Biotechnological Applications of Mushrooms under the Water-Energy-Food Nexus: Crucial Aspects and Prospects from Farm to Pharmacy
Abstract
1. Introduction
2. Methodology of the Review
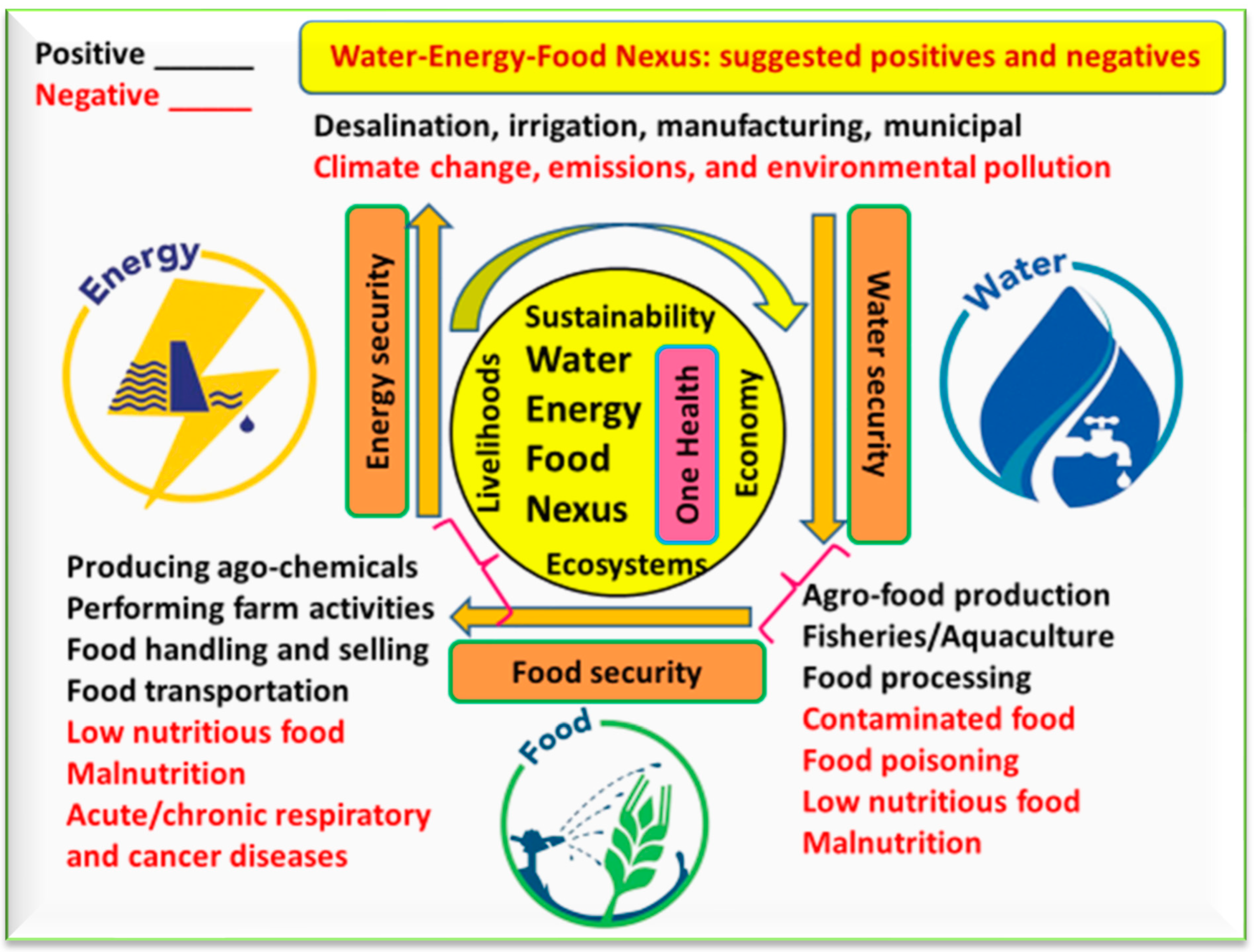
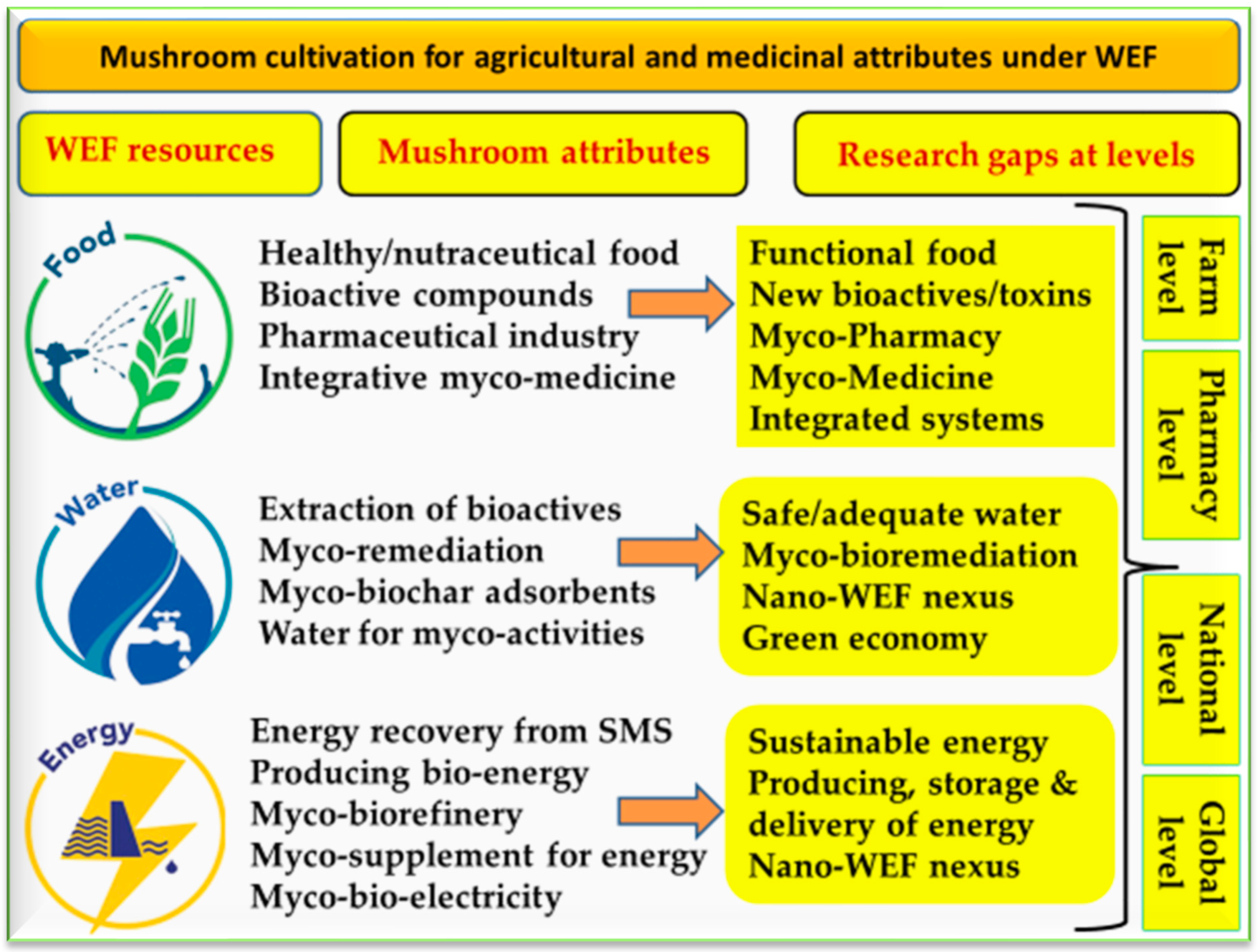
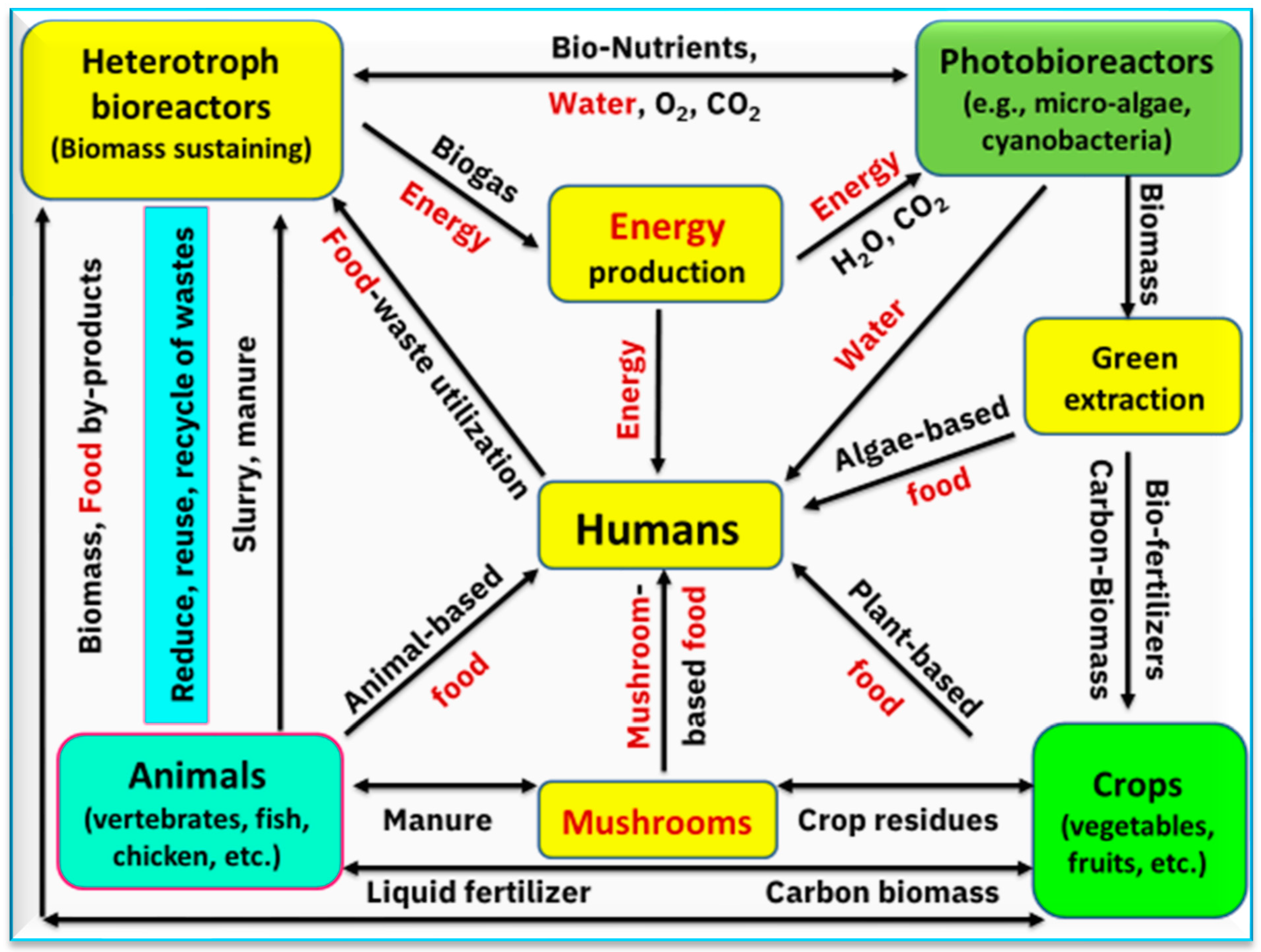
3. Water-Energy-Food Nexus
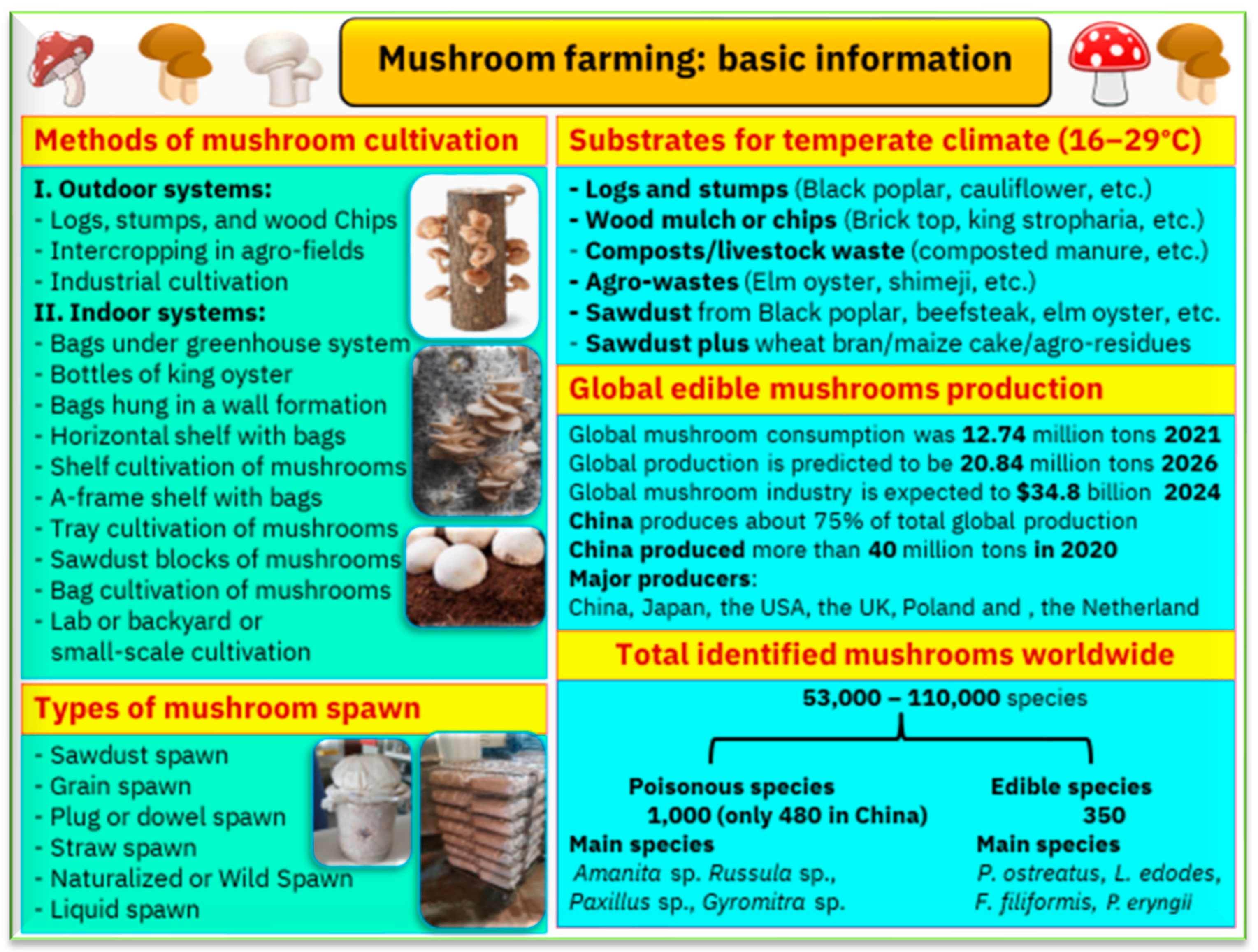
4. Mushroom Farming: Basics and Requirements
5. Mushrooms as a Healthy Food
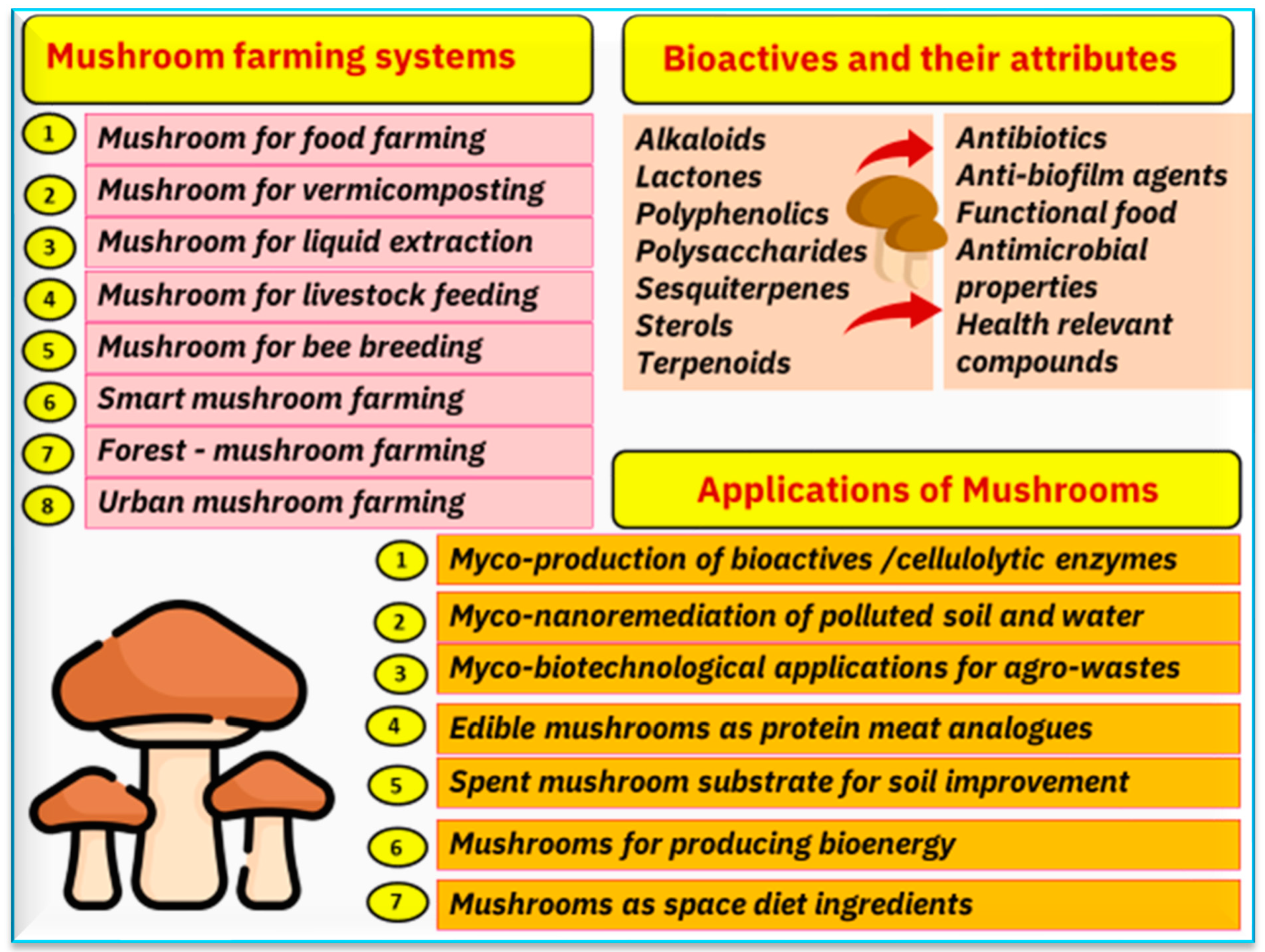
6. Mushroom Biotechnology
6.1. Mushrooms to Produce Nanoparticles
| Mushroom Species | Nanoparticles (NPs) | The Application | References |
|---|---|---|---|
| Enoki mushroom (Flammulina velutipes) | Ag-NPs (~10 nm) | Biodegradable natural biopolymers as active food packaging films. | [78] |
| Oyster mushroom (Pleurotus florida) | Ag-NPs (~12.62 nm) | Effective antimicrobial agents as an alternative to traditional antibiotics to control diseases/microbial infection. | [79] |
| Reishi mushroom (Ganoderma lucidum) | Ag-NPs (15–22 nm) | Ag-NPs can be applied in the pharmaceutical, medical, and cosmetic fields due to their antioxidant, antibacterial, and antifungal activity. | [80] |
| Oyster mushroom (Pleurotus florida) | Gold-platinum (Au-Pt-NPs, 16 nm) | Au-Pt-NPs showed anticancer activity against human colon cancer. | [81] |
| Pleurotus giganteus | Ag-NPs (2–20 nm) | Ag-NPs have antibacterial and α-amylase inhibitory activity. | [82] |
| Macrolepiota procera | Ag-NPs (20–50 nm) | Ag-NPs have antibacterial activity as a green corrosive inhibitor for mild steel in cooling tower water systems. | [83] |
| Termitomyces heimii mushroom | CdS-NPs (<5 nm) | CdS-NPs have potential use in energy (solar panels), biomedical, biofilm, drug delivery, and environmental applications. | [84] |
| Cordyceps militaris mushroom | ZnO-NPs (1.83 nm) | ZnO-NPs can be used for the development of therapeutic drugs and have antioxidant, antidiabetic, and antibacterial activity. | [85] |
