Effects of Co-Modification by Extrusion and Enzymatic Hydrolysis on Physicochemical Properties of Black Wheat Bran and Its Prebiotic Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Pretreatment of Black Wheat Bran (BWB)
2.3. Co-Modification of BWB with Extrusion and Different Enzyme Formulas
2.4. Single-Factor Experiment Design
2.4.1. Factor 1: Xylanase Concentration
2.4.2. Factor 2: Cellulase Concentration
2.4.3. Factor 3: Temperature of Screw Zone III
2.4.4. Factor 4: Screw Speed
2.4.5. Factor 5: Moisture Content of BWB
2.5. Orthogonal Experiment Design
2.6. Preparation of the Co-Modified BWB
2.7. Determination of Nutritional Components and Physicochemical Properties of BWB
2.7.1. WEAX Content and Other Nutrients
2.7.2. Water Holding Capacity (WHC)
2.7.3. Oil Holding Capacity (OHC)
2.7.4. Cholesterol Adsorption Capacity (CAC)
2.7.5. Microscopic Analysis
2.8. In Vitro Digestion
2.9. In Vitro Fermentation and Analysis
2.9.1. Fermentation Medium
2.9.2. Fecal Inoculum
2.9.3. Anaerobic Fermentation
2.9.4. Determination of Bifidobacterium and Lactobacillus
2.9.5. Determination of Short-Chain Fatty Acids (SCFAs) and pH Values
2.10. Statistical Analysis
3. Results and Discussion
3.1. Co-Modification by Extrusion and CXAP Increases WEAX Content in BWB
3.2. Analysis of the Single-Factor Experiment and the Orthogonal Experiment
3.3. Distinct Alteration of Nutrients and Physicochemical Properties of BWB under Co-Modification
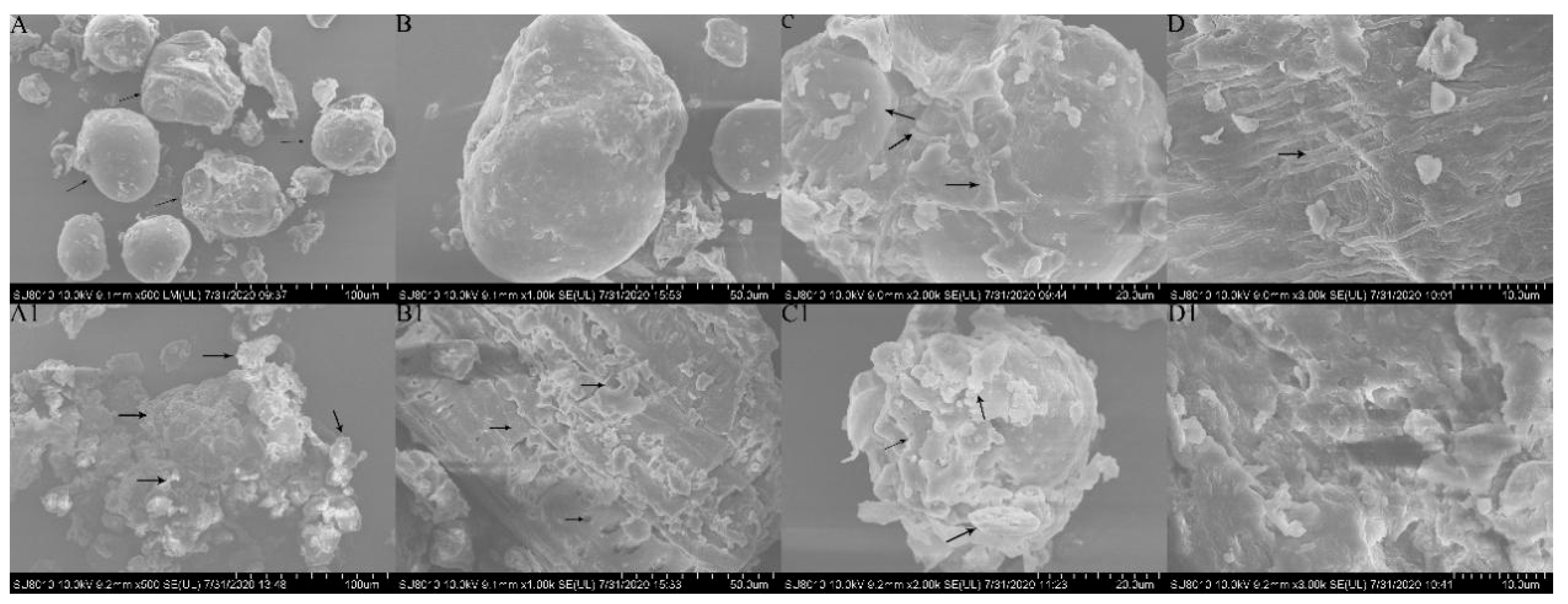
3.4. Physicochemical Structure
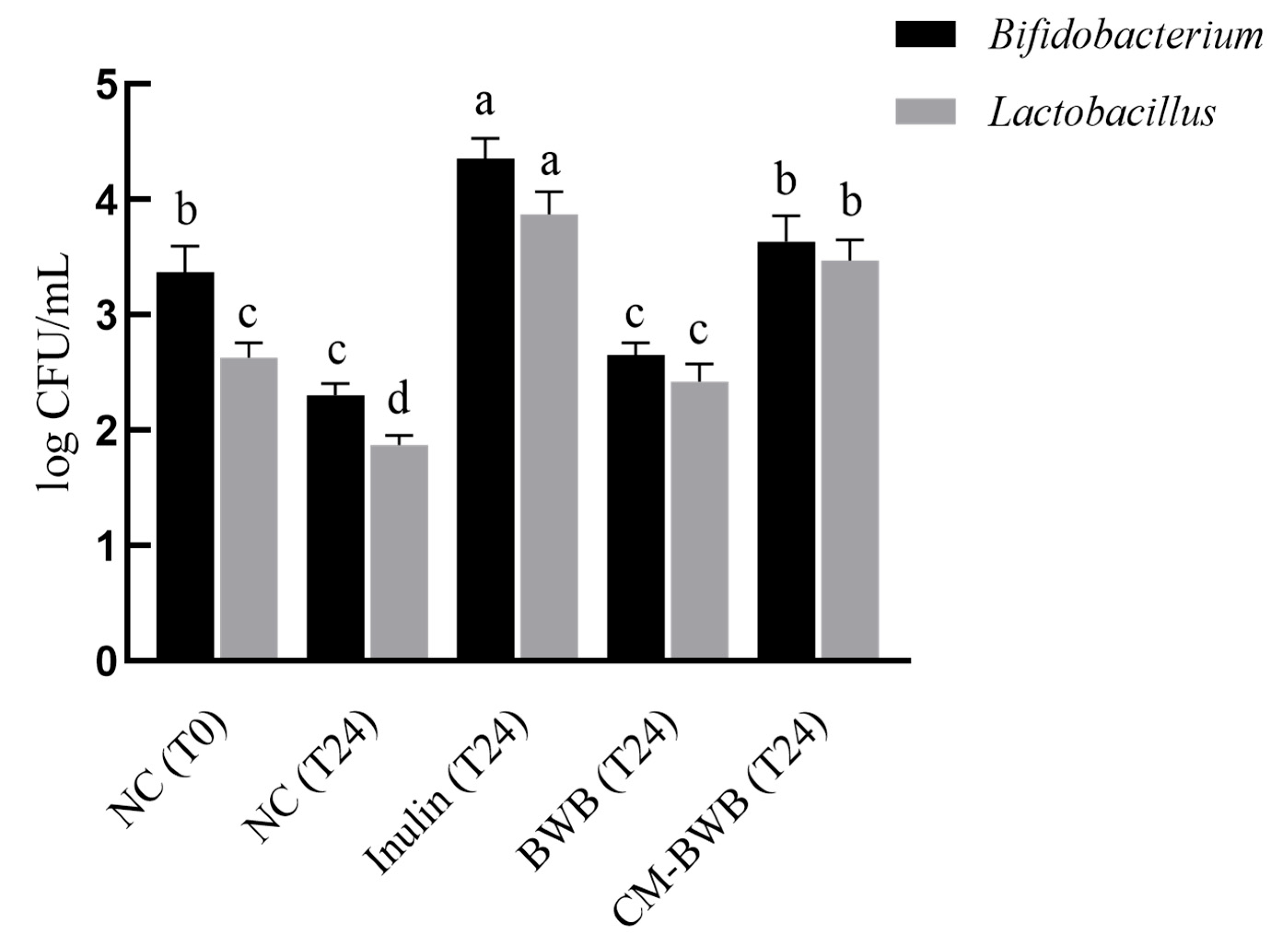
3.5. Inulin, BWB, and Co-Modified BWB Fermentation Increase the Relative Abundance of Bifidobacterium and Lactobacillus
3.6. Changes of SCFA Content and pH in Fermentation Broth In Vitro
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- das Neves, M.A.; Kimura, T.; Shimizu, N.; Shiiba, K. Production of alcohol by simultaneous saccharification and fermentation of low-grade wheat flour. Braz. Arch. Biol. Technol. 2006, 49, 481–490. [Google Scholar] [CrossRef]
- Prückler, M.; Siebenhandl-Ehn, S.; Apprich, S.; Höltinger, S.; Haas, C.; Schmid, E.; Kneifel, W. Wheat bran-based biorefinery 1: Composition of wheat bran and strategies of functionalization. LWT Food Sci. Technol. 2014, 56, 211–221. [Google Scholar] [CrossRef]
- Andersson, A.A.M.; Andersson, R.; Jonsall, A.; Andersson, J.; Fredriksson, H. Effect of Different Extrusion Parameters on Dietary Fiber in Wheat Bran and Rye Bran. J. Food Sci. 2017, 82, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Onipe, O.O.; Jideani, A.I.O.; Beswa, D. Composition and functionality of wheat bran and its application in some cereal food products. Int. J. Food Sci. Technol. 2015, 50, 2509–2518. [Google Scholar] [CrossRef]
- Roye, C.; Bulckaen, K.; De Bondt, Y.; Liberloo, I.; Van De Walle, D.; Dewettinck, K.; Courtin, C.M. Side-by-side comparison of composition and structural properties of wheat, rye, oat, and maize bran and their impact on in vitro fermentability. Cereal Chem. 2019, 97, 20–33. [Google Scholar] [CrossRef]
- Fadel, A.; Ashworth, J.; Plunkett, A.; Mahmoud, A.M.; Ranneh, Y.; Li, W. Improving the extractability of arabinoxylans and the molecular weight of wheat endosperm using extrusion processing. J. Cereal Sci. 2018, 84, 55–61. [Google Scholar] [CrossRef]
