Development of a Sensitive SERS Method for Label-Free Detection of Hexavalent Chromium in Tea Using Carbimazole Redox Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Synthesis of SERS Substrate Au@AgNPs
2.4. Tea Sample Preparation
2.5. Detection of Cr6+ in Tea Samples
2.6. Specific Selectivity and Spike Recovery for Cr6+ Detection in Tea
3. Results
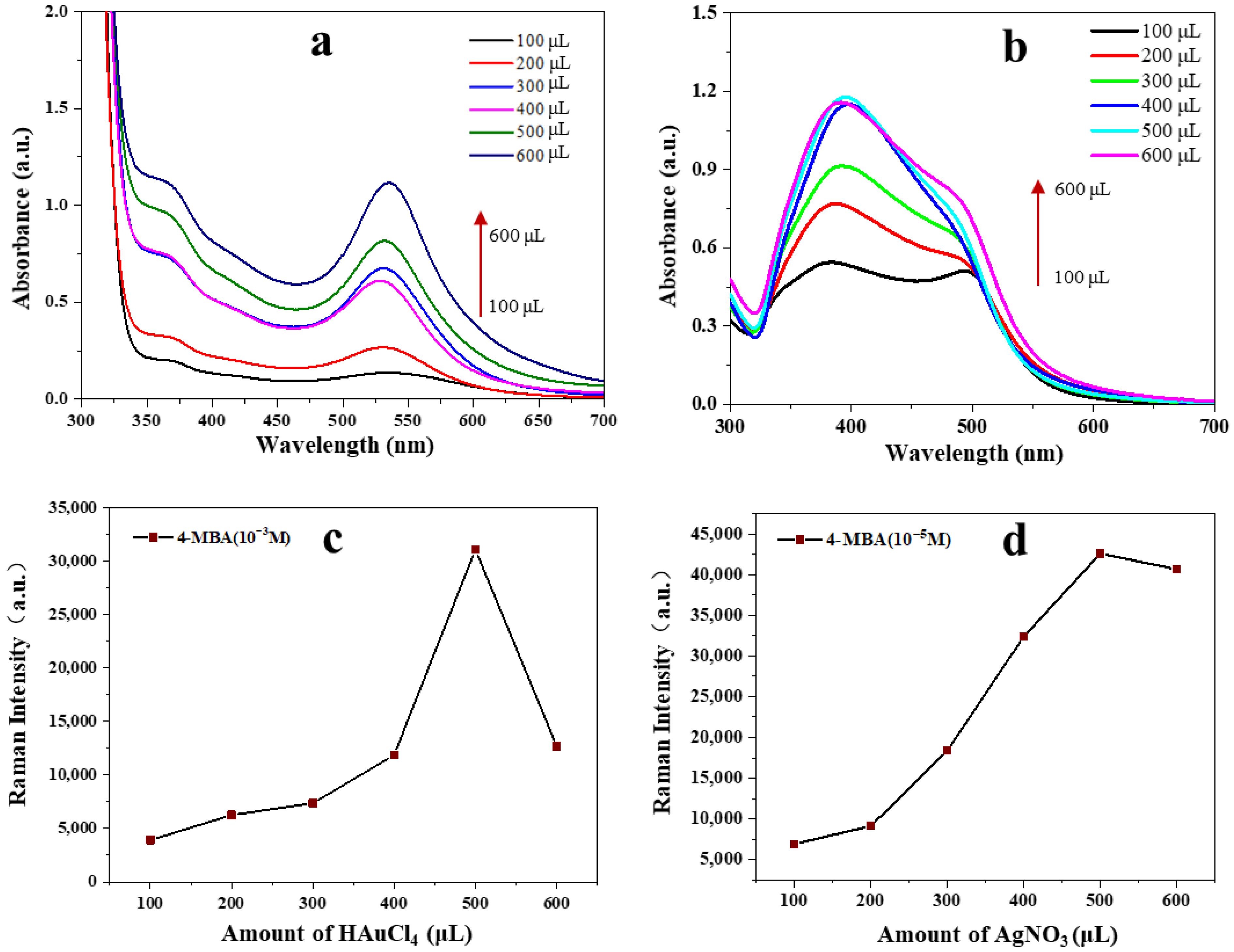
3.1. Synthesis of Au@AgNPs and Optimization
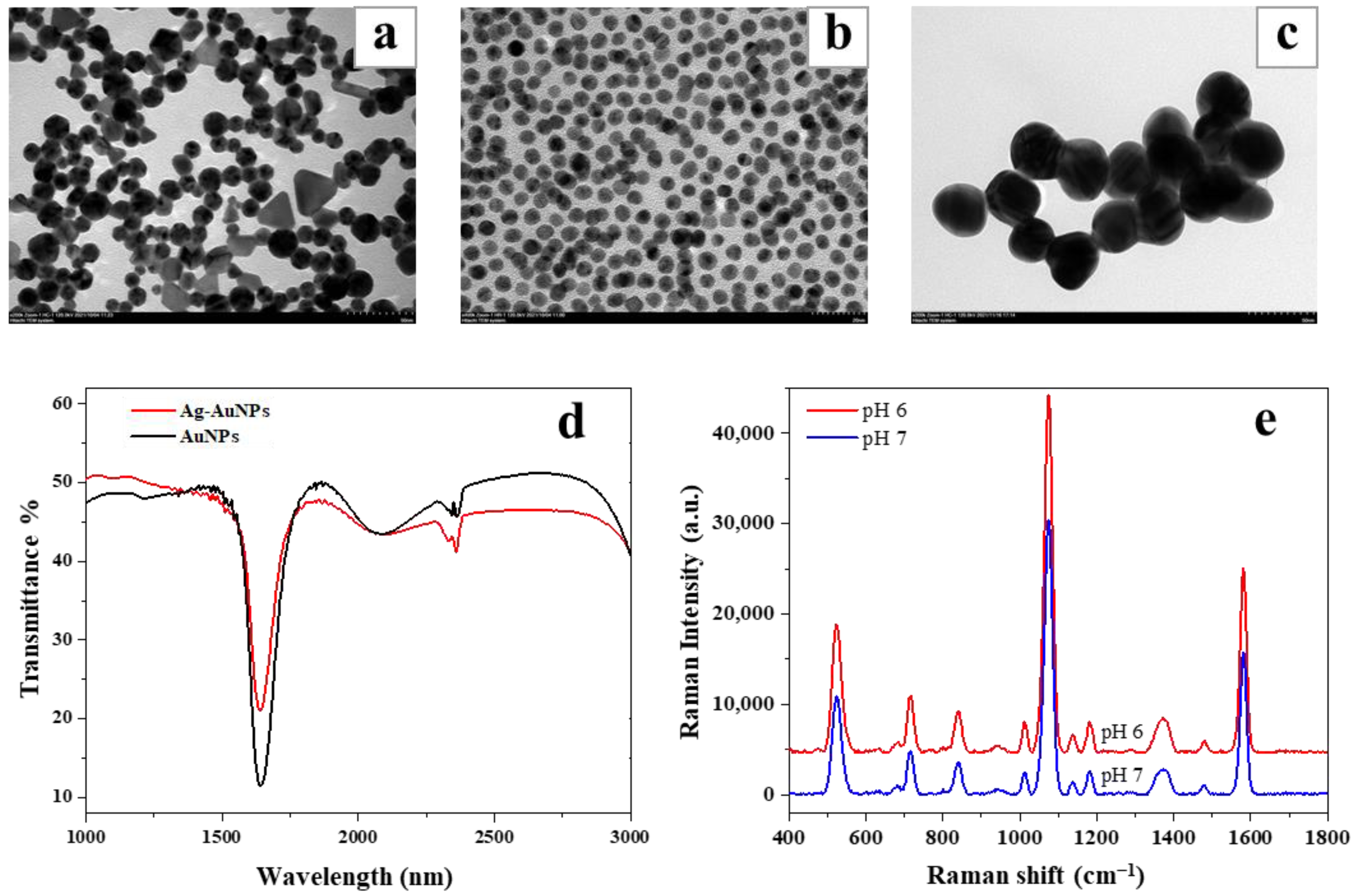
3.2. Characterization of Au@AgNPs
3.3. Quantitative Detection of Cr6+ in Tea Samples
3.4. Specific Selectivity and Recovery for Cr6+ Detection in Spiked Tea
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, X.-L.; He, Y. Challenges and Opportunities in Quantitative Analyses of Lead, Cadmium, and Hexavalent Chromium in Plant Materials by Laser-Induced Breakdown Spectroscopy: A Review. Appl. Spectrosc. Rev. 2017, 52, 605–622. [Google Scholar] [CrossRef]
- Ao, M.; Chen, X.; Deng, T.; Sun, S.; Tang, Y.; Morel, J.L.; Qiu, R.; Wang, S. Chromium Biogeochemical Behaviour in Soil-Plant Systems and Remediation Strategies: A Critical Review. J. Hazard. Mater. 2022, 424, 127233. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.A.; Salem, H. The Toxicology of Chromium with Respect to Its Chemical Speciation: A Review. J. Appl. Toxicol. 1993, 13, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Wang, Y.; Tanabe, I.; Han, X.; Zhao, B.; Ozaki, Y. Semiconductor-Driven “Turn-off” Surface-Enhanced Raman Scattering Spectroscopy: Application in Selective Determination of Chromium(VI) in Water. Chem. Sci. 2014, 6, 342–348. [Google Scholar] [CrossRef]
- Guo, Z.; Barimah, A.O.; Yin, L.; Chen, Q.; Shi, J.; El-Seedi, H.R.; Zou, X. Intelligent Evaluation of Taste Constituents and Polyphenols-to-Amino Acids Ratio in Matcha Tea Powder Using near Infrared Spectroscopy. Food Chem. 2021, 353, 129372. [Google Scholar] [CrossRef]