| Inonotus hispidus mushroom | Ag-NPs (69.24 nm) | Ag-NPs exhibited activity against different pathogenic bacteria and fungi, showing antimicrobial potential. | [86] |
| Ramaria botrytis mushroom | Ag-Au bimetallic composite NPs | The nano-composite was effective for intensive industrial and biomedical applications due to powerful antioxidant properties for DPPH radical scavenging. | [87] |
| Shiitake mushroom (Lentinula edodes) | ZnO-NPs (21–25 nm) | ZnO-NPs degraded methylene blue dye pollution by 90% within 135 min in wastewater. It also showed promise as an antibacterial product. | [88] |
| Portabello mushroom (A. bisporus) | Au-NPs (53 nm) | Au-NPs reduced methylene blue by about 98% in wastewater and decolorized azo dye. | [89] |
| Ganoderma lucidum | ZnO-NPs (using 25 mL for extraction) | ZnO-NPs were used in vitro as nanofertilizer for feeding garden cress (Lepidium sativum). | [90] |
| Edible mushroom (A. bisporus) | Ag-NPs (average 17 nm) | Myco-fabricated Ag-NP had antioxidant/antimicrobial effects without any cytotoxic impacts on human dermal fibroblast cells. | [91] |
6.2. Mushrooms for Bioremediation
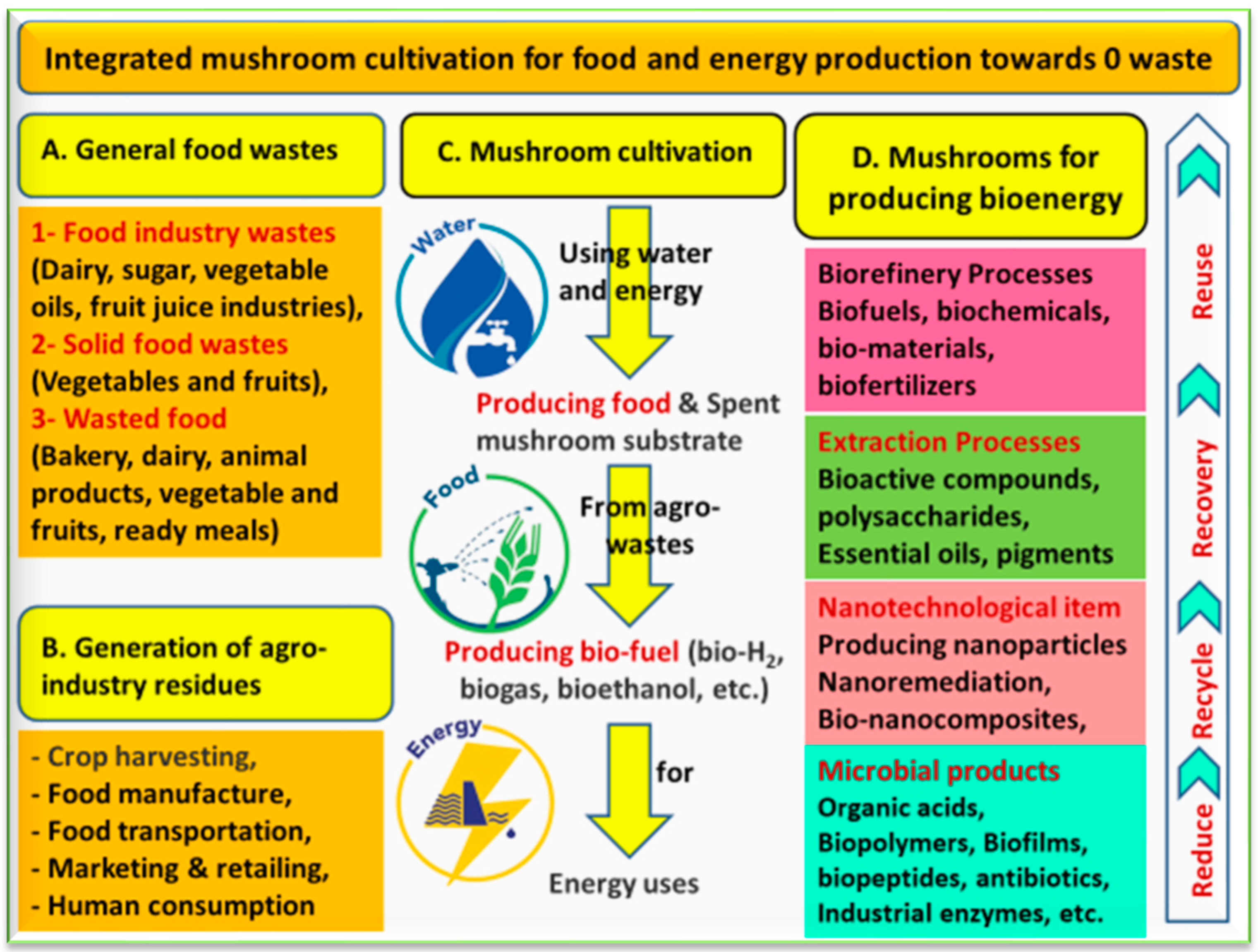
6.3. Mushrooms to Produce Bioenergy
6.4. Mushrooms to Produce Bioactives
7. Mushrooms: Pros and Cons
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nuwayhid, I.; Mohtar, R. The Water, Energy, and Food Nexus: Health is yet Another Resource. Front. Environ. Sci. 2022, 10, 879081. [Google Scholar] [CrossRef]
- Villicaña-García, E.; Cansino-Loeza, B.; Ponce-Ortega, J.M. Applying the “matching law” optimization approach to promote the sustainable use of resources in the water-energy-food nexus. Sustain. Prod. Consum. 2023; in press. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Wang, A.; Liu, T.; Du, S.; Liang, S. Coordinated analysis and evaluation of water–energy–food coupling: A case study of the Yellow River basin in Shandong Province, China. Ecol. Indic. 2023, 148, 110138. [Google Scholar] [CrossRef]
