Glycation Improved the Interfacial Adsorption and Emulsifying Performance of β-Conglycinin to Stabilize the High Internal Phase Emulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Glycated 7S
2.3. Characterization of Untreated and Glycated 7S
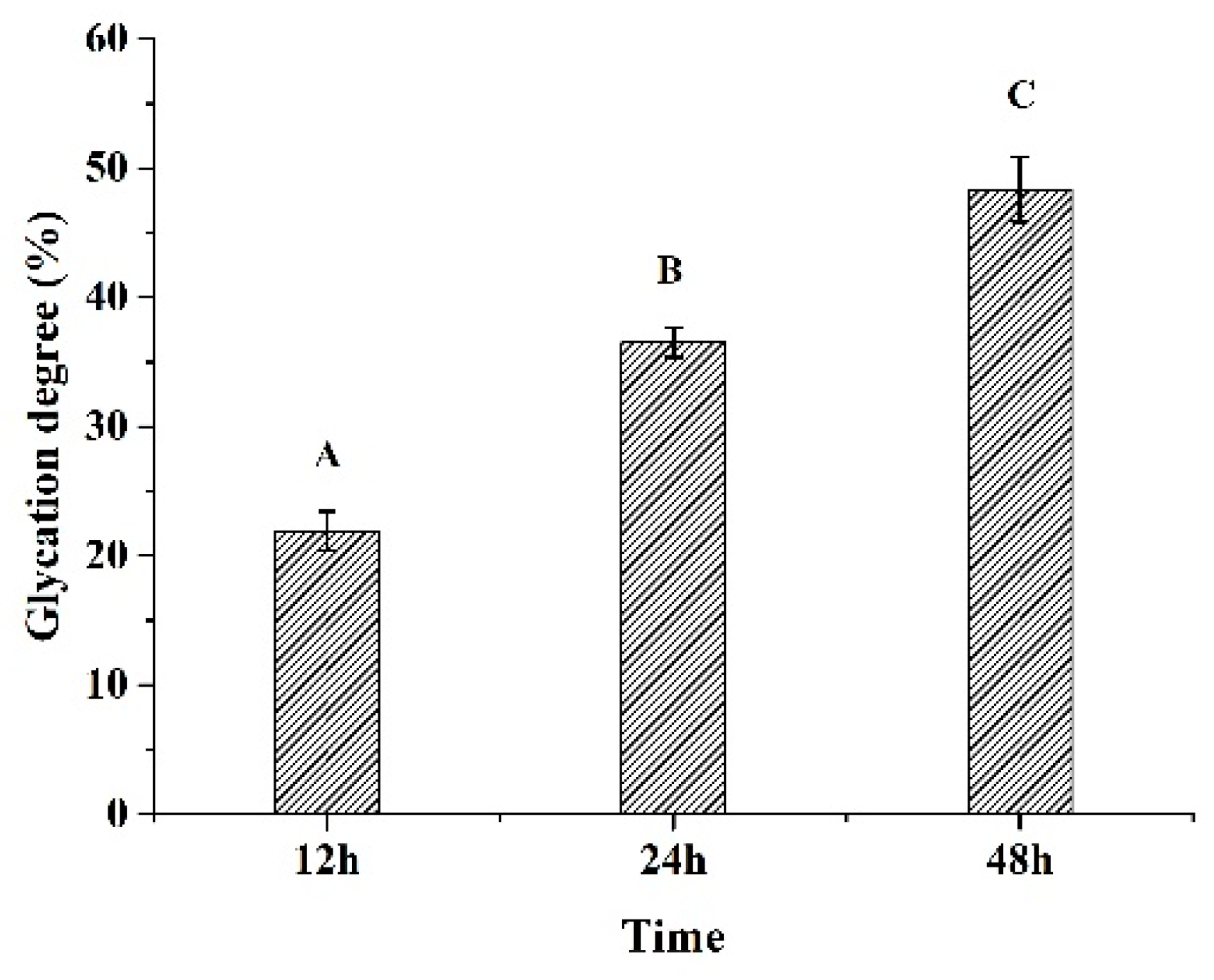
2.3.1. Measurement of Glycation Degree
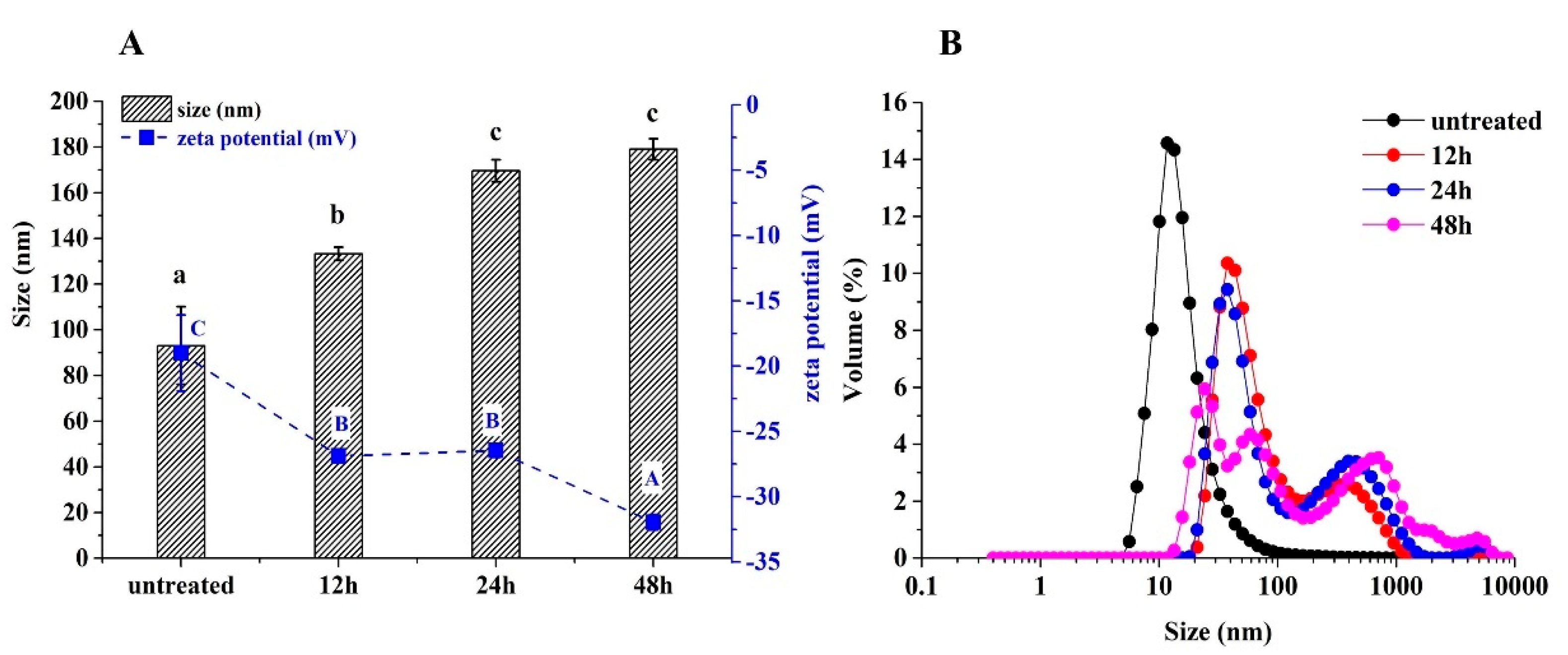
2.3.2. Hydrodynamic Diameter and Zeta Potential
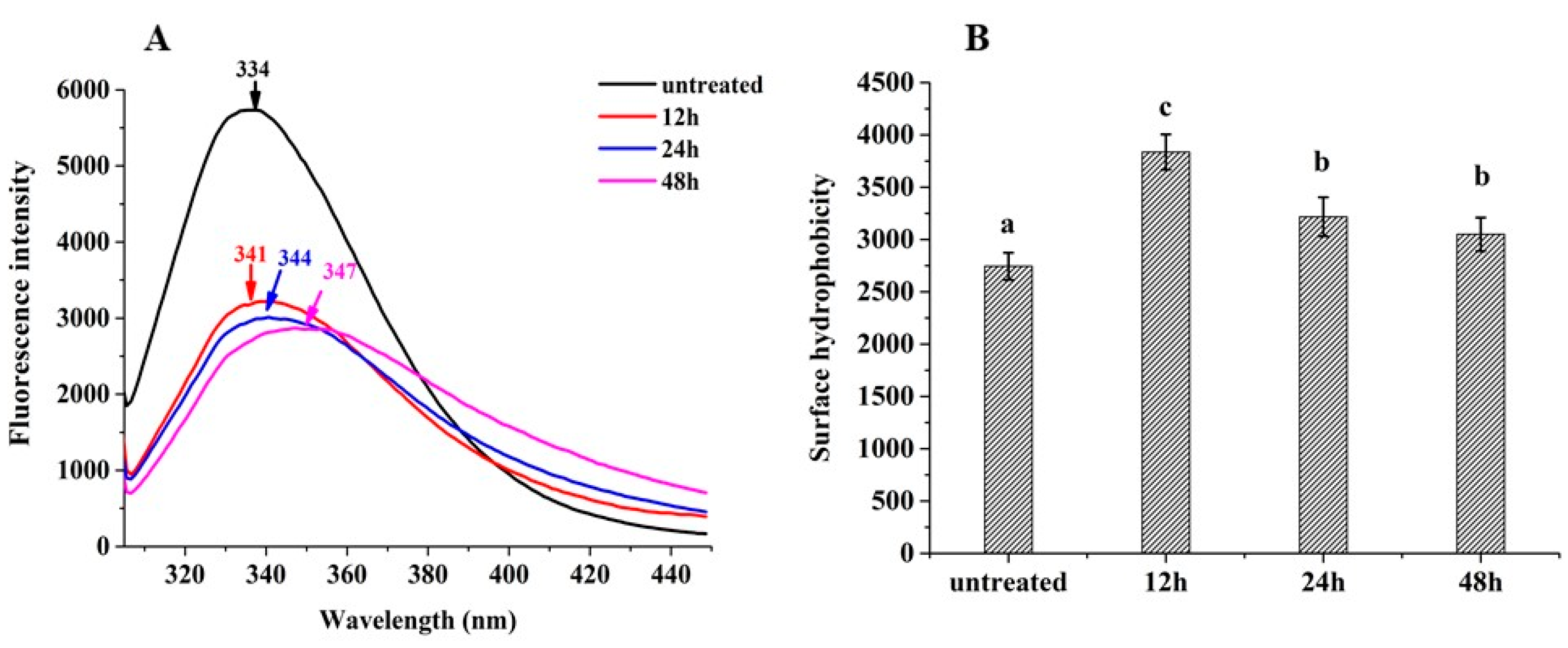
2.3.3. Surface Hydrophobicity (H0)
2.3.4. Intrinsic Fluorescence Spectroscopy
2.3.5. Far-UV Circular Dichroism Spectroscopy
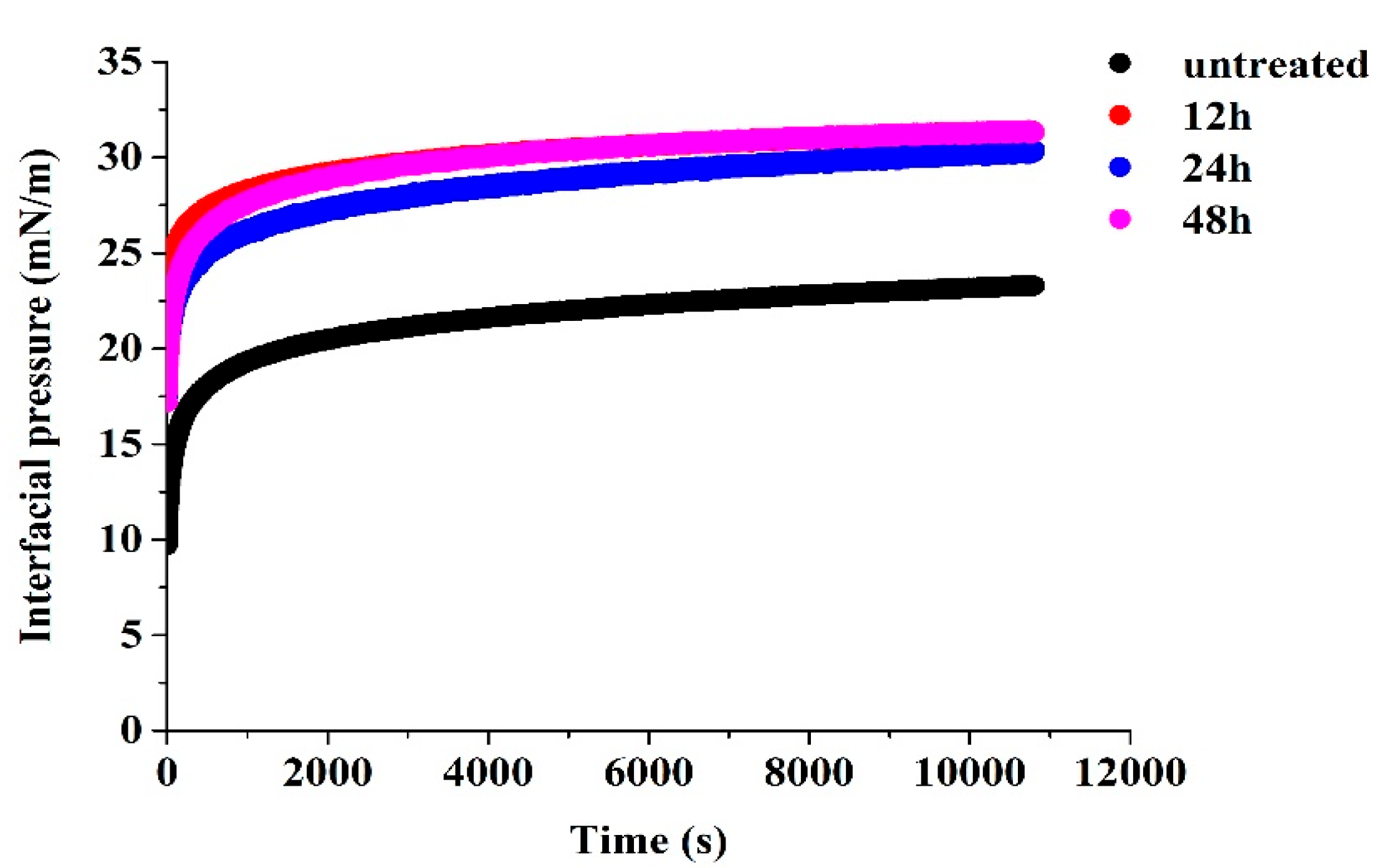
2.3.6. Interfacial Adsorption Kinetics Analysis
2.3.7. Dilatational Viscoelasticity Analysis
2.4. Preparation for HIPE
2.5. Characterization of HIPE
2.5.1. Droplet Size and Zeta Potential
2.5.2. Rheological Behavior Analysis
2.5.3. Stability of HIPE
2.6. Statistic
3. Results and Discussion
3.1. Glycation Degree
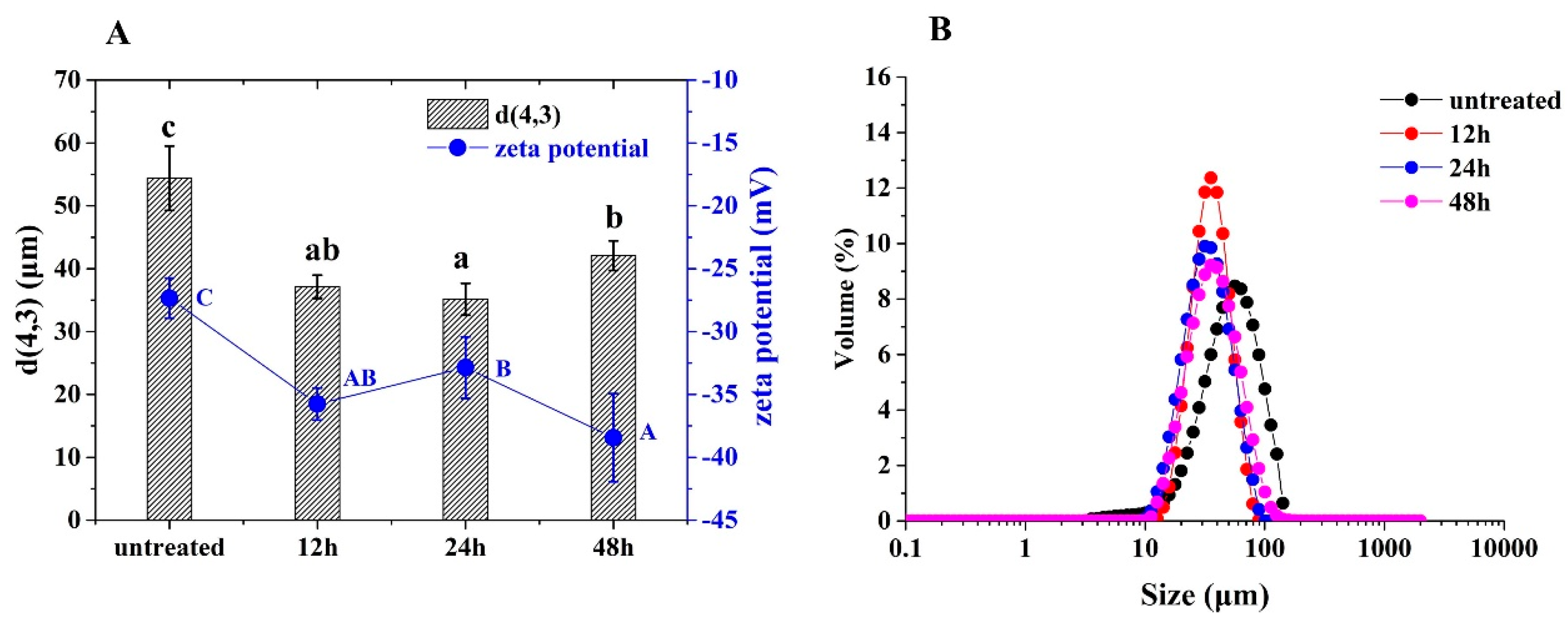
3.2. Particle Sizes and Zeta Potentials
3.3. Intrinsic Fluorescence Spectra
3.4. Hydrophobicity
3.5. Far-UV Circular Dichroism Spectra
3.6. Interfacial Adsorption Behavior
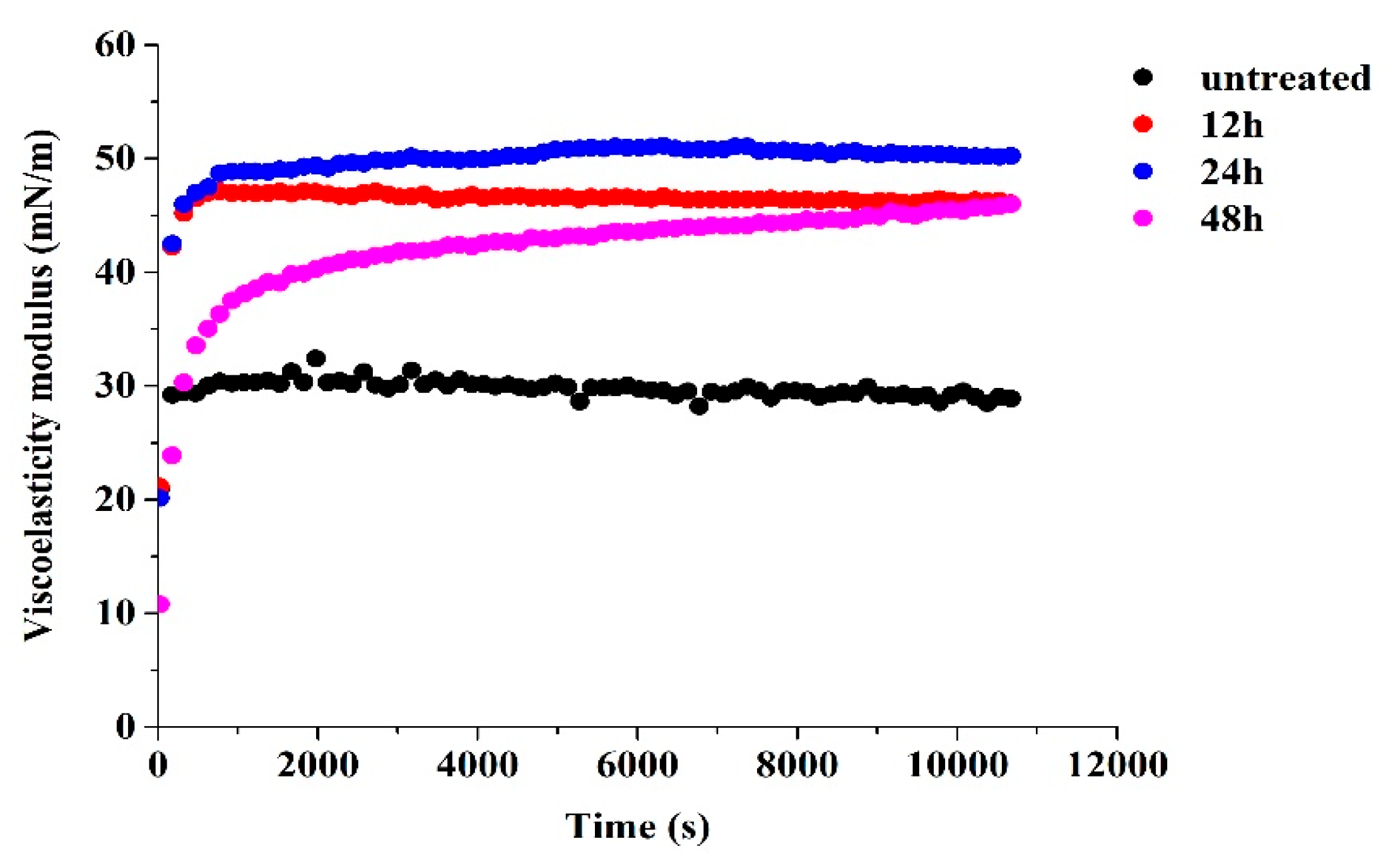
3.7. Dilatational Rheology Properties
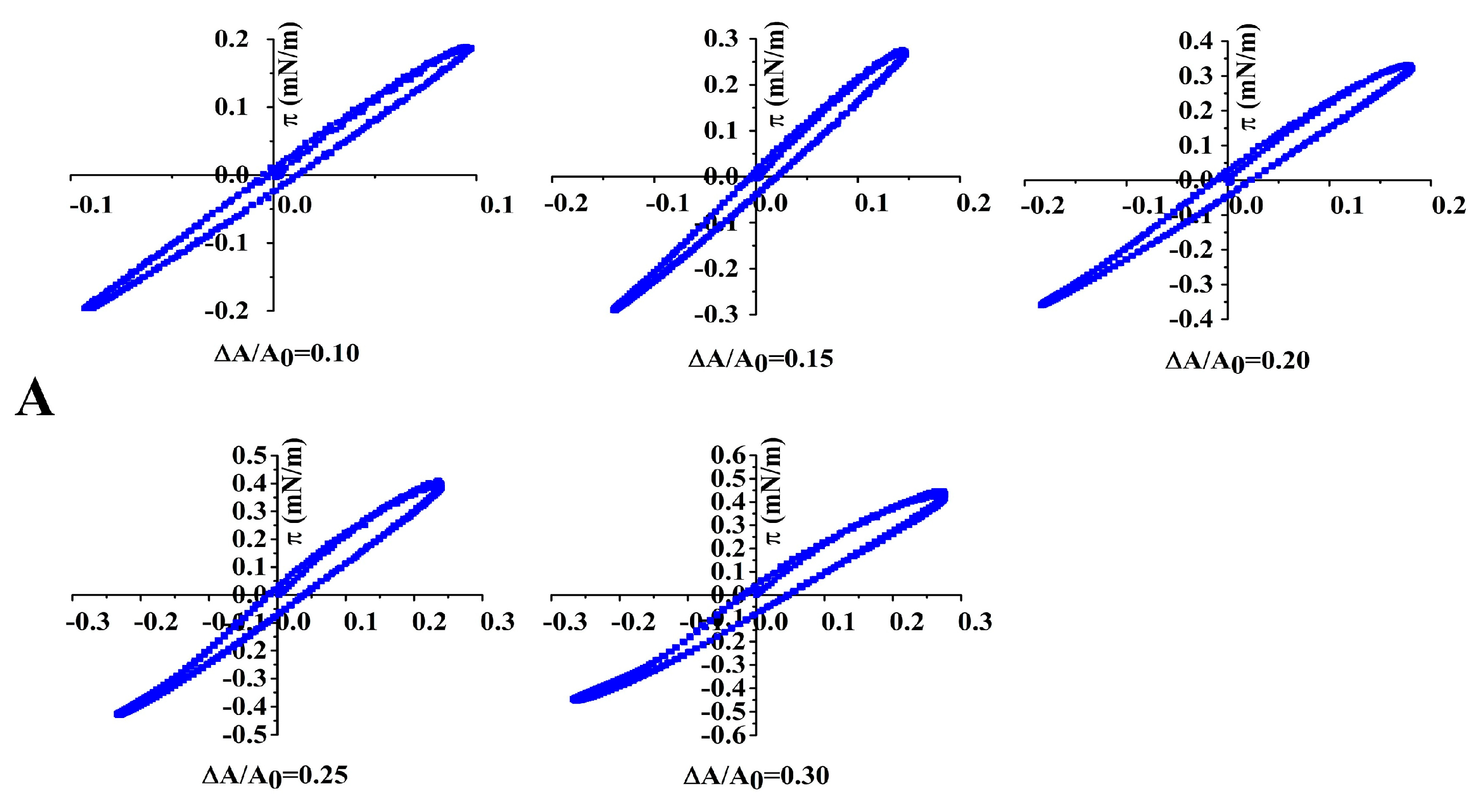
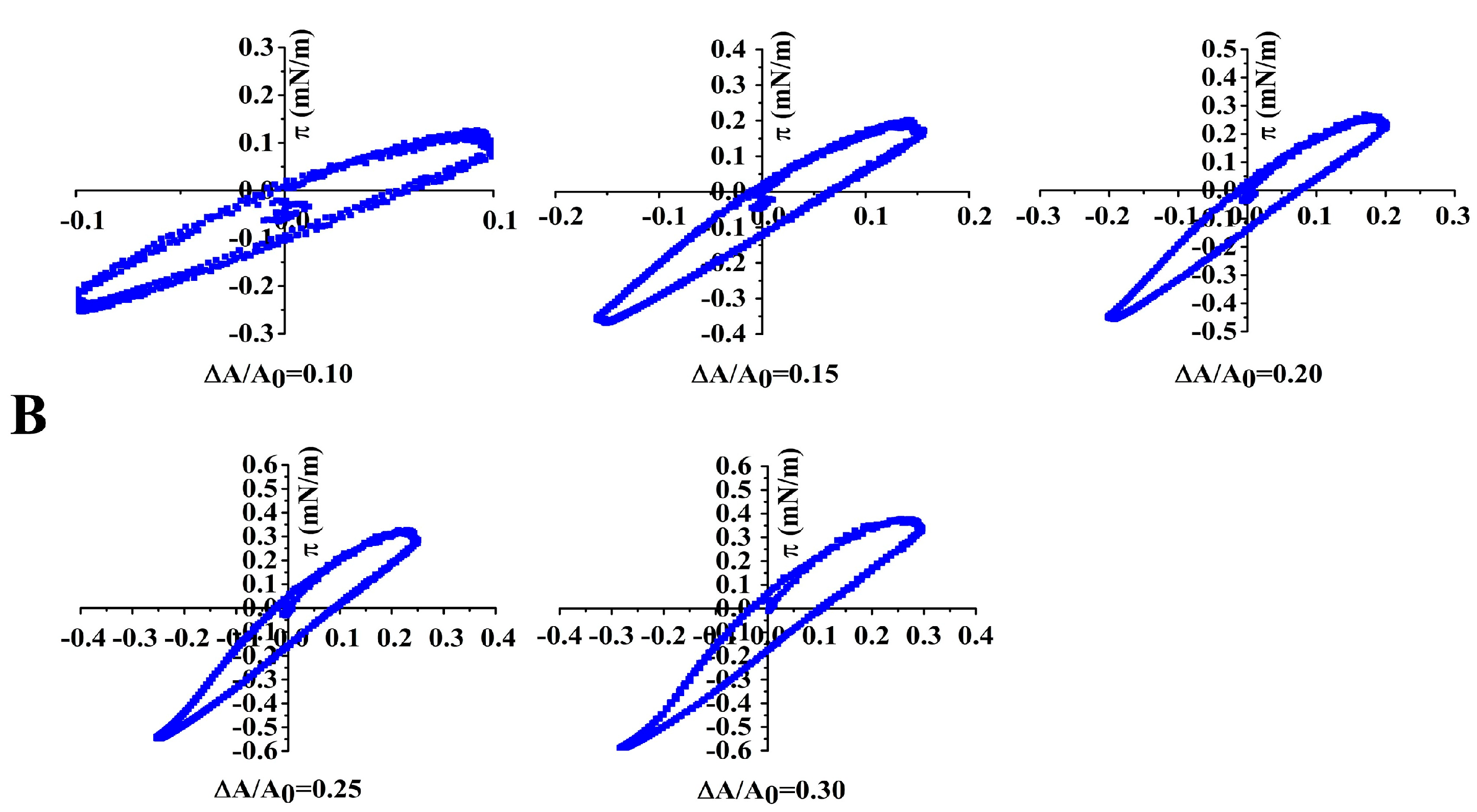
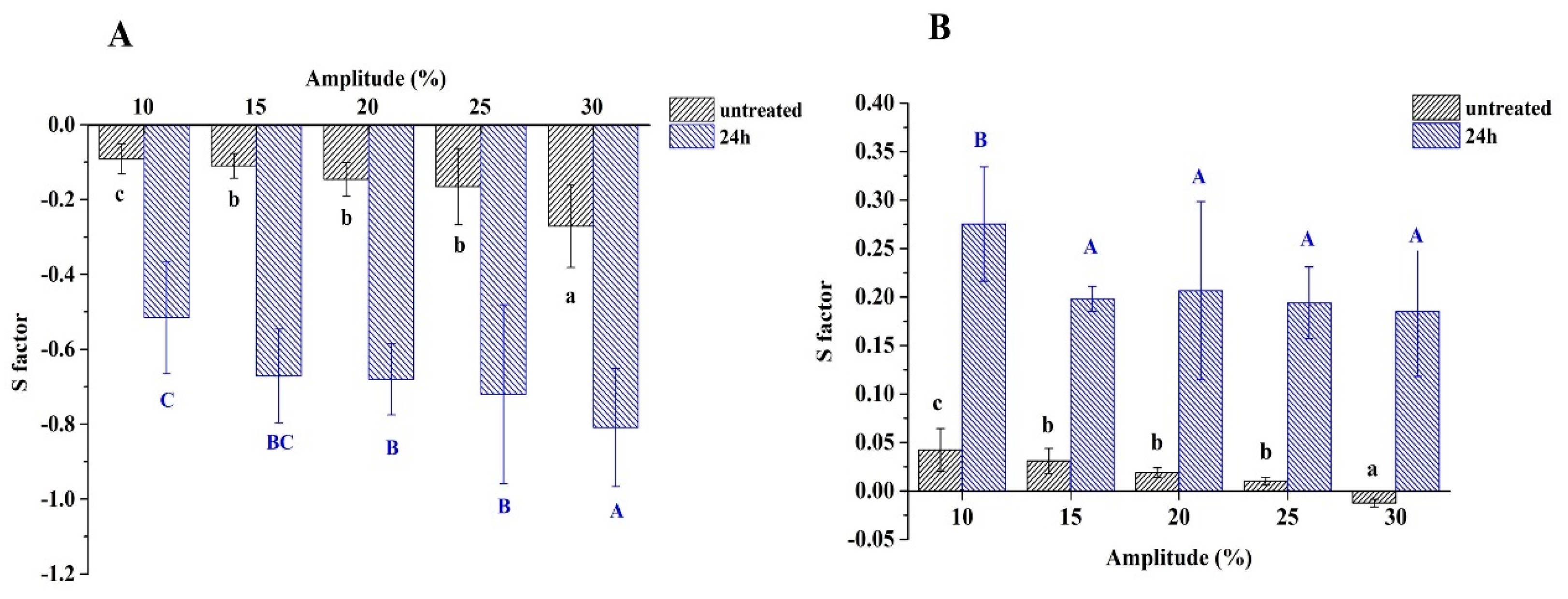
3.8. Non-Linear Dilatational Rheology Properties
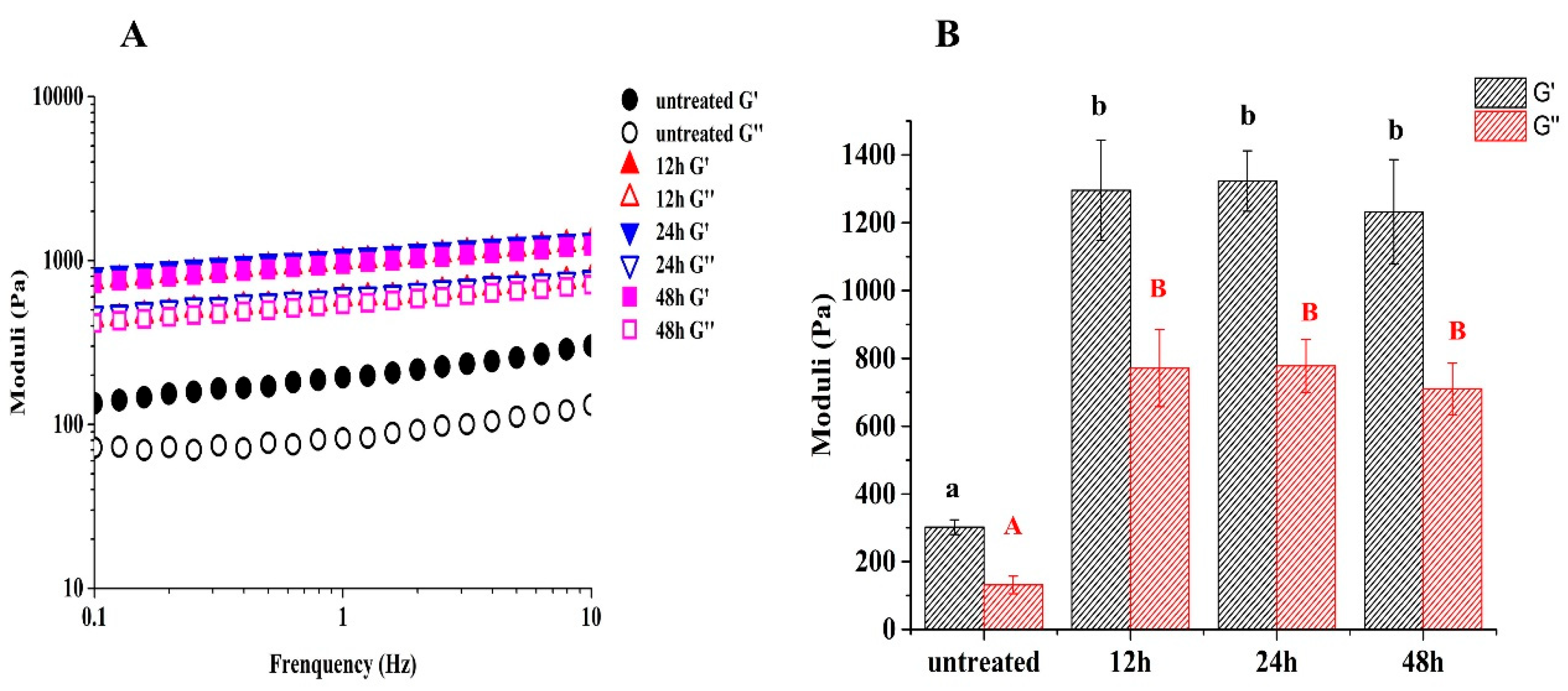
3.9. Droplet Sizes and Zeta Potentials of HIPE
3.10. Rheological Analysis of HIPE
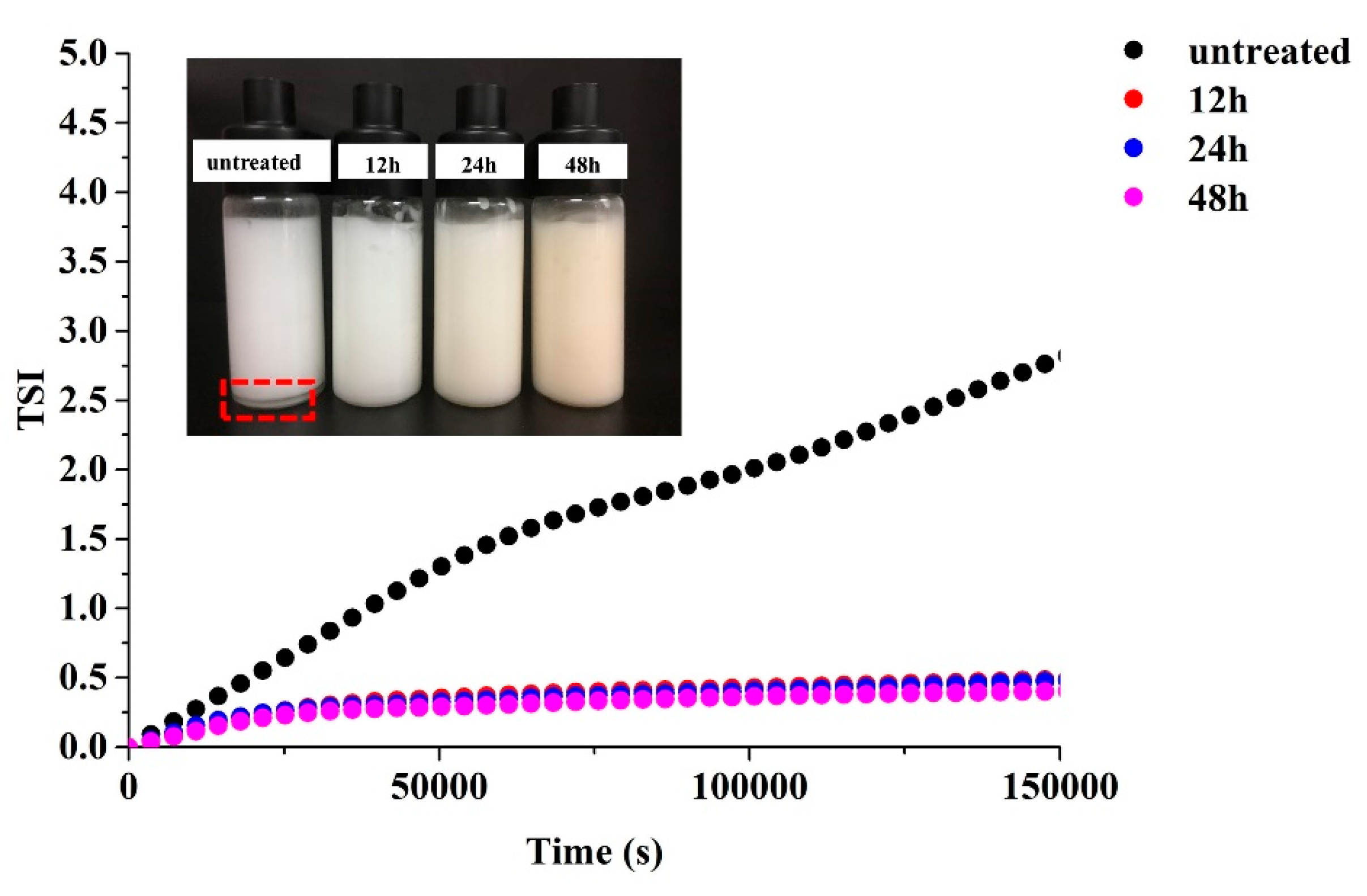
3.11. Stability of HIPE
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Achouri, A.; Boye, J.I.; Belanger, D.; Chiron, T.; Yaylayan, V.A.; Yeboah, F.K. Functional and molecular properties of calcium precipitated soy glycinin and the effect of glycation with κ-carrageenan. Food Res. Int. 2010, 43, 1494–1504. [Google Scholar] [CrossRef]