- Bender, D.; Nemeth, R.; Wimmer, M.; Gotschhofer, S.; Biolchi, M.; Torok, K.; Tomoskozi, S.; D’Amico, S.; Schoenlechner, R. Optimization of Arabinoxylan Isolation from Rye Bran by Adapting Extraction Solvent and Use of Enzymes. J. Food Sci. 2017, 82, 2562–2568. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, R.; Qian, H.; Li, Y.; Zhang, H.; Qi, X.; Wang, L. Investigation the influences of water-extractable and water-unextractable arabinoxylan on the quality of whole wheat you-tiao and its mechanism. Food Chem. 2022, 386, 132809. [Google Scholar] [CrossRef] [PubMed]
- Buksa, K.; Ziobro, R.; Nowotna, A.; Adamczyk, G.; Sikora, M.; Zylewski, M. Water Binding Capacity of Rye Flours with the Addition of Native and Modified Arabinoxylan Preparations. J. Agr. Sci. Technol.-Iran 2014, 16, 1083–1095. [Google Scholar]
- Lamothe, L.M.; Cantu-Jungles, T.M.; Chen, T.; Green, S.; Naqib, A.; Srichuwong, S.; Hamaker, B.R. Boosting the value of insoluble dietary fiber to increase gut fermentability through food processing. Food Funct. 2021, 12, 10658–10666. [Google Scholar] [CrossRef]
- Damen, B.; Verspreet, J.; Pollet, A.; Broekaert, W.F.; Delcour, J.A.; Courtin, C.M. Prebiotic effects and intestinal fermentation of cereal arabinoxylans and arabinoxylan oligosaccharides in rats depend strongly on their structural properties and joint presence. Mol. Nutr. Food Res. 2011, 55, 1862–1874. [Google Scholar] [CrossRef]
- Surampudi, P.; Enkhmaa, B.; Anuurad, E.; Berglund, L. Lipid Lowering with Soluble Dietary Fiber. Curr. Atheroscler. Rep. 2016, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Wu, Y.; Wang, L.; Tan, B.; Shen, W.; Li, X.; Liu, Y.; Tian, X.; Zhang, D. Comparison of six modification methods on the chemical composition, functional properties and antioxidant capacity of wheat bran. Lwt 2021, 149, 111996. [Google Scholar] [CrossRef]
- Zhang, S.; Jia, X.; Xu, L.; Xue, Y.; Pan, Q.; Shen, W.; Wang, Z. Effect of extrusion and semi-solid enzymatic hydrolysis modifications on the quality of wheat bran and steamed bread containing bran. J. Cereal Sci. 2022, 108, 103577. [Google Scholar] [CrossRef]
- Patil, S.S.; Rudra, S.G.; Varghese, E.; Kaur, C. Effect of extruded finger millet (Eleusine coracan L.) on textural properties and sensory acceptability of composite bread. Food Biosci. 2016, 14, 62–69. [Google Scholar] [CrossRef]
- Roye, C.; Chanvrier, H.; Henrion, M.; De Roeck, K.; De Bondt, Y.; Liberloo, I.; King, R.; Courtin, C.M. Single-pass, double-pass and acid twin-screw extrusion-cooking impact physicochemical and nutrition-related properties of wheat bran. Innov. Food Sci. Emerg. Technol. 2020, 66, 102520. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Zhu, X.; Wang, G.; Zhuang, K.; Wang, Y.; Ding, W. Optimization of Extrusion and Ultrasound-Assisted Extraction of Phenolic Compounds from Jizi439 Black Wheat Bran. Processes 2020, 8, 1153. [Google Scholar] [CrossRef]
- Santala, O.; Lehtinen, P.; Nordlund, E.; Suortti, T.; Poutanen, K. Impact of water content on the solubilisation of arabinoxylan during xylanase treatment of wheat bran. J. Cereal Sci. 2011, 54, 187–194. [Google Scholar] [CrossRef]
- Fadel, A.; Plunkett, A.; Ashworth, J.; Mahmoud, A.M.; Ranneh, Y.; El Mohtadi, M.; Li, W. The effect of extrusion screw-speed on the water extractability and molecular weight distribution of arabinoxylans from defatted rice bran. J. Food Sci. Technol. 2018, 55, 1201–1206. [Google Scholar] [CrossRef]
