A Novel Process for One-Step Separation and Cyclodextrin Inclusion of Ginsenoside Rg5 from Ginseng Stem–Leaf Saponins (GSLS): Preparation, Characterization, and Evaluation of Storage Stability and Bioactivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
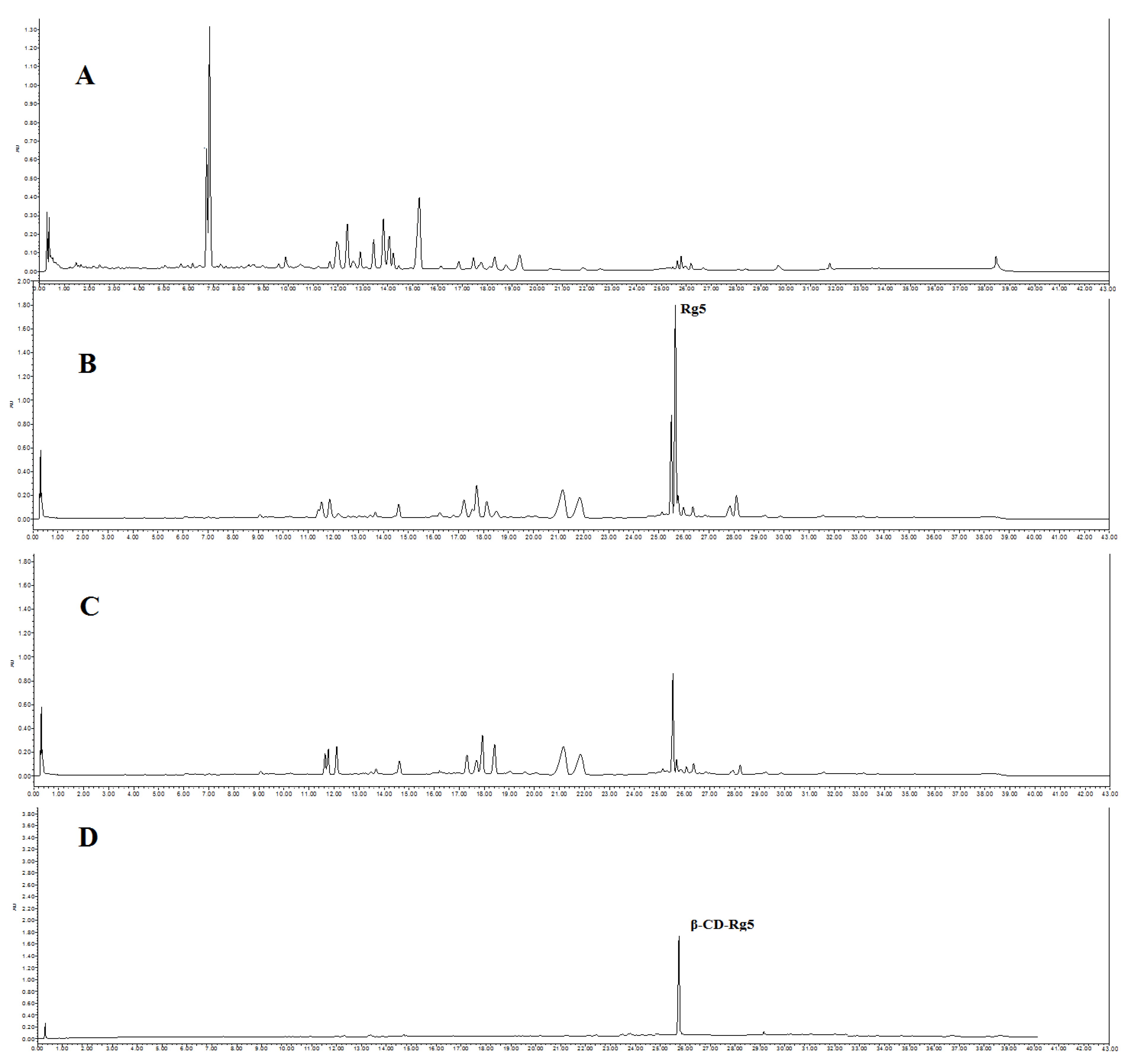
2.2. Rg5 Conversion in GSLS
2.3. Preparation of the β-CD-Rg5 Inclusion Complex from GSLS
2.4. Characterization of β-CD-Rg5
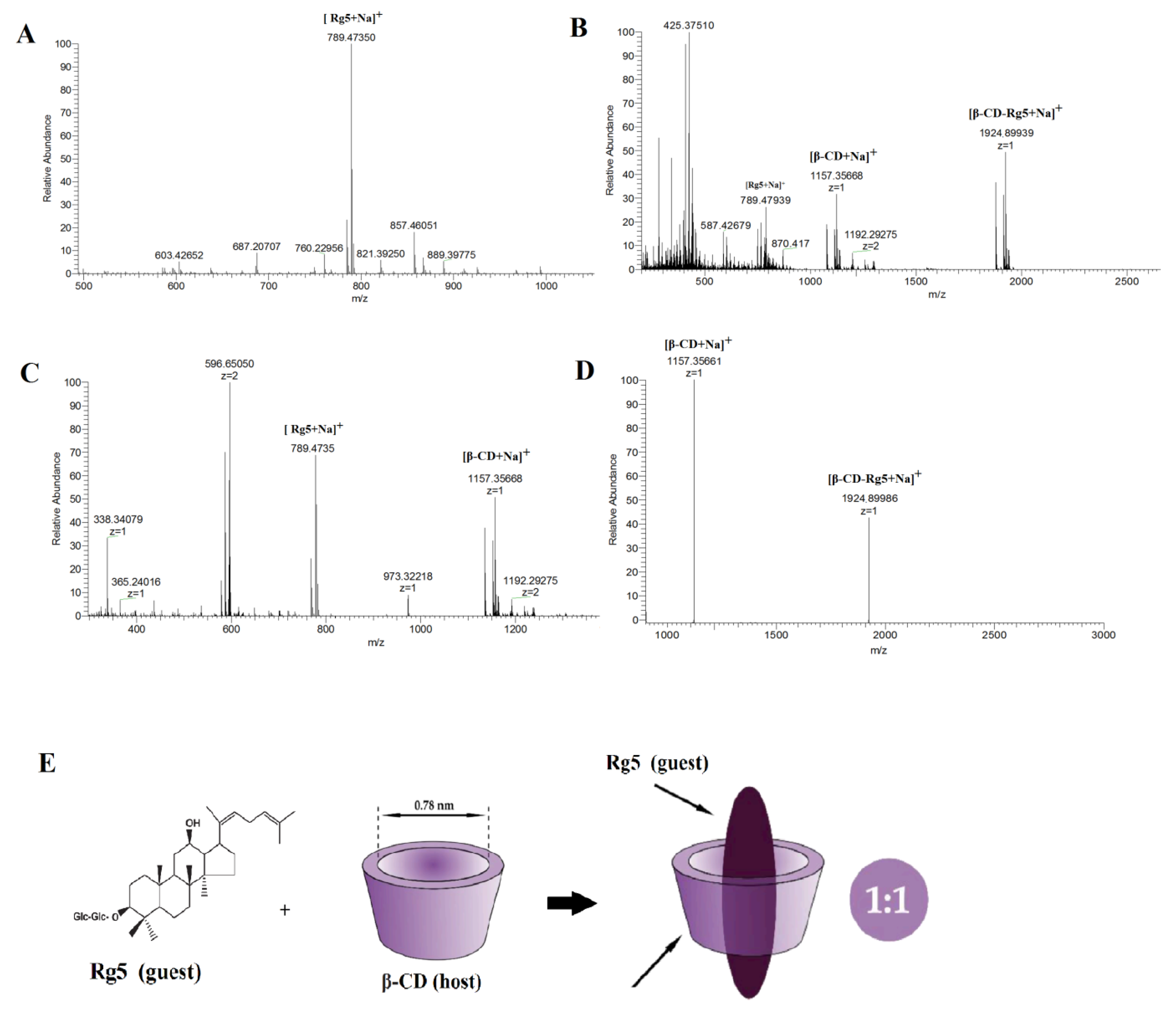
2.4.1. Electrospray Ionization Mass Spectrometry (ESI-MS)
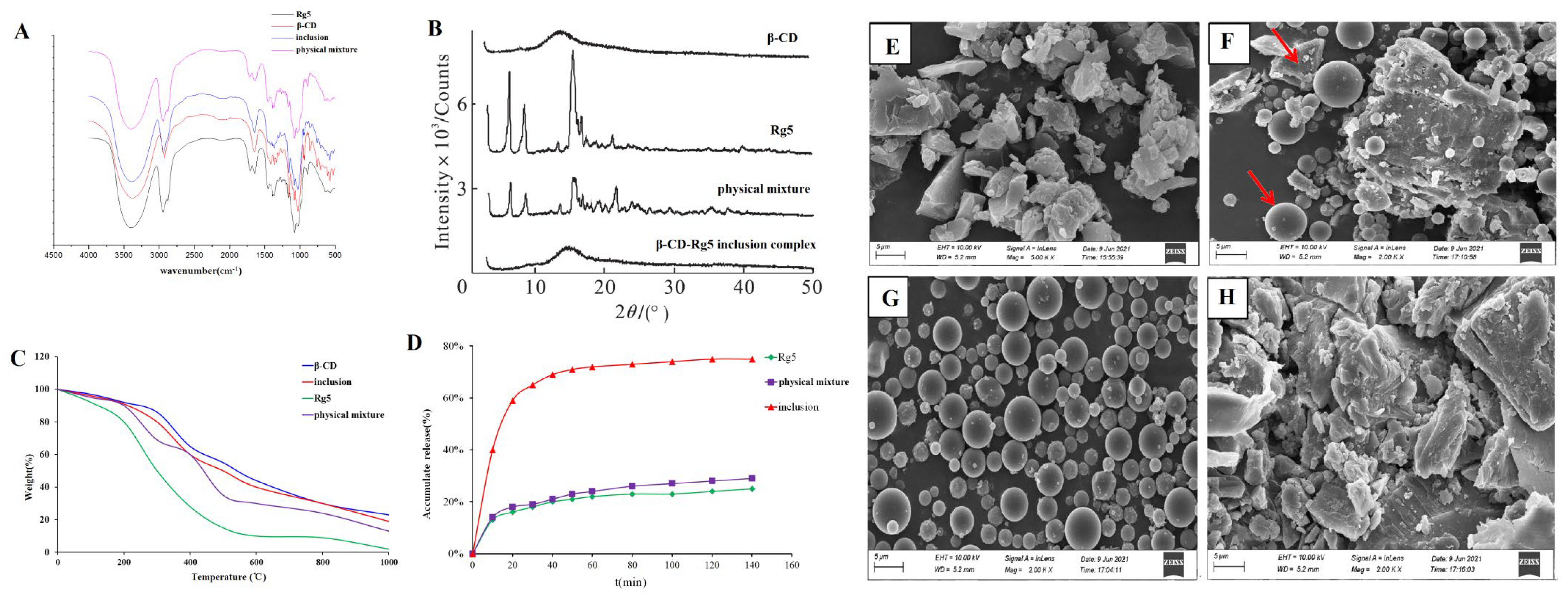
2.4.2. Fourier-Transform Infrared (FTIR) Spectroscopy
2.4.3. X-ray Diffraction Analysis
2.4.4. Thermogravimetric Analysis (TGA)
2.4.5. Scanning Electron Microscopy (SEM)
2.5. Stability of β-CD-Rg5
2.6. Dissolution In Vitro
2.7. Antioxidant Activity
2.7.1. DPPH Free Radical Scavenging Ability
2.7.2. ABTS+ Free Radical Scavenging Ability
2.7.3. Fe2+ Chelating Ability
2.8. Statistical Analysis
3. Results and Discussion
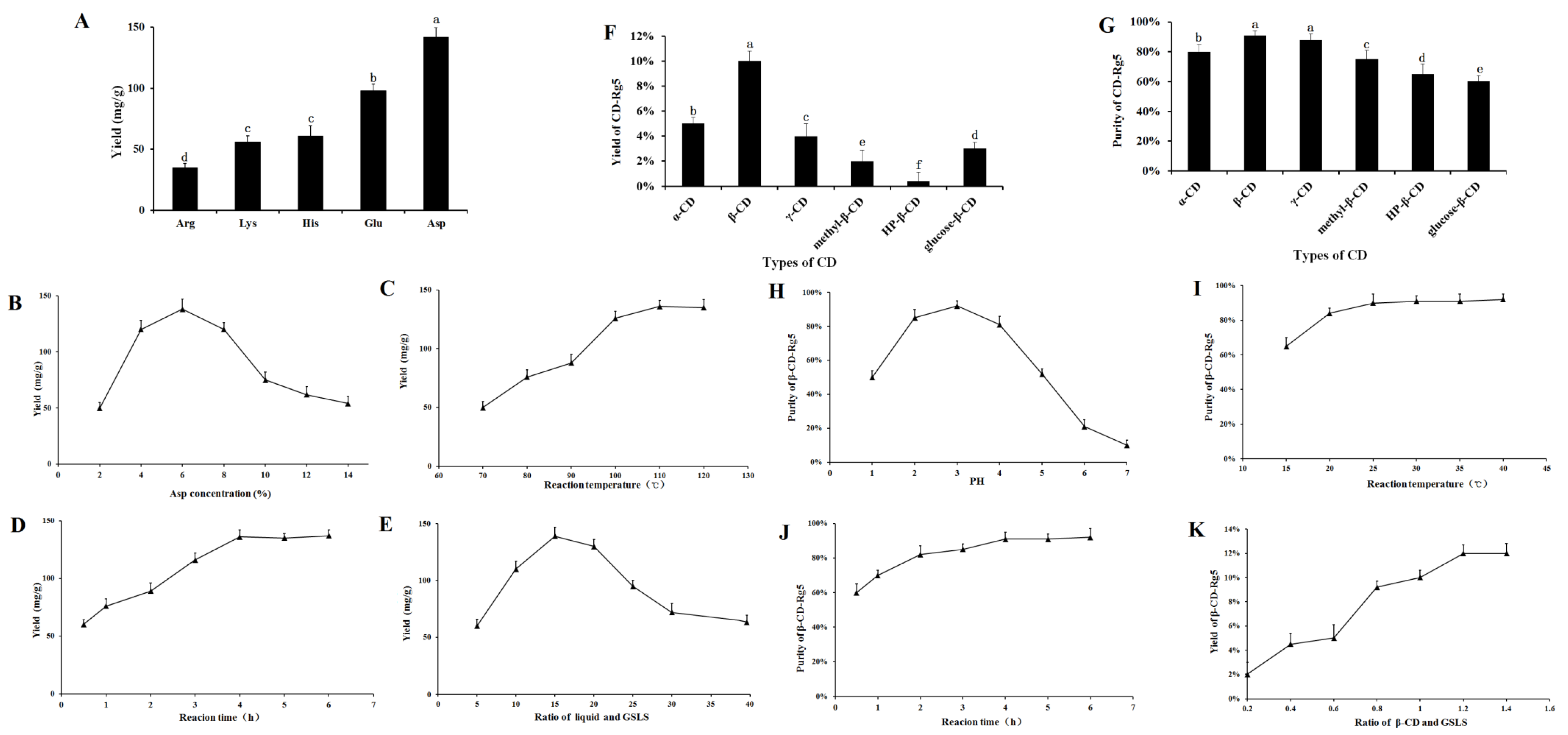
3.1. Effect of Amino Acids on Rg5 Conversion Rates in GSLS
3.2. Optimization and Preparation of β-CD-Rg5 from GSLS
3.3. Characterization of β-CD-Rg5
