± 0.01 | 12.6 ± 0.09 | 74.5 ± 0.07 | 74.1 ± 0.1 | 74.3 ± 0.07 | 74.1 ± 0.01 | 73.1 ± 0.01 |
| 12 | 71.7 ± 0.11 | 71.2 ± 0.04 | 71.5 ± 0.07 | 71.1 ± 0.01 | 70.32 ± 0.07 | 11.9 ± 0.01 | 12.1 ± 0.03 | 11.7 ± 0.06 | 12.1 ± 0.03 | 12.6 ± 0.08 | 74.7 ± 0.07 | 74.1 ± 0.1 | 74.4 ± 0.11 | 74.1 ± 0.04 | 73.1 ± 0.01 |
| 15 | 71.7 ± 0.21 | 71.2 ± 0.03 | 71.7 ± 0.07 | 71.1 ± 0.01 | 70.33 ± 0.06 | 12.0 ± 0.04 | 12.2 ± 0.03 | 11.7 ± 0.03 | 12.1 ± 0.01 | 12.6 ± 0.09 | 74.8 ± 0.14 | 74.2 ± 0.1 | 74.6 ± 0.11 | 74.1 ± 0.01 | 73.1 ± 0.03 |
| 18 | 71.9 ± 0.12 | 71.2 ± 0.01 | 71.8 ± 0.04 | 71.1 ± 0.02 | 70.36 ± 0.04 | 12.0 ± 0.05 | 12.2 ± 0.02 | 11.8 ± 0.04 | 12.2 ± 0.02 | 12.6 ± 0.10 | 75.0 ± 0.07 | 74.3 ± 0.01 | 74.8 ± 0.07 | 74.2 ± 0.06 | 73.1 ± 0.04 |
| 21 | 72.0 ± 0.16 | 71.3 ± 0.04 | 71.9 ± 0.01 | 71.2 ± 0.01 | 70.40 ± 0.10 | 12.1 ± 0.08 | 12.2 ± 0.07 | 11.9 ± 0.05 | 12.2 ± 0.03 | 12.7 ± 0.07 | 75.4 ± 0.07 | 74.4 ± 0.0 | 75.0 ± 0.09 | 74.2 ± 0.01 | 73.2 ± 0.04 |
| Antioxidant Properties | Storage Time (Days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | |
| TPC (mg GAE/L) | ||||||||
| Control | 213.14 ± 3.40 | 203.04 ± 9.31 | 193.18 ± 8.85 | 189.15 ± 8.93 | 181.92 ± 15.01 | 173.29 ± 8.91 | 165.79 ± 14.92 | 160.06 ± 15.68 |
| UV | 136.53 ± 5.45 | 123.37 ± 11.34 | 114.93 ± 10.47 | 110.77 ± 11.34 | 107.45 ± 10.63 | 102.11 ± 6.15 | 95.22 ± 1.75 | 90.24 ± 5.34 |
| NS | 212.64 ± 2.43 | 196.86 ± 15.73 | 187.30 ± 10.80 | 185.31 ± 11.93 | 182.64 ± 10.80 | 176.84 ± 8.10 | 169.59 ± 10.80 | 166.38 ± 10.80 |
| UV + NS | 135.16 ± 11.61 | 124.74 ± 9.18 | 118.81 ± 5.42 | 109.76 ± 10.28 | 105.69 ± 11.88 | 102.98 ± 12.68 | 98.1 ± 13.49 | 86.69 ± 5.78 |
| Pasteurization | 97.39 ± 1.75 | 85.90 ± 4.78 | 76.9 ± 1.86 | 76.55 ± 3.7 | 74.70 ± 2.21 | 71.23 ± 1.40 | 60.45 ± 3.75 | 53.54 ± 1.16 |
| Total Flavonoid Content (mg QE/L) | ||||||||
| Control | 183.18 ± 2.68 | 176.67 ± 1.24 | 166.45 ± 0.78 | 165.38 ± 1.59 | 162.08 ± 1.52 | 151.07 ± 4.58 | 139.23 ± 0.66 | 129.45 ± 7.11 |
| UV | 170.19 ± 2.33 | 161.56 ± 0.08 | 156.48 ± 0.66 | 149.34 ± 1.2 | 141.53 ± 0.58 | 134.14 ± 1.94 | 123.26 ± 5.44 | 104.01 ± 4.78 |
| NS | 179.80 ± 0.78 | 171.12 ± 1.55 | 169.97 ± 1.17 | 168.76 ± 0.78 | 161.29 ± 1.94 | 142.25 ± 0.12 | 136.29 ± 0.39 | 133.40 ± 2.37 |
