Climate Change—A Global Threat Resulting in Increasing Mycotoxin Occurrence
Abstract
1. Introduction
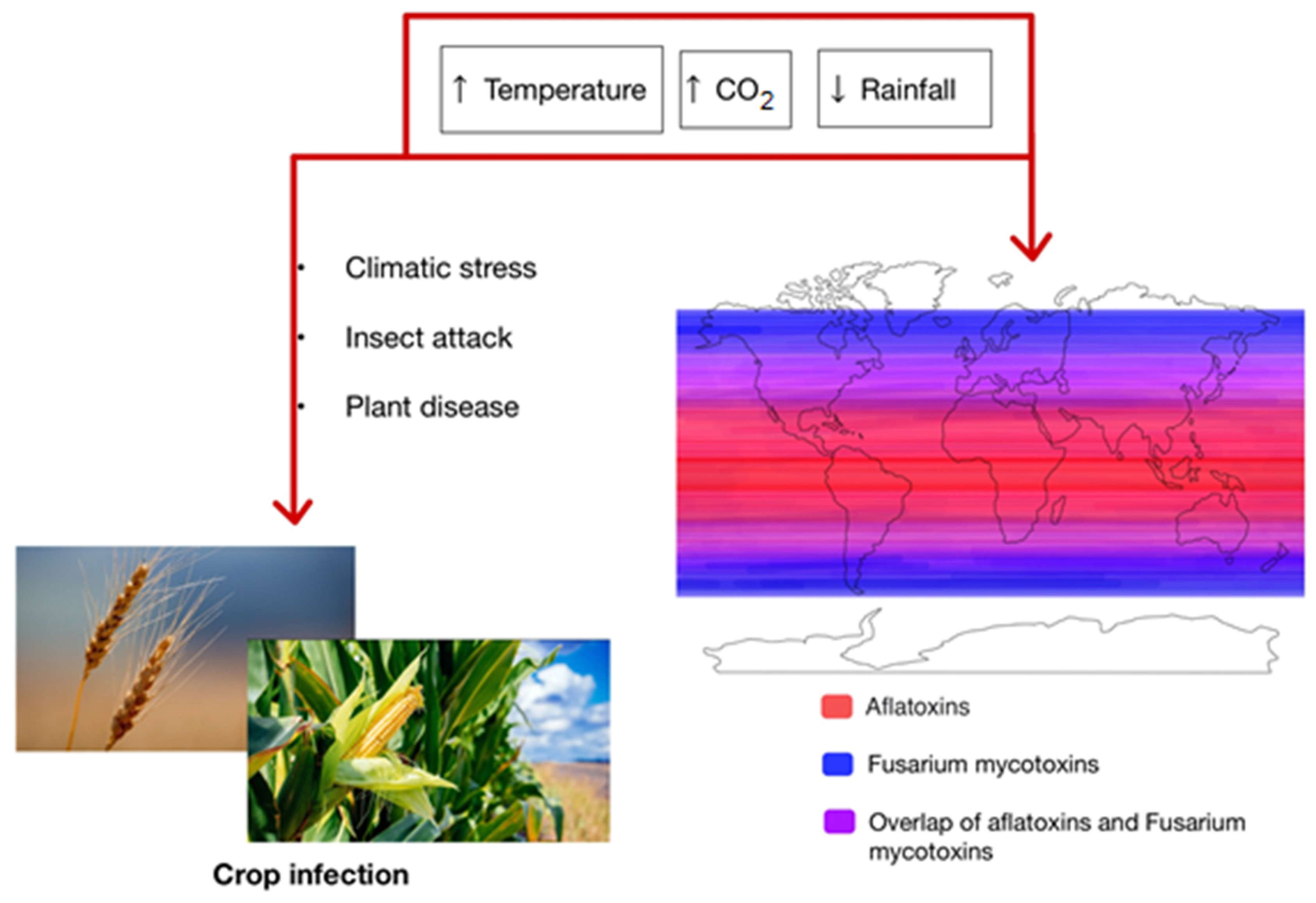
2. Factors Affecting Climate Change
3. Conditions Facilitating Mycotoxin Production
3.1. Aflatoxins
3.2. Ochratoxin A
3.3. Fusarium Mycotoxins
| Mycotoxin | Mould | Temperature Range (°C) | Optimal Temperature (°C) | Water Activity (aw) | pH |
|---|---|---|---|---|---|
| AFs | A. flavus A. parasiticus | 10–48 12–42 | 33 32 | 0.80–0.99 0.80–0.99 | 2–10 3–8 |
| OTA | A. ochraceus P. verrucosum A. niger | 10–40 0–31 6–47 | 37 20 36 | 0.80 0.86 0.77–0.92 | 3–10 6–7 2–6.5 |
| FUM | F. verticilloides F. proliferatum | 2.5–37 5–37 | 25 | 0.90–0.99 | 2.4–3 |
| ZEN | F. culmorum | 0–31 | 21 | 0.96 | 3–9 |
| DON | F. graminearum | 5–37 | 25 | 0.99 | 2.4–3 |
4. Climate Change and Effect on Mycotoxin Occurrence in Europe
4.1. Aspergillus Mycotoxins
4.2. Fusarium Mycotoxins
5. Predictions for Certain Geographic Regions
6. Further Research and Prevention Strategy
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- CAST. Mycotoxins: Risks in Plant, Animal, and Human Systems, Task Force Report, No. 139; Council Agricultural Science and Technology: Ames, IA, USA, 2003. [Google Scholar]
- International Agency for Research on Cancer. Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. In IARC Monograph on the Evaluation of Carcinogenic Risks to Humans; World Health Organization, IARC: Lyon, France, 1993; Volume 56, pp. 1–609. [Google Scholar]
- International Agency for Research on Cancer. Chemical Agents and Related Occupations, a Review of Human Carcinogens in IARC Monograph on the Evaluation of Carcinogenic Risk to Humans; World Health Organization, IARC: Lyon, France, 2012; Volume 100F, pp. 1–628. [Google Scholar]
- Pleadin, J.; Vulić, A.; Perši, N.; Škrivanko, M.; Capek, B.; Cvetnić, Ž. Aflatoxin B1 occurrence in maize sampled from Croatian farms and feed factories during 2013. Food Control. 2014, 40, 286–291. [Google Scholar] [CrossRef]
- Pleadin, J.; Markov, K.; Frece, J.; Vulić, A.; Perši, N. Bio-Prevalence, determination and reduction of aflatoxin B1 in cereals. In Aflatoxins: Food Sources, Occurrence and Toxicological Effects; Faulkner, A.G., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 1–34. [Google Scholar]
- Kos, J.; Hajnal, E.J.; Malachová, A.; Steiner, D.; Stranska, M.; Krska, R.; Poschmaier, B.; Sulyok, M. Mycotoxins in maize harvested in Republic of Serbia in the period 2012–2015. Part 1: Regulated mycotoxins and its derivatives. Food Chem. 2020, 312, 126034. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Janić Hajnal, E.; Radić, B.; Pezo, L.; Malachová, A.; Krska, R.; Sulyok, M. Two years study of Aspergillus metabolites prevalence in maize from the Republic of Serbia. J. Food Process. Preserv. 2022, 46, e15897. [Google Scholar] [CrossRef]
- Pleadin, J.; Kos, J.; Radić, B.; Vulić, A.; Kudumija, N.; Radović, R.; Janić Hajnal, E.; Mandić, A.; Anić, M. Aflatoxins in Maize from Serbia and Croatia: Implications of Climate Change. Foods 2023, 12, 548. [Google Scholar] [CrossRef]
- Pickova, D.; Ostry, V.; Malir, F. A recent overview of producers and important dietary sources of aflatoxins. Toxins 2021, 13, 186. [Google Scholar] [CrossRef]