3.3.1. Confirmation of the β-CD-Rg5 Inclusion Complex Using ESI-MS
3.3.2. FTIR Spectroscopy
3.3.3. X-ray Diffraction Analysis
3.3.4. Thermogravimetric Analysis
3.3.5. SEM
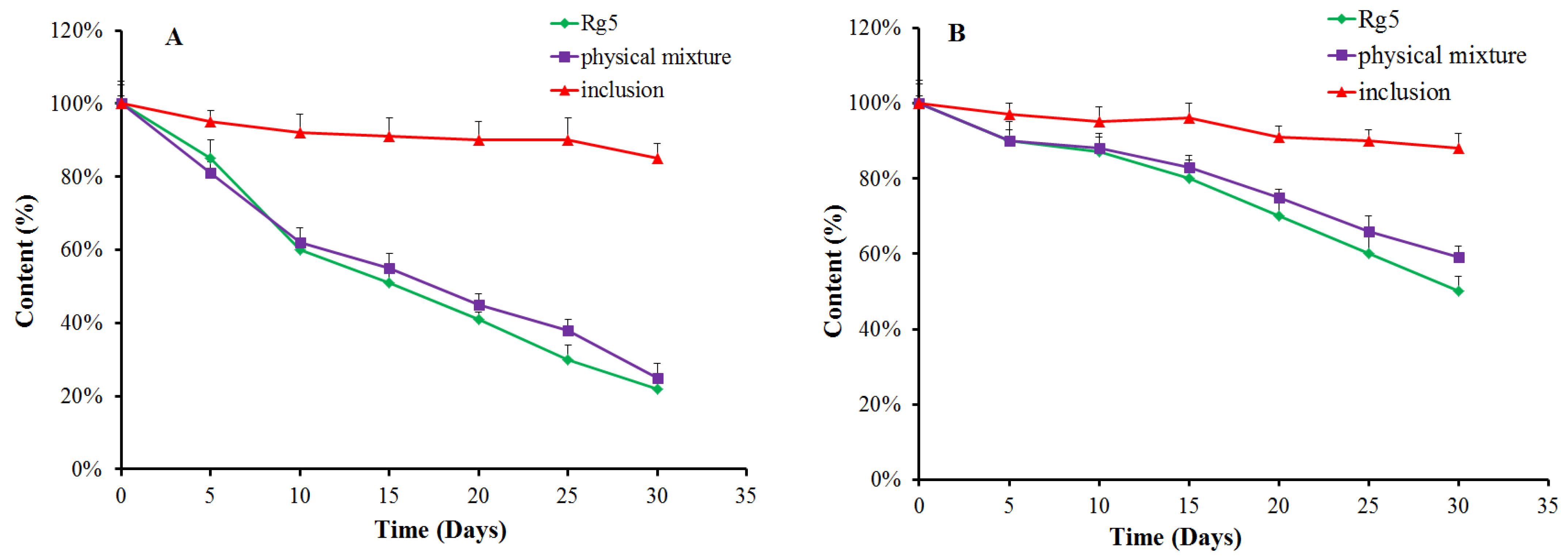
3.4. Stability of β-CD-Rg5
3.5. Dissolution In Vitro
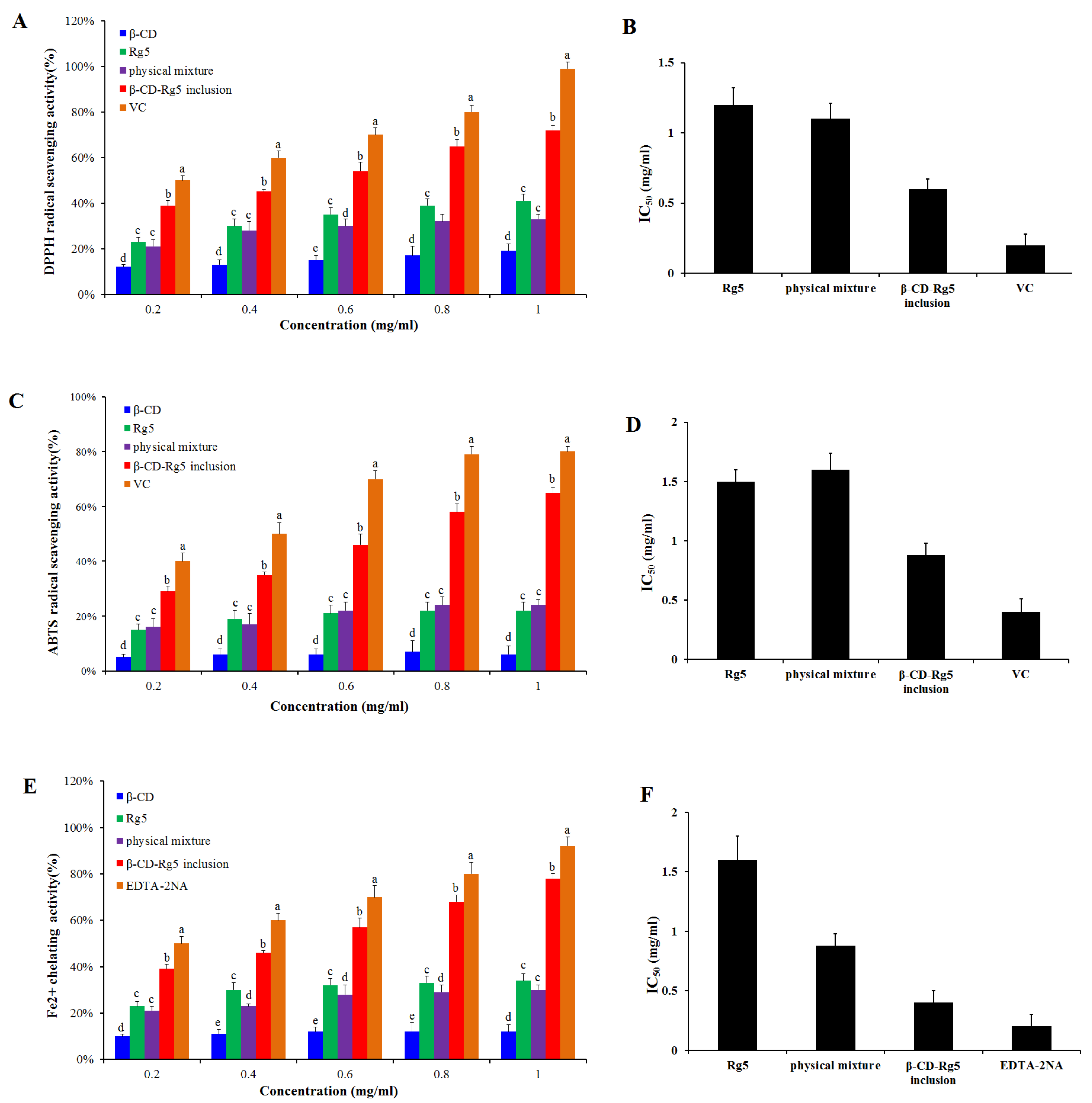
3.6. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shin, B.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Y.; Li, X.; Mao, X.; Jamaldin, K.; Gao, W. Cultivar differencein starch-related physicochemical and functional properties of flours from ginseng (Panax ginseng) roots. Starch 2016, 68, 909. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.H.; Kim, J.E.; Kim, Y.S.; Ryu, C.H.; Lee, H.J.; Lee, J. Micro-/nano-sized delivery systems of ginsenosides for improved systemic bioavailability. J. Ginseng Res. 2018, 42, 361. [Google Scholar] [CrossRef]
- Wang, C.Z.; Zhang, B.; Song, W.X.; Wang, A.; Ni, M.; Luo, X.; Yuan, C.S. Steamed American Ginseng Berry: Ginsenoside analyses and anticancer activities. J. Agric. Food Chem. 2006, 54, 9936. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yan, M.H.; Liu, Y. Ginsenoside Rg5 amelIorates Cisplatin-Induced nephrotoxicity in mice through inhibition of inflammation, oxidative stress, and apoptosis. Nutrients 2016, 8, 566. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; He, T.; Du, T.; Fan, Y.; Chen, D.; Wang, Y. Ginsenoside-Rg5 induces apoptosis and DNA damage in human cervical cancer cells. Mol. Med. Rep. 2015, 11, 940. [Google Scholar] [CrossRef]
- Kim, T.W.; Joh, E.H.; Kim, B. Ginsenoside Rg5 a meliorates lung inflammation in mice by inhibiting thebinding of LPS to toll-like receptor-4 on macrophages. Internat. Immunopharmacol. 2012, 12, 110. [Google Scholar] [CrossRef]
- Yao, H.; Jin, Y.R.; Yang, J. Conversion rule of rare ginsenosides produced from major ginsenosidesby confined microiwave promoted degradation method. Chem. J. Chinese Univ. 2014, 35, 2317. [Google Scholar]
- Kim, S.J.; Kim, A.K. Anti-breast cancer activity of Fine Black ginseng (Panax ginseng Meyer) and ginsenoside Rg5. J. Ginseng Res. 2015, 39, 125. [Google Scholar] [CrossRef]
- Zhu, L.; Luan, X.; Dou, D.; Huang, L. Comparative Analysis of Ginsenosides and Oligosaccharides in White Ginseng (WG), red Ginseng (RG) and Black Ginseng (BG). J. Chromatogr. Sci. 2019, 57, 403. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Taha, A.A.; Ying, Y.; Li, X.; Chen, X. Subcritical water extraction of bioactive components from ginseng roots (Panax ginseng C.A. Mey). Ind. Crop. Prod. 2018, 117, 118. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J.; Yan, M. Caspase-mediated anti-apoptotic effect of ginsenoside Rg5, a main rare ginsenoside, on acetaminophen-induced hepatotoxicity inmice. J. Agric. Food Chem. 2017, 65, 9226. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Yao, C.M.; Yang, X.W. Four new dammarane-type triterpene saponins from the stems and leaves of Panax ginseng and their cytotoxicity on HL-60 cells. Planta Med. 2012, 78, 189. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Yang, X.B.; Yang, X.W. New triterpenoids from the stems and leaves of Panax ginseng. Fitoterapia 2012, 83, 1030. [Google Scholar] [CrossRef]
- Guan, D.P.; Wang, H.; Li, W. Optimization of preparing technology of ginsenoside Rk1 and Rg5 by high-temperature pyrolysis. Shanghai J. Trad. Chin. Med. 2015, 2015, 91. [Google Scholar]
- Lee, S.M.; Shong, H.J.; Choi, C.S. Ginsenosides from heat processed ginseng. Chem. Pharm. Bull. 2009, 57, 92. [Google Scholar] [CrossRef]