- Xia, Y.; Yan, B. Energy-food nexus scarcity risk and the synergic impact of climate policy: A global production network perspective. Environ. Sci. Policy 2022, 135, 26–35. [Google Scholar] [CrossRef]
- Herrera-Franco, G.; Bollmann, H.A.; Lofhagen, J.C.P.; Bravo-Montero, L.; Carrión-Mero, P. Approach on water-energy-food (WEF) nexus and climate change: A tool in decision-making processes. Environ. Dev. 2023, 46, 100858. [Google Scholar] [CrossRef]
- Raya-Tapia, A.Y.; López-Flores, F.J.; Ponce-Ortega, J.M. Incorporating deep learning predictions to assess the water-energy-food nexus security. Environ. Sci. Policy 2023, 144, 99–109. [Google Scholar] [CrossRef]
- Azzam, A.; Samy, G.; Hagras, M.A.; ElKholy, R. Geographic information systems-based framework for water-energy-food nexus assessments. Ain Shams Eng. J. 2023, 102224. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Fu, Q.; Singh, V.P.; Liu, D.; Xu, Y. An optimization approach of water-food-energy nexus in agro-forestry-livestock system under uncertain water supply. J. Clean. Prod. 2023, 407, 137116. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, Y.; Xie, Y.; Wang, Y.; Yang, Z.; Cai, Y. Spatial transmission mechanism of the water, energy and food nexus risks for the Guangdong-Hong Kong-Macao region of China. J. Clean. Prod. 2023, 405, 136906. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Lu, G.; Xu, Z.; Yan, X.; Khu, S.T.; Yang, J.; Zhao, J. Influence of Russia-Ukraine War on the Global Energy and Food Security. Resour. Conserv. Recycl. 2023, 188, 106657. [Google Scholar] [CrossRef]
- Coudard, A.; Corbin, E.; de Koning, J.; Tukker, A.; Mogollón, J.M. Global water and energy losses from consumer avoidable food waste. J. Clean. Prod. 2021, 326, 129342. [Google Scholar] [CrossRef]
- Alexopoulos, C.; Moore, J.; Ahmadjian, D.V. Fungus. Encyclopedia Britannica. Available online: https://www.britannica.com/science/fungus (accessed on 24 May 2023).
