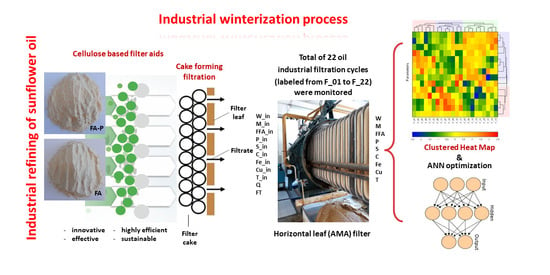
Sunflower Oil Winterization Using the Cellulose-Based Filtration Aid—Investigation of Oil Quality during Industrial Filtration Probe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
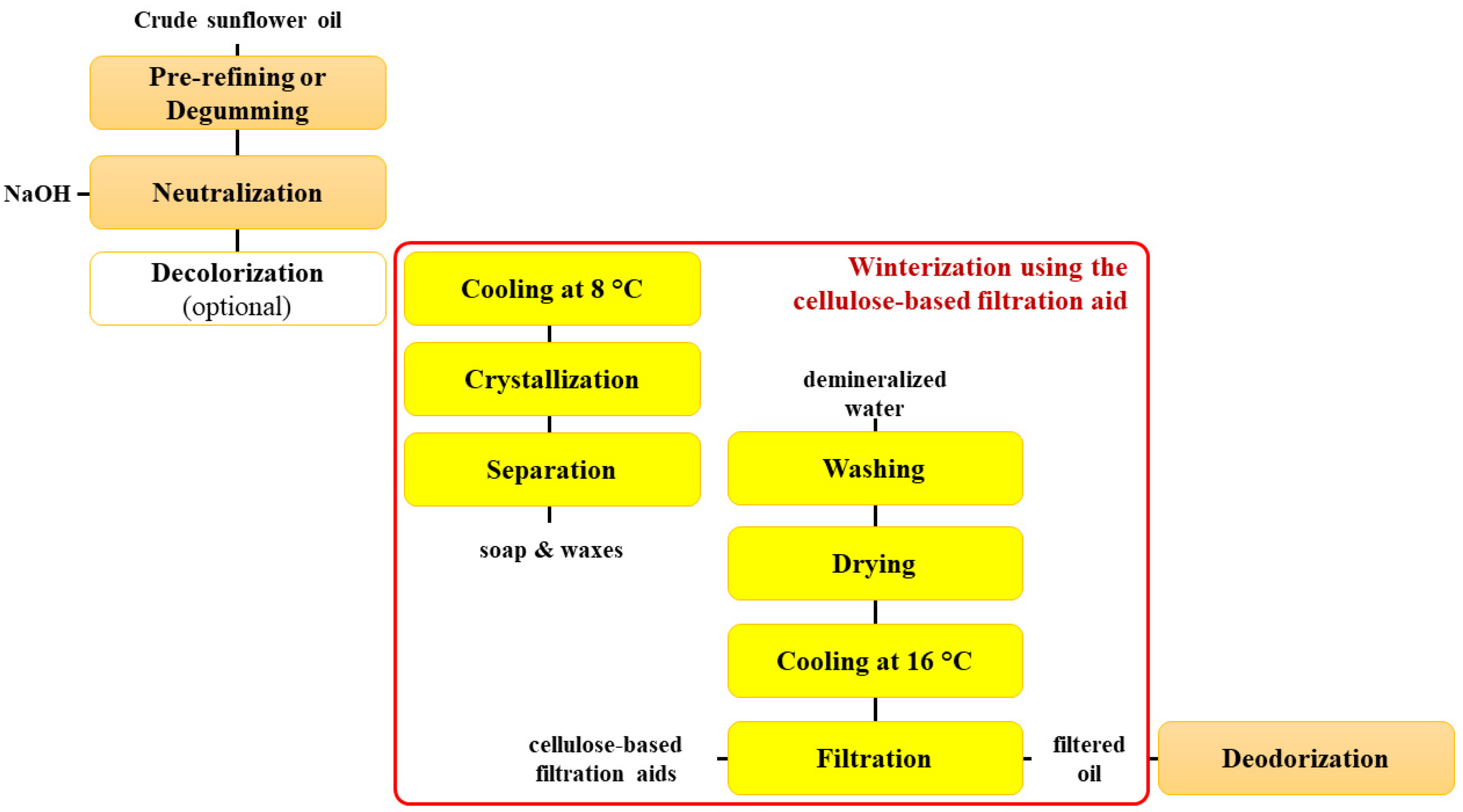
2.1.1. Industrial Winterization Process
2.1.2. Sunflower Oil Samples
2.2. Sunflower Oil Quality
2.2.1. Waxes Content
2.2.2. Moisture Content
2.2.3. Total Phospholipids Content
2.2.4. Soap Content
2.2.5. Free Fatty Acid Content
2.2.6. Total Carotenoids Content
2.2.7. Oil Transparency
2.2.8. Iron and Copper Content
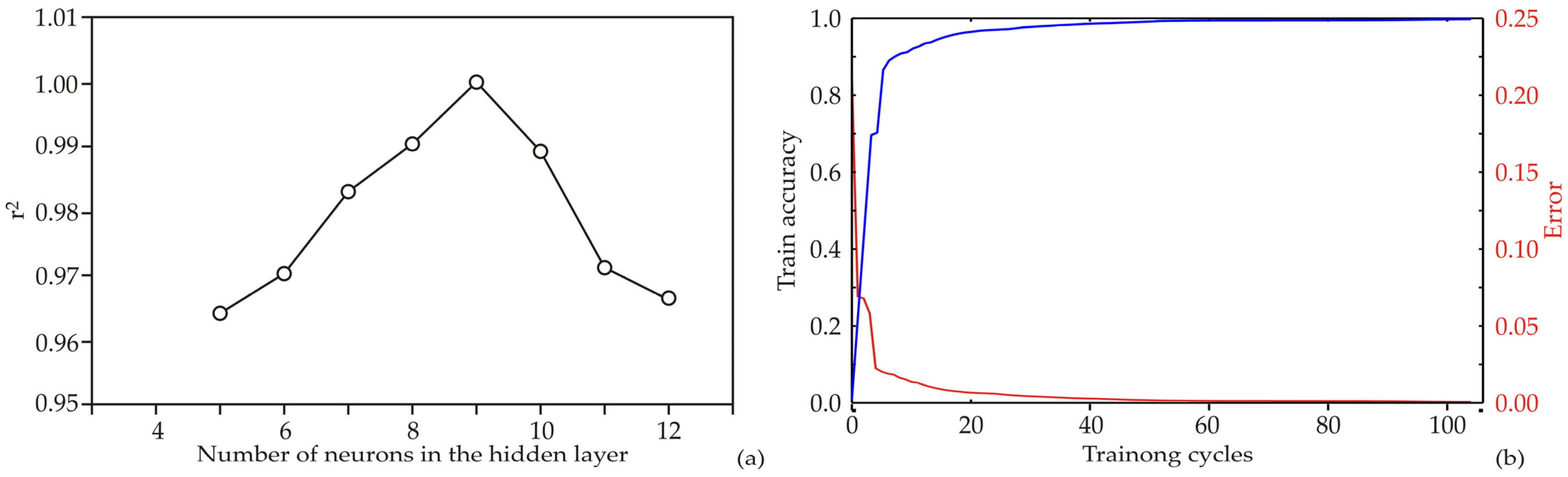
2.3. Machine Learning Model
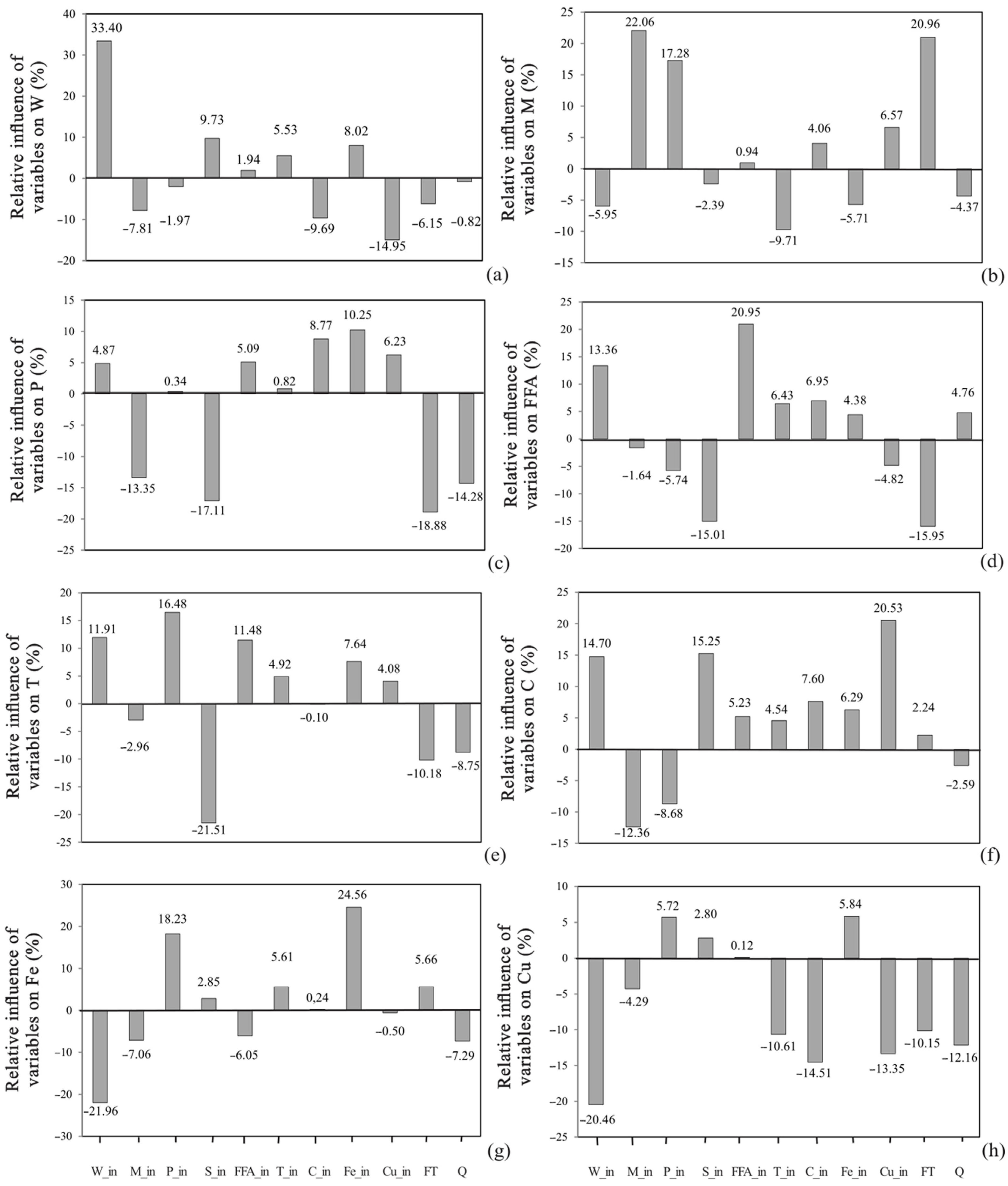
Global Sensitivity Analysis
2.4. Descriptive Statistics
3. Results and Discussion
3.1. Artificial Neural Network Model
3.2. Wax Content
3.3. Total Phospholipids and Soap Content
3.4. Moisture Content and Free Fatty Acids Content
3.5. Total Carotenoids Content and Oil Transparency
3.6. Iron and Copper Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aluyor, E.O.; Aluyor, P.; Ozigagu, C.E. Effect of refining on the quality and composition of groundnut oil. Afr. J. Food Sci. 2009, 3, 201–205. [Google Scholar]
- Ergönül, P.; Nergiz, C. The effect of different filter aid materials and winterization periods on the oxidative stability of sunflower and corn oils. CYTA–J. Food 2015, 13, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M. Practical Guide to Vegetable Oil Processing, 2nd ed.; AOCS Press: Urbana, IL, USA, 2017; pp. 41–78. [Google Scholar]
- Guo, Y.; Jia, Z.; Wan, L.; Cao, J.; Fang, Y.; Zhang, W. Effects of refining process on Camellia vietnamensis oil: Phytochemical composition, antioxidant capacity, and anti-inflammatory activity in THP-1 macrophages. Food Biosci. 2023, 52, 102440. [Google Scholar] [CrossRef]
- Gharby, S. Refining Vegetable Oils: Chemical and Physical Refining. Sci. World J. 2022, 2022, 6627013. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Shen, M.; Liu, G.; Liu, X.; Liang, L.; Li, Y.; Zhang, Y.; Xu, X. Edible vegetable oils from oil crops: Preparation, refining, authenticity identification and application. Process Biochem. 2023, 124, 168–179. [Google Scholar] [CrossRef]
- Farr, W.E.; Proctor, A. Green Vegetable Oil Processing Revised, 1st ed.; Elsevier Inc: Amsterdam, The Netherlands, 2013; pp. 1–294. [Google Scholar]
- Chew, S.C.; Ali, M.A. Recent advances in ultrasound technology applications of vegetable oil refining. Trends Food Sci. Technol. 2021, 116, 468–479. [Google Scholar] [CrossRef]
- Ye, Z.; Liu, Y. Polyphenolic compounds from rapeseeds (Brassica napus L.): The major types, biofunctional roles, bioavailability, and the influences of rapeseed oil processing technologies on the content. Food Res. Int. 2023, 163, 112282. [Google Scholar] [CrossRef]
- Manjula, S.; Subramanian, R. Membrane technology in degumming, dewaxing, deacidifying, and decolorizing edible oils. Crit. Rev. Food Sci. Nutr. 2006, 46, 569–592. [Google Scholar] [CrossRef]
