Evaluating Biological Properties of Stingless Bee Propolis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Reagents and Chemicals
2.3. Maceration of Propolis with Ultrasonic Pretreatment
2.4. Total Phenolic Content
2.5. Total Flavonoid Content
2.6. Total Tannin Content
2.7. DPPH Radical Scavenging Assay
2.8. Oxygen Radical Absorption Capacity
2.9. Antibacterial Assay
2.10. Cultivation of Cancer Cell Lines
2.11. Cell Viability
2.12. Statistical Analysis
3. Results and Discussion
3.1. Extraction of Propolis
3.2. Total Phenolic, Flavonoid and Tannin Content
3.3. Antioxidant Capacity of Propolis Extracts
3.4. Antibacterial Activity
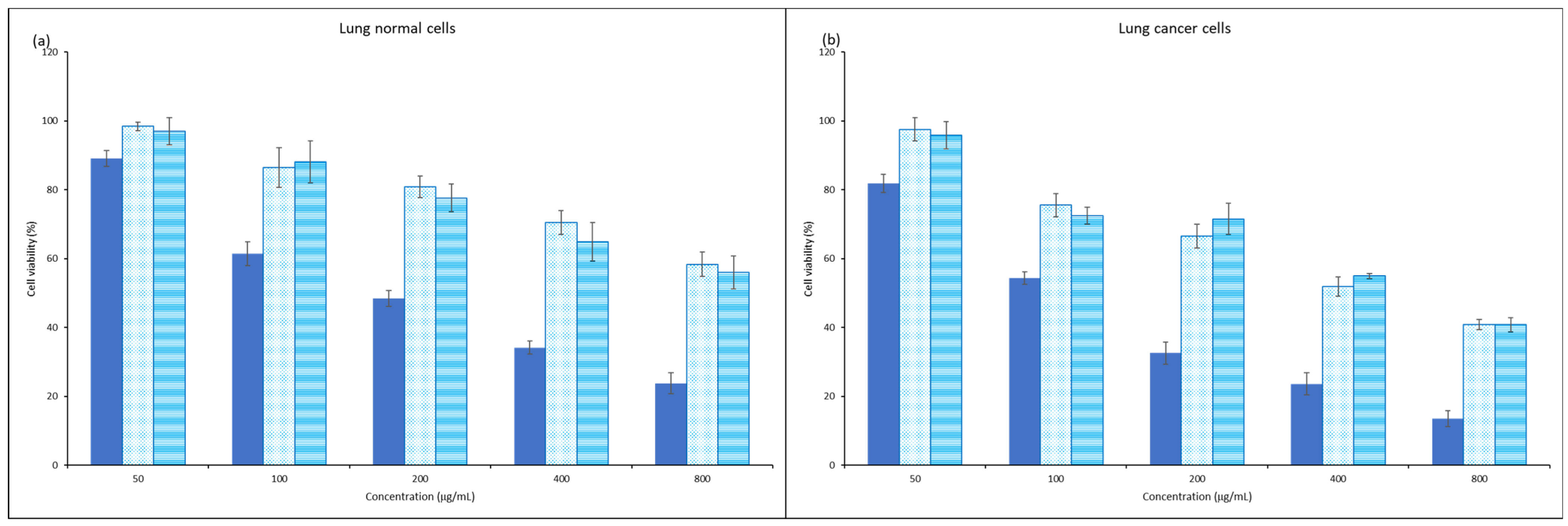
3.5. Anticancer Property of Propolis Extracts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paris, E.H.; Lope, C.P.; Masson, M.A.; Kú, P.C.D.; Ojeda, B.C.E. The organization of stingless beekeeping (Meliponiculture) at Mayapán, Yucatan, Mexico. J. Anthr. Archaeol. 2018, 52, 1–22. [Google Scholar] [CrossRef]
- Syafrizal; Ramadhan, R.; Kusuma, I.W.; Egra, S.; Shimizu, K.; Kanzaki, M.; Tangkearung, E. Diversity and honey properties of stingless bees from meliponiculture in East and North Kalimantan, Indonesia. Biodiversitas J. Biol. Divers. 2020, 21, 4623–4630. [Google Scholar] [CrossRef]
- Zulhendri, F.; Perera, C.O.; Chandrasekaran, K.; Ghosh, A.; Tandean, S.; Abdulah, R.; Herman, H.; Lesmana, R. Propolis of stingless bees for the development of novel functional food and nutraceutical ingredients: A systematic scoping review of the experimental evidence. J. Funct. Foods 2021, 88, 104902. [Google Scholar] [CrossRef]
- Se, K.W.; Ghoshal, S.K.; Wahab, R.A.; Ibrahim, R.K.R.; Lani, M.N. A simple approach for rapid detection and quantification of adulterants in stingless bees (Heterotrigona itama) honey. Food Res. Int. 2018, 105, 453–460. [Google Scholar] [CrossRef]
- Ismail, N.F.; Maulidiani, M.; Omar, S.; Zulkifli, M.F.; Radzi, M.N.F.M.; Ismail, N.; Jusoh, A.Z.; Roowi, S.; Yew, W.M.; Rudiyanto, R.; et al. Classification of stingless bee honey based on species, dehumidification process and geographical origins using physicochemical and ATR-FTIR chemometric approach. J. Food Compos. Anal. 2021, 104, 104126. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Umsza-Guez, M.A.; Rodrigues, D.M.R.; Gálvez-Ruiz, J.C.; Castro, T.L.d.P.; Balderrama-Carmona, A.P. Comparison of the biological potential and chemical composition of Brazilian and Mexican propolis. Appl. Sci. 2021, 11, 11417. [Google Scholar] [CrossRef]
- Salleh, S.N.A.S.; Hanapiah, N.A.M.; Johari, W.L.W.; Ahmad, H.; Osman, N.H. Analysis of bioactive compounds and chemical composition of Malaysian stingless bee propolis water extracts. Saudi J. Biol. Sci. 2021, 28, 6705–6710. [Google Scholar] [CrossRef]
