Comparison of Physicochemical Properties of Noodles Fortified with Commercial Calcium Salts versus Calcium Citrate from Oyster Shells
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Preparation of Calcium Citrate from Oyster Shells
2.3. Fourier Transform Infrared Spectroscopy and Evaluation of Calcium Salt Particle Size Distribution
2.4. Noodle Preparation and Physicochemical Properties of Noodles
2.5. Determination of Calcium Content, Color, and Texture of Noodles
2.6. Sensory Evaluation of Noodle Fortified with Calcium Citrate
2.7. Statistical Analysis
3. Results and Discussion
3.1. Particle Sizes of Calcium Salts
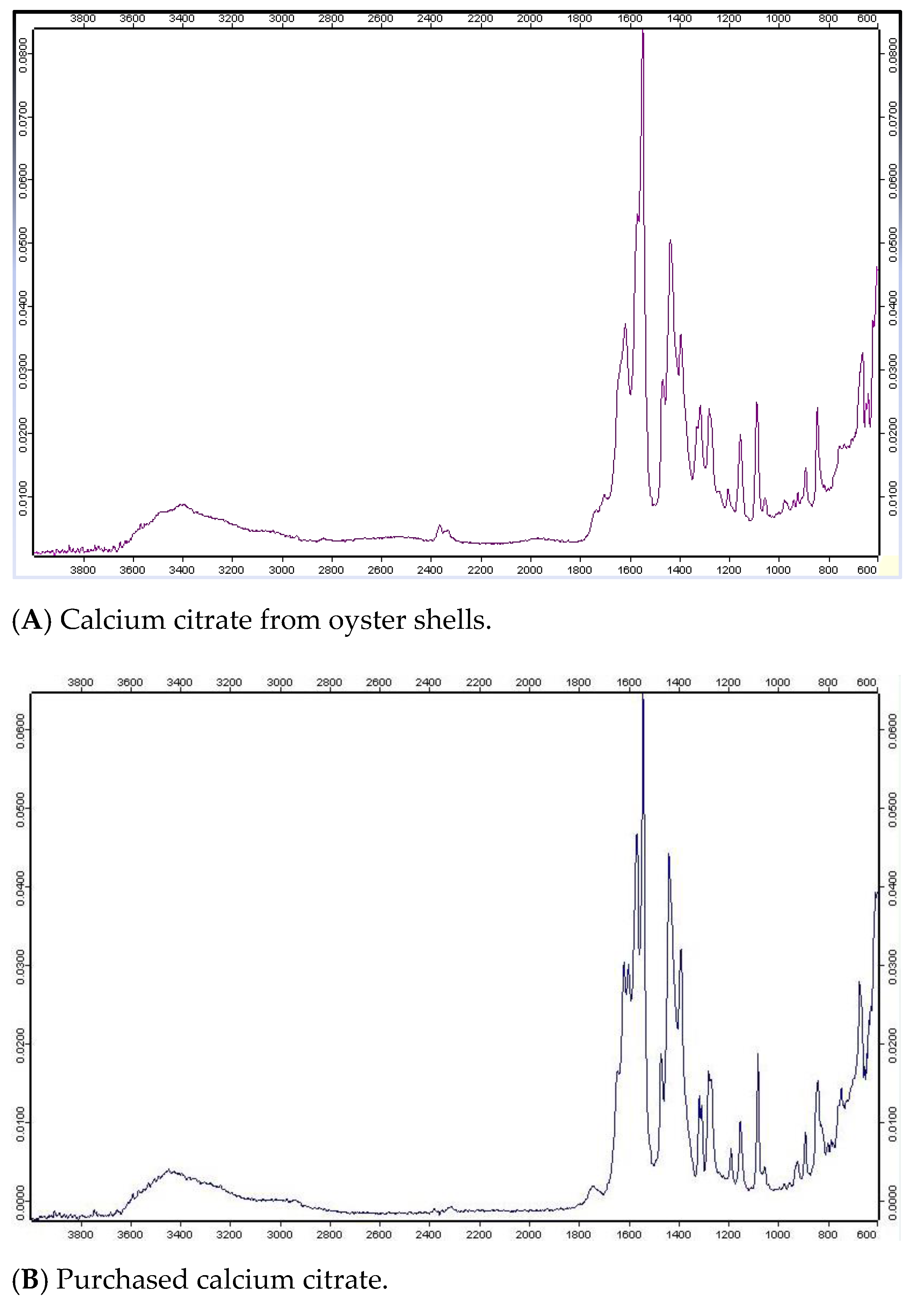
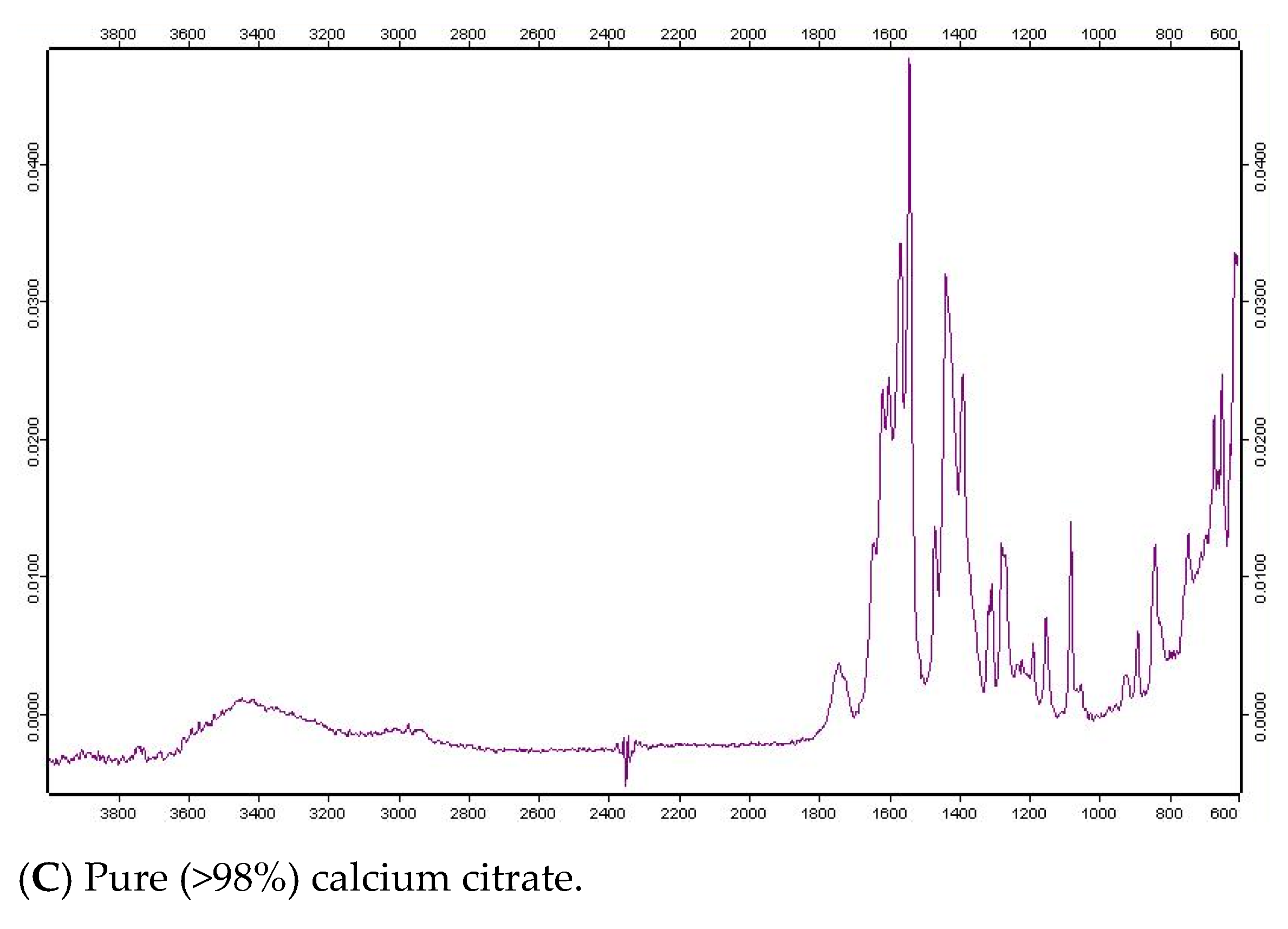
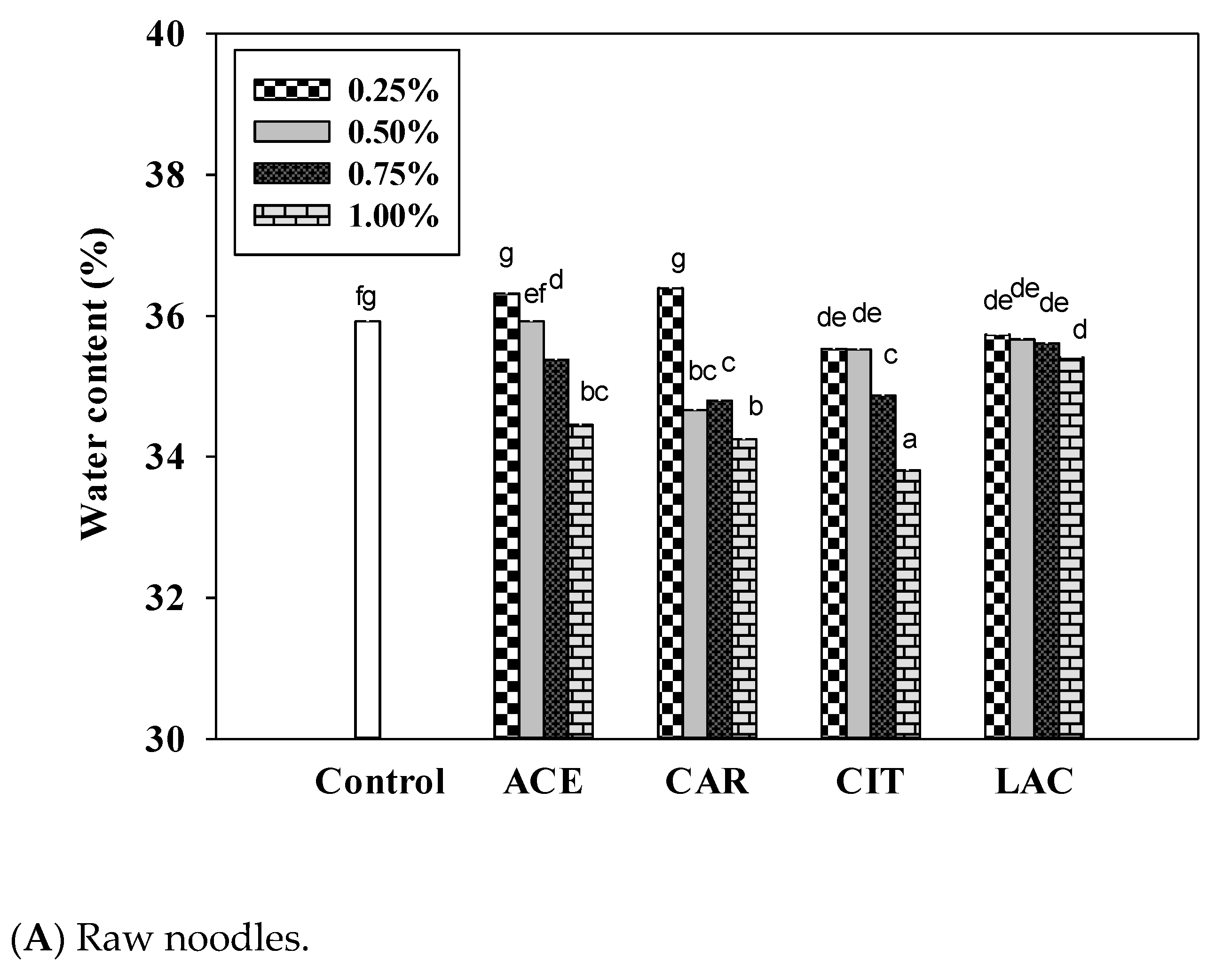
3.2. Fourier Transform Infrared Spectroscopy of Calcium Salts
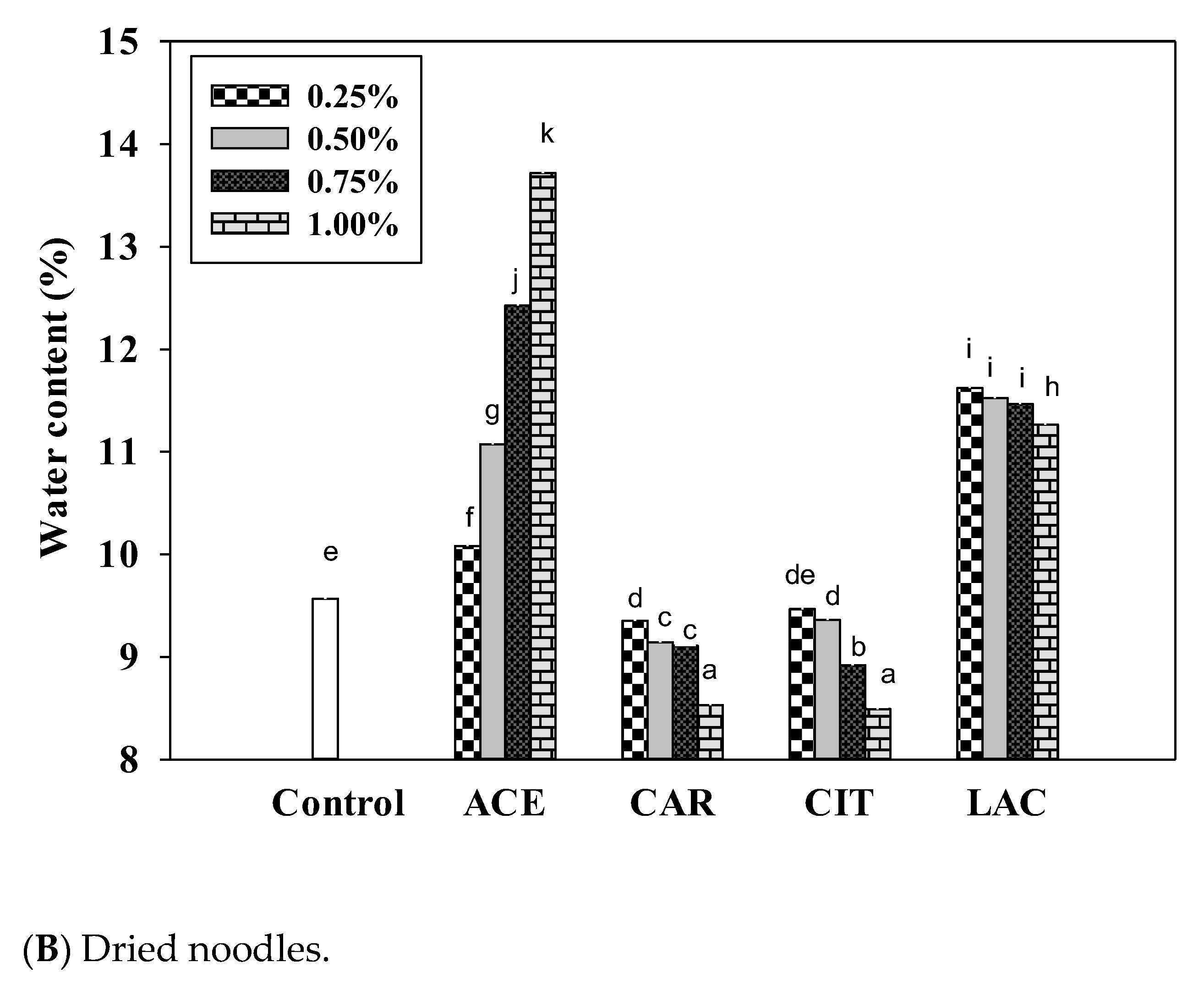
3.3. Moisture Content and Water Activity of White Salted Noodles
3.4. Ash and Calcium Content of White Salted Noodles
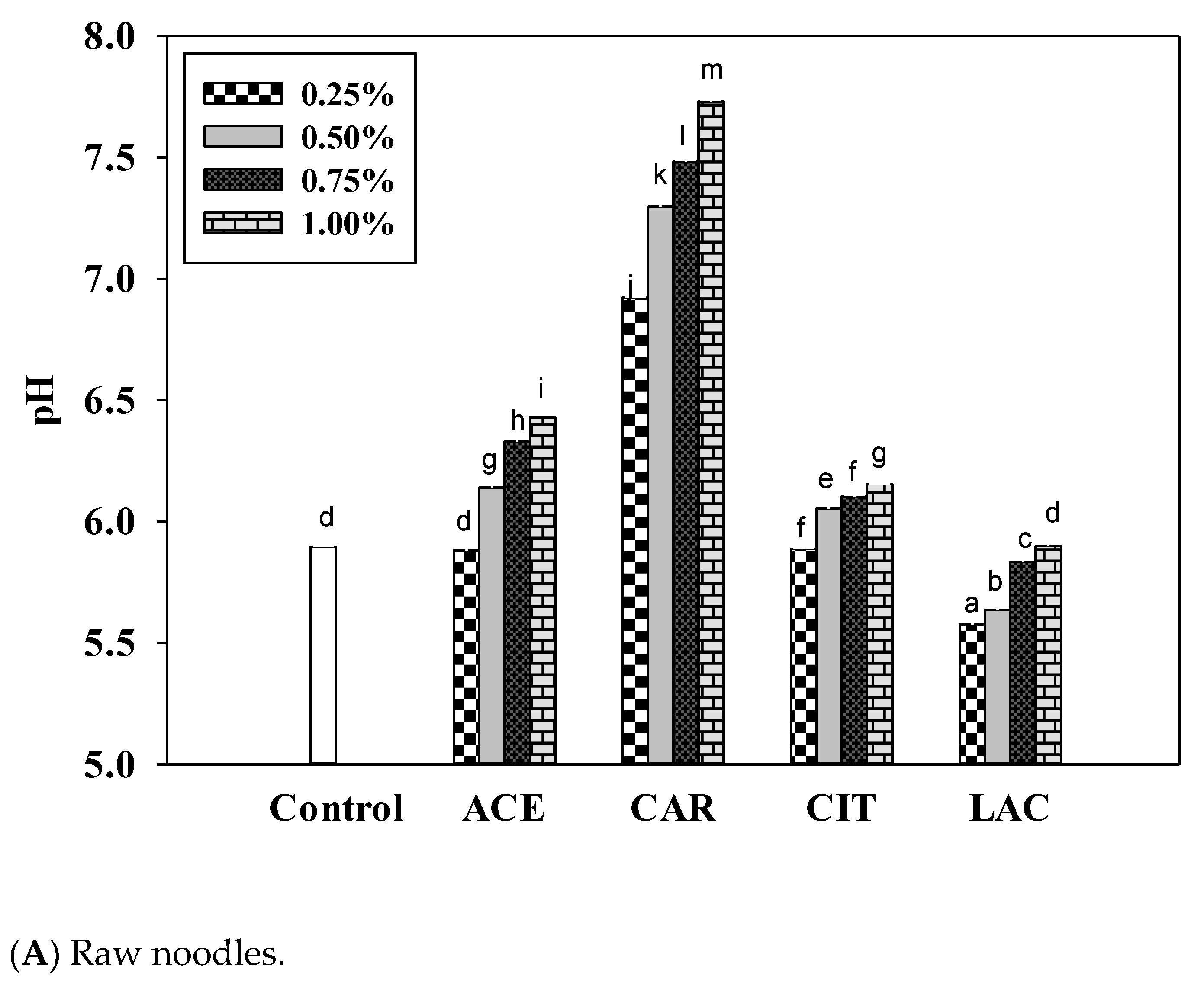
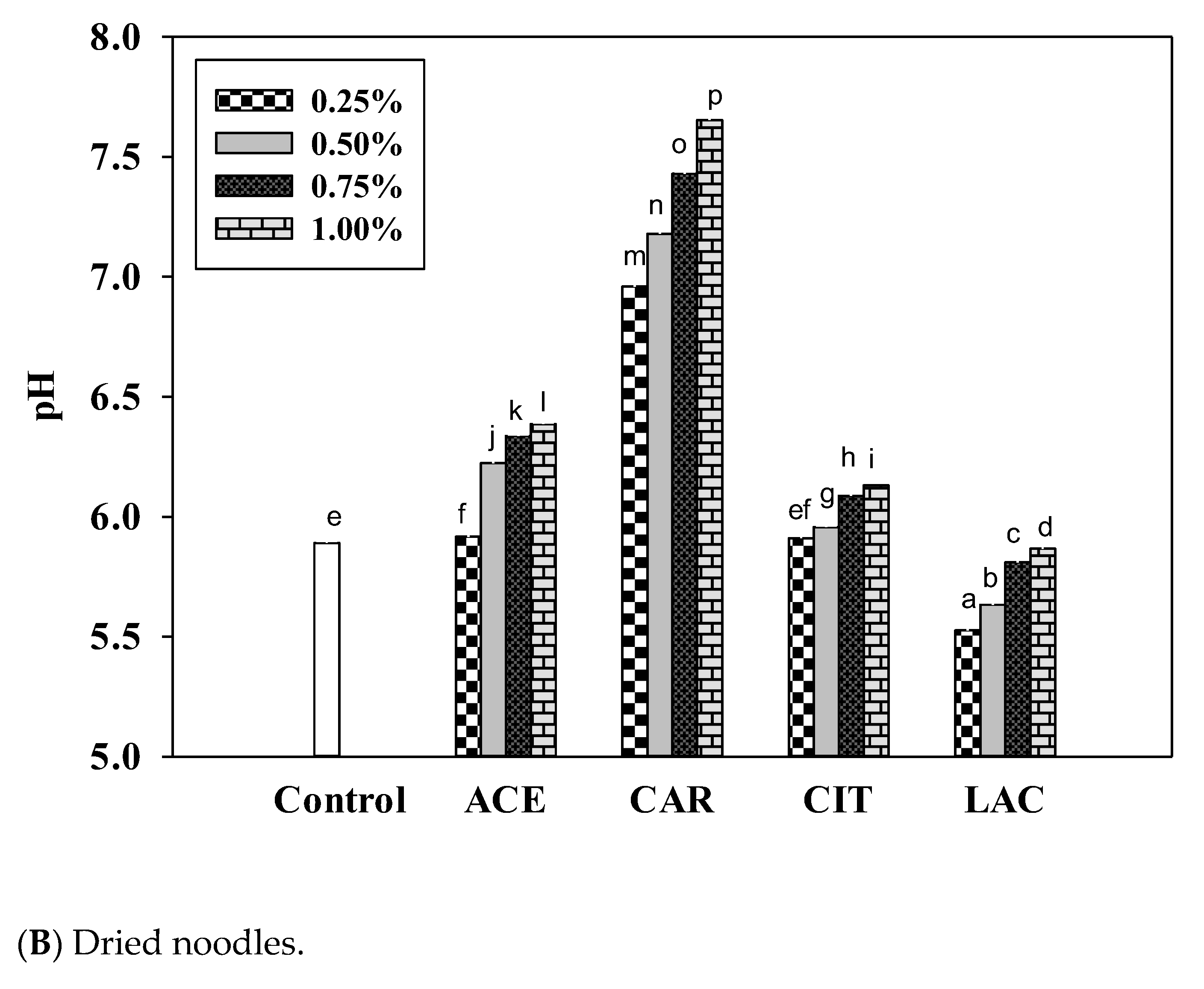
3.5. pH of Noodles
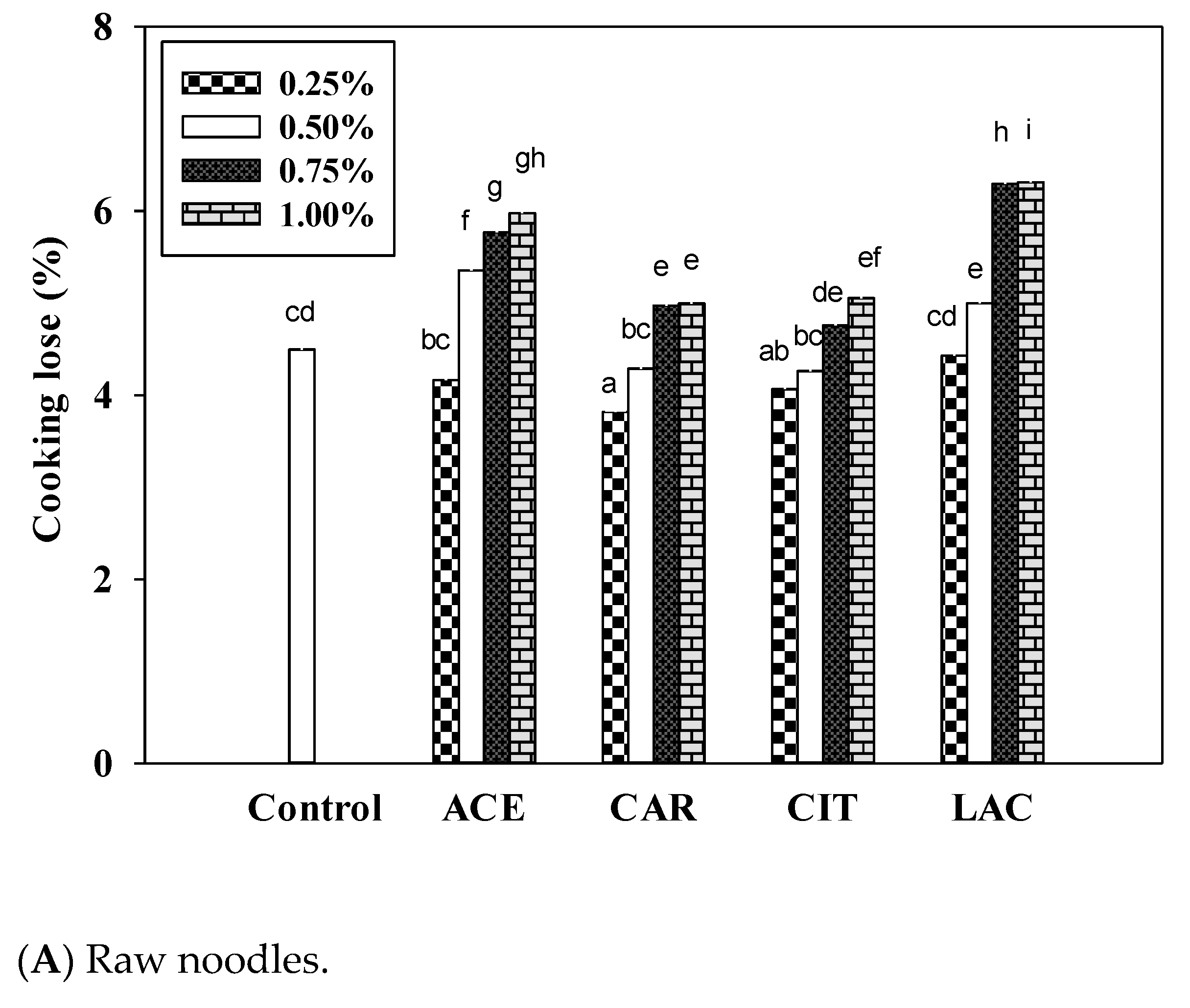
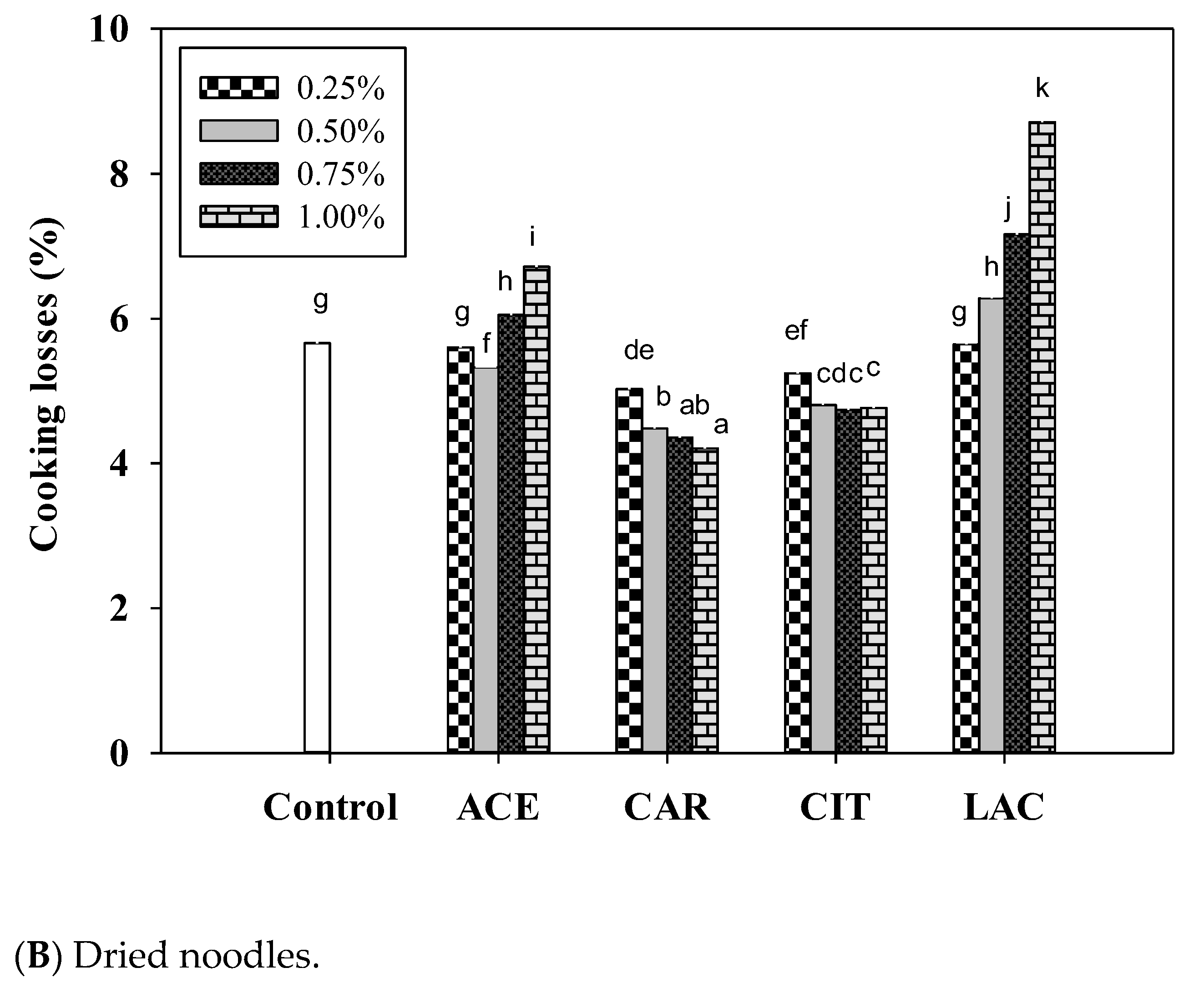
3.6. Cooking Qualities of Noodle
3.7. Physicochemical Properties and Color of Calcium-Fortified Noodles
3.8. Color of Noodles
3.9. Sensory Evaluation of Fortified Noodles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, J.; Li, Q.; Obadi, M.; Qi, Y.; Liu, S.; An, D.; Zhou, X.; Zhang, D.; Xu, B. Quality evaluation systems and methods of the whole making process of Asian noodles: A review. Food Rev. Int. 2021, 12, 1–28. [Google Scholar] [CrossRef]