- Turkulov, J.; Dimić, E.; Karlović, D.; Vukša, V. The effect of temperature and wax content on the appearance of turbidity in sunflowerseed oil. J. Am. Oil Chem. Soc. 1986, 63, 1360–1363. [Google Scholar] [CrossRef]
- Carelli, A.A.; Frizzera, L.M.; Forbito, P.R.; Crapiste, G.H. Wax composition of sunflower seed oils. J. Am. Oil Chem. Soc. 2002, 79, 763–768. [Google Scholar] [CrossRef]
- Martini, S.; Añón, M.C. Crystallization of sunflower oil waxes. J. Am. Oil Chem. Soc. 2003, 80, 525–532. [Google Scholar] [CrossRef]
- Kanya, T.C.S.; Rao, L.J.; Sastry, M.C.S. Characterization of wax esters, free fatty alcohols and free fatty acids of crude wax from sunflower seed oil refineries. Food Chem. 2007, 101, 1552–1557. [Google Scholar] [CrossRef]
- Dijkstra, A.J.; Van Opstal, M. The total degumming processes. J. Am. Oil Chem. Soc. 1989, 66, 1002–1009. [Google Scholar] [CrossRef]
- Kupiec, M.; Zbikowska, A.; Marciniak-Lukasiak, K.; Kowalska, M. Rapeseed oil in new application: Assessment of structure of oleogels based on their physicochemical properties and microscopic observations. Agriculture 2020, 10, 211. [Google Scholar] [CrossRef]
- Morrison, W.H.; Robertson, J.A. Solvent winterization of sunflower seed oil. J. Am. Oil Chem. Soc. 1975, 52, 148–150. [Google Scholar] [CrossRef]
- Rivarola, G.; Añón, M.C.; Calvelo, A. Influence of phospholipids on the crystallization of waxes in sunflower seed oil. J. Am. Oil Chem. Soc. 1988, 65, 1771–1773. [Google Scholar] [CrossRef]
- Orhevba, B.A.; Chukwu, O.; Oguagwu, V.; Osunde, Z.D. Effect of Moisture Content on some Quality Parameters of Mechanically Expressed Neem Seed Kernel Oil. Int. J. Eng. Sci. 2013, 2, 1–7. [Google Scholar]
- Grompone, M.A. Sunflower Oil. In Bailey’s Industrial Oil and Fat Products, 6th ed.; Shahidi, F., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 2, pp. 154–196. [Google Scholar]
- Grompone, M.A. Sunflower and High–Oleic Sunflower Oils. In Bailey’s Industrial Oil and Fat Products, 7th ed.; Shahidi, F., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 1, pp. 1–54. [Google Scholar]
- Kondal Reddy, K.; Subramanian, R.; Kawakatsu, T.; Nakajima, M. Decolorization of vegetable oils by membrane processing. Eur. Food Res. Technol. 2001, 213, 212–218. [Google Scholar] [CrossRef]
- Erten, Y. Use of Domestic Minerals for Vegetable Oil Bleaching. Master’s Thesis, İzmir Institute of Technology, İzmir, Turkey, 2004. [Google Scholar]
- Topkafa, M.; Ayyildiz, H.; Arslana, F.N.; Kucukkolbasi, S.; Durmaz, F.; Sen, S.; Kara, H. Role of Different Bleaching Earths for Sunflower Oil in a Pilot Plant Bleaching System. Polish J. Food Nutr. Sci. 2013, 63, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Wang, Y.; Li, Y.; Zhang, L.; Gao, Y.; Li, Q.; Yu, X. Antioxidant properties of lipid concomitants in edible oils: A review. Food Chem. 2023, 422, 136219. [Google Scholar] [CrossRef]