- Ribeiro, E.; Morell, P.; Nicoletti, V.; Quiles, A.; Hernando, I. Protein- and polysaccharide-based particles used for Pickering emulsion stabilisation. Food Hydrocoll. 2021, 119, 106839. [Google Scholar] [CrossRef]
- Shi, A.; Feng, X.; Wang, Q.; Adhikari, B. Pickering and high internal phase Pickering emulsions stabilized by protein-based particles: A review of synthesis, application and prospective. Food Hydrocoll. 2020, 109, 106117. [Google Scholar] [CrossRef]
- Tirgarian, B.; Farmani, J.; Farahmandfar, R.; Milani, J.M.; Van Bockstaele, F. Ultra-stable high internal phase emulsions stabilized by protein-anionic polysaccharide Maillard conjugates. Food Chem. 2022, 393, 133427. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.-Z.; Peng, X.-Q.; Tang, C.-H. Edible pickering high internal phase emulsions stabilized by soy glycinin: Improvement of emulsification performance and pickering stabilization by glycation with soy polysaccharide. Food Hydrocoll. 2020, 103, 105672. [Google Scholar] [CrossRef]
- Lu, F.; Ma, Y.; Zang, J.; Qing, M.; Ma, Z.; Chi, Y.; Chi, Y. High-temperature glycosylation modifies the molecular structure of ovalbumin to improve the freeze-thaw stability of its high internal phase emulsion. Int. J. Biol. Macromol. 2023, 233, 123560. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Y.; Ahmad, M.; Ying, R.; Wang, Y.; Tan, C. Fabrication of pickering high internal phase emulsions stabilized by pecan protein/xanthan gum for enhanced stability and bioaccessibility of quercetin. Food Chem. 2021, 357, 129732. [Google Scholar] [CrossRef]