- Li, M.; Zhu, K.X.; Guo, X.N.; Brijs, K.; Zhou, H.M. Natural additives in wheat-based pasta and noodle products: Opportunities for enhanced nutritional and functional properties. Compr. Rev. Food Sci. Food Saf. 2014, 13, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Janve, M.; Singhal, R.S. Fortification of puffed rice extrudates and rice noodles with different calcium salts: Physicochemical properties and calcium bioaccessibility. LWT—Food Sci. Technol. 2018, 97, 67–75. [Google Scholar] [CrossRef]
- Gelli, R.; Ridi, F.; Baglioni, P. The importance of being amorphous: Calcium and magnesium phosphates in the human body. Adv. Colloid Interface Sci. 2019, 269, 219–235. [Google Scholar] [CrossRef]
- Maresz, K. Proper calcium use: Vitamin K2 as a promoter of bone and cardiovascular health. Integr. Med. 2015, 14, 34–39. [Google Scholar]
- Srinivasan, P.M.; Harinarayan, C.V. Vitamin D deficiency in India: Fortify or let the sunshine in? J. Clin. Sci. Res. 2015, 4, 220–226. [Google Scholar]
- Lee, Y.K.; Jung, S.K.; Chang, Y.H.; Kwak, H.S. Highly bioavailable nanocalcium from oyster shell for preventing osteoporosis in rats. Int. J. Food Sci. Nutr. 2017, 68, 931–940. [Google Scholar] [CrossRef]
- Greger, J.L.; Krzykowski, E.E.; Khazen, P.P.; Krashoc, C.L. Mineral utilization by rats fed various commercially available calcium supplements or milk. J. Nutr. 1987, 117, 717–724. [Google Scholar] [CrossRef]
- Roland, D.A., Sr. Eggshell quality: Oyster shell versus limestone and the importance of particle size or solubility of calcium source. World Poult. Sci. J. 1986, 42, 166–171. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, K.; Feng, Y.; Jiang, S.; Zhao, Y.; Zeng, M. Preparation, characterization of L-asparatic acid chelated calcium from oyster shell source and its calcium supplementation effect in rats. J. Funct. Foods 2020, 75, 104249. [Google Scholar] [CrossRef]
- Pathomrungsiyounggul, P.; Grandison, A.S.; Lewis, M.J. Effect of calcium carbonate, calcium citrate, tricalcium phosphate, calcium gluconate and calcium lactate on some physicochemical properties of soymilk. Int. J. Food Sci. Technol. 2010, 45, 2234–2240. [Google Scholar] [CrossRef]