- Premović, T.D.; Dimić, E.B.; Takači, A.A.; Romanić, R.S. Influence of impurities and hull content in material for pressing on sensory quality cold-pressed sunflower oil. Acta Period. Technol. 2010, 41, 69–76. [Google Scholar] [CrossRef]
- Ripperger, S.; Gösele, W.; Alt, C.; Loewe, T.; Filtration, I. Fundamentals. In Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; Wiley-VCH, Ed.; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2011; Volume 1, pp. 1–38. [Google Scholar]
- Smith, G. Filter aid filtration. In Filtration in the Biopharmaceutical Industry, 1st ed.; Meltzer, T.H., Jornitz, M.W., Eds.; Marcel Dekker Inc: New York, NY, USA, 1998; Volume 1, pp. 1–69. [Google Scholar]
- Buyel, J.F.; Opdensteinen, P.; Fischer, R. Cellulose-based filter aids increase the capacity of depth filters during the downstream processing of plant-derived biopharmaceutical proteins. Biotechnol. J. 2015, 10, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Anlauf, H. Wet Cake Filtration: Fundamentals, Equipment, and Strategies, 1st ed.; Wiley-VCH: Weinheim, Germany, 2019; pp. 1–349. [Google Scholar]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crop. Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Dahlstrom, D.A.; Bennett, R.C.; Emmett, R.C.; Harriott, P., Jr.; Laros, T.; Leung, W.; McCleary, C.S.; Morey, A.M.B.; Oldshue, J.Y.; Priday, G. Liquid-Solid Operations and Equipment. In Perry’s Chemical Engineers’ Handbook, 7th ed.; Perry, R.H., Green, D.W., Eds.; McGraw-Hill Companies Inc.: New York, NY, USA, 1997; pp. 1–133. [Google Scholar]
- Du, L.; Chen, X.; Li, W.; Zhu, Q. A study on enhancement of filtration process with filter aids diatomaceous earth and wood pulp cellulose. Chin. J. Chem. Eng. 2011, 19, 792–798. [Google Scholar] [CrossRef]
- Nedić Grujin, K.; Romanić, R.; Nikolovski, B. Wax and oil content of filter cake after sunflower oil filtration used cellulose filter aid. Uljarstvo/J. Edible Oil Ind. 2019, 1, 161–167. (In Serbian) [Google Scholar]
- Di Pretoro, A.; Manenti, F. Filtration. In Non-Conventional Unit Operations: Solving Practical Issues, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 47–57. [Google Scholar]
- Oštrić-Matijašević, B.; Turkulov, J. Determination of Waxes in Sunflower Grains, Shells and Oil. Modified Method of Extraction. Rev. Fr. Corps Gras 1973, 20, 5–10. [Google Scholar]
- Chulu, C.L.; Barlow, P.; Hole, M. Cloudiness in Zambian Sunflower Oil: Effect of Storage Temperature and Wax Levels. Trop. Sci. 1989, 29, 33–38. [Google Scholar]
- Botha, I.; Mailer, R.J. Evaluation of cold-test methods for screening cloudy canola oils. J. Am. Oil Chem. Soc. 2001, 78, 395–399. [Google Scholar] [CrossRef]
- AOCS Official Method Cc 11-53; Cold Test. Official Methods and Recommended Practice of the American Oil Chemists’ Society. American Oil Chemists’ Society: Champaign, IL, USA, 1986; p. 1.