- Li, Z.; Xi, J.; Chen, H.; Chen, W.; Chen, W.; Zhong, Q.; Zhang, M. Effect of glycosylation with apple pectin, citrus pectin, mango pectin and sugar beet pectin on the physicochemical, interfacial and emulsifying properties of coconut protein isolate. Food Res. Int. 2022, 156, 111363. [Google Scholar] [CrossRef]
- Sun, C.; Fu, J.-J.; Tan, Z.-F.; Zhang, G.-Y.; Xu, X.-B.; Song, L. Improved thermal and oxidation stabilities of pickering high internal phase emulsions stabilized using glycated pea protein isolate with glycation extent. LWT Food Sci. Technol. 2022, 162, 113465. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, S.; Jiang, M.; Liu, F.; Chen, K.; Zhang, Y. Effects of glycation methods on the interfacial behavior and emulsifying performance of soy protein isolate-gum Arabic conjugates. Int. J. Biol. Macromol. 2023, 233, 123554. [Google Scholar] [CrossRef]
- Tang, C.-H. Emulsifying properties of soy proteins: A critical review with emphasis on the role of conformational flexibility. Crit. Rev. Food Sci. Nutr. 2017, 57, 2636–2679. [Google Scholar] [CrossRef]
- Peng, L.-P.; Tang, C.-H. Outstanding antioxidant pickering high internal phase emulsions by co-assembled polyphenol-soy β-conglycinin nanoparticles. Food Res. Int. 2020, 136, 109509. [Google Scholar] [CrossRef]
- Xu, Y.-T.; Tang, C.-H.; Binks, B.P. High internal phase emulsions stabilized solely by a globular protein glycated to form soft particles. Food Hydrocoll. 2020, 98, 105254. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, J.-R.; Li, K.-K.; Yin, S.-W.; Wang, J.-M.; Zhu, J.-H.; Yang, X.-Q. Characterization of soy β-conglycinin–dextran conjugate prepared by Maillard reaction in crowded liquid system. Food Res. Int. 2012, 49, 648–654. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Z.; Zhang, P.; Taha, A.; Hu, H.; Pan, S. The role of conformational state of pH-shifted β-conglycinin on the oil/water interfacial properties and emulsifying capacities. Food Hydrocoll. 2020, 108, 105990. [Google Scholar] [CrossRef]