- Li, Z.M.; Shao, Z.J.; Qu, D.; Huo, X.H.; Hua, M.; Chen, J.B.; Lu, Y.S.; Sha, J.Y.; Li, S.S.; Sun, Y.S. Transformation mechanism of rareGinsenosides in American ginseng by different processing methods and antitumour effects. Front. Nutr. 2022, 9, 1. [Google Scholar]
- Marques, H.M.C. A review on cyclodextrin encapsulation of essential oils andvolatiles. Flavour Frag. J. 2010, 25, 313. [Google Scholar] [CrossRef]
- Astray, G.; Mejuto, J.C.; Simal-Gandara, J. Latest developments in theapplication of cyclodextrin host-guest complexes in beverage technology processes. Food Hydrocolloid. 2020, 106, 105882. [Google Scholar] [CrossRef]
- Fernandes, A.; Rocha, M.A.A.; Santos, L.M.N.B.F.; Brás, J.; Oliveira, J.; Mateus, N.; de Freitas, V. Blackberry anthocyanins: β-Cyclodextrin fortification for thermal and gastrointestinal stabilization. Food Chem. 2018, 245, 426. [Google Scholar] [CrossRef]
- Cai, R.; Yuan, Y.; Cui, L.; Wang, Z.; Yue, T. Cyclodextrin-assisted extraction of phenolic compounds: Current research and future prospects. Trends Food Sci. Technol. 2018, 4, 19. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry A critical review. Food Chem. 2019, 272, 192. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.K.S.; Denadai, M.; Deoliveira, C.S. Evaluation of bioactive compounds potentialand antioxidant activity of brown, green and red propolis from Brazilian northeast region. Food Res. Int. 2017, 101, 129. [Google Scholar] [CrossRef]
- Aarland, R.C.; Bauelos-Hernndez, A.E.; Fragoso-serrano, M. Studies on phytochemical, antioxidant, anti-inflammatory, hypoglycaemic and antiproliferative activities of Echinacea purpurea and Echinacea angustifolia extracts. Pharm. Biol. 2017, 55, 649. [Google Scholar] [CrossRef]
- Li, D.M.; Na, X.K.; Wang, H.T.; Xie, Y.S.; Cong, S.; Song, Y.K. Fluorescentcarbon dots derived from Maillard reaction products: Their properties, biodistribution, cytotoxicity and antioxidant activity. J. Agric. Food Chem. 2018, 66, 1569. [Google Scholar] [CrossRef]
- Zhang, H.R.; Chen, G.; Wang, L.; Ding, L.; Tian, Y.; Jin, W.Q.; Zhang, H.Q. Study on the inclusion complexes of cyclodextrin and sulphonatedazo dyes by electrospray ionization mass spectrometry. Int. J. Mass Spectrom. 2006, 252, 1. [Google Scholar] [CrossRef]
- Yang, L.J.; Ma, S.X.; Zhou, S.Y. Preparation and characterization of inclusion complexes of naringenin with β-cyclodextrin or its derivative. Carbohyd. Polym. 2013, 98, 861. [Google Scholar] [CrossRef]