- Ortiz-Villeda, B.; Lobos, O.; Aguilar-Zuniga, K.; Carrasco-Sánchez, V. Ochratoxins in Wines: A Review of Their Occurrence in the Last Decade, Toxicity, and Exposure Risk in Humans. Toxins 2021, 13, 478. [Google Scholar] [CrossRef]
- Leitão, A.L. Occurrence of Ochratoxin A in Coffee: Threads and Solutions—A Mini-Review. Beverages 2019, 5, 36. [Google Scholar] [CrossRef]
- Khodaei, D.; Javanmardi, F.; Khaneghah, A.M. The global overview of the occurrence of mycotoxins in cereals: A three-year survey. Curr. Opin. Food Sci. 2021, 39, 36–42. [Google Scholar] [CrossRef]
- Bebber, D.P.; Gurr, S.J. Crop-destroying fungal and oomycete pathogens challenge food security. Fungal Gen. Biol. 2015, 74, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Milićević, D.; Pleadin, J. Mycotoxin incidence in southeastern European countries: Implications for the food supply chain. In Sustainable and Nutrition-Sensitive Food Systems for Healthy Diets and Prevention of Malnutrition in Europe and Central Asia; Fang, C., Gurinović, M., Eds.; FAO: Budapest, Hungary, 2023; pp. 117–149. [Google Scholar]
- Magan, N.; Aldred, D.; Sanchis, V. Role of fungi in seed deterioration. In Fungal Biotechnology in Agricultural, Food and Environmental Applications; Arora, D., Ed.; Marcel Dekker: New York, NY, USA, 2003; pp. 311–323. [Google Scholar]
- Medina, A.; González-Jartín, J.M.; Sainz, M.J. Impact of global warming on mycotoxins. Curr. Opin. Food Sci. 2017, 18, 76–81. [Google Scholar] [CrossRef]
- Pleadin, J.; Vulić, A.; Babić, J.; Šubarić, D. The Incidence of T-2 and HT-2 Toxins in Cereals and Methods of their Reduction Practice by the Food Industry. In Fusarium—Plant Diseases, Pathogen Diversity, Genetic Diversity, Resistance and Molecular Markers; Askun, T., Ed.; IntechOpen: London, UK, 2018; pp. 41–64. [Google Scholar]
- Zingales, V.; Taroncher, M.; Martino, P.A.; Ruiz, M.J.; Caloni, F. Climate Change and Effects on Moldsand Mycotoxins. Toxins 2022, 14, 445. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Climate Change as a Driver of Emerging Risks for Food and Feed Safety, Plant, Animal Health and Nutritional Quality; Maggiore, A., Afonso, A., Barrucci, F., De Sanctis, G., Eds.; EFSA: Parma, Italy, 2020; EN-1881; Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2020.EN-1881 (accessed on 15 January 2022).
- Battilani, P.; Rossi, V.; Giorni, P.; Pietri, A.; Gualla, A.; Van der Fels-Klerx, H.J.; Booij, C.J.H.; Moretti, A.; Logrieco, A.; Miglietta, F.; et al. Modelling, predicting and mapping the emergence of aflatoxins in cereals in the EU due to climate change. EFSA Support. Publ. 2012, 9, 223E. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; der Fels-Klerx, V.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Zadravec, M.; Lešić, T.; Frece, J.; Vasilj, V.; Markov, K. Climate change—A potential threat for increasing occurrences of mycotoxins. Vet. Stanica 2020, 51, 659–671. [Google Scholar] [CrossRef]
- World Meteorological Organization. Provisional State of the Global Climate 2022. Available online: https://public.wmo.int/en/our-mandate/climate/wmo-statement-state-of-global-climate (accessed on 30 January 2022).