- Jia, F.; Ma, Z.; Wang, X.; Li, X.; Liu, L.; Hu, X. Effect of kansui addition on dough rheology and quality characteristics of chickpea-water composit flour-based noodles and the underlying mechanism. Food Chem. 2019, 298, 125081. [Google Scholar] [CrossRef]
- Wang, X.Z.; Ki, X.; Liu, L.; Yin, X.; Zhang, K.; Hu, X. Food additives and technologies used in Chinese traditional staple foods. Chem. Biol. Technol. Agric. 2018, 5, 1. [Google Scholar] [CrossRef]
- Karim, R.; Sultan, M.T. Yellow Alkaline Noodles: Processing Technology and Quality Improvement; Springer: New York, NY, USA, 2014. [Google Scholar]
- Fisheries Agency. Fisheries Agency. Fisheries production. In Fisheries Statistical Yearbook, Taiwan, Kinmen and Matsu Area; Council of Agriculture, Executive Yuang: Taiwan, China, 2012. [Google Scholar]
- Ahmed, S.A.; Gibriel, A.A.; Abdellatif, A.K.; Ebied, H.M. Evaluation of food products fortified with oyster shell for the prevention and treatment of osteoporosis. J. Food Sci. Technol. 2015, 52, 6818–6820. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Ohue, T.; Miyauchi, A.; Takagi, Y. Heated oyster shell-seaweed calcium (AAA Ca) on osteoporosis. Calcif. Tissue Int. 1996, 58, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, H.; Gao, Q.; Liu, X.; Tong, Z. Alginate-calcium carbonate oprous microparticle hybrid hydrogels with versatile drug loading capabilities and variable mechanical strengths. Carbohydr. Polym. 2008, 71, 476–480. [Google Scholar] [CrossRef]
- Hofmann, M.P.; Young, A.M.; Gbureck, U.; Nazhat, S.N.; Barralet, J.F. FTIR-monitoring of a fast setting brushite bone cement: Effect of intermediate phases. J. Mater. Chem. 2006, 16, 3199–3206. [Google Scholar] [CrossRef]