- Feng, J.; Xu, B.; Ma, D.; Hao, Z.; Jia, Y.; Wang, C.; Wang, L. Metabolite identification in fresh wheat grains of different colors and the influence of heat processing on metabolites via targeted and non-targeted metabolomics. Food Res. Int. 2022, 160, 111728. [Google Scholar] [CrossRef]
- Saini, P.; Kumar, N.; Kumar, S.; Mwaurah, P.W.; Panghal, A.; Attkan, A.K.; Singh, V.K.; Garg, M.K.; Singh, V. Bioactive compounds, nutritional benefits and food applications of colored wheat: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3197–3210. [Google Scholar] [CrossRef] [PubMed]
- Dhua, S.; Kumar, K.; Kumar, Y.; Singh, L.; Sharanagat, V.S. Composition, characteristics and health promising prospects of black wheat: A review. Trends Food Sci. Technol. 2021, 112, 780–794. [Google Scholar] [CrossRef]
- Sharma, N.; Tiwari, V.; Vats, S.; Kumari, A.; Chunduri, V.; Kaur, S.; Kapoor, P.; Garg, M. Evaluation of Anthocyanin Content, Antioxidant Potential and Antimicrobial Activity of Black, Purple and Blue Colored Wheat Flour and Wheat-Grass Juice against Common Human Pathogens. Molecules 2020, 25, 5785. [Google Scholar] [CrossRef]
- Menis-Henrique, M.E.C.; Scarton, M.; Piran, M.V.F.; Clerici, M.T.P.S. Cereal fiber: Extrusion modifications for food industry. Curr. Opin. Food Sci. 2020, 33, 141–148. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Kang, J.; Wang, N.; Xiao, M.; Li, Z.; Wang, C.; Guo, Q.; Hu, X. Arabinoxylan from wheat bran: Molecular degradation and functional investigation. Food Hydrocoll. 2020, 107, 105914. [Google Scholar] [CrossRef]
- Zhao, H.M.; Guo, X.N.; Zhu, K.X. Impact of solid state fermentation on nutritional, physical and flavor properties of wheat bran. Food Chem. 2017, 217, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Jin, X.; Gao, J.; Qiu, Z.; Ying, J.; Wang, Y.; Dong, Z.; Zhou, W. Impact of wheat bran micronization on dough properties and bread quality: Part I—Bran functionality and dough properties. Food Chem. 2021, 353, 129407. [Google Scholar] [CrossRef]
- Aktas-Akyildiz, E.; Masatcioglu, M.T.; Köksel, H. Effect of extrusion treatment on enzymatic hydrolysis of wheat bran. J. Cereal Sci. 2020, 93, 102941. [Google Scholar] [CrossRef]
- Kong, F.; Wang, L.; Chen, H.; Zhao, X. Improving storage property of wheat bran by steam explosion. Int. J. Food Sci. Technol. 2020, 56, 287–292. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, S.; Fan, B.; Tong, L.; Wang, L.; Guo, Y.; Wang, F.; Liu, L. The influence of xylanase and thermal treatment on the composition and interfacial rheology properties of whole wheat dough liquor. Int. J. Food Sci. Technol. 2022, 57, 3128–3141. [Google Scholar] [CrossRef]
- Jureviciute, I.; Kersiene, M.; Basinskiene, L.; Leskauskaite, D.; Jasutiene, I. Characterization of Berry Pomace Powders as Dietary Fiber-Rich Food Ingredients with Functional Properties. Foods 2022, 11, 716. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Venkitasamy, C.; Zhang, W.; Khir, R.; Upadhyaya, S.; Pan, Z. Effective moisture diffusivity and drying simulation of walnuts under hot air. Int. J. Heat Mass Transf. 2020, 150, 119283. [Google Scholar] [CrossRef]
- Guo, P.; Yu, J.; Wang, S.; Wang, S.; Copeland, L. Effects of particle size and water content during cooking on the physicochemical properties and in vitro starch digestibility of milled durum wheat grains. Food Hydrocoll. 2018, 77, 445–453. [Google Scholar] [CrossRef]