- Peng, X.-Q.; Xu, Y.-T.; Liu, T.-X.; Tang, C.-H. Molecular Mechanism for Improving Emulsification Efficiency of Soy Glycinin by Glycation with Soy Soluble Polysaccharide. J. Agric. Food Chem. 2018, 66, 12316–12326. [Google Scholar] [CrossRef]
- Tian, Y.; Taha, A.; Zhang, P.; Zhang, Z.; Hu, H.; Pan, S. Effects of protein concentration, pH, and NaCl concentration on the physicochemical, interfacial, and emulsifying properties of β-conglycinin. Food Hydrocoll. 2021, 118, 106784. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Z.; Taha, A.; Chen, Y.; Hu, H.; Pan, S. Interfacial and emulsifying properties of β-conglycinin/pectin mixtures at the oil/water interface: Effect of pH. Food Hydrocoll. 2020, 109, 106145. [Google Scholar] [CrossRef]
- Sagis, L.M.C.; Fischer, P. Non-linear rheology of complex fluid–fluid interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 520–529. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, H.; Muhoza, B.; Hayat, K.; Hussain, S.; Tahir, M.U.; Zhang, X.; Ho, C.-T. Whey protein isolate-dextran conjugates: Decisive role of glycation time dependent conjugation degree in size control and stability improvement of colloidal nanoparticles. LWT Food Sci. Technol. 2021, 148, 111766. [Google Scholar] [CrossRef]
- Li, R.; Cui, Q.; Wang, G.; Liu, J.; Chen, S.; Wang, X.; Wang, X.; Jiang, L. Relationship between surface functional properties and flexibility of soy protein isolate-glucose conjugates. Food Hydrocoll. 2019, 95, 349–357. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, X.-H. Structure and property changes of the soy protein isolate glycated with maltose in an ionic liquid through the Maillard reaction. Food Funct. 