- El-Ramady, H.; Brevik, E.C.; Bayoumi, Y.; Shalaby, T.A.; El-Mahrouk, M.E.; Taha, N.; Elbasiouny, H.; Elbehiry, F.; Amer, M.; Abdalla, N.; et al. An Overview of Agro-Waste Management in Light of the Water-Energy-Waste Nexus. Sustainability 2022, 14, 15717. [Google Scholar] [CrossRef]
- Leong, Y.K.; Varjani, S.; Lee, D.J.; Chang, J.C. Valorization of spent mushroom substrate for low-carbon biofuel production: Recent advances and developments. Bioresour. Technol. 2022, 363, 128012. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.; Ghanekar, S.; Kumar, D.S. Current trends in health-promoting potential and biomaterial applications of edible mushrooms for human wellness. Food Biosci. 2023, 51, 102290. [Google Scholar] [CrossRef]
- Kour, H.; Kour, D.; Kour, S.; Singh, S.; Hashmi, S.A.J.; Yadav, A.N.; Kumar, K.; Sharma, Y.P.; Ahluwalia, A.S. Bioactive compounds from mushrooms: Emerging bioresources of food and nutraceuticals. Food Biosci. 2022, 50 Pt B, 102124. [Google Scholar] [CrossRef]
- Ahmad, I.; Arif, M.; Xu, M.; Zhang, J.; Ding, Y.; Lyu, F. Therapeutic values and nutraceutical properties of shiitake mushroom (Lentinula edodes): A review. Trends Food Sci. Technol. 2023, 134, 123–135. [Google Scholar] [CrossRef]
- Lee, A.M.L.; Chin, C.F.S.; Seelan, J.S.S.; Chye, F.Y.; Lee, H.H.; Rakib, M.R.M. Metabolites profiling of protein enriched oyster mushroom (Pleurotus ostreatus (Jacq.) P. Kumm.) grown on oil palm empty fruit bunch substrate. LWT 2023, 181, 114731. [Google Scholar] [CrossRef]
- Hultberg, M.; Ahrens, L.; Golovko, O. Use of lignocellulosic substrate colonized by oyster mushroom (Pleurotus ostreatus) for removal of organic micropollutants from water. J. Environ. Manag. 2020, 272, 111087. [Google Scholar] [CrossRef]
- Li, H.; Chai, L.; Cui, J.; Zhang, F.; Wang, F.; Li, S. Polypyrrole-modified mushroom residue activated carbon for sulfate and nitrate removal from water: Adsorption performance and mechanism. J. Water Process Eng. 2022, 49, 102916. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S. Biotechnological production of sugar-alcohols: Focus on Yarrowia lipolytica and edible/medicinal mushrooms. Process Biochem. 2023, 124, 113–131. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Fawzy, Z.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Green Biotech-nology of Oyster Mushroom (Pleurotus ostreatus L.): A Sustainable Strategy for Myco-Remediation and Bio-Fermentation. Sustainability 2022, 14, 3667. [Google Scholar] [CrossRef]
- Xu, S.; Gao, M.; Peng, Z.; Sui, K.; Li, Y.; Li, C. Upcycling from chitin-waste biomass into bioethanol and mushroom via solid-state fermentation with Pleurotus ostreatus. Fuel 2022, 326, 125061. [Google Scholar] [CrossRef]
- Ma, X.; Gao, M.; Li, Y.; Wang, Q.; Sun, X. Production of cellulase by Aspergillus niger through fermentation of spent mushroom substance: Glucose inhibition and elimination approaches. Process Biochem. 2022, 122 Pt 2, 26–35. [Google Scholar] [CrossRef]
- Shan, G.; Li, W.; Bao, S.; Hu, X.; Liu, J.; Zhu, L.; Tan, W. Energy and nutrient recovery by spent mushroom substrate-assisted hydrothermal carbonization of sewage sludge. Waste Manag. 2023, 155, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guan, W.; Sun, H.; Zhao, P.; Wang, W.; Gao, M.; Sun, X.; Wang, Q. Intermittent energization improves microbial electrolysis cell-assisted thermophilic anaerobic co-digestion of food waste and spent mushroom substance. Bioresour. Technol. 2023, 370, 128577. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.B.; Jewitt, G.P.W. The Development of the Water-Energy-Food Nexus as a Framework for Achieving Resource Security: A Review. Front. Environ. Sci. 2019, 7, 8. [Google Scholar] [CrossRef]
- Orimoloye, I.R. Water, Energy and Food Nexus: Policy Relevance and Challenges. Front. Sustain. Food Syst. 2022, 5, 824322. [Google Scholar] [CrossRef]
- Ding, T.; Chen, J.; Fang, L.; Ji, J.; Fang, Z. Urban ecosystem services supply-demand assessment from the perspective of the water-energy-food nexus. Sustain. Cities Soc. 2023, 90, 104401. [Google Scholar] [CrossRef]
- Cui, S.; Wu, M.; Huang, X.; Wang, X.; Cao, X. Sustainability and assessment of factors driving the water-energy-food nexus in pumped irrigation systems. Agric. Water Manag. 2022, 272, 107846. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, D.; Guo, S.; Xiong, L.; Liu, P.; Chen, J.; Yin, J.; Wu, Z.; Zhou, W. Assessing the effects of water resources allocation on the uncertainty propagation in the water–energy–food–society (WEFS) nexus. Agric. Water Manag. 2023, 282, 108279. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Xu, Y.; Zheng, L.; Fan, Y. A factorial inexact copula stochastic programming (FICSP) approach for water-energy- food nexus system management. Agric. Water Manag. 2023, 277, 108069. [Google Scholar] [CrossRef]
- Muhirwa, F.; Shen, L.; Elshkaki, A.; Chiaka, J.C.; Zhong, S.; Bönecke, E.; Hirwa, H.; Seka, A.M.; Habiyakare, T.; Tuyishimire, A.; et al. Alert in the dynamics of water-energy-food production in African countries from a nexus perspective. Resour. Conserv. Recycl. 2023, 194, 106990. [Google Scholar] [CrossRef]
- Meng, F.; Yuan, Q.; Bellezoni, R.A.; de Oliveira, J.A.P.; Cristiano, S.; Shah, A.M.; Liu, G.; Yang, Z.; Seto, K.C. Quantification of the food-water-energy nexus in urban green and blue infrastructure: A synthesis of the literature. Resour., Conserv. Recycl. 2023, 188, 106658. [Google Scholar] [CrossRef]
- Yin, D.; Yu, H.; Shi, Y.; Zhao, M.; Zhang, J.; Li, X. Matching supply and demand for ecosystem services in the Yellow River Basin, China: A perspective of the water-energy-food nexus. J. Clean. Prod. 2023, 384, 135469. [Google Scholar] [CrossRef]
- Yuxi, Z.; Jingke, H.; Wen, Q.; Yang, C.; Danfei, N. Managing water-land-food nexus towards resource efficiency improvement: A superedge-based analysis of China. J. Environ. Manag. 2023, 325 Pt A, 116607. [Google Scholar] [CrossRef]
- Balaican, D.; Nichersu, I.; Nichersu, I.I.; Pierce, A.; Wilhelmi, O.; Laborgne, P.; Bratfanof, E. Creating knowledge about food-water-energy nexus at a local scale: A participatory approach in Tulcea, Romania. Environ. Sci. Policy 2023, 141, 23–32. [Google Scholar] [CrossRef]
- Barati, A.A.; Pour, M.D.; Sardooei, M.A. Water crisis in Iran: A system dynamics approach on water, energy, food, land and climate (WEFLC) nexus. Sci. Total. Environ. 2023, 882, 163549. [Google Scholar] [CrossRef]
- Morlet-Espinosa, J.; Flores-Tlacuahuac, A.; Fuentes-Cortes, L.F. A combined variational encoding and optimization framework for design of the water–energy–food nexus. Comput. Chem. Eng. 2023, 170, 108076. [Google Scholar] [CrossRef]
- Corriero, A.C.; Aborode, A.T.; Reggio, M.; Shatila, N. The impact of COVID-19 and the economic crisis on Lebanese public health: Food insecurity and healthcare disintegration. Ethics Med. Public. Health 2022, 24, 100802. [Google Scholar] [CrossRef]
- Kaicker, N.; Gupta, A.; Gaiha, R. COVID-19 pandemic and food security in India: Can authorities alleviate the disproportionate burden on the disadvantaged? J. Policy Model. 2022, 44, 963–980. [Google Scholar] [CrossRef]
- Chitrakar, B.; Zhang, M.; Bhesh Bhandari, B. Improvement strategies of food supply chain through novel food processing technologies during COVID-19 pandemic. Food Control 2021, 125, 108010. [Google Scholar] [CrossRef] [PubMed]
- Srinivash, M.; Krishnamoorthi, R.; Mahalingam, P.U.; Malaikozhundan, B.; Bharathakumar, S.; Gurushankar, K.; Dhanapal, K.; Samy, K.K.; Perumal, A.B. Nanomedicine for drug resistant pathogens and COVID-19 using mushroom nanocomposite inspired with bacteriocin—A review. Inorg. Chem. Commun. 2023, 152, 110682. [Google Scholar] [CrossRef] [PubMed]
- Saputera, A.; Sofyan, A.; Saputra, R.A.; Sari, N. Effect of Watering Frequency on the Growth and Yield of Oyster Mushrooms (Pleurotus ostreatus). Agrosainstek 2020, 4, 155–160. [Google Scholar] [CrossRef]
- Robinson, B.; Winans, K.; Kendall, A.; Dlott, J.; Dlott, F. A life cycle assessment of Agaricus bisporus mushroom production in the USA. Int. J. Life Cycle Assess. 2019, 24, 456–467. [Google Scholar] [CrossRef]
- Clune, S.; Crossin, E.; Verghese, K. Systematic review of greenhouse gas emissions for different fresh food categories. J. Clean. Product. 2017, 140 Pt 2, 766–783. [Google Scholar] [CrossRef]
- Hanafi, F.H.M.; Rezania, S.; Taib, S.M.; Din, M.F.M.; Yamauchi, M.; Sakamoto, M.; Hara, H.; Park, J.; Ebrahimi, S.S. Environmentally sustainable applications of agro-based spent mushroom substrate (SMS): An overview. J. Mater. Cycles Waste Manag. 2018, 20, 1383–1396. [Google Scholar] [CrossRef]
- Zied, D.C.; Sánchez, J.E.; Noble, R.; Pardo-Giménez, A. Use of Spent Mushroom Substrate in New Mushroom Crops to Promote the Transition towards A Circular Economy. Agronomy 2020, 10, 1239. [Google Scholar] [CrossRef]
- Raman, J.; Jang, K.Y.; Oh, Y.L.; Oh, M.; Im, J.H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and Nutritional Value of Prominent Pleurotus spp.: An Overview. Mycobiology 2021, 49, 1–14. [Google Scholar] [CrossRef]
- Bhambri, A.; Srivastava, M.; Mahale, V.G.; Mahale, S.; Karn, S.K. Mushrooms as Potential Sources of Active Metabolites and Medicines. Front. Microbiol. 2022, 13, 837266. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Badgar, K.; Llanaj, X.; Töros, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Edible Mushrooms for Sustainable and Healthy Human Food: Nutritional and Medicinal Attributes. Sustainability 2022, 14, 4941. [Google Scholar] [CrossRef]