- Chen, J.; Zou, Y.; Zheng, T.; Huang, S.; Guo, L.; Lin, J.; Zheng, Q. The in Vitro Fermentation of Cordyceps militaris Polysaccharides Changed the Simulated Gut Condition and Influenced Gut Bacterial Motility and Translocation. J. Agric. Food Chem. 2022, 70, 14193–14204. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kim, D.Y. Characterization, Antioxidant Activities, and Functional Properties of Mucilage Extracted from Corchorus olitorius L. Polymers 2022, 14, 2488. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.M.; Zhang, J.; Yang, Z.L.; Huang, J.H.; Pan, L.; Hou, Y.C.; Li, X.X.; Zhao, P.H.; Dong, Y.Q.; Hu, Z.Y.; et al. Structural, Physicochemical, and Functional Properties of Wheat Bran Insoluble Dietary Fiber Modified With Probiotic Fermentation. Front. Nutr. 2022, 9, 803440. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Vasanthan, T. Modification of rice bran dietary fiber concentrates using enzyme and extrusion cooking. Food Hydrocoll. 2019, 89, 773–782. [Google Scholar] [CrossRef]
- Han, X.; Zhou, Q.; Gao, Z.; Lin, X.; Zhou, K.; Cheng, X.; Chitrakar, B.; Chen, H.; Zhao, W. In vitro digestion and fecal fermentation behaviors of polysaccharides from Ziziphus Jujuba cv. Pozao and its interaction with human gut microbiota. Food Res. Int. 2022, 162 Pt A, 112022. [Google Scholar] [CrossRef]
- Yu, C.; Hu, X.; Ahmadi, S.; Wu, D.; Xiao, H.; Zhang, H.; Ding, T.; Liu, D.; Ye, X.; Chen, S.; et al. Structure and In Vitro Fermentation Characteristics of Polysaccharides Sequentially Extracted from Goji Berry (Lycium barbarum) Leaves. J. Agric. Food Chem. 2022, 70, 7535–7546. [Google Scholar] [CrossRef] [PubMed]
- Zeyneb, H.; Pei, H.; Cao, X.; Wang, Y.; Win, Y.; Gong, L. In vitro study of the effect of quinoa and quinoa polysaccharides on human gut microbiota. Food Sci. Nutr. 2021, 9, 5735–5745. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, T.; Zhang, Y.; Chen, Y.; Ge, X.; Sui, W.; Zhu, Q.; Geng, J.; Zhang, M. Release of bound polyphenols from wheat bran soluble dietary fiber during simulated gastrointestinal digestion and colonic fermentation in vitro. Food Chem. 2023, 402, 134111. [Google Scholar] [CrossRef] [PubMed]
- Rinttila, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.; De Grandi, R.; Stronati, L.; De Vecchi, E.; Drago, L. Effect of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 on the healthy gut microbiota composition at phyla and species level: A preliminary study. World J. Gastroenterol. 2017, 23, 2696–2704. [Google Scholar] [CrossRef]
- Ma, G.; Xu, Q.; Du, H.; Muinde Kimatu, B.; Su, A.; Yang, W.; Hu, Q.; Xiao, H. Characterization of polysaccharide from Pleurotus eryngii during simulated gastrointestinal digestion and fermentation. Food Chem. 2022, 370, 131303. [Google Scholar] [CrossRef]
- Escarnot, E.; Aguedo, M.; Agneessens, R.; Wathelet, B.; Paquot, M. Extraction and characterization of water-extractable and water-unextractable arabinoxylans from spelt bran: Study of the hydrolysis conditions for monosaccharides analysis. J. Cereal Sci. 2011, 53, 45–52. [Google Scholar] [CrossRef]
- Demuth, T.; Betschart, J.; Nystrom, L. Structural modifications to water-soluble wheat bran arabinoxylan through milling and extrusion. Carbohydr. Polym. 2020, 240, 116328. [Google Scholar] [CrossRef]
- Ma, F.; Li, X.; Yin, J.; Ma, L.; Li, D. Optimisation of double-enzymatic extraction of arabinoxylan from fresh corn fibre. J. Food Sci. Technol. 2020, 57, 4649–4659. [Google Scholar] [CrossRef]