- The Intergovernmental Panel on Climate Change: Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; p. 3056. [Google Scholar] [CrossRef]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant phenology and global climate change: Current progresses and challenges. Glob. Chang. Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; McVicar, T.R.; Miralles, D.G.; Yang, Y.; Tomas-Burguera, M. Unraveling the influence of atmospheric evaporative demand on drought and its response to climate change. WIREs Clim. Change 2020, 11, e632. [Google Scholar] [CrossRef]
- Van der Fels-Klerx, H.J.; Liu, C.; Battilani, P. Modelling climate change impacts on mycotoxin contamination. World Mycotoxin J. 2016, 9, 717–726. [Google Scholar] [CrossRef]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate change, food security and mycotoxins: Do we know enough? Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef]
- Kovalsky, P.; Kos, G.; Nährer, K.; Schwab, C.; Jenkins, T.; Schatzmayr, G.; Sulyok, M.; Krska, R. Co-occurrence of regulated, masked and emerging mycotoxins and secondary metabolites in finished feed and maize—An extensive survey. Toxins 2016, 8, 363. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantifica-tion of >500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. Anal. Bioanal. Chem. 2020, 412, 2607–2620. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Parra, R.; Geisen, R.; Magan, N. Modelling the relationship between environmental factors, transcriptional genes and deoxynivalenol mycotoxin production by strains of two Fusarium species. J. Royal Soc. Interface 2011, 8, 117–126. [Google Scholar] [CrossRef]
- Medina, A.; Rodriguez, A.; Magan, N. Climate change and mycotoxigenic fungi: Impacts on mycotoxin production. Curr. Opin. Food Sci. 2015, 5, 99–104. [Google Scholar] [CrossRef]
- Medina, A.; Rodríguez, A.; Sultan, Y.; Magan, N. Climate change factors and A. flavus: Effects on gene expression, growth and aflatoxin production. World Mycotoxin J. 2015, 8, 171–179. [Google Scholar] [CrossRef]
- Paris, M.P.K.; Liu, Y.J.; Nahrer, K.; Binder, E.M. Climate change impacts on mycotoxin production. In Climate Change and Mycotoxins; Botana, L.M., Sainz, M.J., Eds.; De Gruyter: Berlin, Germany, 2015; pp. 133–152. [Google Scholar]
- Paterson, R.R.M.; Lima, N. Thermophilic Fungi to Dominate Aflatoxigenic/Mycotoxigenic Fungi on Food under Global Warming. Int. J. Environ. Res. Public Health 2017, 14, 199. [Google Scholar] [CrossRef]
- Bebber, D.P.; Holmes, T.; Gurr, S.J. The global spread of crop pests and pathogens. Global Ecol. Biogeogr. 2014, 23, 1398–1407. [Google Scholar] [CrossRef]
- Medina, A.; Rodriguez, A.; Magan, N. Changes in environmental factors driven by climate change: Effects on the ecophysiology of mycotoxigenic fungi. In Climate Change and Mycotoxins; Botana, L.M., Sainz, M.J., Eds.; De Gruyter: Berlin, Germany, 2015; pp. 71–85. [Google Scholar]
- Ramos, A.J.; Labernia, N.; Marın, S.; Sanchis, V.; Magan, N. Effect of water activity and temperature on growth and ochratoxin production by three strains of Aspergillus ochraceus on a barley extract medium and on barley grains. Int. J. Food Microbiol. 1998, 44, 133–140. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Papenbrock, J. Mycotoxins: Producing Fungi and Mechanisms of Phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef]