2019, 10, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, Q.; Tang, C.; Luo, J.; Wu, X.; Lu, L.; Cai, Z. Study on structural, rheological and foaming properties of ovalbumin by ultrasound-assisted glycation with xylose. Ultrason. Sonochemistry 2019, 58, 104644. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.X.; Xiao, Y.; Wickham, E.D. Changes in physical, chemical and functional properties of whey protein isolate (WPI) and sugar beet pectin (SBP) conjugates formed by controlled dry-heating. Food Hydrocoll. 2017, 69, 86–96. [Google Scholar] [CrossRef]
- Zhang, Q.; Long, X.; Xie, J.; Xue, B.; Li, X.; Gan, J.; Bian, X.; Sun, T. Effect of d-galactose on physicochemical and functional properties of soy protein isolate during Maillard reaction. Food Hydrocoll. 2022, 133, 107914. [Google Scholar] [CrossRef]
- Zhou, X.; Sala, G.; Sagis, L.M. Bulk and interfacial properties of milk fat emulsions stabilized by whey protein isolate and whey protein aggregates. Food Hydrocoll. 2020, 109, 106100. [Google Scholar] [CrossRef]
- Cui, Q.; Zhang, A.; Li, R.; Wang, X.; Sun, L.; Jiang, L. Ultrasonic treatment affects emulsifying properties and molecular flexibility of soybean protein isolate-glucose conjugates. Food Biosci. 2020, 38, 100747. [Google Scholar] [CrossRef]
- Zhao, C.; Chu, Z.; Mao, Y.; Xu, Y.; Fei, P.; Zhang, H.; Xu, X.; Wu, Y.; Zheng, M.; Liu, J. Structural characteristics and acid-induced emulsion gel properties of heated soy protein isolate–soy oligosaccharide glycation conjugates. Food Hydrocoll. 2023, 137, 108408. [Google Scholar] [CrossRef]
- Xu, C.H.; Yu, S.J.; Yang, X.-Q.; Qi, J.-R.; Lin, H.; Zhao, Z.-G. Emulsifying properties and structural characteristics of β-conglycinin and dextran conjugates synthesised in a pressurised liquid system. Int. J. Food Sci. Technol. 2010, 45, 995–1001. [Google Scholar] [CrossRef]