- Li, P.H.; Huang, C.C.; Yang, M.Y.; Wang, C.C.R. Textural and sensory properties of salted noodles containing purple flour. Food Res. Int. 2012, 47, 223–228. [Google Scholar] [CrossRef]
- Zang, Y.Q.; Hui, Y.; Wang, Y.; Zhang, B.; Guo, B.L.; Zhang, G.O.; Wei, Y.M. Effects of drying temperature and relative humidity on quality properties of Chinese dried noodles. J. Food Qual. 2020, 2020, 8843974. [Google Scholar]
- Hwang, J.Y.; Sung, W.C.; Shyu, Y.S. Effect of mulberry lees addition on dough mixing characteristics and the quality of mulberry toast. J. Mar. Sci. Tech.-Taiw. 2008, 16, 103–108. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 21st ed.; Sidney, W., Ed.; AOAC: Washington, DC, USA, 2019. [Google Scholar]
- Koh, W.Y.; Matanjun, P.; Lim, X.X.; Kobun, R. Sensory, physicochemical, and cooking qualities of instant noodles incorporated with red seaweed (Eucheuma denticulatum). Foods 2022, 11, 2669. [Google Scholar] [CrossRef] [PubMed]
- AACC. Approved Methods of the American Association of Cereal Chemists, 10th ed.; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Choo, C.L.; Aziz, N.A.A. Effects of banana flour and β-glucan on the nutritional and sensory evaluation of noodles. Food Chem. 2010, 119, 34–40. [Google Scholar] [CrossRef]
- Good, H. Measurement of color in cereal products. Cereal Foods World 2002, 47, 5–6. [Google Scholar]
- Reungmaneepaitoon, S.; Sikkhamondhol, C.; Tiangpook, C. Nutritive improvement of instant fried noodles with oat bran. Songklanakarin J. Sci. Technol. 2006, 28, 89–97. [Google Scholar]
- Casanova, H.; Higuita, L.P. Synthesis of calcium carbonate nanoparticles by reactive precipitation using a high pressure jet homogenizer. Chem. Eng. J. 2011, 175, 569–578. [Google Scholar] [CrossRef]
- Heller, H.J.; Stewart, A.; Haynes, S.; Pak, C.Y. Pharmacokinetics of calcium absorption from two commercial calcium supplements. J. Clin. Pharmacol. 1999, 39, 1151–1154. [Google Scholar] [CrossRef]