- ISO 662; Animal and Vegetable Fats and Oils—Determination of Moisture and Volatile Matter Content. International Organization for Standardization: Geneva, Switzerland, 2016.
- AOCS Official Method Ca 12-55; Phosphorus in Oil. Official Methods and Recommended Practices of the American Oil Chemists’ Society. American Oil Chemists’ Society: Champaign, IL, USA, 1989; pp. 1–2.
- AOCS Official Method Cc 17-79; Soap in Oil-Titrimetric Method, Official Methods and Recommended Practice of the American Oil Chemists’ Society. American Oil Chemists’ Society: Champaign, IL, USA, 1985; p. 1.
- ISO 660; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. International Organization for Standardization: Geneva, Switzerland, 2020.
- British Standard BS 684-2.20:1977; British Standard Method of Analysis of Fats and Fatty Oils—Determination of Carotene in Vegetable Oils. British Standard Illustration: London, UK, 1977.
- Dimić, E.; Turkulov, J. Quality Control in Edible Oil Technology; University of Novi Sad, Faculty of Technology: Novi Sad, Serbia, 2000; pp. 17–26, 135–137. (In Serbian) [Google Scholar]
- Ma, H.; Ding, F.; Wang, Y. A novel multi-innovation gradient support vector machine regression method. ISA Trans. Press 2022, 130, 343–359. [Google Scholar] [CrossRef]
- Wang, C.; Peng, G.; De Baets, B. Embedding metric learning into an extreme learning machine for scene recognition. Expert Syst. Appl. 2022, 203, 117505. [Google Scholar] [CrossRef]
- Su, J.; Wang, Y.; Niu, X.; Shaa, S.; Yu, J. Prediction of ground surface settlement by shield tunneling using XGBoost and Bayesian optimization. Eng. Appl. Artif. Intel. 2022, 114, 105020. [Google Scholar] [CrossRef]
- Mahmood, J.; Mustafa, G.E.; Ali, M. Accurate estimation of tool wear levels during milling, drilling and turning operations by designing novel hyperparameter tuned models based on LightGBM and stacking. Measurement 2022, 190, 110722. [Google Scholar] [CrossRef]
- Dutta, J.; Roy, S. Occupancy Sense: Context-based indoor occupancy detection & prediction using CatBoost model. Appl. Soft Comput. 2022, 119, 108536. [Google Scholar]
- Wang, X.; Zhang, R.; Wang, Z.; Li, T. How Does Digital Competence Preserve University Students’ Psychological Well-Being During the Pandemic? An Investigation from Self-Determined Theory. Front. Psychol. 2021, 12, 652594. [Google Scholar] [CrossRef]
- Yang, C.; Chen, A.; Chen, Y. College students’ stress and health in the COVID-19 pandemic: The role of academic workload, separation from school, and fears of contagion. PLoS ONE 2021, 16, e0246676. [Google Scholar] [CrossRef]
- Kleijnen, J.P.C. Design and analysis of simulation experiments. In Springer Proceedings in Mathematics and Statistics; Springer: New York, NY, USA, 2018; Volume 231, pp. 3–22. [Google Scholar]
- Kujawa, S.; Niedbała, G. Artificial neural networks in agriculture. Agriculture 2021, 11, 497. [Google Scholar] [CrossRef]
- Kollo, T.; von Rosen, D. Advanced Multivariate Statistics with Matrices; Springer: Amsterdam, The Netherlands, 2005; pp. 355–472. [Google Scholar]
- Doumpos, M.; Zopounidis, C. Preference disaggregation and statistical learning for multicriteria decision support: A review. Eur. J. Oper. Res. 2011, 209, 203–214. [Google Scholar] [CrossRef]
- Pezo, L.; Curcic, B.; Filipovic, V.; Nicetin, M.; Koprivica, G.; Misljenovic, N.; Levic, L. Artificial neural network model of pork meat cubes osmotic dehydration. Hem. Ind. 2013, 67, 465–475. [Google Scholar] [CrossRef]
- Yoon, Y.; Swale, G.; Margavio, T.M. A Comparison of Discriminant Analysis versus Artificial Neural Networks. J. Oper. Res. Soc. 2017, 44, 51–60. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1984; p. 978. [Google Scholar]
- Chattopadhyay, P.B.; Rangarajan, R. Application of ANN in sketching spatial nonlinearity of unconfined aquifer in agricultural basin. Agric. Water Manag. 2014, 133, 81–91. [Google Scholar] [CrossRef]