- Zadravec, M.; Vahčić, N.; Brnić, D.; Markov, K.; Frece, J.; Beck, R.; Lešić, T.; Pleadin, J. A study of surface moulds and mycotoxins in Croatian traditional dry-cured meat products. Int. J. Food Microbiol. 2020, 317, 108459. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, V.; Magan, N. Environmental conditions affecting mycotoxins. In Mycotoxins in Food; Magan, N., Olsen, M., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Soston, UK, 2004; pp. 174–189. [Google Scholar]
- Abarca, M.L.; Bragulat, M.R.; Castella, G.; Cabanes, F. Ochratoxin A production by strains of Aspergillus niger var. niger. Appl. Environ. Microbiol. 1994, 60, 2650–2652. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Kevei, E.; Rinyu, E.; Téren, J.; Kozakiewicz, Z. Ochratoxin production by Aspergillus species. Appl. Environ. Microbiol. 1996, 62, 4461–4464. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Zadravec, M.; Lešić, T.; Vahčić, N.; Frece, J.; Mitak, M.; Markov, K. Co-occurrence of ochratoxin A and citrinin in unprocessed cereals established during a three-year investigation period. Food Addit. Contam Part B 2018, 11, 20–25. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Microbiological Specification for Foods. Toxigenic fungi: Aspergillus in ICMSF, Microorganisms in foods. Characteristics of Food Pathogens; Blackie Academic and Professional: London, UK, 1996; pp. 347–381. [Google Scholar]
- Mitchell, D.; Aldred, D.; Magan, N. Impact of ecological factors on growth and ochratoxin A production by Aspergillus carbonarius from different regions of Europe. Asp. Appl. Biol. 2003, 68, 109–116. [Google Scholar]
- Battilani, P.; Pietri, A.; Giorni, P.; Bertuzzi, T.; Barbano, C. Growth and ochratoxin A production of Aspergillus section Nigri isolates from Italian grapes. Asp. Appl. Biol. 2003, 68, 175–180. [Google Scholar]
- Cairns, V.; Hope, R.; Magan, N. Environmental factors and competing mycoflora affect growth and ochratoxin production by Penicillium verrucosum on wheat grain. Asp. Appl. Biol. 2003, 68, 81–90. [Google Scholar]
- Binder, E.M.; Tan, L.M.; Chin, L.J.; Handl, J.; Richard, J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- Magan, N.; Lacey, J. The effect of temperature and pH on the water relations of field and storage fungi. Trans. Br. Mycol. Soc. 1984, 82, 71–81. [Google Scholar] [CrossRef]
- Pleadin, J.; Vahčić, N.; Perši, N.; Ševelj, D.; Markov, K.; Frece, J. Fusarium mycotoxins’ occurrence in cereals harvested from Croatian fields. Food Control 2013, 32, 49–54. [Google Scholar] [CrossRef]
- Pleadin, J.; Staver, M.M.; Markov, K.; Frece, J.; Zadravec, M.; Jaki, V.; Krupić, N.; Vahčić, N. Mycotoxins in organic and conventional cereals and cereal products grown and marketed in Croatia. Mycotoxin Res. 2017, 33, 219–227. [Google Scholar] [CrossRef]
- Radić, B.; Kos, J.; Janić Hajnal, E.; Malachová, A.; Krska, R.; Sulyok, M. Fusarium metabolites in maize from regions of Northern Serbia in 2016–2017. Food Addit. Contam. Part B 2021, 14, 295–305. [Google Scholar] [CrossRef]