- Abdullah, N.A.; Ja’Afar, F.; Yasin, H.M.; Taha, H.; Petalcorin, M.I.; Mamit, M.H.; Kusrini, E.; Usman, A. Physicochemical analyses, antioxidant, antibacterial, and toxicity of propolis particles produced by stingless bee Heterotrigona itama found in Brunei Darussalam. Heliyon 2019, 5, e02476. [Google Scholar] [CrossRef]
- Ahmed, R.; Tanvir, E.M.; Hossen, S.; Afroz, R.; Ahmmed, I.; Rumpa, N.-E.; Paul, S.; Gan, S.H.; Sulaiman, S.A.; Khalil, I. Antioxidant properties and cardioprotective mechanism of Malaysian propolis in rats. Evid.-Based Complement. Altern. Med. 2017, 2017, 5370545. [Google Scholar] [CrossRef]
- Ramli, N.; Ali, N.; Hamzah, S.; Yatim, N. Physicochemical characteristics of liposome encapsulation of stingless bees’ propolis. Heliyon 2021, 7, e06649. [Google Scholar] [CrossRef]
- Cavalaro, R.I.; da Cruz, R.G.; Dupont, S.; de Moura Bell, J.; Vieira, T. In vitro and in vivo antioxidant properties of bioactive compounds from green propolis obtained by ultrasound-assisted extraction. Food Chem. X 2019, 4, 100054. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.A.; Zullkiflee, N.; Zaini, S.N.Z.; Taha, H.; Hashim, F.; Usman, A. Phytochemicals, mineral contents, antioxidants, and antimicrobial activities of propolis produced by Brunei stingless bees Geniotrigona thoracica, Heterotrigona itama, and Tetrigona binghami. Saudi J. Biol. Sci. 2020, 27, 2902–2911. [Google Scholar] [CrossRef] [PubMed]
- Nna, V.U.; Abu Bakar, A.B.; Lazin, R.M.L.M.; Mohamed, M. Antioxidant, anti-inflammatory and synergistic anti-hyperglycemic effects of Malaysian propolis and metformin in streptozotocin–induced diabetic rats. Food Chem. Toxicol. 2018, 120, 305–320. [Google Scholar] [CrossRef]
- Ibrahim, M.E.E.-D.; Alqurashi, R.M. Anti-fungal and antioxidant properties of propolis (bee glue) extracts. Int. J. Food Microbiol. 2021, 361, 109463. [Google Scholar] [CrossRef] [PubMed]
- Arung, E.T.; Ramadhan, R.; Khairunnisa, B.; Amen, Y.; Matsumoto, M.; Nagata, M.; Kusuma, I.W.; Paramita, S.; Tandirogang, N.; Takemoto, N.; et al. Cytotoxicity effect of honey, bee pollen, and propolis from seven stingless bees in some cancer cell lines. Saudi J. Biol. Sci. 2021, 28, 7182–7189. [Google Scholar] [CrossRef] [PubMed]
- Laaroussi, H.; Ferreira-Santos, P.; Genisheva, Z.; Bakour, M.; Ousaaid, D.; Teixeira, J.A.; Lyoussi, B. Unraveling the chemical composition, antioxidant, α-amylase and α-glucosidase inhibition of Moroccan propolis. Food Biosci. 2021, 42, 101160. [Google Scholar] [CrossRef]
- Peixoto, M.; Freitas, A.S.; Cunha, A.; Oliveira, R.; Almeida-Aguiar, C. Antioxidant and antimicrobial activity of blends of propolis samples collected in different years. LWT 2021, 145, 111311. [Google Scholar] [CrossRef]