- Habila, M.; Unsal, Y.E.; Alothman, Z.A.; Shabaka, A.; Tuzen, M.; Soylak, M. Speciation of Chromium in Natural Waters, Tea, and Soil with Membrane Filtration Flame Atomic Absorption Spectrometry. Anal. Lett. 2015, 48, 2258–2271. [Google Scholar] [CrossRef]
- Wu, J.; Lu, G.; Huang, X. Fabrication of Monolith-Based Solid-Phase Microextraction for Effective Extraction of Total Chromium in Milk and Tea Samples Prior to HPLC/DAD Analysis. Microchem. J. 2020, 159, 105549. [Google Scholar] [CrossRef]
- Ling, S.; Zhao, Q.; Iqbal, M.N.; Dong, M.; Li, X.; Lin, M.; Wang, R.; Lei, F.; He, C.; Wang, S. Development of Immunoassay Methods Based on Monoclonal Antibody and Its Application in the Determination of Cadmium Ion. J. Hazard. Mater. 2021, 411, 124992. [Google Scholar] [CrossRef]
- Zheng, M.; Xie, Z.; Qu, D.; Li, D.; Du, P.; Jing, X.; Sun, Z. On–off–on Fluorescent Carbon Dot Nanosensor for Recognition of Chromium(VI) and Ascorbic Acid Based on the Inner Filter Effect. ACS Appl. Mater. Interfaces 2013, 5, 13242–13247. [Google Scholar] [CrossRef]
- Areo, O.M.; Njobeh, P.B. Risk assessment of heavy metals in rooibos (Aspalathus linearis) tea consumed in South Africa. Environ. Sci. Pollut. Res. 2021, 28, 59687–59695. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, P.; Yosri, N.; Chen, Q.; Elseedi, H.R.; Zou, X.; Yang, H. Detection of Heavy Metals in Food and Agricultural Products by Surface-Enhanced Raman Spectroscopy. Food Rev. Int. 2023, 39, 1440–1461. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, M.; Agyekum, A.A.; Wu, J.; Chen, Q.; Zuo, M.; El-Seedi, H.R.; Tao, F.; Shi, J.; Ouyang, Q.; et al. Quantitative Detection of Apple Watercore and Soluble Solids Content by near Infrared Transmittance Spectroscopy. J. Food Eng. 2020, 279, 109955. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.I.; Lyu, D.; Lu, Z.; Liu, G.; Ren, B. Surface-Enhanced Raman Spectroscopy: Benefits, Trade-offs and Future Developments. Chem. Sci. 2020, 11, 4563–4577. [Google Scholar] [CrossRef]