- Broughton, R.; Ruíz-Lopez, N.; Hassall, K.L.; Martínez-Force, E.; Garcés, R.; Salas, J.J.; Beaudoin, F. New insights in the composition of wax and sterol esters in common and mutant sunflower oils revealed by ESI-MS/MS. Food Chem. 2018, 269, 70–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitrović, S.; Dimić, E.; Grujin, K.; Muc, S. New technological process of removing of waxes from the oil in Dijamant, A.D. Uljarstvo/J. Edible Oil Ind. 2009, 1, 67–72. (In Serbian) [Google Scholar]
- Stojanović, Z.; Šarac, V.; Kojčin, A. Monitoring of waxes content change in sunflower oil during the refining process by turbidimetric method. Uljarstvo/J. Edible Oil Ind. 2018, 49, 55–60. (In Serbian) [Google Scholar]
- Lamas, D.L.; Constenla, D.T.; Raab, D. Effect of degumming process on physicochemical properties of sunflower oil. Biocatal. Agric. Biotechnol. 2016, 6, 138–143. [Google Scholar] [CrossRef]
- Smith, K.W.; Bhaggan, K.; Talbot, G.; Van Malssen, K.F. Crystallization of fats: Influence of minor components and additives. J. Am. Oil Chem. Soc. 2011, 88, 1085–1101. [Google Scholar] [CrossRef]
- Lamas, D.L.; Crapiste, G.H.; Constenla, D.T. Changes in quality and composition of sunflower oil during enzymatic degumming process. LWT 2014, 58, 71–76. [Google Scholar] [CrossRef]
- Codex Alimentarius. Standard for Named Vegetable Oils. Codex Stan 1999, 210, 1–13. [Google Scholar]
- Bockisch, M. Fats and Oils Handbook, 1st ed.; Elsevier: Urbana, IL, USA, 1998; pp. 345–445. [Google Scholar]
- Tynek, M.; Hazuka, Z.; PawŁowicz, R.; Dudek, M. Changes in the frying medium during deep-frying of food rich in proteins and carbohydrates. J. Food Lipids 2001, 8, 251–261. [Google Scholar] [CrossRef]
- Mistry, B.S.; Min, D.B. Effects of Fatty Acids on the Oxidative Stability of Soybean Oil. J. Food Sci. 1987, 52, 831–832. [Google Scholar] [CrossRef]
- Merhan, O. The Biochemistry and Antioxidant Properties of Carotenoids. In Carotenoids; Cvetkovic, D., Nikolic, G., Eds.; InTech: London, UK, 2017; pp. 51–66. [Google Scholar]
- Romanić, R. Chemometric Approach to the Optimization of the Production of Cold Pressed Oil from High-Oleic Sunflower Seeds. Ph.D. Thesis, University of Novi Sad, Faculty of Technology Novi Sad, Novi Sad, Serbia, 2015. [Google Scholar]
- Matos Reyes, M.N.; Campos, R.C. Determination of copper and nickel in vegetable oils by direct sampling graphite furnace atomic absorption spectrometry. Talanta 2006, 70, 929–932. [Google Scholar] [CrossRef]
- Tokay, F.; Baʇdat, S. Determination of iron and copper in edible oils by flame atomic absorption spectrometry after liquid-liquid extraction. J. Am. Oil Chem. Soc. 2015, 92, 317–322. [Google Scholar] [CrossRef]

| Filtration Cycle | Parameter | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| W_in (mg kg−1) | M_in (%) | FFA_in (%) | P_in (mg kg−1) | S_in (mg kg−1) | C_in (mg kg−1) | Fe_in (mg kg−1) | Cu_in (mg kg−1) | T_in (%) | FT (h) | Q (kg) | |
| F_01 | 382 ± 8 e,f | 0.20 ± 0.01 c,d,e,f,g | 0.10 ± 0.02 b,c,d,e | 45 ± 3 e,f | 105 ± 5 j,k | 5.38 ± 0.01 d,e | 2.67 ± 0.22 j | 0.03 ± 0.00 a,b,c | 54.8 ± 0.1 i | 17 | 455 |
| F_02 | 366 ± 7 d,e | 0.18 ± 0.01 b,c,d,e,f | 0.11 ± 0.01 d,e | 12 ± 2 a,b | 119 ± 3 l | 4.83 ± 0.03 a,b | 0.79 ± 0.08 b,c,d,e,f | 0.05 ± 0.01 e | 58.6 ± 0.1 l | 17 | 430 |
| F_03 | 509 ± 9 j,k | 0.19 ± 0.02 b,c,d,e,f,g | 0.08 ± 0.02 a,b | 72 ± 3 h,i,j | 72 ± 3 f,g | 5.13 ± 0.02 c | 0.88 ± 0.02 c,d,e,f,g | 0.03 ± 0.00 a,b,c | 56.4 ± 0.1 j,k | 13 | 405 |
| F_04 | 547 ± 9 l | 0.12 ± 0.01 a,b,c,d | 0.08 ± 0.00 a,b,c | 79 ± 1 j | 109 ± 3 k | 4.82 ± 0.01 a,b | 0.99 ± 0.09 g,h | 0.03 ± 0.00 a,b | 58.2 ± 0.1 l | 11 | 330 |
| F_05 | 403 ± 8 f | 0.21 ± 0.01 d,e,f,g | 0.10 ± 0.01 c,d,e | nd | 56 ± 4 e | 4.75 ± 0.01 a | 0.58 ± 0.01 a | 0.03 ± 0.00 a,b,c,d | 56.2 ± 0.1 j | 16 | 455 |
| F_06 | 326 ± 7 b | 0.23 ± 0.01 e,f,g | 0.09 ± 0.01 b,c,d,e | 17 ± 2 b | 54 ± 3 d,e | 6.53 ± 0.04 k | 0.76 ± 0.03 b,c,d,e,f | 0.05 ± 0.01 c,d,e | 47.4 ± 0.1 a,b | 29 | 455 |
| F_07 | 295 ± 7 a | 0.15 ± 0.01 a,b,c,d,e | 0.11 ± 0.01 d,e | 31 ± 2 c | 49 ± 2 c,d,e | 6.43 ± 0.02 j | 0.72 ± 0.03 a,b,c,d,e | 0.05 ± 0.00 b,c,d,e | 48.1 ± 0.1 c | 31 | 450 |