- Moussa, A.A.; Fayez, S.; Xiao, H.; Xu, B. New insights into antimicrobial and antibiofilm effects of edible mushrooms. Food Res. Inter. 2022, 162 Pt A, 111982. [Google Scholar] [CrossRef]
- Al-Obaidi, J.R.; Jambari, N.N.; Ahmad-Kamil, E.I. Mycopharmaceuticals and Nutraceuticals: Promising Agents to Improve Human Well-Being and Life Quality. J. Fungi 2021, 7, 503. [Google Scholar] [CrossRef] [PubMed]
- You, S.W.; Hoskin, R.T.; Komarnytsky, S.; Marvin Moncada, M. Mushrooms as Functional and Nutritious Food Ingredients for Multiple Applications. ACS Food Sci. Technol. 2022, 2, 1184–1195. [Google Scholar] [CrossRef]
- Tan, Y.; Zeng, N.K.; Xu, B. Chemical profiles and health-promoting effects of porcini mushroom (Boletus edulis): A narrative review. Food Chem. 2022, 390, 133199. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.S.; Araújo-Rodrigues, H.; Pintado, M.E. The health-promoting potential of edible mushroom proteins. Curr. Pharm. Des. 2022, 9, 66. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Z.; Song, K.; Li, L.; Chen, M. Medicinal value of edible mushroom polysaccharides: A review. J. Future Foods 2023, 3, 16–23. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Mahmoud, G.A.; Hefzy, M.; Liu, Z.; Changyang Ma, C. Overview on the edible mushrooms in Egypt. J. Future Foods 2023, 3, 8–15. [Google Scholar] [CrossRef]
- Rizzo, G.; Goggi, S.; Giampieri, F.; Baroni, L. A review of mushrooms in human nutrition and health. Trend Food Sci. Technol. 2021, 117, 60–73. [Google Scholar] [CrossRef]
- Martinez-Medina, G.A.; Chávez-González, M.L.; Verma, D.K.; Prado-Barragán, L.A.; Martínez-Hernández, J.L.; Flo-res-Gallegos, A.C.; Thakur, M.; Srivastav, P.P.; Aguilar, C.N. Bio-functional components in mushrooms, a health opportunity: Ergothionine and huitlacohe as recent trends. J. Funct. Foods 2021, 77, 104326. [Google Scholar] [CrossRef]
- Xhensila, L.; Törős, G.; Hajdú, P.; El-Ramady, H.; Peles, F.; Prokisch, J. Mushroom Cultivation Systems: Exploring Anti-microbial and Prebiotic Benefits. Environ. Biodivers. Soil Secur. 2023, 7, 101–120. [Google Scholar] [CrossRef]
- Tör˝os, G.; El-Ramady, H.; Prokisch, J.; Velasco, F.; Llanaj, X.; Nguyen, D.H.H.; Peles, F. Modulation of the Gut Microbiota with Prebiotics and Antimicrobial Agents from Pleurotus ostreatus Mushroom. Foods 2023, 12, 2010. [Google Scholar] [CrossRef]
- Sunil, C.; Xu, B. Mycochemical profile and health-promoting effects of morel mushroom Morchella esculenta (L.)—A review. Food Res. Int. 2022, 159, 111571. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, H.; Cai, M.; Liang, X.; Wu, X.; Wang, A.; Chen, X.; Li, X.; Xiao, C.; Huang, L.; et al. Whole genome sequencing of an edible and medicinal mushroom, Russula griseocarnosa, and its association with mycorrhizal characteristics. Gene 2022, 808, 145996. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, X.; Wang, G.; Kong, L.; Guan, Q.; Yang, R.; Jin, Y.; Liu, X.; Qu, J. Remediation of cadmium contaminated soil by composite spent mushroom substrate organic amendment under high nitrogen level. J. Hazard Mater. 2022, 430, 128345. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Zhong, R.; Chen, J.; Luo, Z.G. Structural characterization, anticancer, hypoglycemia and immune activities of polysaccharides from Russula virescens. Int. J. Biol. Macromol. 2021, 184, 380–392. [Google Scholar] [CrossRef]
- Petre, M.; Petre, V. Biotechnology of Mushroom Growth Through Submerged Cultivation. In Mushroom Biotechnology; Marian, P., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 1–18. [Google Scholar]
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; De Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 2020, 7, 5. [Google Scholar] [CrossRef]
- Balan, V.; Zhu, W.; Krishnamoorthy, H.; Benhaddou, D.; Mowrer, J.; Husain, H.; Eskandari, A. Challenges and opportunities in producing high-quality edible mushrooms from lignocellulosic biomass in a small scale. Appl. Microbiol. Biotechnol. 2022, 106, 1355–1374. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Queirós, J.M.; Martins, P.M.; de Luis, R.F.; Fidalgo-Marijuan, A.; Vilas-Vilela, J.L.; Lanceros-Méndez, S.; Reguera, J. Au-sensitised TiO2 and ZnO nanoparticles for broadband pharmaceuticals photocatalytic degradation in water remediation. Colloids Surf. 2023, 671, 131594. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Zhou, J.; Dai, X.; Peng, Y.; Zhong, Y.; Ho, H.P.; Gao, B.Z.; Zhang, H.; Qu, J.; et al. Advances in inorganic nanoparticles trapping stiffness measurement: A promising tool for energy and environmental study. Energy Rev. 2023, 2, 100018. [Google Scholar] [CrossRef]
- Ahmad, A.; Qurashi, A.; Sheehan, D. Nano packaging—Progress and future perspectives for food safety, and sustainability. Food Packag. Shelf Life 2023, 35, 100997. [Google Scholar] [CrossRef]
- Javier, K.R.A.; Camacho, D.H. Dataset on the optimization by response surface methodology for the synthesis of silver nanoparticles using Laxitextum bicolor mushroom. Data Br. 2022, 45, 108631. [Google Scholar] [CrossRef] [PubMed]
- Owaid, M.N. Green synthesis of silver nanoparticles by Pleurotus (oyster mushroom) and their bioactivity: Review. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100256. [Google Scholar] [CrossRef]
- Qamar, S.U.R.; Ahmad, J.N. Nanoparticles: Mechanism of biosynthesis using plant extracts, bacteria, fungi, and their applications. J. Mol. Liq. 2021, 334, 116040. [Google Scholar] [CrossRef]
- Šebesta, M.; Vojtková, H.; Cyprichová, V.; Ingle, A.P.; Urík, M.; Kolencík, M. Mycosynthesis of Metal-Containing Nanoparticles—Fungal Metal Resistance and Mechanisms of Synthesis. Int. J. Mol. Sci. 2022, 23, 14084. [Google Scholar] [CrossRef]
- Elsakhawy, T.; Omara, A.E.-D.; Abowaly, M.; El-Ramady, H.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Prokisch, J. Green Synthesis of Nanoparticles by Mushrooms: A Crucial Dimension for Sustainable Soil Management. Sustainability 2022, 14, 4328. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Starch/agar-based functional films integrated with enoki mushroom-mediated silver nanoparticles for active packaging applications. Food Biosci. 2022, 49, 101867. [Google Scholar] [CrossRef]
- Priyadarshni, K.C.; Krishnamoorthi, R.; Mumtha, C.; Mahalingam, P.U. Biochemical analysis of cultivated mushroom, Pleurotus florida and synthesis of silver nanoparticles for enhanced antimicrobial effects on clinically important human pathogens. Inorg. Chem. Commun. 2022, 142, 109673. [Google Scholar] [CrossRef]
- Aygün, A.; Özdemir, S.; Gülcan, M.; Cellat, K.; Şen, F. Synthesis and characterization of Reishi mushroom-mediated green synthesis of silver nanoparticles for the biochemical applications. J. Pharm. Biomed. Anal. 2020, 178, 112970. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Yadav, N.; Rai, N.K.; Bohara, R.A.; Rai, S.N.; Aleya, L.; Singh, M.P. Two birds with one stone: Oyster mushroom mediated bimetallic Au-Pt nanoparticles for agro-waste management and anticancer activity. Environ. Sci. Pollut. Res. 2021, 28, 13761–13775. [Google Scholar] [CrossRef]
- Debnath, G.; Das, P.; Saha, A.S. Green Synthesis of Silver Nanoparticles Using Mushroom Extract of Pleurotus giganteus: Characterization, Antimicrobial, and α-Amylase Inhibitory Activity. BioNanoSci 2019, 9, 611–619. [Google Scholar] [CrossRef]
- Preethi, P.S.; Suganya, M.; Narenkumar, J.; AlSalhi, M.S.; Devanesan, S.; Nanthini, A.U.R.; Kamalakannan, S.; Rajasekar, A. Macrolepiota-mediated synthesized silver nanoparticles as a green corrosive inhibitor for mild steel in re-circulating cooling water system. Bioprocess Biosyst. Eng. 2022, 45, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Tudu, S.C.; Zubko, M.; Kusz, Z.; Bhattacharjee, E. CdS nanoparticles (<5 nm): Green synthesized using Termitomyces heimii mushroom–structural, optical and morphological studies. Appl. Phys. A 2021, 127, 85. [Google Scholar]
- Dias, C.; Ayyanar, M.; Amalraj, S.; Khanal, P.; Subramaniyan, V.; Das, S.; Gandhale, P.; Biswa, V.; Ali, R.; Gurav, N.; et al. Biogenic synthesis of zinc oxide nanoparticles using mushroom fungus Cordyceps militaris: Characterization and mechanistic insights of therapeutic investigation. J. Drug Deliv. Sci. Technol. 2022, 73, 103444. [Google Scholar] [CrossRef]
- Jaloot, A.S.; Owaid, M.N.; Naeem, G.A.; Muslim, R.F. Mycosynthesizing and characterizing silver nanoparticles from the mushroom Inonotus hispidus (Hymenochaetaceae), and their antibacterial and antifungal activities. Environ. Nanotechnol Monit. Manag. 2020, 14, 100313. [Google Scholar] [CrossRef]
- Bhanja, S.K.; Samanta, S.K.; Mondal, B.; Jana, S.; Ray, J.; Pandey, A.; Tripathy, T. Green synthesis of Ag@Au bimetallic composite nanoparticles using a polysaccharide extracted from Ramaria botrytis mushroom and performance in catalytic reduction of 4-nitrophenol and antioxidant, antibacterial activity. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100341. [Google Scholar] [CrossRef]
- Chauhan, N.; Thakur, N.; Kumari, A.; Khatana, C.; Sharma, R. Mushroom and silk sericin extract mediated ZnO nanoparticles for removal of organic pollutants and microorganisms. S. Afr. J. Bot. 2023, 153, 370–381. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Owaid, M.N.; Rabeea, M.A.; Abdul Aziz, A.; Jameel, M.S. Mycosynthesis of gold nanoparticles by the Portabello mushroom extract, Agaricaceae, and their efficacy for decolorization of Azo dye. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100312. [Google Scholar] [CrossRef]