- Hope, R.; Magan, N. Two dimensional environmental profiles of growth, deoxynivalenol and nivalenol production by Fusarium culmorum on a wheat-based substrate. Lett. Appl. Microbiol. 2003, 37, 70–74. [Google Scholar] [CrossRef]
- Wu, L.; Qiu, L.; Zhang, H.; Sun, J.; Hu, X.; Wang, B. Optimization for the Production of Deoxynivalenoland Zearalenone by Fusarium graminearum Using Response Surface Methodology. Toxins 2017, 9, 57. [Google Scholar] [CrossRef]
- Marin, S.; Magan, N.; Serra, J.; Ramos, A.J.; Canela, R.; Sanchis, V. Fumonisin B1 production and growth of Fusarium moniliforme and Fusarium proliferatum on maize, wheat, and barley grain. J. Food Sci. 1999, 64, 921–924. [Google Scholar] [CrossRef]
- Marın, S.; Magan, N.; Bellı, N.; Ramos, A.J.; Canela, R.; Sanchis, V. Two-dimensional profiles of fumonisin B1 production by Fusarium moniliforme and Fusarium proliferatum inrelation to environmental factors and potential for modelling toxin formation in maize grain. Int. J. Food Microbiol. 1999, 51, 159–167. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: New York, NY, USA, 2009. [Google Scholar]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Copernicus Climate Change Servise. European State of The Climate. Available online: https://climate.copernicus.eu/ESOTC (accessed on 30 January 2023).
- Luo, S.; Du, H.; Kebede, H.; Liu, Y.; Xing, F. Contamination status of major mycotoxins in agricultural product and food stuff in Europe. Food Control 2021, 127, 108120. [Google Scholar] [CrossRef]
- Kos, J.; Lević, J.; Đuragić, O.; Kokić, B.; Miladinović, I. Occurrence and estimation of aflatoxin M1 exposure in milk in Serbia. Food Control 2014, 38, 41–46. [Google Scholar] [CrossRef]
- Janić Hajnal, E.; Kos, J.; Radić, B.; Anić, M.; Radović, R.; Kudumija, N.; Anić, M.; Pleadin, J. Impact of Climate Changes on the Natural Prevalence of Fusarium Mycotoxins in Maize Harvested in Serbia and Croatia. Foods 2023, 12, 1002. [Google Scholar] [CrossRef]
- Loi, M.; Logrieco, A.F.; Pusztahelyi, T.; Leiter, É.; Hornok, L.; Pócsi, I. Advanced mycotoxin control and decontamination techniques in view of an increased aflatoxin risk in Europe due to climate change. Front. Microbiol. 2023, 13, 1085891. [Google Scholar] [CrossRef] [PubMed]
- Van der Fels-Klerx, H.J.; Vermeulen, L.C.; Gavai, A.K.; Liu, C. Climate change impacts on aflatoxin B1 in maize and aflatoxin M1 in milk: A case study of maize grown in Eastern Europe and imported to the Netherlands. PLoS ONE 2019, 14, e0218956. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Vulić, A.; Perši, N.; Škrivanko, M.; Capek, B.; Cvetnić, Ž. Annual and regional variations of aflatoxin B1 levels seen in grains and feed coming from Croatian dairy farms over a 5-year period. Food Control 2015, 47, 221–225. [Google Scholar] [CrossRef]
- Milićević, D.; Petronijević, R.; Petrovic, Z.; Đjinović-Stojanović, J.; Jovanovic, J.; Baltić, T.; Janković, S. Impact of climate change on aflatoxin M1 contamination of raw milk with special focus on climate conditions in Serbia. J. Sci. Food Agric. 2019, 99, 5202–5210. [Google Scholar] [CrossRef]
- Republic Hydrometeorological Service of Serbia. Available online: http://www.hidmet.gov.rs/ciril/meteorologija/agro.php (accessed on 1 January 2022).