- Vongsak, B.; Kongkiatpaiboon, S.; Jaisamut, S.; Machana, S.; Pattarapanich, C. In vitro alpha glucosidase inhibition and free-radical scavenging activity of propolis from Thai stingless bees in mangosteen orchard. Rev. Bras. Farm. 2015, 25, 445–450. [Google Scholar] [CrossRef]
- Biscaia, D.; Ferreira, S.R. Propolis extracts obtained by low pressure methods and supercritical fluid extraction. J. Supercrit. Fluids 2009, 51, 17–23. [Google Scholar] [CrossRef]
- Pellati, F.; Prencipe, F.P.; Bertelli, D.; Benvenuti, S. An efficient chemical analysis of phenolic acids and flavonoids in raw propolis by microwave-assisted extraction combined with high-performance liquid chromatography using the fused-core technology. J. Pharm. Biomed. Anal. 2013, 81–82, 126–132. [Google Scholar] [CrossRef]
- Song, M.; Wang, K.; Lu, H.; Yan, S.; Wu, L.; Xue, X. Composition and distribution of α-dicarbonyl compounds in propolis from different plant origins and extraction processing. J. Food Compos. Anal. 2021, 104, 104141. [Google Scholar] [CrossRef]
- Oroian, M.; Ursachi, F.; Dranca, F. Influence of ultrasonic amplitude, temperature, time and solvent concentration on bioactive compounds extraction from propolis. Ultrason. Sonochemistry 2020, 64, 105021. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Dranca, F.; Ursachi, F. Comparative evaluation of maceration, microwave and ultrasonic-assisted extraction of phenolic compounds from propolis. J. Food Sci. Technol. 2019, 57, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Suárez, G.A.P.; Galindo, N.J.P.; Cuervo, O.H.P. Obtaining Colombian propolis extracts using modern methods: A determination of its antioxidant capacity and the identification of its bioactive compounds. J. Supercrit. Fluids 2022, 182, 105538. [Google Scholar] [CrossRef]
- Kara, Y.; Can, Z.; Kolaylı, S. Applicability of phenolic profile analysis method developed with RP-HPLC-PDA to some bee product. Braz. Arch. Biol. Technol. 2022, 65, e22210384. [Google Scholar] [CrossRef]
- Ahn, M.-R.; Kumazawa, S.; Usui, Y.; Nakamura, J.; Matsuka, M.; Zhu, F.; Nakayama, T. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem. 2007, 101, 1383–1392. [Google Scholar] [CrossRef]
- Abduh, M.Y.; Adam, A.; Fadhlullah, M.; Putra, R.E.; Manurung, R.; Abduh, M.Y.; Adam, A.; Fadhlullah, M.; Putra, R.E.; Manurung, R. Production of propolis and honey from Tetragonula laeviceps cultivated in Modular Tetragonula Hives. Heliyon 2020, 6, e05405. [Google Scholar] [CrossRef]
- Monteiro, J.M.; de Souza, J.S.; Neto, E.M.L.; Scopel, K.; Trindade, E.F. Does total tannin content explain the use value of spontaneous medicinal plants from the Brazilian semi-arid region? Rev. Bras. Farm. 2014, 24, 116–123. [Google Scholar] [CrossRef]