- Sedefoglu, N.; Zalaoglu, Y.; Bozok, F. Green synthesized ZnO nanoparticles using Ganoderma lucidum: Characterization and In Vitro Nanofertilizer effects. J. Alloys Compd. 2022, 918, 165695. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Mahalingam, P.U.; Malaikozhundan, B. Edible mushroom extract engineered Ag NPs as safe antimicrobial and antioxidant agents with no significant cytotoxicity on human dermal fibroblast cells. Inorg. Chem. Commun. 2022, 139, 109362. [Google Scholar] [CrossRef]
- Khandel, P.; Shahi, S.K. Mycogenic Nanoparticles and Their Bio-Prospective Applications: Current Status and Future Challenges. J. Nanostruct. Chem. 2018, 8, 369–391. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; Lima, R. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef] [PubMed]
- Sudheer, S.; Bai, R.G.; Muthoosamy, K.; Tuvikene, R.; Gupta, V.K.; Manickam, S. Biosustainable Production of Nanoparticles via Mycogenesis for Biotechnological Applications: A Critical Review. Environ. Res. 2022, 204, 111963. [Google Scholar] [CrossRef] [PubMed]
- Sahithya, K.; Mouli, T.; Biswas, A.; Mercy Scorlet, M.T. Remediation potential of mushrooms and their spent substrate against environmental contaminants: An overview. Biocatal. Agric. Biotechnol. 2022, 42, 102323. [Google Scholar]
- El-Ramady, H.; Brevik, E.C.; Fawzy, Z.F.; Elsakhawy, T.; Omara, A.E.-D.; Amer, M.; Faizy, S.E.-D.; Abowaly, M.; El-Henawy, A.; Kiss, A.; et al. Nano-Restoration for Sustaining Soil Fertility: A Pictorial and Diagrammatic Review Article. Plants 2022, 11, 2392. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, K.; Olabi, A.G.; Abdel-Wahab, A.; Elkamel, A.; Alami, A.; Inayat, A.; Chae, K.J.; Abdelkareem, M.A. Membrane processes for environmental remediation of nanomaterials: Potentials and challenges. Sci. Total Environ. 2023, 879, 162569. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.; Mitra, S. Spent waste from edible mushrooms offers innovative strategies for the remediation of persistent organic micropollutants: A review. Environ. Pollut. 2022, 305, 119285. [Google Scholar] [CrossRef]
- Yu, H.; Liu, P.; Shan, W.; Teng, Y.; Rao, D.; Zou, L. Remediation potential of spent mushroom substrate on Cd pollution in a paddy soil. Environ. Sci. Pollut. Res. 2021, 28, 36850–36860. [Google Scholar] [CrossRef]
- Alhujaily, A.; Yu, H.; Zhang, X.; Ma, F. Adsorptive removal of anionic dyes from aqueous solutions using spent mushroom waste. Appl. Water Sci. 2020, 10, 183. [Google Scholar] [CrossRef]
- Wei, Y.; Jin, Z.; Zhang, M.; Li, Y.; Huang, S.; Liu, X.; Jin, Y.; Wang, H.; Qu, J. Impact of spent mushroom substrate on Cd immobilization and soil property. Environ. Sci. Pollut. Res. 2020, 27, 3007–3022. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Q.; Su, Y.; Xue, Q.; Sun, L. Cobalt speciation and phytoavailability in fluvo-aquic soil under treatments of spent mushroom substrate from Pleurotus ostreatus. Environ. Sci. Pollut. Res. 2019, 26, 7486–7496. [Google Scholar] [CrossRef]
- Mayans, B.; Camacho-Arévalo, R.; García-Delgado, C.; Antón-Herrero, R.; Escolástico, C.; Segura, M.L.; Eymar, E. An assessment of Pleurotus ostreatus to remove sulfonamides, and its role as a biofilter based on its own spent mushroom substrate. Environ. Sci. Pollut. Res. 2021, 28, 7032–7042. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.N.; Redington, W.; Holland, L.B. Remediation of Metal Contaminated Simulated Acid Mine Drainage Using a Lab-Scale Spent Mushroom Substrate Wetland. Water Air Soil Pollut. 2021, 232, 220. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, M.; Li, R.; Zhang, X.; Wang, G.; Liu, X.; Qu, J.; Jin, Y. Spent mushroom substrate combined with alkaline amendment passivates cadmium and improves soil property. Environ. Sci. Pollut. Res. 2020, 27, 16317–16325. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zou, D.; Xu, Z.; Chen, B.; Zhang, X.; Chen, F.; Zhang, M. Combined effects of spent mushroom substrate and dicyandiamide on carbendazim dissipation in soils: Double-edged sword effects and potential risk controls. Environ. Pollut. 2023, 319, 120992. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, W.; Luo, L.; Li, Y.; Chen, Z.; Gu, Y.; Chen, Q.; Deng, O.; Xu, X.; Lan, T.; et al. Short-term responses of soil nutrients, heavy metals and microbial community to partial substitution of chemical fertilizer with spent mushroom substrates (SMS). Sci. Total Environ. 2022, 844, 157064. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Harirchi, S.; Sar, T.; Vigneswaran, V.S.; Rajendran, K.; Gómez-García, R.; Hellwig, C.; Binod, B.; Sindhu, R.; Madhavan, A.; et al. Myco-biorefinery approaches for food waste valorization: Present status and future prospects. Bioresour. Technol. 2022, 360, 127592. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ryu, D.-y.; Jang, K.H.; Lee, J.W.; Kim, D. Influence of Different Pretreatment Methods and Conditions on the Anaerobic Digestion Efficiency of Spent Mushroom Substrate. Sustainability 2022, 14, 15854. [Google Scholar] [CrossRef]
- Qian, X.; Bi, X.; Xu, Y.; Yang, Z.; Wei, T.; Xi, M.; Li, J.; Chen, L.; Li, H.; Sun, S. Variation in community structure and network characteristics of spent mushroom substrate (SMS) compost microbiota driven by time and environmental conditions. Bioresour. Technol. 2022, 364, 127915. [Google Scholar] [CrossRef]
- Huang, Z.; Guan, H.; Zheng, H.; Wang, M.; Xu, P.; Dong, S.; Yang, Y.; Xiao, J. Novel liquid organic fertilizer: A potential way to effectively recycle spent mushroom substrate. J. Clean. Prod. 2022, 376, 134368. [Google Scholar] [CrossRef]
- Rao, W.; Zhang, D.; Guan, X.; Pan, X. Recycling of spent mushroom substrate biowaste as an Anti-UV agent for Bacillus thuringiensis. Sustain. Chem. Pharm. 2022, 30, 100811. [Google Scholar] [CrossRef]
- Arshadi, N.; Nouri, H.; Moghimi, H. Increasing the production of the bioactive compounds in medicinal mushrooms: An omics perspective. Microb. Cell Fact. 2023, 22, 11. [Google Scholar] [CrossRef]
- Pathak, M.P.; Pathak, K.; Saikia, R.; Gogoi, U.; Ahmad, M.Z.; Patowary, P.; Das, A. Immunomodulatory effect of mushrooms and their bioactive compounds in cancer: A comprehensive review. Biomed. Pharma 2022, 149, 112901. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Shang, Y.; Xu, H.; Xia, R.; Hou, Z.; Pan, S.; Li, L.; Bian, Y.; Zhu, J.; et al. Sexual spores in edible mushroom: Bioactive components, discharge mechanisms and effects on fruiting bodies quality. Food Sci. Hum. Wellness 2023, 12, 2111–2123. [Google Scholar] [CrossRef]
- Tiwari, A.; Kumar, S.; Choudhir, G.; Singh, G.; Gangwar, U.; Sharma, V.; Srivastava, R.K.; Sharma, S. Bioactive metabolites of edible mushrooms efficacious against androgenic alopecia: Targeting SRD5A2 using computational approach. J. Herb. Med. 2022, 36, 100611. [Google Scholar] [CrossRef]
- Yadav, D.; Negi, P.S. Bioactive components of mushrooms: Processing effects and health benefits. Food Res. Int. 2021, 148, 110599. [Google Scholar] [CrossRef]
- Marçal, S.; Sousa, A.S.; Taofiq, O.; Antunes, F.; Morais, A.M.M.B.; Freitas, A.C.; Barros, L.; Ferreira, I.C.F.R.; Pintado, M. Impact of postharvest preservation methods on nutritional value and bioactive properties of mushrooms. Trends Food Sci. Technol. 2021, 110, 418–431. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Feng, X.-l.; Liu, C.; Gao, J.-M.; Qi, J. Diverse Metabolites and Pharmacological Effects from the Basidiomycetes Inonotus hispidus. Antibiotics 2022, 11, 1097. [Google Scholar] [CrossRef]
- Qi, J.; Gao, Y.Q.; Kang, S.J.; Liu, C.; Gao, J.M. Secondary Metabolites of Bird’s Nest Fungi: Chemical Structures and Biological Activities. J. Agric. Food Chem. 2023, 71, 6513–6524. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, M.; Wu, S.; Liao, X.; Wang, J.; Wu, Q.; Zhuang, M.; Ding, Y. A review on mushroom-derived bioactive peptides: Preparation and biological activities. Food Res. Int. 2020, 134, 109230. [Google Scholar] [CrossRef]
- Goswami, B.; Majumdar, S.; Das, A.; Barui, A.; Bhowal, J. Evaluation of bioactive properties of Pleurotus ostreatus mushroom protein hydrolysate of different degree of hydrolysis. LWT 2021, 149, 111768. [Google Scholar] [CrossRef]
- Garcia, J.; Rodrigues, F.; Saavedra, M.J.; Nunes, F.M.; Marques, G. Bioactive polysaccharides from medicinal mushrooms: A review on their isolation, structural characteristics and antitumor activity. Food Biosci. 2022, 49, 101955. [Google Scholar] [CrossRef]
- Lin, G.; Li, Y.; Chen, X.; Zhang, F.; Linhardt, R.J.; Zhang, A. Extraction, structure and bioactivities of polysaccharides from Sanghuangporus spp.: A review. Food Biosci. 2023, 53, 102587. [Google Scholar] [CrossRef]