- Dev, M.J.; Pandit, A.B.; Singhal, R.S. Ultrasound assisted vis-à-vis classical heating for the conjugation of whey protein isolate-gellan gum: Process optimization, structural characterization and physico-functional evaluation. Innov. Food Sci. Emerg. Technol. 2021, 72, 102724. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Shao, G.; Liu, J.; Wang, J.; Yang, L.; Li, J.; Liu, H.; Zhu, D.; Li, Y. pH-induced conformational changes and interfacial dilatational rheology of soy protein isolated/soy hull polysaccharide complex and its effects on emulsion stabilization. Food Hydrocoll. 2020, 109, 106075. [Google Scholar] [CrossRef]
- Zhou, Y.; Niu, H.; Luo, T.; Yun, Y.; Zhang, M.; Chen, W.; Zhong, Q.; Zhang, H.; Chen, H.; Chen, W. Effect of glycosylation with sugar beet pectin on the interfacial behaviour and emulsifying ability of coconut protein. Int. J. Biol. Macromol. 2021, 183, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Shanshan, W.; Meigui, H.; Chunyang, L.; Zhi, C.; Li, C.; Wuyang, H.; Ying, L.; Jin, F. Fabrication of ovalbumin-burdock polysaccharide complexes as interfacial stabilizers for nanostructured lipid carriers: Effects of high-intensity ultrasound treatment. Food Hydrocoll. 2021, 111, 106407. [Google Scholar] [CrossRef]
- Bu, G.; Zhao, C.; Wang, M.; Yu, Z.; Yang, H.; Zhu, T. The development and properties of nanoemulsions stabilized with glycated soybean protein for carrying β-carotene. J. Food Eng. 2023, 345, 111411. [Google Scholar] [CrossRef]
- Peng, D.; Jin, W.; Arts, M.; Yang, J.; Li, B.; Sagis, L.M. Effect of CMC degree of substitution and gliadin/CMC ratio on surface rheology and foaming behavior of gliadin/CMC nanoparticles. Food Hydrocoll. 2020, 107, 105955. [Google Scholar] [CrossRef]