- Fan, J.P.; Fan, C.; Dong, W.M. Free radical scavenging and anti-oxidative activities of an ethanol-soluble pigment extract prepared from fermented Zi-juan Pu-erh tea. Food Chem. Toxicol. 2013, 59, 527. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ying, D.; Cai, Y.; Le, X. Improved antioxidant activity and physicochemical properties of curcumin by adding ovalbumin and its structural characterization. Food Hydrocolloid. 2017, 72, 304. [Google Scholar] [CrossRef]
- Ge, X.Z.; Jing, L.Z.; Zhao, K. The phenolic compounds profile, quantitative analysis and antioxidant activity of four naked barley grains with differentcolor. Food Chem. 2021, 335, 127655. [Google Scholar] [CrossRef]
- Purushothaman, A.; Sheeja, A.A.; Janardanan, D. Hydroxyl radical scavenging activity of melatonin and its related indolamines. Free Radical Res. 2020, 54, 373. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Li, M.; Huo, X.; Li, Z.; Qu, D.; Sha, J.; Sun, Y. A Novel Process for One-Step Separation and Cyclodextrin Inclusion of Ginsenoside Rg5 from Ginseng Stem–Leaf Saponins (GSLS): Preparation, Characterization, and Evaluation of Storage Stability and Bioactivity. Foods 2023, 12, 2349. https://doi.org/10.3390/foods12122349