- Shan, D.; Zhu, M.; Han, E.; Xue, H.; Sier, S. Calcium carbonate nanoparticles: A host matrix for the construction of highly sensitive amperometric phenol biosensor. Biosens. Bioelectron. 2007, 23, 648–654. [Google Scholar] [CrossRef]
- Clydesdale, F.M. Minerals: Their Chemistry and Fate in Food. In Trace Minerals in Food; Smith, K., Ed.; Marcel Dekker: New York, NY, USA, 1988; pp. 57–94. [Google Scholar]
- Bao, J.; Corke, H. Pasting properties of γ-irradiated rice starches as affected by pH. J. Agric. Food Chem. 2002, 50, 336–341. [Google Scholar] [CrossRef]

| Sample | Breaking Force * (N) | Breaking Point (mm) | Work of Breaking (N × mm) | |
|---|---|---|---|---|
| Control | 10.88 ± 0.47 a | 0.56 ± 0.03 a | 6.69 ± 0.09 | |
| ACE ** | 0.25% | 18.39 ± 0.71 e | 0.60 ± 0.03 b | 11.08 ± 0.27 |
| 0.50% | 20.22 ± 0.83 h | 0.61 ± 0.02 b | 12.25 ± 0.91 | |
| 0.75% | 22.91 ± 0.85 j | 0.78 ± 0.04 e | 17.75 ± 0.64 | |
| 1.00% | 23.04 ± 0.53 j | 0.98 ± 0.05 h | 22.57 ± 1.30 | |
| CAR | 0.25% | 14.10 ± 0.61 b | 0.79 ± 0.04 e | 11.15 ± 0.83 |
| 0.50% | 15.81 ± 0.79 c | 0.85 ± 0.03 fg | 13.39 ± 1.15 | |
| 0.75% | 17.62 ± 0.60 d | 0.83 ± 0.04 f | 14.59 ± 0.81 | |
| 1.00% | 18.43 ± 0.92 e | 0.97 ± 0.05 h | 17.94 ± 1.66 | |
| CIT | 0.25% | 17.34 ± 0.78 d | 0.84 ± 0.03 fg | 14.59 ± 0.79 |
| 0.50% | 18.72 ± 0.36 ef | 0.87 ± 0.03 g | 16.24 ± 0.49 | |
| 0.75% | 19.05 ± 0.21 ef | 0.95 ± 0.02 h | 18.05 ± 0.44 | |
| 1.00% | 19.37 ± 0.85 fg | 0.97 ± 0.02 h | 18.85 ± 1.05 | |
| LAC | 0.25% | 21.49 ± 0.86 i | 0.68 ± 0.03 c | 14.67 ± 0.97 |
| 0.50% | 21.50 ± 0.91 i | 0.73 ± 0.03 d | 15.61 ± 1.37 | |
| 0.75% | 19.73 ± 0.78 gh | 0.71 ± 0.03 cd | 13.99 ± 0.62 | |
| 1.00% | 20.39 ± 0.95 h | 0.72 ± 0.03 d | 14.76 ± 0.55 | |
| Oyster CIT | 0.50% | 18.94 ± 0.25 c | 0.79 ± 0.04 b | 14.89 ± 0.75 |
| Sample | Tensile Force * (N) | Tensile Elongation (mm) | |