- Assunção, R.; Martins, C.; Viegas, S.; Viegas, C.; Jakobsen, L.S.; Pires, S.; Alvito, P. Climate change and the health impact of aflatoxins exposure in Portugal–an overview. Food Addit. Contam. Part A 2018, 35, 1610–1621. [Google Scholar] [CrossRef]
- European Food Safety Authority. EFSA CONTAM Panel, 2020. Scientific opinion—Risk assessment of aflatoxins in food. EFSA J. 2020, 18, 6040. [Google Scholar]
- Bryła, M.; Pierzgalski, A.; Zapaśnik, A.; Uwineza, P.A.; Ksieniewicz-Woźniak, E.; Modrzewska, M.; Waśkiewicz, A. Recent Research on Fusarium Mycotoxins in Maize—A Review. Foods 2022, 11, 3465. [Google Scholar] [CrossRef]
- World Mycotoxin Survey. Available online: https://www.biomin.net/science-hub/world-mycotoxin-survey/ (accessed on 15 January 2022).
- Pleadin, J.; Perši, N.; Mitak, M.; Zadravec, M.; Sokolović, M.; Vulić, A.; Jaki, V.; Brstilo, M. The natural occurrence of T-2 toxin and fumonisins in maize samples in Croatia. Bull. Environ. Contam. Toxicol. 2012, 88, 863–866. [Google Scholar] [CrossRef]
- Pleadin, J.; Sokolović, M.; Perši, N.; Zadravec, M.; Jaki, V.; Vulić, A. Contamination of maize with deoxynivalenol and zearalenone in Croatia. Food Control 2012, 28, 94–98. [Google Scholar] [CrossRef]
- Pleadin, J.; Zadravec, M.; Perši, N.; Vulić, A.; Jaki, V.; Mitak, M. Mould and mycotoxin contamination of pig feed in northwest Croatia. Mycotoxin Res. 2012, 28, 157–162. [Google Scholar] [CrossRef]
- DHMZ. Odabrana Poglavlja Osmog Nacionalnog Izvješća Republike Hrvatske Prema Okvirnoj Konvenciji Ujedinjenih Naroda o Promjeni Klime (UNFCCC); DHMZ: Zagreb, Croatia, 2023; p. 43. (In Croatian) [Google Scholar]
- Koletsi, P.; Schrama, J.W.; Graat, E.A.; Wiegertjes, G.F.; Lyons, P.; Pietsch, C. The Occurrence of Mycotoxins in Raw Materials and Fish Feeds in Europe and the Potential Effects of Deoxynivalenol on the Health and Growth of Farmed Fish Species—A Review. Toxins 2021, 13, 403. [Google Scholar] [CrossRef]
- Janić Hajnal, E.; Kos, J.; Malachová, A.; Steiner, D.; Stranska, M.; Krska, R.; Sulyok, M. Mycotoxins in maize harvested in Serbia in the period 2012–2015. Part 2: Non-regulated mycotoxins and other fungal metabolites. Food Chem. 2020, 317, 126409. [Google Scholar] [CrossRef]
- Speijers, G.J.A.; Speijers, M.H.M. Combined toxic effects of mycotoxins. Toxicol. Lett. 2004, 153, 91–98. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3489–3507. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The Impact of Climate Change on Agricultural Insect Pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Bebber, D.P.; Ramotowski, M.A.T.; Gurr, S.J. Crop pests and pathogens move poleward in a warming world. Nat. Clim. Change 2013, 3, 985–988. [Google Scholar] [CrossRef]
- Medina, A.; Rodriguez, A.; Magan, N. Effect of climate change on Aspergillus flavus and aflatoxins. Front. Microbiol. 2014, 5, 348. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. Further mycotoxin effects from climate change. Food Res. Int. 2011, 44, 2555–2566. [Google Scholar] [CrossRef]