| UV + NS | 166.26 ± 3.15 | 161.48 ± 1.13 | 153.43 ± 2.33 | 147.41 ± 4.47 | 144.20 ± 0.47 | 133.15 ± 7.23 | 127.11 ± 7.77 | 99.42 ± 1.55 |
| Pasteurization | 147.55 ± 1.01 | 146.18 ± 0.16 | 144.61 ± 0.51 | 133.98 ± 0.62 | 125.71 ± 4.47 | 117.03 ± 1.59 | 106.09 ± 1.75 | 88.84 ± 0.74 |
| Total Carotenoid Content (μg/100 mL) | ||||||||
| Control | 16.6 ± 0.96 | 16.05 ± 1.01 | 15.18 ± 1.18 | 14.33 ± 2.11 | 13.96 ± 1.91 | 13.13 ± 1.57 | 12.8 ± 1.51 | 12.08 ± 1.28 |
| UV | 13.82 ± 0.98 | 13.13 ± 0.78 | 12.44 ± 1.14 | 11.62 ± 1.09 | 11.19 ± 1.14 | 10.31 ± 0.82 | 9.20 ± 0.49 | 8.67 ± 0.38 |
| NS | 16.22 ± 0.24 | 15.86 ± 0.39 | 14.86 ± 0.06 | 14.41 ± 0.28 | 13.9 ± 0.39 | 13.31 ± 0.26 | 12.49 ± 0.2 | 11.73 ± 0.10 |
| UV + NS | 14.16 ± 1.27 | 13.67 ± 0.84 | 12.66 ± 1.05 | 12.09 ± 0.76 | 11.52 ± 0.66 | 10.67 ± 0.09 | 10.04 ± 0.28 | 8.82 ± 0.55 |
| Pasteurization | 7.64 ± 1.06 | 7.04 ± 1.21 | 6.49 ± 1.17 | 6.00 ± 1.11 | 5.50 ± 0.98 | 4.65 ± 0.93 | 4.07 ± 1.08 | 3.56 ± 0.92 |
| Vitamin C (mg/100 mL) | ||||||||
| Control | 46.32 ± 2.63 | 42.06 ± 2.26 | 41.05 ± 2.26 | 36.48 ± 2.26 | 33.9 ± 0.11 | 32.36 ± 0.64 | 30.95 ± 0.38 | 28.77 ± 0.30 |
| UV | 35.15 ± 1.88 | 33.77 ± 1.28 | 31.32 ± 2.03 | 26.37 ± 1.50 | 24.27 ± 1.62 | 22.06 ± 2.26 | 19.96 ± 3.27 | 17.99 ± 1.62 |
| NS | 47.25 ± 0.94 | 43.02 ± 2.11 | 40.71 ± 1.77 | 36.90 ± 0.15 | 34.4 ± 1.05 | 30.31 ± 0.75 | 29.03 ± 0.75 | 26.45 ± 1.47 |
| UV + NS | 36.05 ± 1.50 | 34.62 ± 0.15 | 32.84 ± 0.04 | 27.97 ± 1.05 | 25.31 ± 1.28 | 21.74 ± 1.20 | 20.55 ± 2.29 | 17.62 ± 1.77 |
| Pasteurization | 28.37 ± 1.84 | 26.08 ± 1.02 | 24.56 ± 1.73 | 22.81 ± 0.60 | 18.45 ± 0.75 | 16.27 ± 0.75 | 15.31 ± 0.75 | 12.01 ± 0.75 |
| DPPH (mM Trolox/100 mL) | ||||||||
| Control | 392.18 ± 3.64 | 361.45 ± 1.82 | 355.18 ± 3.09 | 340.45 ± 7.82 | 323.55 ± 7.82 | 297.09 ± 5.45 | 290.82 ± 18.36 | 256.00 ± 1.09 |
| UV | 347.82 ± 2.31 | 332.91 ± 21.86 | 302.27 ± 1.16 | 278.82 ± 1.41 | 263.91 ± 5.01 | 260.45 ± 3.73 | 248.18 ± 1.54 | 226.09 ± 3.99 |
| NS | 381.27 ± 7.97 | 353.45 ± 3.60 | 347.73 ± 3.99 | 328.55 ± 3.60 | 303.64 ± 2.57 | 292.91 ± 3.34 | 283.82 ± 3.34 | 249.27 ± 6.43 |