|---|---|---|---|
| Control | 0.54 ± 0.06 gh | 19.08 ± 0.58 i | |
| ACE ** | 0.25% | 0.46 ± 0.05 ef | 8.47 ± 0.30 b |
| 0.50% | 0.45 ± 0.03 ef | 12.39 ± 1.42 d | |
| 0.75% | 0.53 ± 0.11 gh | 15.82 ± 0.34 f | |
| 1.00% | 0.56 ± 0.08 ghi | 17.98 ± 0.43 h | |
| CAR | 0.25% | 0.40 ± 0.08 cde | 18.31 ± 0.71 h |
| 0.50% | 0.45 ± 0.03 ef | 11.51 ± 0.54 c | |
| 0.75% | 0.39 ± 0.05 cde | 12.43 ± 0.26 d | |
| 1.00% | 0.38 ± 0.04 bcde | 13.41 ± 0.37 e | |
| CIT | 0.25% | 0.42 ± 0.07 def | 13.53 ± 0.49 e |
| 0.50% | 0.58 ± 0.05 h | 16.85 ± 0.94 g | |
| 0.75% | 0.50 ± 0.02 fg | 17.53 ± 0.70 gh | |
| 1.00% | 0.50 ± 0.07 fg | 17.59 ± 1.21 gh | |
| LAC | 0.25% | 0.29 ± 0.04 a | 6.28 ± 0.41 a |
| 0.50% | 0.35 ± 0.03 abc | 6.44 ± 0.48 a | |
| 0.75% | 0.35 ± 0.04 abcd | 5.77 ± 0.41 a | |
| 1.00% | 0.31 ± 0.02 ab | 5.68 ± 0.52 a | |
| Oyster CIT | 0.50% | 0.60 ± 0.03 a | 17.98 ± 0.12 b |
| Sample | L* | a* | b* | ΔE | WI | |
|---|---|---|---|---|---|---|
| Control | 69.99 ± 0.06 b | −8.21 ± 0.03 m | 32.15 ± 0.03 ef | 0.00 | 55.26 | |
| ACE ** | 0.25% | 68.83 ± 0.02 a | −8.32 ± 0.03 l | 31.94 ± 0.06 bcd | 1.20 | 54.58 |
| 0.50% | 70.07 ± 0.06 c | −8.39 ± 0.01 k | 31.91 ± 0.10 bc | 0.27 | 55.46 | |
| 0.75% | 72.06 ± 0.04 g | −8.46 ± 0.01 j | 33.40 ± 0.02 k | 2.43 | 55.65 | |
| 1.00% | 73.96 ± 0.07 fg | −8.72 ± 0.02 f | 34.70 ± 0.03 n | 4.75 | 55.75 | |
| CAR | 0.25% | 68.58 ± 0.24 a | −8.46 ± 0.01 j | 31.85 ± 0.03 ab | 1.46 | 54.47 |
| 0.50% | 72.42 ± 0.23 d | −8.56 ± 0.01 h | 33.20 ± 0.20 j | 2.67 | 56.00 | |
| 0.75% | 72.46 ± 0.08 e | −8.67 ± 0.01 g | 33.32 ± 0.08 k | 2.77 | 55.92 | |
| 1.00% | 74.49 ± 0.05 fg | −9.09 ± 0.02 b | 34.23 ± 0.04 m | 5.04 | 56.36 | |
| CIT | 0.25% | 70.21 ± 0.05 fg | −8.52 ± 0.02 i | 32.18 ± 0.03 fg | 0.38 | 55.32 |
| 0.50% | 70.15 ± 0.05 b | −8.73 ± 0.02 f | 32.01 ± 0.06 cd | 0.60 | 55.34 | |