| F_08 | 549 ± 11 l | 0.11 ± 0.01 a,b,c | 0.10 ± 0.01 c,d,e | 80 ± 5 j | 87 ± 4 h,i | 6.55 ± 0.04 k | 1.13 ± 0.11 i | 0.02 ± 0.00 a | 47.7 ± 0.1 b,c | 16 | 455 |
| F_09 | 288 ± 7 a | 0.19 ± 0.07 e,f,g | 0.10 ± 0.01 d,e | 41 ± 6 d,e | 40 ± 3 b,c | 5.43 ± 0.01 e,f | 0.65 ± 0.02 a,b | 0.44 ± 0.02 f,g | 54.0 ± 0.1 h | 28 | 455 |
| F_10 | 526 ± 6 k,l | 0.20 ± 0.01 f,g | 0.10 ± 0.01 d,e | 76 ± 8 i,j | 100 ± 3 j | 5.30 ± 0.01 d | 0.73 ± 0.03 b,c,d,e,f | 0.42 ± 0.01 f | 56.0 ± 0.1 k | 11 | 355 |
| F_11 | 355 ± 8 c,d | 0.23 ± 0.08 f,g | 0.11 ± 0.00 b,c,d,e | 40 ± 9 d,e | 50 ± 6 d,e | 5.88 ± 0.01 h | 0.85 ± 0.05 c,d,e,f,g | 0.44 ± 0.01 g | 52.0 ± 0.1 f | 20 | 455 |
| F_12 | 490 ± 12 i,j | 0.21 ± 0.02 g | 0.10 ± 0.01 d,e | 60 ± 1 g | 75 ± 11 f,g | 5.80 ± 0.01 g | 0.73 ± 0.03 b,c,d,e,f | 0.04 ± 0.00 b,c,d,e | 53.0 ± 0.1 g | 10 | 285 |
| F_13 | 520 ± 10 k | 0.19 ± 0.03 e,f,g | 0.10 ± 0.01 d,e | 80 ± 6 j | 90 ± 12 i | 5.47 ± 0.01 f | 0.64 ± 0.02 a,b | 0.04 ± 0.00 b,c,d,e | 55.0 ± 0.1 i | 7 | 250 |
| F_14 | 494 ± 8 j | 0.19 ± 0.05 f,g | 0.09 ± 0.01 e | 79 ± 4 i,j | 75 ± 6 f,g | 5.54 ± 0.05 g | 2.67 ± 0.22 a,b,c,d,e | 0.04 ± 0.00 b,c,d,e | 51.0 ± 0.1 e | 9 | 310 |
| F_15 | 287 ± 4 a | 0.15 ± 0.02 a,b,c,d,e | 0.12 ± 0.01 e | 5 ± 1 a | 30 ± 4 a | 5.70 ± 0.01 g | 0.79 ± 0.08 e,f,g | 0.05 ± 0.00 c,d,e | 53.0 ± 0.1 g | 37 | 355 |
| F_16 | 281 ± 8 a | 0.12 ± 0.04 a,b,c,d | 0.09 ± 0.01 a,b,c,d | 50 ± 12 f | 33 ± 6 a,b | 5.34 ± 0.01 d | 0.71 ± 0.01 f,g | 0.05 ± 0.00 d,e | 51.2 ± 0.1 e | 39 | 455 |
| F_17 | 340 ± 6 b,c | 0.20 ± 0.05 c,d,e,f,g | 0.09 ± 0.01 a,b,c,d | 60 ± 7 g | 40 ± 5 b,c | 5.87 ± 0.01 h | 0.91 ± 0.03 c,d,e,f,g | 0.04 ± 0.00 b,c,d,e | 49.8 ± 0.1 d | 27 | 455 |
| F_18 | 351 ± 9 b,c,d | 0.08 ± 0.03 a | 0.11 ± 0.02 d,e | 36 ± 4 c,d | 45 ± 4 c,d | 6.17 ± 0.01 i | 0.92 ± 0.03 d,e,f,g | 0.04 ± 0.00 b,c,d,e | 47.0 ± 0.1 a | 30 | 450 |
| F_19 | 443 ± 5 g,h | 0.09 ± 0.02 a,b | 0.07 ± 0.01 a | 65 ± 2 g,h | 70 ± 3 f | 5.40 ± 0.01 e | 0.88 ± 0.02 c,d,e,f,g | 0.04 ± 0.00 b,c,d,e | 53.0 ± 0.1 g | 13 | 375 |
| F_20 | 466 ± 10 h,i | 0.18 ± 0.05 b,c,d,e,f,g | 0.08 ± 0.01 a,b,c | 75 ± 8 i,j | 100 ± 6 j | 5.30 ± 0.01 d | 0.89 ± 0.03 a,b,c | 0.04 ± 0.01 b,c,d,e | 53.0 ± 0.1 g | 12 | 375 |
| F_21 | 441 ± 9 g | 0.08 ± 0.01 a | 0.08 ± 0.01 a,b | 70 ± 1 h,i | 80 ± 2 g,h | 5.20 ± 0.01 cd | 0.87 ± 0.02 a,b,c,d | 0.05 ± 0.00 c,d,e | 54.0 ± 0.1 h | 14 | 405 |
| F_22 | 328 ± 7 b | 0.06 ± 0.01 a | 0.09 ± 0.01 a,b,c,d | 10 ± 3 a,b | 45 ± 4 c,d | 4.90 ± 0.01 b | 0.68 ± 0.02 c,d,e,f,g | 0.04 ± 0.00 b,c,d,e | 55.0 ± 0.1 i | 21 | 435 |
| Filtration Cycle | Parameter | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| W_out (mg kg−1) | M_out (%) | FFA_out (%) | P_out (mg kg−1) | S_out (mg kg−1) | C_out (mg kg−1) | Fe_out (mg kg−1) | Cu_out (mg kg−1) | T_out (%) | |
| F_01 | 2.94 ± 0.05 a,b,c | 0.18 ± 0.01 d,e,f,g,h | 0.10 ± 0.01 a,b,c,d | 21 ± 1 c,d | nd | 5.02 ± 0.04 d,e | 0.93 ± 0.02 a | 0.02 ± 0.00 a,b | 58.6 ± 0.0 h,i |
| F_02 | 3.01 ± 0.08 a,b,c,d,e,f | 0.21 ± 0.01 g,h | 0.10 ± 0.01 a,b,c,d | 4 ± 2 a | nd | 4.60 ± 0.03 a | 0.56 ± 0.02 f,g | 0.04 ± 0.00 f,g,h,i | 61.7 ± 0.0 l |
| F_03 | 3.19 ± 0.08 e,f | 0.19 ± 0.01 e,f,g,h | 0.08 ± 0.01 a,b | 12 ± 3 b | nd | 4.83 ± 0.06 c | 0.75 ± 0.03 d,e,f | 0.03 ± 0.00 c,d,e | 58.6 ± 0.5 h,i |
| F_04 | 3.16 ± 0.07 d,e,f | 0.28 ± 0.01 i | 0.08 ± 0.01 a | 38 ± 4 f | nd | 4.70 ± 0.01 b | 0.64 ± 0.03 h | 0.03 ± 0.00 b,c,d | 59.6 ± 0.1 j,k |
| F_05 | 3.00 ± 0.13 a,b,c,d,e,f | 0.27 ± 0.01 i | 0.08 ± 0.01 a,b | nd | nd | 4.57 ± 0.05 a | 1.01 ± 0.01 b | 0.02 ± 0.00 a,b,c | 59.0 ± 0.1 i,j |
| F_06 | 2.95 ± 0.07 a,b,c,d | 0.11 ± 0.01 a,b,c | 0.08 ± 0.01 a,b | nd | nd | 5.51 ± 0.04 h | 0.52 ± 0.01 f,g | 0.04 ± 0.01 g,h,i | 53.7 ± 0.1 c,d |
| F_07 | 2.95 ± 0.07 a,b,c,d | 0.12 ± 0.01 a,b,c,d | 0.09 ± 0.01 a,b,c | nd | nd | 5.72 ± 0.01 j | 0.71 ± 0.01 c | 0.04 ± 0.01 e,f,g,h,i | 52.4 ± 0.1 b |
| F_08 | 3.21 ± 0.06 f | 0.10 ± 0.01 a,b | 0.09 ± 0.01 a,b,c,d | 20 ± 4 c,d | nd | 5.98 ± 0.02 k | 0.79 ± 0.01 i | 0.02 ± 0.00 a | 50.4 ± 0.1 a |
| F_09 | 2.89 ± 0.04 a | 0.18 ± 0.06 c,d,e,f,g,h | 0.10 ± 0.01 d,e,f | 10 ± 1 b | trace | 5.00 ± 0.01 d,e | 0.65 ± 0.02 a,b | 0.03 ± 0.00 b,c,d | 58.0 ± 0.1 h |