- Yang, J.; Roozalipour, S.P.L.; Berton-Carabin, C.C.; Nikiforidis, C.V.; van der Linden, E.; Sagis, L.M. Air-water interfacial and foaming properties of whey protein—Sinapic acid mixtures. Food Hydrocoll. 2021, 112, 106467. [Google Scholar] [CrossRef]
- Wan, Z.-L.; Wang, L.-Y.; Wang, J.-M.; Yuan, Y.; Yang, X.-Q. Synergistic Foaming and Surface Properties of a Weakly Interacting Mixture of Soy Glycinin and Biosurfactant Stevioside. J. Agric. Food Chem. 2014, 62, 6834–6843. [Google Scholar] [CrossRef]
- Wan, Z.; Yang, X.; Sagis, L.M. Non-linear surface dilatational rheology and foaming behavior of protein and protein fibrillar aggregates in the presence of natural surfactant. Langmuir 2016, 32, 3679–3690. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, H.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Modification of soy protein hydrolysates by Maillard reaction: Effects of carbohydrate chain length on structural and interfacial properties. Colloids Surfaces B Biointerfaces 2016, 138, 70–77. [Google Scholar] [CrossRef]
- Feng, T.; Wang, X.; Wang, X.; Xia, S.; Huang, Q. Plant protein-based antioxidant Pickering emulsions and high internal phase Pickering emulsions against broad pH range and high ionic strength: Effects of interfacial rheology and microstructure. LWT Food Sci. Technol. 2021, 150, 111953. [Google Scholar] [CrossRef]
- Xu, Y.-T.; Tang, C.-H.; Liu, T.-X.; Liu, R.H. Ovalbumin as an Outstanding Pickering Nanostabilizer for High Internal Phase Emulsions. J. Agric. Food Chem. 2018, 66, 8795–8804. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-T.; Liu, T.-X.; Tang, C.-H. Novel pickering high internal phase emulsion gels stabilized solely by soy β-conglycinin. Food Hydrocoll. 2019, 88, 21–30. [Google Scholar] [CrossRef]