- Luan, F.; Peng, X.; Zhao, G.; Zeng, J.; Zou, J.; Rao, Z.; Liu, Y.; Zhang, X.; Ma, H.; Zeng, N. Structural diversity and bioactivity of polysaccharides from medicinal mushroom Phellinus spp.: A review. Food Chem. 2022, 397, 133731. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yu, J.S.; Lee, S.R.; Kim, K.H. Non-peptide secondary metabolites from poisonous mushrooms: Overview of chemistry, bioactivity, and biosynthesis. Nat. Prod. Rep. 2022, 39, 512–559. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Li, B.; Wang, Z.; Qiao, X.; Ye, M. Terpenoids from the medicinal mushroom Antrodia camphorata: Chemistry and medicinal potential. Nat. Prod. Rep. 2021, 38, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Guan, M.; Tang, W.; Walayat, N.; Ding, Y.; Liu, J. Structural diversity, fermentation production, bioactivities and applications of triterpenoids from several common medicinal fungi: Recent advances and future perspectives. Fitoterapia 2023, 166, 105470. [Google Scholar] [CrossRef]
- Citores, L.; Ragucci, S.; Ferreras, J.M.; Maro, A.D.; Iglesias, R.; Citores, L.; Ferreras, J.M.; Iglesias, R. Ageritin, a Ribotoxin from Poplar Mushroom (Agrocybe aegerita) with Defensive and Antiproliferative Activities. ACS Chem. Biol. 2019, 14, 1319–1327. [Google Scholar] [CrossRef]
- Luo, A.; Luo, A.; Huang, J.; Fan, Y. Purification, Characterization and Antioxidant Activities in Vitro and in Vivo of the Polysaccharides from Boletus edulis Bull. Molecules 2012, 17, 8079–8090. [Google Scholar] [CrossRef]
- Liu, G.; Ye, J.; Li, W.; Zhang, J.; Wang, Q.; Zhu, X.; Miao, J.; Huang, Y.; Chen, Y.; Cao, Y. Extraction, Structural Charac-terization, and Immunobiological Activity of ABP Ia Polysaccharide from Agaricus bisporus. Int. J. Biol. Macromol. 2020, 162, 975–984. [Google Scholar] [CrossRef]
- Su, S.; Ding, X.; Fu, L.; Hou, Y. Structural Characterization and Immune Regulation of a Novel Polysaccharide from Maerkang Lactarius deliciosus Gray. Int. J. Mol. Med. 2019, 44, 713–724. [Google Scholar] [CrossRef]
- Nowakowski, P.; Naliwajko, S.K.; Markiewicz-Zukowska, R.; Borawska, M.H.; Socha, K. The Two Faces of Coprinus comatus—Functional Properties and Potential Hazards. Phytother. Res. 2020, 34, 2932–2944. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, Y.; Qiang, Z. A Potent Pharmacological Mushroom: Pleurotus eryngii. Fungal Genom. Biol. 2016, 6, 1–5. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, D.; Lee, H.-J.; Noh, H.J.; Jung, K.; Kang, K.S.; Kim, K.H. Renoprotective Chemical Constituents from an Edible Mushroom, Pleurotus cornucopiae in Cisplatin-Induced Nephrotoxicity. Bioorg. Chem. 2017, 71, 67–73. [Google Scholar] [CrossRef]
- Chen, H.-P.; Zhao, Z.-Z.; Li, Z.-H.; Huang, Y.; Zhang, S.-B.; Tang, Y.; Yao, J.-N.; Chen, L.; Isaka, M.; Feng, T.; et al. An-ti-Proliferative and Anti-Inflammatory Lanostane Triterpenoids from the Polish Edible Mushroom Macrolepiota procera. J. Agric. Food Chem. 2018, 66, 3146–3154. [Google Scholar] [CrossRef]
- He, M.Q.; Wang, M.Q.; Chen, Z.H.; Deng, W.Q.; Li, T.H.; Vizzini, A.; Jeewon, R.; Hyde, K.D.; Zhao, R.L. Potential benefits and harms: A review of poisonous mushrooms in the world. Fungal Biol. Rev. 2022, 42, 56–68. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, L.W.; Yang, Z.L.; Bau, T.; Li, T.H.; Dai, Y.C. Resource diversity of Chinese macrofungi: Edible, medicinal and poisonous species. Fungal Divers. 2019, 98, 1–76. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, P.; Zhang, Z. Investigation and analysis of 102 mushroom poisoning cases in Southern China from 1994 to 2012. Fungal Divers. 2014, 64, 123–131. [Google Scholar] [CrossRef]
- Zhuk, O.; Jasicka-Misiak, I.; Poliwoda, A.; Kazakova, A.; Godovan, V.V.; Halama, M.; Wieczorek, P.P. Research on Acute Toxicity and the Behavioral Effects of Methanolic Extract from Psilocybin Mushrooms and Psilocin in Mice. Toxins 2015, 7, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Feng, T.; Shang, J.H.; Zhao, Y.L.; Wang, F.; Li, Z.H.; Dong, Z.J.; Luo, X.D.; Liu, J.K. Chemical and Toxicological Investigations of a Previously Unknown Poisonous European Mushroom Tricholoma terreum. Chem. Eur. J. 2014, 20, 7001–7009. [Google Scholar] [CrossRef] [PubMed]
- Leudang, S.; Sikaphan, S.; Parnmen, S.; Nantachaiphong, N.; Polputpisatkul, D.; Ramchiun, S.; Teeyapant, P. DNA-based identification of gastrointestinal irritant mushrooms in the genus Chlorophyllum: A food poisoning case in Thailand. J. Health Res. 2017, 31, 41–49. [Google Scholar]
- Stover, A.; Haberl, B.; Helmreich, C.; Muller, W.; Musshoff, F.; Fels, H.; Graw, M.; Groth, O. Fatal immunohaemolysis after the consumption of the poison pax mushroom: A focus on the diagnosis of the paxillus syndrome with the aid of two case reports. Diagnostics 2019, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fan, T.; Hengchao, E.; Zhang, Y.; Li, X.; Yang, X.; Tian, E.; Chen, A.; Zhao, X.; Zhou, C. A simple derivatization method for simultaneous determination of four amino group-containing mushroom toxins in mushroom and urine by UPLC-MS/MS. Food Control 2022, 137, 108720. [Google Scholar] [CrossRef]
- Yoshioka, N.; Hayakawa, I.; Minatani, T.; Tomozawa, J.; Akiyama, H.; Yomo, H. Quantitative analysis of the Tricholoma ustale-derived toxin, ustalic acid, in mushroom and food samples by LC–MS/MS. Forensic Sci. Int. 2020, 317, 110554. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H.; Li, T.; Wang, Y. Edibility and species discrimination of wild bolete mushrooms using FT-NIR spectroscopy combined with DD-SIMCA and RF models. LWT 2023, 180, 114701. [Google Scholar] [CrossRef]
- Janatolmakan, M.; Jalilian, M.; Rezaeian, S.; Abdi, A.; Khatony, A. Mortality rate and liver transplant in patients with mushroom poisoning: A systematic review & meta-analysis. Heliyon 2023, 9, e12759. [Google Scholar] [PubMed]
- Cateni, F.; Gargano, M.L.; Procida, G.; Venturella, G.; Cirlincione, F.; Ferraro, V. Mycochemicals in wild and cultivated mushrooms: Nutrition and health. Phytochem. Rev. 2022, 21, 339–383. [Google Scholar] [CrossRef]
- Okuda, Y. Sustainability perspectives for future continuity of mushroom production: The bright and dark sides. Front. Sustain. Food Syst. 2022, 6, 1026508. [Google Scholar] [CrossRef]
- De Cianni, R.; Pippinato, L.; Mancuso, T. A systematic review on drivers influencing consumption of edible mushrooms and innovative mushroom-containing products. Appetite 2023, 182, 106454. [Google Scholar] [CrossRef]
- Diaz, J.H. Nephrotoxic Mushroom Poisoning: Global Epidemiology, Clinical Manifestations, and Management. Wilderness Environ. Med. 2021, 32, 537–544. [Google Scholar] [CrossRef]
- El-Ramady, H.; Töros, G.; Badgar, K.; Llanaj, X.; Hajdú, P.; El-Mahrouk, M.E.; Abdalla, N.; Prokisch, J. A Comparative Photographic Review on Higher Plants and Macro-Fungi: A Soil Restoration for Sustainable Production of Food and Energy. Sustainability 2022, 14, 7104. [Google Scholar] [CrossRef]
- Kazige, O.K.; Chuma, G.B.; Lusambya, A.S.; Mondo, J.M.; Balezi, A.Z.; Mapatano, S.; Mushagalusa, G.N. Valorizing Staple Crop Residues through Mushroom Production to Improve Food Security in Eastern Democratic Republic of Congo. J. Agric. Food Res. 2022, 8, 100285. [Google Scholar] [CrossRef]
- Otieno, O.D.; Mulaa, F.J.; Obiero, G.; Midiwo, J. Utilization of Fruit Waste Substrates in Mushroom Production and Manipulation of Chemical Composition. Biocatal. Agric. Biotechnol. 2022, 39, 102250. [Google Scholar] [CrossRef]
- Ivarsson, E.; Grudén, M.; Södergren, J.; Hultberg, M. Use of Faba Bean (Vicia faba L.) Hulls as Substrate for Pleurotus ostreatus—Potential for Combined Mushroom and Feed Production. J. Clean. Prod. 2021, 313, 127969. [Google Scholar] [CrossRef]
- Chouhan, P.; Koreti, D.; Kosre, A.; Chauhan, R.; Jadhav, S.K.; Chandrawanshi, N.K. Production and Assessment of Stick-Shaped Spawns of Oyster Mushroom from Banana Leaf-Midribs. Proc. Natl. Acad. Sci. India Sect. B. Biol. Sci. 2022, 92, 405–414. [Google Scholar] [CrossRef]
- Vieira, V.O.; Conceição, A.A.; Cunha, J.R.B.; Machado, A.E.V.; de Almeida, E.G.; Dias, E.S.; Alcantara, L.M.; Miller, R.N.G.; de Siqueira, F.G. A new circular economy approach for integrated production of tomatoes and mushrooms. Saudi J. Biol. Sci. 2022, 29, 2756–2765. [Google Scholar] [CrossRef] [PubMed]
- Stoknes, K.; Wojciechowska, E.; Jasinska, A.; Noble, R. Amelioration of Composts for Greenhouse Vegetable Plants Using Pasteurised Agaricus Mushroom Substrate. Sustainability 2019, 11, 6779. [Google Scholar] [CrossRef]
- Jung, D.-H.; Son, J.-E. CO2 Utilization Strategy for Sustainable Cultivation of Mushrooms and Lettuces. Sustainability 2021, 13, 5434. [Google Scholar] [CrossRef]
- Grimm, D.; Kuenz, A.; Rahmann, G. Integration of Mushroom Production into Circular Food Chains. Org. Agric. 2021, 11, 309–317. [Google Scholar] [CrossRef]
- Sharma, M.; Sridhar, K.; Gupta, V.K.; Dikkala, P.K. Greener technologies in agri-food wastes valorization for plant pigments: Step towards circular economy. Curr. Res. Green. Sustain. Chem. 2022, 5, 100340. [Google Scholar] [CrossRef]





| Mushroom Species | Health Benefits | References |
|---|---|---|
| Shiitake mushroom (Lentinula edodes) | Therapeutic potential that prevents diseases due to antiviral, antimicrobial, anticancer, antidiabetic, antiobesity, and antioxidant activities. | Ahmad et al. [17] |
| Ganoderma spp. | Have anti-inflammatory, antitumor, antioxidant, immune regulation, and other functions due to high polysaccharides content. | Yu et al. [57] |
| Agaricus bisporus | Protect against several human diseases due to many bioactive compounds (e.g., anticancer, anti-inflammation, and antioxidant agents). | Ahmed et al. [58] |
| Lion’s mane mushroom (Hericium erinaceus) | Antimicrobial action due to high content of corrinoids in the form of vitamin B12. | Rizzo et al. [59] |