- Yang, M.; Li, N.; Wang, A.; Tong, L.; Wang, L.; Yue, Y.; Yao, J.; Zhou, S.; Liu, L. Evaluation of rheological properties, microstructure and water mobility in buns dough enriched in aleurone flour modified by enzyme combinations. Int. J. Food Sci. Technol. 2021, 56, 5913–5922. [Google Scholar] [CrossRef]
- Vangsøe, C.T.; Sørensen, J.F.; Bach Knudsen, K.E. Aleurone cells are the primary contributor to arabinoxylan oligosaccharide production from wheat bran after treatment with cell wall-degrading enzymes. Int. J. Food Sci. Technol. 2019, 54, 2847–2853. [Google Scholar] [CrossRef]
- Chien, H.I.; Tsai, Y.H.; David Wang, H.M.; Dong, C.D.; Huang, C.Y.; Kuo, C.H. Extrusion puffing pretreated cereals for rapid production of high-maltose syrup. Food Chem. X 2022, 15, 100445. [Google Scholar] [CrossRef]
- Snelders, J.; Dornez, E.; Delcour, J.A.; Courtin, C.M. Ferulic Acid content and appearance determine the antioxidant capacity of arabinoxylanoligosaccharides. J. Agric. Food Chem. 2013, 61, 10173–10182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhan, A.; Ye, Y.; Liu, C.; Hang, F.; Li, K.; Li, J. Molecular modification, structural characterization, and biological activity of xylans. Carbohydr. Polym. 2021, 269, 118248. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Srivastava, T.; Saxena, D.C. Valorization of deoiled rice bran by development and process optimization of extrudates. Eng. Agric. Environ. Food 2019, 12, 173–180. [Google Scholar] [CrossRef]
- Yadav, G.P.; Dalbhagat, C.G.; Mishra, H.N. Effects of extrusion process parameters on cooking characteristics and physicochemical, textural, thermal, pasting, microstructure, and nutritional properties of millet-based extruded products: A review. J. Food Process Eng. 2022, 45, e14106. [Google Scholar] [CrossRef]
- Ciardullo, K.; Donner, E.; Thompson, M.R.; Liu, Q. Influence of Extrusion Mixing on Preparing Lipid Complexed Pea Starch for Functional Foods. Starch Stärke 2018, 71. [Google Scholar] [CrossRef]
- Arora, B.; Yoon, A.; Sriram, M.; Singha, P.; Rizvi, S.S.H. Reactive extrusion: A review of the physicochemical changes in food systems. Innov. Food Sci. Emerg. Technol. 2020, 64, 1800196. [Google Scholar] [CrossRef]
- Krishnan, R.; Dharmaraj, U.; Sai Manohar, R.; Malleshi, N.G. Quality characteristics of biscuits prepared from finger millet seed coat based composite flour. Food Chem. 2011, 129, 499–506. [Google Scholar] [CrossRef]
- Yin, Z.; Wu, W.; Sun, C.; Lei, Z.; Chen, H.; Liu, H.; Chen, W.; Ma, J.; Min, T.; Zhang, M.; et al. Comparison of releasing bound phenolic acids from wheat bran by fermentation of three Aspergillus species. Int. J. Food Sci. Technol. 2018, 53, 1120–1130. [Google Scholar] [CrossRef]
- Chen, X.; Tang, W.; Li, X.; Zhuang, K.; Lyu, Q.; Ding, W. Effect of extrusion on phenolics from Jizi439 black wheat bran: The profile, structure, and bioactivities. Lwt 2023, 177, 114369. [Google Scholar] [CrossRef]
- Kamau, E.H.; Nkhata, S.G.; Ayua, E.O. Extrusion and nixtamalization conditions influence the magnitude of change in the nutrients and bioactive components of cereals and legumes. Food Sci. Nutr. 2020, 8, 1753–1765. [Google Scholar] [CrossRef]
- Qiao, H.; Shao, H.; Zheng, X.; Liu, J.; Liu, J.; Huang, J.; Zhang, C.; Liu, Z.; Wang, J.; Guan, W. Modification of sweet potato (Ipomoea batatas Lam.) residues soluble dietary fiber following twin-screw extrusion. Food Chem. 2021, 335, 127522. [Google Scholar] [CrossRef] [PubMed]