| α-Helix | β-Sheet | β-Turn | Random Coil | |
|---|---|---|---|---|
| untreated | 18.3 ± 0.9 b | 37.3 ± 2.5 a | 14.2 ± 2.2 b | 30.2 ± 1.6 a |
| 12 h | 16.4 ± 0.9 b | 40.6 ± 2.0 ab | 11.4 ± 2.7 a | 31.6 ± 1.4 a |
| 24 h | 12.3 ± 1.1 a | 41.2 ± 0.9 ab | 10.5 ± 2.1 a | 36.0 ± 1.6 b |
| 48 h | 11.7 ± 2.7 a | 43.3 ± 4.0 b | 10.3 ± 1.9 a | 34.6 ± 2.2 b |
| Kdif (mN/m s0.5) | Kpen × 10−4 (s−1) | Krea × 10−4 (s−1) | |
|---|---|---|---|
| untreated | 0.39 ± 0.02 a | −2.85 ± 0.00 b | −17.49 ± 0.00 b |
| 12 h | 0.49 ± 0.06 b | −3.29 ± 0.00 a | −21.77 ± 0.00 b |
| 24 h | 0.55 ± 0.03 bc | −2.76 ± 0.00 b | −34.44 ± 0.00 a |
| 48 h | 0.64 ± 0.08 c | −3.69 ± 0.00 a | −39.54 ± 0.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Tian, Y.; Pan, S.; Zheng, L. Glycation Improved the Interfacial Adsorption and Emulsifying Performance of β-Conglycinin to Stabilize the High Internal Phase Emulsions. Foods 2023, 12, 2706. https://doi.org/10.3390/foods12142706
Zhang H, Tian Y, Pan S, Zheng L. Glycation Improved the Interfacial Adsorption and Emulsifying Performance of β-Conglycinin to Stabilize the High Internal Phase Emulsions. Foods. 2023; 12(14):2706. https://doi.org/10.3390/foods12142706
Chicago/Turabian StyleZhang, Hongjian, Yan Tian, Siyi Pan, and Lianhe Zheng. 2023. "Glycation Improved the Interfacial Adsorption and Emulsifying Performance of β-Conglycinin to Stabilize the High Internal Phase Emulsions" Foods 12, no. 14: 2706. https://doi.org/10.3390/foods12142706
APA StyleZhang, H., Tian, Y., Pan, S., & Zheng, L. (2023). Glycation Improved the Interfacial Adsorption and Emulsifying Performance of β-Conglycinin to Stabilize the High Internal Phase Emulsions. Foods, 12(14), 2706. https://doi.org/10.3390/foods12142706