- Saidan, N.H.; Hamil, M.S.R.; Memon, A.H.; Abdelbari, M.M.; Hamdan, M.R.; Mohd, K.S.; Majid, A.M.S.A.; Ismail, Z. Selected metabolites profiling of Orthosiphon stamineus Benth leaves extracts combined with chemometrics analysis and correlation with biological activities. BMC Complement. Altern. Med. 2015, 15, 350. [Google Scholar] [CrossRef]
- Andrade, J.K.S.; Denadai, M.; de Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of bioactive compounds potential and antioxidant activity of brown, green and red propolis from Brazilian northeast region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef]
- M07; 11th ed. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2018. Available online: https://clsi.org/media/1928/m07ed11_sample.pdf (accessed on 28 January 2023).
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, J.-G.; Li, W.-F.; Chen, J.; Wang, D.-Y.; Zhu, L. Optimum extraction process of polyphenols from the bark of Phyllanthus emblica L. based on the response surface methodology. J. Sep. Sci. 2009, 32, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Dutra, R.P.; de Barros Abreu, B.V.d.B.; Cunha, M.S.; Batista, M.C.A.; Torres, L.M.B.; Nascimento, F.R.F.; Ribeiro, M.N.S.; Guerra, R.N.M. Phenolic acids, hydrolyzable tannins, and antioxidant activity of geopropolis from the stingless bee Melipona fasciculata Smith. J. Agric. Food Chem. 2014, 62, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.H.; Chua, L.S.; Lee, C.T.; Aziz, R. Optimization and kinetic modeling of rosmarinic acid extraction from Orthosiphon stamineus. Curr. Bioact. Compd. 2015, 10, 271–285. [Google Scholar] [CrossRef]
- Chua, L.S.; Abdullah, F.I.; Azlah, M.A.F. Phytochemical profile of Andrographis paniculata extract from solvent partition and precipitation. J. Biol. Act. Prod. Nat. 2019, 9, 238–249. [Google Scholar] [CrossRef]
- Asem, N.; Gapar, N.A.A.; Hapit, N.H.A.; Omar, E.A. Correlation between total phenolic and flavonoid contents with antioxidant activity of Malaysian stingless bee propolis extract. J. Apic. Res. 2019, 59, 437–442. [Google Scholar] [CrossRef]
- Farida, S.; Pratami, D.K.; Sahlan, M.; Laksmitawati, D.R.; Rohmatin, E.; Situmorang, H. In-Vitro antioxidant, in-vivo anti-inflammatory, and acute toxicity study of Indonesian propolis capsule from Tetragonula sapiens. Saudi J. Biol. Sci. 2021, 29, 2489–2500. [Google Scholar] [CrossRef]
- Awang, N.; Ali, N.; Abd Majid, F.A.; Hamzah, S.; Abd Razak, S.B. Total flavonoids and phenolic contents of sticky and hard propolis from 10 species of indo-malayan stingless bees. Malays. J. Anal. Sci. 2018, 22, 877–884. [Google Scholar] [CrossRef]