- Dalbhagat, C.G.; Mishra, H.N. Effects of extrusion process conditions on system parameters; physicochemical properties and cooking characteristics of extruded fortified rice kernels. J. Cereal Sci. 2019, 89, 102782. [Google Scholar] [CrossRef]
- Li, S.; Hu, N.; Zhu, J.; Zheng, M.; Liu, H.; Liu, J. Influence of modification methods on physicochemical and structural properties of soluble dietary fiber from corn bran. Food Chem. X 2022, 14, 100298. [Google Scholar] [CrossRef]
- Ma, Q.; Yu, Y.; Zhou, Z.; Wang, L.; Cao, R. Effects of different treatments on composition, physicochemical and biological properties of soluble dietary fiber in buckwheat bran. Food Biosci. 2023, 53, 102517. [Google Scholar] [CrossRef]
- Chu, J.; Zhao, H.; Lu, Z.; Lu, F.; Bie, X.; Zhang, C. Improved physicochemical and functional properties of dietary fiber from millet bran fermented by Bacillus natto. Food Chem. 2019, 294, 79–86. [Google Scholar] [CrossRef]
- Yang, C.; Si, J.; Chen, Y.; Xie, J.; Tian, S.; Cheng, Y.; Hu, X.; Yu, Q. Physicochemical structure and functional properties of soluble dietary fibers obtained by different modification methods from Mesona chinensis Benth. residue. Food Res. Int. 2022, 157, 111489. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Zhang, Q.; Xu, L.; Xiao, X. Dietary Supplementation with Inulin Modulates the Gut Microbiota and Improves Insulin Sensitivity in Prediabetes. Int. J. Endocrinol. 2021, 2021, 5579369. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, B.; Chavez, A.; Forero, A.; Garcia-Huante, Y.; Romero, A.; Sanchez, M.; Rocha, D.; Sanchez, B.; Rodriguez-Sanoja, R.; Sanchez, S.; et al. Production of microbial secondary metabolites: Regulation by the carbon source. Crit. Rev. Microbiol. 2010, 36, 146–167. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, J.; Wang, L.; Meng, A.; Wei, S.; Cui, J.; Hu, X.; Yan, L. Short-Chain Inulin Modulates the Cecal Microbiota Structure of Leptin Knockout Mice in High-Fat Diet. Front. Microbiol. 2021, 12, 703929. [Google Scholar] [CrossRef]
- Demuth, T.; Edwards, V.; Bircher, L.; Lacroix, C.; Nystrom, L.; Geirnaert, A. In vitro Colon Fermentation of Soluble Arabinoxylan Is Modified Through Milling and Extrusion. Front. Nutr. 2021, 8, 707763. [Google Scholar] [CrossRef] [PubMed]
- LaBouyer, M.; Holtrop, G.; Horgan, G.; Gratz, S.W.; Belenguer, A.; Smith, N.; Walker, A.W.; Duncan, S.H.; Johnstone, A.M.; Louis, P.; et al. Higher total faecal short-chain fatty acid concentrations correlate with increasing proportions of butyrate and decreasing proportions of branched-chain fatty acids across multiple human studies. Gut Microbiome 2022, 3, 1–14. [Google Scholar] [CrossRef]
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.M.; Van den Mooter, G.; De Vuyst, L.; Courtin, C.M.; et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, P.; Maiolini, M.; Alnafoosi, O.; Hussein, S.; Alnafoosi, H.; Umbela, S.; Richardson, T.; Alla, N.; Lamichhane, N.; Subhadra, B.; et al. Colorectal Cancer and Probiotics: Are Bugs Really Drugs? Cancers 2020, 12, 1162. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopec, P.; Slizewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]

| Raw Material | Modification Method | Modification Conditions | Physicochemical Functions | Biological Functions | References |
|---|---|---|---|---|---|
| Wheat bran | Ultra-sonication | Time: 0.5 h, 1.0 h, 2.0 h; Power: 200 w, 400 w, 600 w | Molecular weight and viscosity of AX↓; | None | [25] |