| UV + NS | 333.27 ± 5.66 | 317.00 ± 7.07 | 307.45 ± 29.31 | 265.09 ± 7.71 | 254.00 ± 4.37 | 248.00 ± 3.60 | 236.82 ± 1.93 | 227.82 ± 5.40 |
| Pasteurization | 292.18 ± 4.37 | 287.09 ± 3.34 | 282.00 ± 5.14 | 233.45 ± 9.77 | 224.55 ± 2.57 | 211.82 ± 2.57 | 202.55 ± 4.37 | 170.64 ± 4.24 |
| FRAP (mM Trolox/100 mL) | ||||||||
| Control | 367.75 ± 3.51 | 331.09 ± 12.63 | 322.49 ± 0.35 | 304.25 ± 3.16 | 295.04 ± 1.23 | 289.68 ± 0.35 | 283.28 ± 0.53 | 272.4 ± 2.28 |
| UV | 325.65 ± 0.50 | 303.11 ± 2.61 | 296.70 ± 0.99 | 280.47 ± 1.12 | 268.19 ± 1.12 | 263.46 ± 2.61 | 255.21 ± 4.84 | 239.33 ± 6.2 |
| NS | 358.81 ± 2.23 | 331.96 ± 3.97 | 319.51 ± 11.91 | 310.74 ± 9.68 | 287.32 ± 3.6 | 280.21 ± 6.45 | 275.21 ± 10.3 | 265.21 ± 9.06 |
| UV + NS | 320.47 ± 3.10 | 316.61 ± 2.61 | 292.05 ± 2.61 | 273.28 ± 5.09 | 263.81 ± 0.37 | 268.19 ± 25.18 | 253.89 ± 11.66 | 226.79 ± 1.61 |
| Pasteurization | 290.56 ± 3.23 | 274.51 ± 6.57 | 268.19 ± 3.6 | 249.16 ± 3.47 | 244.33 ± 3.85 | 223.81 ± 5.58 | 220.56 ± 6.45 | 207.23 ± 2.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kijpatanasilp, I.; Shiekh, K.A.; Jafari, S.; Worobo, R.W.; Assatarakul, K. Microbial Inhibition by UV Radiation Combined with Nisin and Shelf-Life Extension of Tangerine Juice during Refrigerated Storage. Foods 2023, 12, 2725. https://doi.org/10.3390/foods12142725
Kijpatanasilp I, Shiekh KA, Jafari S, Worobo RW, Assatarakul K. Microbial Inhibition by UV Radiation Combined with Nisin and Shelf-Life Extension of Tangerine Juice during Refrigerated Storage. Foods. 2023; 12(14):2725. https://doi.org/10.3390/foods12142725
Chicago/Turabian StyleKijpatanasilp, Isaya, Khursheed Ahmad Shiekh, Saeid Jafari, Randy W. Worobo, and Kitipong Assatarakul. 2023. "Microbial Inhibition by UV Radiation Combined with Nisin and Shelf-Life Extension of Tangerine Juice during Refrigerated Storage" Foods 12, no. 14: 2725. https://doi.org/10.3390/foods12142725
APA StyleKijpatanasilp, I., Shiekh, K. A., Jafari, S., Worobo, R. W., & Assatarakul, K. (2023). Microbial Inhibition by UV Radiation Combined with Nisin and Shelf-Life Extension of Tangerine Juice during Refrigerated Storage. Foods, 12(14), 2725. https://doi.org/10.3390/foods12142725