- Mayworm, M.A.S.; Lima, C.A.; Tomba, A.C.B.; Fernandes-Silva, C.C.; Salatino, M.L.F.; Salatino, A. Does propolis contain tannins? Evid. Based Complement. Altern. Med. 2014, 2014, 613647. [Google Scholar] [CrossRef]
- Kiziltas, H.; Erkan, C. The effects of different beehives on propolis production and quality. Food Sci. Technol. 2021, 41, 877–883. [Google Scholar] [CrossRef]
- Miguel, M.D.G.; Doughmi, O.; Aazza, S.; Antunes, D.; Lyoussi, B. Antioxidant, anti-inflammatory and acetylcholinesterase inhibitory activities of propolis from different regions of Morocco. Food Sci. Biotechnol. 2013, 23, 313–322. [Google Scholar] [CrossRef]
- Boulechfar, S.; Zellagui, A.; Bensouici, C.; Asan-Ozusaglam, M.; Tacer, S.; Hanene, D. Anticholinesterase, anti-α-glucosidase, antioxidant and antimicrobial effects of four Algerian propolis. J. Food Meas. Charact. 2021, 16, 793–803. [Google Scholar] [CrossRef]
- Selvaraju, G.D.; Umapathy, V.R.; SumathiJones, C.; Cheema, M.S.; Jayamani, D.R.; Dharani, R.; Sneha, S.; Yamuna, M.; Gayathiri, E.; Yadav, S. Fabrication and characterization of surgical sutures with propolis silver nano particles and analysis of its antimicrobial properties. J. King Saud Univ. Sci. 2022, 34, 102082. [Google Scholar] [CrossRef]
- Mafra, J.F.; de Santana, T.S.; Cruz, A.I.C.; Ferreira, M.A.; Miranda, F.M.; Araújo, F.M.; Ribeiro, P.R.; Evangelista-Barreto, N.S. Influence of red propolis on the physicochemical, microbiological and sensory characteristics of tilapia (Oreochromis niloticus) salami. Food Chem. 2022, 394, 133502. [Google Scholar] [CrossRef]
- Mizuno, S.; Miyata, R.; Mukaide, K.; Honda, S.; Sukito, A.; Sahlan, M.; Taniguchi, T.; Kumazawa, S. New compound from the plant origin of propolis from Lombok, Indonesia and its antibacterial activity. Results Chem. 2021, 4, 100276. [Google Scholar] [CrossRef]
- Guzelmeric, E.; Sipahi, H.; Özhan, Y.; Hamitoğlu, M.; Helvacıoğlu, S.; Düz, G.; Akyıldız, E.; Yaman, B.K.; Hazar, M.; Dilsiz, S.A.; et al. Comprehensive estrogenic/anti-estrogenic, anticancer, mutagenic/anti-mutagenic, and genotoxic/anti-genotoxic activity studies on chemically characterized black poplar and Eurasian aspen propolis types. J. Pharm. Biomed. Anal. 2023, 226, 115241. [Google Scholar] [CrossRef]
- Brihoum, H.; Maiza, M.; Sahali, H.; Boulmeltout, M.; Barratt, G.; Benguedouar, L.; Lahouel, M. Dual effect of Algerian propolis on lung cancer: Antitumor and chemopreventive effects involving antioxidant activity. Braz. J. Pharm. Sci. 2018, 54, e17396. [Google Scholar] [CrossRef]
- Frion, Y.; Díaz-García, A.; Ruiz-Fuentes, J.; Rodríguez-Sánchez, H.; Sforcin, J.M. Brazilian green propolis induced apoptosis in human lung cancer A549 cells through mitochondrial-mediated pathway. J. Pharm. Pharmacol. 2015, 67, 1448–1456. [Google Scholar] [CrossRef]