| Enzymatic (xylanase) | Time: 5 min, 10 min, 15 min; Xylanase content: 1 mg/L, 5 mg/L, 10 mg/L | Solubility and degree of branching↑ | None | ||
| Trifluoroacetic acid (TFA) | Time: 0.5 h, 1.0 h, 2.0 h; TFA content: 0.025 M, 0.05 M, 0.075 M | Solubility and degree of branching↑ | None | ||
| Crude wheat bran | Microwaving | Time: 1 min; Power: 1680 W | Folic acid↑, Vitamin B2↓, WEAX↑, TPC↑, Ferulic acid↑, | ABTS radical scavenging capacity↑ | [13] |
| Steam | Time: 10 min; Temperature: 100 °C | Folic acid↑, Vitamin B2↓, TPC↑, Ferulic acid↑ | None | ||
| Extrusion | Temperature: 60 °C, 90 °C, 120 °C, 150 °C Moisture content: 17 g/100 g; Screw speed: 275 rpm | Folic acid↑, Vitamin B2↓, WEAX↑, Swelling force↑, TPC↑, Ferulic acid↑, | Oxygen radical absorbance capacity↑ | ||
| Superheated steam | Temperature: 220 °C; Time: 90 s | Folic acid↑, Vitamin B2↓, TPC↑, Ferulic acid↑ | None | ||
| Fermentation (yeast) | Temperature: 37 °C; Time: 24 h | SDF↑, WEAX↑, TPC↑, Ferulic acid↑ | None | ||
| Enzymatic treatment (xylanase) | pH: 5.0 | Folic acid↑, Vitamin B2↓, TPC↑, Ferulic acid↑, | DPPH radical scavenging capacity↓ | ||
| Wheat bran | Fermentation (yeast and lactic acid bacteria) | Temperature: 37 °C; Time: 24 h; Moisture content: 50% | WEAX↑, TDF↑, SDF↑, Phytic acid↓, Protein↓, WHC↑, Water Retention Capacity↑ | None | [26] |
| Coarse wheat bran | Micronization | Screw speed: 960 rpm; Power: 25 Hz, 50 Hz | SDF↑, Ferulic acid↑, TPC↑, ABTS and DPPH radical scavenging capacity↑, Water Retention Capacity↓, IDF↓ | None | [27] |
| Wheat bran | Extrusion | Moisture content: 24%; Screw speed: 400 rpm; Temperature: 130 °C | AX extractability↑, TDF-, Total AX-, β-glucan extractability↑ | None | [3] |
| Coarse wheat bran | Extrusion and enzymatic hydrolysis | Screw speed: 100 rpm, 200 rpm; Moisture content: 12%, 14%, 16%; Temperature: 105 °C, 120 °C, 135 °C Enzymatic hydrolysis time: 0, 30, 60, 120 min; Temperature: 45 °C | Solubility of fiber↑, Water Binding Capacity↓, Microstructure disruption | None | [28] |
| Wheat bran | Steam explosion | Pressure: 0.3 MPa, 0.5 MPa, 0.8 MPa; Time: 5 min | Total flavonoids↑, TPC↑, SDF↑,Fatty acid value↓, Peroxide value↓, IDF↓, Rancidity↓ | DPPH free radical scavenging activity↑ | [29] |
| Enzyme Formula | Concentration (%) |
|---|---|
| Enzyme-free | 0 |
| Cellulase | 0.4 |
| Xylanase | 0.9 |
| Cellulase, Xylanase | 0.4, 0.9 |
| High-temperature α-amylase, Acid protease | 0.4, 0.4 |
| CXAP 1 | 0.6, 0.9, 0.4, 0.4 |
| Level | Factor | ||||
|---|---|---|---|---|---|
| Xylanase (%) | Cellulase (%) | Temperature of Screw Zone III (°C) | Screw Speed (Hz) | Moisture Content (%) | |
| 1 | 0.3 | 0.4 | 130 | 14 | 20 |
| 2 | 0.6 | 0.6 | 140 | 15 | 22 |
| 3 | 0.9 | 0.8 | 150 | 16 | 24 |
| Method | Enzyme | WEAX (g/100 g) |
|---|---|---|
| Control | None (Native) | 0.312 ± 0.04 d |
| None (Extrusion) | 0.519 ± 0.05 c | |
| Co-modification by extrusion with a single enzyme | Cellulase | 0.818 ± 0.04 b |
| Xylanase | 0.912 ± 0.05 b | |
| Co-modification by extrusion with a dual enzyme | Cellulase, Xylanase | 1.183 ± 0.04 a |
| Thermostable α-amylase, Acid protease | 0.592 ± 0.02 c | |
| Co-modification by extrusion with CXAP | Cellulase, Xylanase, Thermostable α-amylase, Acid protease | 1.210 ± 0.05 a |
| Co-modification by extrusion with a single enzyme | Cellulase | 0.818 ± 0.04 b |
| A | B | C | D | E | WEAX Content (g/100 g) | |
|---|---|---|---|---|---|---|
| 1 | 0.3 | 0.4 | 130 | 14 | 20 | 1.68 ± 0.07 |