| 0.75% | 71.96 ± 0.16 d | −8.78 ± 0.01 e | 32.43 ± 0.05 h | 2.07 | 56.23 | |
| 1.00% | 72.19 ± 0.20 e | −8.81 ± 0.02 e | 32.30 ± 0.03 g | 2.27 | 56.49 | |
| LAC | 0.25% | 67.86 ± 0.03 d | −8.37 ± 0.02 k | 31.75 ± 0.01 a | 2.18 | 54.05 |
| 0.50% | 70.59 ± 0.10 f | −8.87 ± 0.03 d | 32.04 ± 0.03 de | 0.94 | 55.61 | |
| 0.75% | 72.74 ± 0.16 h | −8.92 ± 0.01 c | 32.69 ± 0.12 i | 2.88 | 56.52 | |
| 1.00% | 77.07 ± 0.10 i | −9.63 ± 0.02 a | 34.01 ± 0.03 l | 7.46 | 57.87 | |
| Sample | L* | a* | b* | ΔE | WI | |
|---|---|---|---|---|---|---|
| Control | 61.55 ± 0.03 a | −7.22 ± 0.01 b | 29.45 ± 0.01 d | 0.00 | 51.03 | |
| ACE ** | 0.25% | 61.82 ± 0.08 b | −7.13 ± 0.04 a | 29.50 ± 0.06 d | 0.29 | 51.22 |
| 0.50% | 62.75 ± 0.07 c | −8.33 ± 0.02 f | 29.11 ± 0.03 c | 1.66 | 51.99 | |
| 0.75% | 64.60 ± 0.04 e | −8.33 ± 0.01 f | 30.12 ± 0.03 ef | 3.31 | 52.78 | |
| 1.00% | 66.67 ± 0.14 f | −9.60 ± 0.01 m | 30.75 ± 0.02 g | 5.79 | 53.65 | |
| CAR | 0.25% | 61.58 ± 0.02 ab | −7.69 ± 0.02 d | 28.89 ± 0.03 b | 0.73 | 51.32 |
| 0.50% | 62.58 ± 0.09 c | −8.14 ± 0.01 e | 28.03 ± 0.13 a | 1.98 | 52.54 | |
| 0.75% | 64.75 ± 0.01 e | −8.45 ± 0.05 g | 30.23 ± 0.11 f | 3.52 | 52.80 | |
| 1.00% | 67.49 ± 0.31 g | −9.22 ± 0.02 k | 31.24 ± 0.06 i | 6.52 | 53.98 | |
| CIT | 0.25% | 69.14 ± 0.21 i | −8.64 ± 0.03 h | 31.00 ± 0.06 h | 7.88 | 55.41 |
| 0.50% | 70.19 ± 0.09 j | −8.84 ± 0.02 i | 29.19 ± 0.02 c | 8.80 | 57.36 | |
| 0.75% | 71.15 ± 0.25 k | −9.15 ± 0.03 j | 31.42 ± 0.13 j | 11.71 | 56.37 | |
| 1.00% | 72.66 ± 0.18 l | −9.58 ± 0.09 m | 32.31 ± 0.16 k | 13.82 | 56.60 | |
| LAC | 0.25% | 69.27 ± 0.02 d | −7.43 ± 0.01 c | 30.09 ± 0.02 e | 7.75 | 56.35 |
| 0.50% | 69.39 ± 0.03 h | −8.60 ± 0.01 h | 30.78 ± 0.01 g | 8.08 | 55.75 | |
| 0.75% | 74.30 ± 0.07 m | −9.30 ± 0.03 l | 32.87 ± 0.04 l | 13.37 | 57.25 | |
| 1.00% | 74.54 ± 0.06 n | −9.55 ± 0.02 m | 33.08 ± 0.02 m | 13.69 | 57.18 | |