| F_10 | 3.12 ± 0.07 b,c,d,e,f | 0.23 ± 0.01 h,i | 0.10 ± 0.02 f | 30 ± 3 e | nd | 4.80 ± 0.01 c | 0.57 ± 0.03 d,e,f | 0.04 ± 0.00 g,h,i | 59.0 ± 0.1 i,j |
| F_11 | 2.98 ± 0.03 a,b,c,d | 0.20 ± 0.09 f,g,h | 0.09 ± 0.01 a,b,c,d,e | 5 ± 2 a | nd | 5.30 ± 0.01 g | 0.64 ± 0.02 g | 0.04 ± 0.00 e,f,g,h,i | 54.1 ± 0.1 d |
| F_12 | 3.12 ± 0.05 b,c,d,e,f | 0.23 ± 0.01 h,i | 0.10 ± 0.01 d,e,f | nd | nd | 5.20 ± 0.01 f | 0.72 ± 0.02 c,d,e | 0.03 ± 0.00 b,c,d | 55.4 ± 0.1 e,f |
| F_13 | 3.21 ± 0.05 f | 0.21 ± 0.02 g,h | 0.11 ± 0.01 f | 5 ± 1 a | nd | 4.80 ± 0.01 c | 0.70 ± 0.02 b | 0.03 ± 0.00 c,d,e,f,g,h | 56.0 ± 0.1 f |
| F_14 | 3.13 ± 0.07 c,d,e,f | 0.19 ± 0.01 e,f,g,h | 0.10 ± 0.01 d,e,f | nd | nd | 5.00 ± 0.01 d,e | 0.73 ± 0.03 c,d | 0.03 ± 0.01 c,d,e,f,g | 53.0 ± 0.1 b,c |
| F_15 | 2.91 ± 0.02 a,b | 0.14 ± 0.03 a,b,c,d,e | 0.11 ± 0.01 e,f | nd | nd | 5.00 ± 0.01 d,e | 0.93 ± 0.02 f,g | 0.05 ± 0.00 i | 56.0 ± 0.1 f |
| F_16 | 2.89 ± 0.05 a | 0.14 ± 0.01 b,c,d,e,f,g | 0.11 ± 0.01 b,c,d,e | 20 ± 3 c | trace | 5.05 ± 0.01 e | 0.56 ± 0.02 d,e,f | 0.04 ± 0.00 e,f,g,h,i | 55.0 ± 0.1 e |
| F_17 | 2.97 ± 0.03 a,b,c,d | 0.14 ± 0.00 a,b,c,d,e,f | 0.10 ± 0.01 a,b,c,d,e | 32 ± 1 e | nd | 5.60 ± 0.01 i | 0.75 ± 0.03 f,g | 0.04 ± 0.00 d,e,f,g,h,i | 53.0 ± 0.1 b,c |
| F_18 | 2.98 ± 0.04 a,b,c,d,e | 0.08 ± 0.01 a | 0.10 ± 0.00 a,b,c,d,e | nd | nd | 5.78 ± 0.01 j | 0.71 ± 0.02 e,f | 0.04 ± 0.01 e,f,g,h,i | 50.0 ± 0.1 a |
| F_19 | 3.08 ± 0.08 a,b,c,d,e,f | 0.23 ± 0.05 h,i | 0.10 ± 0.01 a,b,c,d,e | 25 ± 2 d | nd | 4.90 ± 0.01 d | 0.79 ± 0.01 g | 0.04 ± 0.00 h,i | 57.0 ± 0.1 g |
| F_20 | 3.11 ± 0.10 b,c,d,e,f | 0.10 ± 0.02 a,b | 0.11 ± 0.01 b,c,d,e | 30 ± 3 e | trace | 5.00 ± 0.01 d,e | 0.51 ± 0.02 a,b | 0.03 ± 0.00 c,d,e,f | 56.0 ± 0.1 f |
| F_21 | 3.09 ± 0.10 a,b,c,d,e,f | 0.14 ± 0.02 a,b,c,d,e,f | 0.11 ± 0.01 b,c,d,e | 20 ± 1 c | nd | 4.80 ± 0.01 c | 0.54 ± 0.01 a,b | 0.03 ± 0.00 c,d,e,f,g,h | 57.0 ± 0.1 g |
| F_22 | 2.96 ± 0.09 a,b,c,d | 0.14 ± 0.01 a,b,c,d,e | 0.11 ± 0.01 c,d,e,f | nd | nd | 4.60 ± 0.01 a | 0.68 ± 0.01 c,d,e,f | 0.03 ± 0.00 b,c | 60.0 ± 0.1 k |
| Filtration Cycle | Parameter | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| W (mg kg−1) | M (%) | FFA (%) | P (mg kg−1) | S (mg kg−1) | C (mg kg−1) | Fe (mg kg−1) | Cu [mg kg−1) | T [%) | |
| F_01 | −99.23 ± 0.02 f,g | −10.09 ± 4.87 a,b | 2.27 ± 21.68 a,b,c,d,e | −54.28 ± 3.68 g,h | −100.00 ± 0.00 a | −6.70 ± 0.56 g,h | −82.01 ± 2.92 a | −41.08 ± 2.32 b | 7.00 ± 0.11 g,h,i |
| F_02 | −99.18 ± 0.03 g,h | 15.26 ± 12.60 b | −9.09 ± 9.09 a,b,c | −69.40 ± 9.53 e,f | −100.00 ± 0.00 a | −4.63 ± 0.12 i,j | −5.52 ± 8.74 d,e,f | −20.33 ± 10.88 b,c,d | 5.35 ± 0.10 c,d,e,f |
| F_03 | −99.37 ± 0.01 a,b,c | 2.46 ± 13.15 a,b | 11.57 ± 22.24 a,b,c,d,e,f | −82.95 ± 2.77 c,d | −100.00 ± 0.00 a | −5.85 ± 1.35 h,i | −19.50 ± 3.36 b,c,d | −4.20 ± 19.29 c,d | 3.90 ± 0.92 b,c |
| F_04 | −99.42 ± 0.02 a | 143.18 ± 10.69 e | 0.00 ± 0.00 a,b,c,d,e | −52.28 ± 5.04 g,h | −100.00 ± 0.00 a | −2.49 ± 0.20 k | −6.41 ± 8.23 d,e,f | −0.75 ± 7.73 d | 2.41 ± 0.17 a,b |
| F_05 | −99.26 ± 0.05 e,f | 30.28 ± 4.14 b,c | −19.09 ± 8.67 a | 0.00 ± 0.00 i | −100.00 ± 0.00 a | −3.86 ± 0.75 j,k | −3.42 ± 5.28 e,f | −29.77 ± 13.93 b,c,d | 4.99 ± 0.18 c,d,e,f |
| F_06 | −99.09 ± 0.02 i | −53.56 ± 3.30 a | −10.74 ± 0.64 a,b,c | −100.00 ± 0.00 a | −100.00 ± 0.00 a | −15.52 ± 0.99 a | −1.64 ± 0.40 f | −14.57 ± 2.93 b,c,d | 13.44 ± 0.26 l |
| F_07 | −99.00 ± 0.02 j | −20.32 ± 6.20 a,b | −15.45 ± 10.24 a,b | −100.00 ± 0.00 a | −100.00 ± 0.00 a | −10.99 ± 0.20 c,d | −11.84 ± 7.52 b,c,d,e,f | −12.62 ± 14.10 b,c,d | 9.08 ± 0.13 j,k |
| F_08 | −99.42 ± 0.01 a | −6.36 ± 5.53 a,b | −9.39 ± 9.11 a,b,c | −74.50 ± 5.04 d,e | −100.00 ± 0.00 a | −8.60 ± 0.72 e,f | −10.13 ± 8.78 c,d,e,f | −2.03 ± 21.84 d | 5.61 ± 0.24 d,e,f,g |
| F_09 | −99.00 ± 0.01 j | −16.52 ± 50.32 a,b | 9.39 ± 0.52 a,b,c,d,e,f | −74.93 ± 2.59 d,e | −100.00 ± 0.00 a | −7.86 ± 1.11 f,g | −19.30 ± 1.38 b,c,d | −93.95 ± 0.80 a | 7.61 ± 0.17 i,j |