- European Commission. Adapting to climate change in Europe—Options for EU action. In Green Paper from the Commission to the Council, the European Parliament, the European Economic and Social Committee and the Committee of the Regions, COM; European Commission: Brussels, Belgium, 2007; 354 final, SEC, 849. [Google Scholar]
- Olesen, J.E.; Trnka, M.; Kersebaum, K.C.; Skjelvåg, A.O.; Seguin, B.; Peltonen-Sainio, P.; Kozyra, J.; Micale, F. Impacts and adaptation of European crop production systems to climate change. Eur. J. Argon. 2011, 34, 96–112. [Google Scholar] [CrossRef]
- Marroquín-Cardona, A.G.; Johnson, N.M.; Phillips, T.D.; Hayes, A.W. Mycotoxins in a changing global environment—A review. Food Chem. Toxicol. 2014, 69, 220–230. [Google Scholar] [CrossRef]
- Chakraborty, S.; Murray, G.M.; Magarey, P.A.; Yonow, T.; O’Brien, R.G.; Croft, B.J.; Barbetti, M.J.; Sivasithamparam, K.; Old, K.M.; Dudzinski, M.J.; et al. Potential impact of climate change on plant diseases of economic significance to Australia. Australas. Plant Path. 1988, 27, 15–35. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Paterson, R.R.M.; Bhatti, I.A.; Asi, M.R. Comparing aflatoxin contamination in chilies from Punjab, Pakistan produced in summer and winter. Mycotoxin Res. 2011, 27, 75–80. [Google Scholar] [CrossRef] [PubMed]
- The Intergovernmental Panel on Climate Change. Intergovernmental Panel on Climate Change Report, Climate Change 2007; Synthesis Report, 52; The Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2007. [Google Scholar]
- Pritchard, S.G. Soil organisms and global climate change. Plant Pathol. 2011, 60, 82–99. [Google Scholar] [CrossRef]
- Matumba, L.; Namaumbo, S.; Ngoma, T.; Meleke, N.; De Boevre, M.; Logrieco, A.F.; De Saeger, S. Five keys to prevention and control of mycotoxins in grains: A proposal. Glob. Food Secur. 2021, 30, 100562. [Google Scholar] [CrossRef]
- Liu, Y.; Galani Yamdeu, J.H.; Gong, Y.Y.; Orfila, C. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1521–1560. [Google Scholar] [CrossRef]
- Zadravec, M.; Markov, K.; Lešić, T.; Frece, J.; Petrović, D.; Pleadin, J. Biocontrol Methods in Avoidance and Downsizing of Mycotoxin Contamination of Food Crops. Processes 2022, 10, 655. [Google Scholar] [CrossRef]
- Yousaf, Z.; Saleh, N.; Ramazan, A.; Aftab, A. Postharvesting Techniques and Maintenance of Seed Quality. In New Chal-lenges in Seed Biology—Basic and Translational Research Driving Seed Technology; Araújo, S., Ed.; IntechOpen: London, UK, 2016; pp. 114–135. [Google Scholar]
- Steen, M. Greenhouse Gas Emissions from Fossil Fuel Fired Power Generation Systems; Institute for Advanced Materials, Joint Research Centre, European Commission: Parma, Italy, 2000; pp. 1–61, EUR 19754 EN; Available online: https://core.ac.uk/download/pdf/38616493.pdf (accessed on 30 January 2022).