| Ganoderma applanatum | Prevent oxidative stress (antioxidant) due to high ergothioneine content. | Martinez-Medina et al. [60] |
| Oyster mushroom (Pleurotus ostreatus) | Antimicrobial and prebiotic benefits for human health. | Xhensila et al. [61] |
| Pleurotus ostreatus Mushroom | Rich in chitin and glucan prebiotics, which enhance beneficial gut bacteria activity as antimicrobial agents. | Tör˝os et al. [62] |
| Morel mushroom (Morchella esculenta L.) | Health-promoting due to bioactives (polysaccharides and polynucleotides) that provide antidiabetic, antitumor, cardiovascular protective, antiparasitic, hepatoprotective, antibacterial, and antiviral properties. | Sunil and Xu [63] |
| Porcini mushroom (Boletus edulis) | Health-promoting effects from antineoplastic, antioxidant, antibacterial, anti-inflammatory, antiviral, and hepato-protective properties due to bioactive compounds. | Tan et al. [55] |
| Russula griseocarnosa | Health promoting actions include delaying aging and therapeutic functions (e.g., immune regulation, anticancer, antioxidant, hypoglycemic, and hypolipidemic activities). | Liu et al. [64] |
| Tremella fuciformis | Exopolysaccharides have may health benefits such as immune-enhancing, antitumor, and hypoglycemic properties. | Li et al. [65] |
| Russula virescens | Polysaccharides have potential anticancer, hypoglycemia, and immune-boosting activities by inhibiting α-glucosidase and α-amylase and mediating cellular immune response. | Li et al. [66] |
| Mushroom Species | Polluted Medium | Main Finding of the Application | Reference |
|---|---|---|---|
| SMS from Pleurotus eryngii and A. bisporus | Cd-polluted paddy soil (total Cd, 72.87 mg kg−1) | Applied SMS improved the biomass of root and straw at different growth stages by reducing the uptake of Cd and accumulation in rice parts. | [99] |
| SMW of Pleurotus ostreatus | Anionic dyes with initial dose of 100–1300 mg g−1 | Max. adsorption capacities of SMW were found to be 15.46, 18, 14.62, and 20.19 mg g−1 for DB22, DR5B, RB5, and DB71, respectively. | [100] |
| Spent mushroom substrate compost (SMSC) or biochar (SMSB) | Added 0.6, 1.2, 1.8, and 2.4 mg kg−1 Cd to soil | About 4% SMS can be used for amending Cd-polluted soils by Cd immobilization and improving chemical and biological soil properties. | [101] |
| SMS from Pleurotus ostreatus | Soil contained 8.535 SMS, and its applied rate was 20–40 mg kg−1 | Optimum applied into the SMS is 8.86–9.51 g kg−1 soil when growing pak choi (Brassica chinensis L.). | [102] |
| SMS of Pleurotus ostreatus | Wastewater polluted with sulfonamides | Up to 83–91% of sulfonamides were removed over 14 days sulfamethoxazole, sulfathiazole, sulfadiazine, sulfapyridine, etc. | [103] |
| Spent mushroom substrate | Constructed wetland with simulated acid mine drainage | Removal rate of metal-burdened wastewater by SMS was Al, Zn, Cu (99%), Fe (97%), and Pb (97%) over a period of 800 days. | [104] |
| Spent mushroom substrate 0.5% (w/w) | Cd polluted soil, level at 0.6 mg kg−1 | Applied SMS and biochar was more efficient than lime in reducing Cd content and increasing organic matter and enzyme activity after 4 weeks. | [105] |
| Spent mushroom substrate | Soil contaminated with carbendazim | SMS applied to fungicide-polluted soil reduced soil carbendazim residues and significantly increased the total-N, OM, and microbial biomass in the soil. | [106] |
| Substrates of Enoki,A. bisporus, and Auricularia auricula (AAR) | Soil polluted with chemical fertilizer | AAR recorded the highest level of soil nutrients among the 3 SMS replacements (mineral fertilizer by 25%); reduced heavy metals contamination. | [107] |
| Spent mushroom substrate and its biochar | Cd polluted soil, level at 0.6 mg kg−1 | Applied SMS and its biochar alleviated the adverse effects of Cd and N and increased pH, CEC, and OM content in the soil. | [65] |
| Mushroom Species | Main Groups of Bioactive Compounds | Refs. | |||
|---|---|---|---|---|---|
| Phenolics | Polysaccharides | Proteins | Triterpenoids | ||
| Cordyceps aegerita | Proto-catechuic acid | Fucogalactan | Ageritin | Bovistols A-C | Citores et al. [129] |
| Boletus edulis | Gallic acid | Polysaccharides (BEBP-1) | β-Trefoil lectin | Boledulins A-C | Luo et al. [130] |
| Agaricus bisporus | Gallocatechin | Heteropolysaccharide ABP | Protein type FIIb-1 | Ergosterol | Liu et al. [131] |
| Lactarius deliciosus | Syringic acid, vanillic acid | Polysaccharide (LDG-M) | Laccase | Azulene-type sesquiterpen | Su et al. [132] |
| Coprinus comatus | Flavones and flavonols | Modified polysaccharide | Laccases | Terpenoids | Nowakowski et al. [133] |
| Pleurotus ostreatus | Caffeic acid and ferulic acid | Mycelium polysaccharides | Concanavalin A | Ergosterol | Fu et al. [134] |
| Pleurotus cornucopiae | Gallic acid | β-glucan | Oligopeptides | Ergostane-type sterols | Lee et al. [135] |
| Macrolepiota procera | Proto-catechuic acid | Polysaccharides | β-Trefoil lectin | Lanostane triterpenoids | Chen et al. [136] |
| Poisonous Mushroom Group | Target Organ(s) or Symptoms | Mushroom Species | Toxic Dose * | Main Mushroom Toxins | Ref. |
|---|---|---|---|---|---|
| 1. Cytotoxic mushrooms | Liver and kidneys | Amanita bisporigera | LD50 0.4–0.8 mg kg−1 | Amanitin (amatoxins, phallotoxins, and virotoxins) | [139] |
| 2. Neurotoxic mushrooms | Neuroexcitatory effects | Amanita, Clitocybe, Inocybe, Psilocybe | 400 mg/kg psilocin | Psilocybins, muscarines, and isoxazole | [140] |
| 3. Myotoxic mushrooms | Symptoms of rhabdomyolysis | Russula subnigricans and Tricholoma equestre | LD50 63.7–88.3 mg/kg | Russuphelins and cycloprop-2-ene carboxylic acid | [141] |
| 4. Metabolic or endocrine mushrooms | Disulfiram-like symptoms | Coprinus, Coprinopsis, and Ampulloclitocybe | 10–50 mg kg−1 gyromitrin | Trichothecene and gyromitrin | [137] |
| 5. Gastrointestinal irritant mushroom | Gastrointestinal poisoning | Agaricus, Entoloma, Gomphus, Hebeloma, etc. | ** Poisoning is rarely fatal | Specific toxins did not identify, but toxic phenolic compounds may Agaricus sp. | [142] |
| 6. Miscellaneous adverse mushrooms | Hemolytic poisoning | Example of Paxillus involutus (Batsch) Fr. | Symptoms after 2–3 h take to death | The toxin is unknown at present | [143] |
| Mushroom Species | Plant Species | Food or Energy | The Main Purpose of Study | Reference |
|---|---|---|---|---|
| Pleurotus ostreatus | Crop residues (cassava, common bean, maize, banana) | Food and mushroom production | Cropping yield first and using crop residues for mushroom production, besides fodder and compost by farmers. | [153] |
| Pleurotus sajor-caju, P. ostreatus, and Pleurotus eryngii | Peels from the processing of fruits (mango, bananas, pineapple, avocado, orange, and watermelon | Mushroom production | Using fruit waste materials as a low-cost method to produce edible mushrooms as a source for health-promoting compounds such as antioxidants. | [154] |
| Oyster mushrooms (Pleurotus ostreatus) | Faba bean (Vicia faba L.) hulls | Combined mushroom and feed production | Faba bean hulls as a substrate for mushroom production led to higher protein levels and feed production. | [155] |
| Oyster mushrooms (Pleurotus ostreatus) | Banana leaf-midrib sticks | Mushroom production | Banana sticks were submerged in a liquid mycelium culture to produce spawn as a promising alternative industrial application. | [156] |
| Pleurotus ostreatus or A. bisporus | Tomato (Solanum lycopersicum) | Integrated tomato and mushroom production | Spent mushroom substrate was applied as a nutrient source to feed tomato seedlings in an integrated co-production system. | [157] |
| Agaricus subrufescens or A. bisporus | Lettuce, cucumber, and tomato | Integrated vegetables and mushroom cultivation | Spent mushrooms were used as compost combined with vermicompost, green waste compost, and fertigation with liquid digestate of food waste. | [158] |
| King oyster (Pleurotus eryngii) | Romaine lettuces (Lactuca sativa L.) | Food production | Sustainable agro-system using CO2 from mushrooms to cultivate lettuce in a continuous system. | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llanaj, X.; Törős, G.; Hajdú, P.; Abdalla, N.; El-Ramady, H.; Kiss, A.; Solberg, S.Ø.; Prokisch, J. Biotechnological Applications of Mushrooms under the Water-Energy-Food Nexus: Crucial Aspects and Prospects from Farm to Pharmacy. Foods 2023, 12, 2671. https://doi.org/10.3390/foods12142671
Llanaj X, Törős G, Hajdú P, Abdalla N, El-Ramady H, Kiss A, Solberg SØ, Prokisch J. Biotechnological Applications of Mushrooms under the Water-Energy-Food Nexus: Crucial Aspects and Prospects from Farm to Pharmacy. Foods. 2023; 12(14):2671. https://doi.org/10.3390/foods12142671
Chicago/Turabian StyleLlanaj, Xhensila, Gréta Törős, Péter Hajdú, Neama Abdalla, Hassan El-Ramady, Attila Kiss, Svein Ø. Solberg, and József Prokisch. 2023. "Biotechnological Applications of Mushrooms under the Water-Energy-Food Nexus: Crucial Aspects and Prospects from Farm to Pharmacy" Foods 12, no. 14: 2671. https://doi.org/10.3390/foods12142671
APA StyleLlanaj, X., Törős, G., Hajdú, P., Abdalla, N., El-Ramady, H., Kiss, A., Solberg, S. Ø., & Prokisch, J. (2023). Biotechnological Applications of Mushrooms under the Water-Energy-Food Nexus: Crucial Aspects and Prospects from Farm to Pharmacy. Foods, 12(14), 2671. https://doi.org/10.3390/foods12142671