- Prakash, J. Fundamentals and Applications of Recyclable SERS Substrates. Int. Rev. Phys. Chem. 2019, 38, 201–242. [Google Scholar] [CrossRef]
- Tian, S.; Hu, Y.; Chen, X.; Liu, C.; Xue, Y.; Han, B. Green Synthesis of Silver Nanoparticles Using Sodium Alginate and Tannic Acid: Characterization and Anti-S. Aureus Activity. Int. J. Biol. Macromol. 2022, 195, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.M. A Kinetic Method for the Determination of Carbimazole in Pharmaceutical Preparations by Oxidation with Bichromate in Sulfuric Acid. Anal. Sci. 1992, 8, 503–506. [Google Scholar] [CrossRef]
- Bu, X.; Zhang, Z.; Zhang, L.; Li, P.; Wu, J.; Zhang, H.; Tian, Y. Highly Sensitive SERS Determination of Chromium(VI) in Water Based on Carbimazole Functionalized Alginate-Protected Silver Nanoparticles. Sens. Actuators B Chem. 2018, 273, 1519–1524. [Google Scholar] [CrossRef]
- Huang, X.; Wu, H.; Pu, S.; Zhang, W.; Liao, X.; Shi, B. One-Step Room-Temperature Synthesis of Au@Pd Core–Shell Nanoparticles with Tunable Structure Using Plant Tannin as Reductant and Stabilizer. Green Chem. 2011, 13, 950–957. [Google Scholar] [CrossRef]
- Gangwar, C.; Yaseen, B.; Kumar, I.; Singh, N.K.; Naik, R.M. Growth Kinetic Study of Tannic Acid Mediated Monodispersed Silver Nanoparticles Synthesized by Chemical Reduction Method and Its Characterization. ACS Omega 2021, 6, 22344–22356. [Google Scholar] [CrossRef]
- Ren, Z.; Li, Z.; Chen, Z.; Zhang, Y.; Lin, X.; Weng, W.; Yang, H.; Li, B. Characteristics and Application of Fish Oil-in-Water Pickering Emulsions Structured with Tea Water-Insoluble Proteins/κ-Carrageenan Complexes. Food Hydrocoll. 2021, 114, 106562. [Google Scholar] [CrossRef]