- Campa, R.D.L.; Hooker, D.C.; Miller, J.D.; Schaafsma, A.W.; Hammond, B.G. Modeling effects of environment, insect damage, and Bt genotypes on fumonisin accumulation in maize in Argentina and the Philippines. Mycopathologia 2005, 159, 539–552. [Google Scholar] [CrossRef]
- Schaafsma, A.W.; Hooker, D.C. Climatic models to predict occurrence of Fusarium toxins in wheat and maize. Int. J. Food Microbiol. 2007, 119, 116–125. [Google Scholar] [CrossRef]
- Chauhan, Y.; Wright, G.; Rachaputi, N. Modelling climatic risks of aflatoxin contamination in maize. Aust. J. Exp. Agric. 2008, 48, 358–366. [Google Scholar] [CrossRef]
- Maiorano, A.; Reyneri, A.; Sacco, D.; Magni, A.; Ramponi, C. A dynamic risk assessment model (FUMAgrain) of fumonisin synthesis by Fusarium verticillioides in maize grain in Italy. J. Crop. Prot. 2009, 28, 243–256. [Google Scholar] [CrossRef]
- Rossi, V.; Scandolara, A.; Battilani, P. Effect of environmental conditions on spore production by Fusarium verticillioides, the causal agent of maize ear rot. Eur. J. Plant Pathol. 2009, 123, 159–169. [Google Scholar] [CrossRef]
- Wu, F.; Bhatnagar, D.; Bui-Klimke, T.; Carbone, I.; Hellmich, R.; Munkvold, G.; Paul, P.; Payne, G.; Takle, E. Climate change impacts on mycotoxin risks in US maize. World Mycotoxin J. 2011, 4, 79–93. [Google Scholar] [CrossRef]
| Continent | Warming | Precipitation |
|---|---|---|
| Africa | Increased mean air temperatures; increased annual number of hot days and warm nights; heatwaves became longer and more intensive | Aridity increase due to decrease in precipitation in North Africa; increased trend of heavy rainfall followed by more frequent droughts in West Africa; more frequent and prolonged droughts in East and Southern Africa; increased number and intensity of extreme precipitation events in Southern Africa |
| Asia | Increased mean air temperatures followed by increased number of hot days and warm nights throughout continent; more frequent and longer heatwaves in South and East Asia | Observed annual precipitation trends are different depending on region; decreased precipitation and increased evapotranspiration are observed in West and Central Asia; increase in heavy precipitation in South, Southeast, and East Asia |
| Australia | Increased mean air temperature; heatwaves have increased in frequency and duration in most regions; increased number of extremely hot days | Increase in rainfall over northern Australia; decreased rainfall in southwestern and southeastern Australia in the period April–October; extreme rainfall intensities increased in many locations; less frequent droughts in northern and central Australia; more frequent droughts in southwest |
| Europe | Increased mean and maximum air temperatures in all regions followed by more frequent and prolonged heatwaves; increased occurrence of hot days and warm nights; cold extremes have decreased | Mean precipitation increased over Northern, Eastern, Western, and Central Europe; precipitation extremes increased in Northern and Eastern Europe; decreasing trend of precipitation observed in Mediterranean; more frequent droughts in Mediterranean; less frequent droughts in Northern Europe |
| Central and South America | Increase in the frequency and intensity of warm extremes and heatwave duration; increased mean and maximum temperatures; decrease in the intensity and frequency of cold extremes | Decreasing trend in precipitation in Central America; intensity and frequency of droughts have increased in many parts of Southern America; increasing trend of precipitation was observed in southeastern part of South America |
| North America | Increased mean and maximum air temperatures; increased occurrence of hot extreme events | Annual precipitation has increased in northern and eastern parts and decreased in western parts; increased frequency and intensity of heavy precipitation events in USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kos, J.; Anić, M.; Radić, B.; Zadravec, M.; Janić Hajnal, E.; Pleadin, J. Climate Change—A Global Threat Resulting in Increasing Mycotoxin Occurrence. Foods 2023, 12, 2704. https://doi.org/10.3390/foods12142704
Kos J, Anić M, Radić B, Zadravec M, Janić Hajnal E, Pleadin J. Climate Change—A Global Threat Resulting in Increasing Mycotoxin Occurrence. Foods. 2023; 12(14):2704. https://doi.org/10.3390/foods12142704
Chicago/Turabian StyleKos, Jovana, Mislav Anić, Bojana Radić, Manuela Zadravec, Elizabet Janić Hajnal, and Jelka Pleadin. 2023. "Climate Change—A Global Threat Resulting in Increasing Mycotoxin Occurrence" Foods 12, no. 14: 2704. https://doi.org/10.3390/foods12142704
APA StyleKos, J., Anić, M., Radić, B., Zadravec, M., Janić Hajnal, E., & Pleadin, J. (2023). Climate Change—A Global Threat Resulting in Increasing Mycotoxin Occurrence. Foods, 12(14), 2704. https://doi.org/10.3390/foods12142704