| F_10 | −99.41 ± 0.02 a | −13.72 ± 7.65 a,b | 17.73 ± 7.51 b,c,d,e,f | −60.53 ± 1.15 f,g | −100.00 ± 0.00 a | −13.46 ± 0.47 b | −2.40 ± 4.52 e,f | −90.48 ± 0.41 a | 4.11 ± 0.57 c,d |
| F_11 | −99.16 ± 0.02 g,h,i | −23.57 ± 20.79 a,b | 0.00 ± 10.00 a,b,c,d,e | −87.22 ± 3.13 b,c | −100.00 ± 0.00 a | −9.86 ± 0.30 d,e | −6.96 ± 6.18 d,e,f | −91.06 ± 0.24 a | 3.91 ± 0.42 b,c |
| F_12 | −99.36 ± 0.02 a,b,c,d | −16.04 ± 13.17 a,b | 6.36 ± 5.53 a,b,c,d,e,f | −100.00 ± 0.00 a | −100.00 ± 0.00 a | −10.29 ± 0.50 d,e | −11.07 ± 0.20 c,d,e,f | −25.19 ± 1.05 b,c,d | 4.59 ± 0.30 c,d,e |
| F_13 | −99.38 ± 0.01 a,b | −12.47 ± 2.93 a,b | 18.28 ± 1.67 b,c,d,e,f | −93.73 ± 1.41 a,b | −100.00 ± 0.00 a | −12.30 ± 0.45 b,c | −11.47 ± 7.51 b,c,d,e,f | −28.98 ± 4.62 b,c,d | 1.76 ± 0.38 a |
| F_14 | −99.37 ± 0.01 a,b,c,d | −28.84 ± 10.29 a,b | 0.25 ± 8.71 a,b,c,d,e | −100.00 ± 0.00 a | −100.00 ± 0.00 a | −9.74 ± 0.77 d,e | −10.06 ± 2.29 c,d,e,f | −22.50 ± 16.82 b,c,d | 3.99 ± 0.82 c |
| F_15 | −98.98 ± 0.01 j | −6.29 ± 29.19 a,b | 5.81 ± 5.04 a,b,c,d,e,f | −100.00 ± 0.00 a | −100.00 ± 0.00 a | −13.74 ± 0.20 a,b | −20.00 ± 0.22 b,c,d | −6.49 ± 6.61 c,d | 5.66 ± 0.50 d,e,f,g |
| F_16 | −98.97 ± 0.03 j | 30.56 ± 39.38 b,c | 23.61 ± 13.25 c,d,e,f,g | −60.04 ± 3.20 f,g | −100.00 ± 0.00 a | −5.49 ± 0.21 h,i,j | −23.61 ± 0.77 b,c | −22.94 ± 11.58 b,c,d | 7.49 ± 0.49 h,i |
| F_17 | −99.13 ± 0.01 h,i | −28.23 ± 18.86 a,b | 19.44 ± 7.35 b,c,d,e,f,g | −46.69 ± 2.45 h | −100.00 ± 0.00 a | −4.60 ± 0.17 i,j | −16.47 ± 1.68 b,c,d,e | −7.54 ± 2.44 c,d | 6.35 ± 1.11 f,g,h,i |
| F_18 | −99.15 ± 0.02 h,i | 6.67 ± 30.55 a,b | −4.88 ± 14.36 a,b,c,d | −100.00 ± 0.00 a | −100.00 ± 0.00 a | −6.27 ± 0.18 g,h,i | −19.65 ± 4.55 b,c,d | −7.51 ± 5.48 c,d | 6.38 ± 0.43 f,g,h,i |
| F_19 | −99.31 ± 0.01 c,d,e | 160.13 ± 16.37 e | 55.56 ± 11.98 g | −61.55 ± 0.95 f,g | −100.00 ± 0.00 a | −4.56 ± 0.22 i,j | −9.31 ± 1.02 c,d,e,f | −7.49 ± 3.33 c,d | 7.55 ± 0.68 h,i,j |
| F_20 | −99.33 ± 0.03 b,c,d | −46.67 ± 5.77 a | 34.79 ± 19.41 e,f,g | −59.54 ± 1.27 g | −100.00 ± 0.00 a | −2.35 ± 0.20 k | −25.85 ± 3.93 b | −32.97 ± 2.04 b,c | 5.98 ± 0.77 e,f,g,h |
| F_21 | −99.30 ± 0.04 d,e,f | 82.14 ± 15.57 c,d | 39.29 ± 3.09 f,g | −71.60 ± 2.24 e | −100.00 ± 0.00 a | 6.90 ± 0.01 m | −23.42 ± 0.22 b,c | −23.49 ± 1.40 b,c,d | 5.86 ± 0.25 e,f,g |
| F_22 | −99.10 ± 0.03 i | 142.22 ± 15.40 d,e | 27.78 ± 20.03 d,e,f,g | −100.00 ± 0.00 a | −100.00 ± 0.00 a | 3.29 ± 0.57 l | −16.78 ± 0.28 b,c,d,e | −29.37 ± 4.96 b,c,d | 9.58 ± 0.31 k |
| Average | −99.22 ± 0.15 | 15.01 ± 61.33 | 9.25 ± 18.93 | −74.97 ± 25.08 | −100.00 ± 0.00 | −6.77 ± 5.37 | −16.22 ± 16.37 | −27.97 ± 28.20 | 6.03 ± 2.56 |
| Network Name | Performance Train | Error Train | Training Algorithm | Error Function | Hidden Activation | Output Activation |
|---|---|---|---|---|---|---|
| MLP 2-9-9 | 1.000 | 0.455 | BFGS 153 | SOS | Logistic | Logistic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedić Grujin, K.; Lužaić, T.; Pezo, L.; Nikolovski, B.; Maksimović, Z.; Romanić, R. Sunflower Oil Winterization Using the Cellulose-Based Filtration Aid—Investigation of Oil Quality during Industrial Filtration Probe. Foods 2023, 12, 2291. https://doi.org/10.3390/foods12122291
Nedić Grujin K, Lužaić T, Pezo L, Nikolovski B, Maksimović Z, Romanić R. Sunflower Oil Winterization Using the Cellulose-Based Filtration Aid—Investigation of Oil Quality during Industrial Filtration Probe. Foods. 2023; 12(12):2291. https://doi.org/10.3390/foods12122291
Chicago/Turabian StyleNedić Grujin, Katarina, Tanja Lužaić, Lato Pezo, Branislava Nikolovski, Zoran Maksimović, and Ranko Romanić. 2023. "Sunflower Oil Winterization Using the Cellulose-Based Filtration Aid—Investigation of Oil Quality during Industrial Filtration Probe" Foods 12, no. 12: 2291. https://doi.org/10.3390/foods12122291
APA StyleNedić Grujin, K., Lužaić, T., Pezo, L., Nikolovski, B., Maksimović, Z., & Romanić, R. (2023). Sunflower Oil Winterization Using the Cellulose-Based Filtration Aid—Investigation of Oil Quality during Industrial Filtration Probe. Foods, 12(12), 2291. https://doi.org/10.3390/foods12122291