| 2 | 0.6 | 0.6 | 130 | 15 | 22 | 1.41 ± 0.05 |
| 3 | 0.9 | 0.8 | 130 | 16 | 24 | 1.30 ± 0.04 |
| 4 | 0.6 | 0.4 | 140 | 15 | 20 | 2.01 ± 0.06 |
| 5 | 0.9 | 0.6 | 140 | 16 | 22 | 3.16 ± 0.03 |
| 6 | 0.3 | 0.8 | 140 | 14 | 24 | 1.84 ± 0.05 |
| 7 | 0.3 | 0.6 | 150 | 16 | 20 | 2.70 ± 0.06 |
| 8 | 0.6 | 0.8 | 150 | 14 | 22 | 2.13 ± 0.02 |
| 9 | 0.9 | 0.4 | 150 | 15 | 24 | 2.76 ± 0.07 |
| 10 | 0.9 | 0.8 | 130 | 15 | 20 | 1.35 ± 0.03 |
| 11 | 0.3 | 0.4 | 130 | 16 | 22 | 1.57 ± 0.05 |
| 12 | 0.6 | 0.6 | 130 | 14 | 24 | 1.20 ± 0.04 |
| 13 | 0.9 | 0.6 | 140 | 14 | 20 | 1.84 ± 0.04 |
| 14 | 0.3 | 0.8 | 140 | 15 | 22 | 2.38 ± 0.02 |
| 15 | 0.6 | 0.4 | 140 | 16 | 24 | 2.20 ± 0.06 |
| 16 | 0.6 | 0.8 | 150 | 16 | 20 | 1.41 ± 0.03 |
| 17 | 0.9 | 0.4 | 150 | 14 | 22 | 2.68 ± 0.07 |
| 18 | 0.3 | 0.6 | 150 | 15 | 24 | 2.06 ± 0.03 |
| K1 | 1.42 | 1.83 | 2.15 | 2.04 | 1.89 | None |
| K2 | 2.24 | 2.22 | 2.06 | 1.73 | 2.00 | None |
| K3 | 2.29 | 1.89 | 1.74 | 2.18 | 2.06 | None |
| Range | 0.87 | 0.39 | 0.41 | 0.46 | 0.16 | None |
| Index | Native | Co-Modification |
|---|---|---|
| Fat (g/100 g) | 3.19 ± 0.10 a | 2.77 ± 0.06 b |
| Starch (g/100 g) | 18.47 ± 0.20 a | 16.24 ± 0.33 b |
| Protein (g/100 g) | 19.32 ± 0.51 a | 19.37 ± 0.12 a |
| TP (mg/g) | 2.83 ± 0.18 b | 3.09 ± 0.20 a |
| TDF (g/100 g) | 41.97 ± 3.25 b | 46.04 ± 3.05 a |
| SDF (g/100 g) | 2.43 ± 0.13 b | 8.45 ± 0.22 a |
| WHC (g/g) | 3.12 ± 0.13 b | 6.25 ± 0.21 a |
| OHC (g/g) | 4.82 ± 0.18 b | 8.24 ± 0.26 a |
| CAC (pH = 2.0, mg/g) | 7.31 ± 0.23 b | 16.87 ± 0.35 a |
| CAC (pH = 7.0, mg/g) | 23.63 ± 0.84 b | 55.16 ± 1.12 a |
| SCFA (mmol/L) | pH | |||||
|---|---|---|---|---|---|---|
| Acetic Acid | Propionic Acid | Butyric Acid | Isobutyric Acid | Isovaleric Acid | ||
| C0 | 1.21 ± 0.05 e | 0.62 ± 0.01 d | 0.15 ± 0.01 d | 0.031 ± 0.002 e | 0.025 ± 0.001 e | 7.83 ± 0.03 a |
| C24 | 11.31 ± 0.13 d | 0.73 ± 0.01 c | 0.19 ± 0.02 d | 0.260 ± 0.02 a | 0.49 ± 0.03 a | 7.05 ± 0.07 b |
| Inulin | 47.43 ± 0.76 a | 2.67 ± 0.05 b | 0.29 ± 0.01 c | 0.057 ± 0.001 d | 0.037 ± 0.003 d | 4.76 ± 0.02 e |
| BWB | 28.67 ± 0.85 c | 3.09 ± 0.03 a | 1.31 ± 0.01 b | 0.104 ± 0.002 b | 0.109 ± 0.002 b | 6.25 ± 0.02 c |
| CM-BWB | 40.93 ± 0.49 b | 2.62 ± 0.03 b | 2.33 ± 0.01 a | 0.072 ± 0.001 c | 0.097 ± 0.001 bc | 5.11 ± 0.06 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, C.; Duan, C.; Zhang, S.; Liu, R.; Sun, Y.; Zhou, S. Effects of Co-Modification by Extrusion and Enzymatic Hydrolysis on Physicochemical Properties of Black Wheat Bran and Its Prebiotic Potential. Foods 2023, 12, 2367. https://doi.org/10.3390/foods12122367
Kong C, Duan C, Zhang S, Liu R, Sun Y, Zhou S. Effects of Co-Modification by Extrusion and Enzymatic Hydrolysis on Physicochemical Properties of Black Wheat Bran and Its Prebiotic Potential. Foods. 2023; 12(12):2367. https://doi.org/10.3390/foods12122367
Chicago/Turabian StyleKong, Chunli, Caiping Duan, Shunzhi Zhang, Rui Liu, Yuanlin Sun, and Sumei Zhou. 2023. "Effects of Co-Modification by Extrusion and Enzymatic Hydrolysis on Physicochemical Properties of Black Wheat Bran and Its Prebiotic Potential" Foods 12, no. 12: 2367. https://doi.org/10.3390/foods12122367
APA StyleKong, C., Duan, C., Zhang, S., Liu, R., Sun, Y., & Zhou, S. (2023). Effects of Co-Modification by Extrusion and Enzymatic Hydrolysis on Physicochemical Properties of Black Wheat Bran and Its Prebiotic Potential. Foods, 12(12), 2367. https://doi.org/10.3390/foods12122367