| Sample | Cooked Noodle ** | |||||
|---|---|---|---|---|---|---|
| Color | Tissue | Adhesiveness | Springiness | Flavor | Overall Acceptability | |
| Control | 4.45 ± 1.02 a | 4.75 ± 1.03 b | 4.57 ± 1.12 b | 4.83 ± 1.21 b | 4.51 ± 0.94 b | 4.92 ± 1.02 b |
| 0.25% | 4.22 ± 1.07 a | 4.54 ± 1.16 ab | 4.12 ± 1.23 a | 4.29 ± 1.33 a | 4.15 ± 1.29 ab | 4.23 ± 1.14 a |
| 0.50% | 4.58 ± 1.01 a | 4.34 ± 1.12 ab | 4.28 ± 1.05 ab | 4.34 ± 1.31 a | 4.25 ± 1.06 b | 4.34 ± 1.03 a |
| 0.75% | 4.51 ± 0.97 a | 4.29 ± 1.35 ab | 3.95 ± 1.23 a | 4.15 ± 1.39 a | 4.17 ± 1.15 ab | 4.14 ± 1.14 a |
| 1.00% | 4.55 ± 1.09 a | 4.12 ± 1.27 a | 3.88 ± 0.96 a | 4.11 ± 1.29 a | 3.80 ± 1.02 a | 4.08 ± 1.25 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-T.V.; Chen, G.-W.; Chang, K.-L.B.; Bo, Y.-J.; Sung, W.-C. Comparison of Physicochemical Properties of Noodles Fortified with Commercial Calcium Salts versus Calcium Citrate from Oyster Shells. Foods 2023, 12, 2696. https://doi.org/10.3390/foods12142696
Lin H-TV, Chen G-W, Chang K-LB, Bo Y-J, Sung W-C. Comparison of Physicochemical Properties of Noodles Fortified with Commercial Calcium Salts versus Calcium Citrate from Oyster Shells. Foods. 2023; 12(14):2696. https://doi.org/10.3390/foods12142696
Chicago/Turabian StyleLin, Hong-Ting Victor, Guan-Wen Chen, Ke-Liang Bruce Chang, Yi-Jun Bo, and Wen-Chieh Sung. 2023. "Comparison of Physicochemical Properties of Noodles Fortified with Commercial Calcium Salts versus Calcium Citrate from Oyster Shells" Foods 12, no. 14: 2696. https://doi.org/10.3390/foods12142696
APA StyleLin, H.-T. V., Chen, G.-W., Chang, K.-L. B., Bo, Y.-J., & Sung, W.-C. (2023). Comparison of Physicochemical Properties of Noodles Fortified with Commercial Calcium Salts versus Calcium Citrate from Oyster Shells. Foods, 12(14), 2696. https://doi.org/10.3390/foods12142696