- Zuo, Q.; Chen, Y.; Chen, Z.P.; Yu, R.Q. Quantification of Cadmium in Rice by Surface-Enhanced Raman Spectroscopy Based on a Ratiometric Indicator and Conical Holed Enhancing Substrates. Anal. Sci. 2018, 34, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Hertaeg, M.J.; Rees-Zimmerman, C.; Tabor, R.F.; Routh, A.F.; Garnier, G. Predicting Coffee Ring Formation upon Drying in Droplets of Particle Suspensions. J. Colloid Interface Sci. 2021, 591, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Welna, M.; Szymczycha-Madeja, A.; Pohl, P. Novel ICP-OES-Based Method for the Reliable Determination of the Total Content of 15 Elements in Yerba Mate Drinks along with the Determination of Caffeine and the in Vitro Bioaccessibility of the Compounds. Molecules 2023, 28, 3374. [Google Scholar] [CrossRef] [PubMed]
- Grembecka, M. Assessment of the Mineral Composition and the Selected Physicochemical Parameters of Dietary Supplements Containing Green Tea Extracts. Foods 2022, 11, 3580. [Google Scholar] [CrossRef]
- Guo, Z.; Barimah, A.O.; Guo, C.; Agyekum, A.A.; Annavaram, V.; El-Seedi, H.R.; Zou, X.; Chen, Q. Chemometrics Coupled 4-Aminothiophenol Labelled Ag-Au Alloy SERS off-Signal Nanosensor for Quantitative Detection of Mercury in Black Tea. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 242, 118747. [Google Scholar] [CrossRef]
- Huang, H.; Toit, H.D.; Besenhard, M.O.; Ben-Jaber, S.; Dobson, P.; Parkin, I.; Gavriilidis, A. Continuous Flow Synthesis of Ultrasmall Gold Nanoparticles in a Microreactor Using Trisodium Citrate and Their SERS Performance. Chem. Eng. Sci. 2018, 189, 422–430. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV-Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Chen, P.; Yin, L.; El-Seedi, H.R.; Zou, X.; Guo, Z. Green reduction of silver nanoparticles for cadmium detection in food using surface-enhanced Raman spectroscopy coupled multivariate calibration. Food Chem. 2022, 394, 133481. [Google Scholar] [CrossRef]
- Wu, W.; Tan, Z.; Chen, X.; Chen, X.; Cheng, L.; Wu, H.; Li, P.; Zhang, Z. Carnation-like Morphology of BiVO4-7 Enables Sensitive Photoelectrochemical Determination of Cr(VI) in the Food and Environment. Biosensors 2022, 12, 130. [Google Scholar] [CrossRef]