- Teerasripreecha, D.; Phuwapraisirisan, P.; Puthong, S.; Kimura, K.; Okuyama, M.; Mori, H.; Kimura, A.; Chanchao, C. In vitro antiproliferative/cytotoxic activity on cancer cell lines of a cardanol and a cardol enriched from Thai Apis mellifera propolis. BMC Complement. Altern. Med. 2012, 12, 27. [Google Scholar] [CrossRef]
- Eklund, P.C.; Backman, M.J.; Kronberg, L.; Smeds, A.I.; Sjöholm, R.E. Identification of lignans by liquid chromatography-electrospray ionization ion-trap mass spectrometry. J. Mass Spectrom. 2007, 43, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.R.; Chua, L.S.; Soo, J. Study of stingless bee (Heterotrigona itama) propolis using LC-MS/MS and TGA-FTIR. Appl. Food Res. 2023, 3, 100252. [Google Scholar] [CrossRef]
- Peuhu, E.; Paul, P.; Remes, M.; Holmbom, T.; Eklund, P.; Sjöholm, R.; Eriksson, J.E. The antitumor lignan Nortrachelogenin sensitizes prostate cancer cells to TRAIL-induced cell death by inhibition of the Akt pathway and growth factor signaling. Biochem. Pharmacol. 2013, 86, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-X.; Liu, C.; Dong, S.-L.; Ou, C.-S.; Lu, J.-L.; Ye, J.-H.; Liang, Y.-R.; Zheng, X.-Q. Anticarcinogenic potentials of tea catechins. Front. Nutr. 2022, 9, 1060783. [Google Scholar] [CrossRef] [PubMed]



| Propolis Extract | DPPH (IC50, mg/g) | ORAC (mg Trolox Equivalent/g Propolis) |
|---|---|---|
| 100% water | 96.28 ± 15.13 | 0.623 ± 0.284 |
| 20% ethanol | 30.77 ± 3.17 | 3.821 ± 3.444 |
| Samples | Minimum Inhibitory Concentration (MIC, mg/g) | ||
|---|---|---|---|
| Escherichia coli | Pseudomonas aeruginosa | Staphylococcus aureus | |
| Polymyxin B (µg/g) | 25 | 25 | NA |
| Vancomycin (µg/g) | NA | NA | 12.5 |
| 100% water | 267.75 | 267.75 | 8.36 |
| 20% ethanol | 320.75 | 320.75 | 5.01 |
| Propolis Extract | Normal Cells (µg/mL) | Cancer Cells (µg/mL) |
|---|---|---|
| Cisplatin | 226.452 ± 7.610 | 153.705 ± 6.101 |
| 100% water | 862.500 ± 18.128 | 334.516 ± 15.932 |
| 20% ethanol | 669.501 ± 15.508 | 434.600 ± 9.281 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.R.; Chua, L.S.; Dawood, D.A.S. Evaluating Biological Properties of Stingless Bee Propolis. Foods 2023, 12, 2290. https://doi.org/10.3390/foods12122290
Lim JR, Chua LS, Dawood DAS. Evaluating Biological Properties of Stingless Bee Propolis. Foods. 2023; 12(12):2290. https://doi.org/10.3390/foods12122290
Chicago/Turabian StyleLim, Jin Ru, Lee Suan Chua, and Dawood Ali Salim Dawood. 2023. "Evaluating Biological Properties of Stingless Bee Propolis" Foods 12, no. 12: 2290. https://doi.org/10.3390/foods12122290
APA StyleLim, J. R., Chua, L. S., & Dawood, D. A. S. (2023). Evaluating Biological Properties of Stingless Bee Propolis. Foods, 12(12), 2290. https://doi.org/10.3390/foods12122290