Chen J, Li M, Huo X, Li Z, Qu D, Sha J, Sun Y. A Novel Process for One-Step Separation and Cyclodextrin Inclusion of Ginsenoside Rg5 from Ginseng Stem–Leaf Saponins (GSLS): Preparation, Characterization, and Evaluation of Storage Stability and Bioactivity. Foods. 2023; 12(12):2349. https://doi.org/10.3390/foods12122349
Chicago/Turabian StyleChen, Jianbo, Meijia Li, Xiaohui Huo, Zhiman Li, Di Qu, Jiyue Sha, and Yinshi Sun. 2023. "A Novel Process for One-Step Separation and Cyclodextrin Inclusion of Ginsenoside Rg5 from Ginseng Stem–Leaf Saponins (GSLS): Preparation, Characterization, and Evaluation of Storage Stability and Bioactivity" Foods 12, no. 12: 2349. https://doi.org/10.3390/foods12122349
APA StyleChen, J., Li, M., Huo, X., Li, Z., Qu, D., Sha, J., & Sun, Y. (2023). A Novel Process for One-Step Separation and Cyclodextrin Inclusion of Ginsenoside Rg5 from Ginseng Stem–Leaf Saponins (GSLS): Preparation, Characterization, and Evaluation of Storage Stability and Bioactivity. Foods, 12(12), 2349. https://doi.org/10.3390/foods12122349