| Raman Shift (cm−1) | Band Assignment |
|---|---|
| 412.63 | Ring rotation, C–N–S bend |
| 539.54 | S=S stretching |
| 595.85 | C–N–S bend |
| 708.48 | Ring rotation, CH(NH) bend |
| 872.67 | Ring rotation, CH(NH) bend, C–N–S bend |
| 1014.35 | C–N stretching, CH(NH) bend |
| 1370.14 | C–N stretching, bend and rotation |
| 1469.72 | C–S stretching, CN stretching, NH bend |
| 1593.76 | C–C stretching, CH(NH) bend |
| Spiked Value (mg/kg) | SERS | ICP-MS | ||||
|---|---|---|---|---|---|---|
| Obtained Value (mg/kg) | Recovery Percentage (%) | RSD (%, n = 3) | Obtained Value (mg/kg) | Recovery Percentage (%) | RSD (%, n = 3) | |
| 2.5 | 2.621 | 104.84 | 2.06 | 2.528 | 101.12 | 1.07 |
| 5 | 4.686 | 93.72 | 1.39 | 4.8825 | 97.65 | 0.96 |
| 10 | 9.162 | 91.62 | 2.12 | 10.386 | 103.86 | 0.89 |
| 20 | 20.526 | 102.63 | 3.07 | 19.638 | 98.19 | 1.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, L.; Jayan, H.; Cai, J.; El-Seedi, H.R.; Guo, Z.; Zou, X. Development of a Sensitive SERS Method for Label-Free Detection of Hexavalent Chromium in Tea Using Carbimazole Redox Reaction. Foods 2023, 12, 2673. https://doi.org/10.3390/foods12142673
Yin L, Jayan H, Cai J, El-Seedi HR, Guo Z, Zou X. Development of a Sensitive SERS Method for Label-Free Detection of Hexavalent Chromium in Tea Using Carbimazole Redox Reaction. Foods. 2023; 12(14):2673. https://doi.org/10.3390/foods12142673
Chicago/Turabian StyleYin, Limei, Heera Jayan, Jianrong Cai, Hesham R. El-Seedi, Zhiming Guo, and Xiaobo Zou. 2023. "Development of a Sensitive SERS Method for Label-Free Detection of Hexavalent Chromium in Tea Using Carbimazole Redox Reaction" Foods 12, no. 14: 2673. https://doi.org/10.3390/foods12142673
APA StyleYin, L., Jayan, H., Cai, J., El-Seedi, H. R., Guo, Z., & Zou, X. (2023). Development of a Sensitive SERS Method for Label-Free Detection of Hexavalent Chromium in Tea Using Carbimazole Redox Reaction. Foods, 12(14), 2673. https://doi.org/10.3390/foods12142673







